Short abstract
Chondrosarcoma is characterized by the presence of histologically aggressive behavior, and commonly involves the scapula. Currently, limb salvage surgery is the recommended surgical treatment. Owing to the irregularity of the tumor, the suitability of an implant after tumor resection is a challenge for surgeons. Three-dimensional (3D) printing technology has the potential to make personalized limb salvage surgery a reality. We report the case of a 53-year-old man who was diagnosed with chondrosarcoma of the scapula. Considering the low-grade malignancy and lack of invasion of the glenoid, we agreed upon segmental scapula replacement as the treatment protocol. Nevertheless, reconstruction of the irregular bony defect remaining after tumor resection can be complicated. Therefore, a personalized prosthesis and navigation template corresponding to tumor was designed with 3D printing technique, and tumor resection, prosthesis implantation, and rotator cuff reconstruction were completed. The affected shoulder achieved satisfactory function during a 32-month follow-up with no tumor recurrence. 3D printing technique can help implement the individualized design of the implant and accurate reconstruction after tumor resection, simplify complicated operations, improve operational efficiency, and allow early functional recovery.
Keywords: Three-dimensional printing, scapula, chondrosarcoma, tumor, prosthesis, implant
Background
Chondrosarcoma, which frequently presents with histologically aggressive behavior, accounts for 20% of all malignant bone tumors.1 It accounts for the highest incidence of malignant tumors in the scapula2 and greatly inhibits shoulder function, distresses patients, and shortens their lifespan. Surgical resection is the standard of care for chondrosarcoma, as chemotherapy and radiation therapy have limited efficacy.3 The functional outcome following resection of a scapular neoplasm is an important concern for patients. Before the mid-1970s, the treatment of scapula tumors frequently involved forequarter amputation. In 1977, Marcove et al.4 published the first report of a large series of patients with malignant tumors of the proximal humerus and scapula who received resection in lieu of forequarter amputation. According to his publication, local recurrence and survival rates in limb salvage patients were similar to those achieved with forequarter amputation. Furthermore, cosmesis and function were much better with preservation of elbow motion and hand dexterity. For limb-sparing resections of the tumor, patients’ achieving similar survival and better function than amputations have motivated the investigation of a less aggressive surgical approach. Since then, several limb salvage procedures and different indications for scapula tumors have been published in small series and case reports.5–8
Among the treatment methods, scapular endoprosthesis is considered the most effective method owing to its acceptable cosmesis, good postoperative function, and relatively low incidence of complications.9 Yet the major challenge for clinicians is to reconstruct the bone and soft tissue after resection of the tumor. Three-dimensional (3D) printing is a kind of rapid prototyping technology that can construct objects based on digital model files and reproduce tissues and organs in different materials, and satisfy the individualization and precision of medical treatment. 3D-printed models represent a new concept for preoperative planning with real scale probe testing of a specific implant.10 With the application of 3D printing in clinical settings, surgery has become more precise and individualized. Liu et al.11 described the 3D-printed total scapula prosthesis for the treatment of scapula tumors, but segmental prosthesis replacement for scapula tumors has not been mentioned. We report the case of a 53-year-old man diagnosed with chondrosarcoma in the left scapula. Assisted by 3D planning, we obtained a customized segmental prosthesis belonging to the patient and completed an individualized procedure.
Case presentation
A 53-year-old man presented with a history of a progressive swelling and pain in his left shoulder for 5 months. The protuberance grew rapidly, the pain had increased without any inciting factors over the past 1 month, and it restricted his shoulder joint movement. Prior to the development of his discomfort, he had no history of injury to his shoulder.
First, we completed the patient’s physical examination. He had a marked ridge at his left scapula that was approximately 4 × 6 cm. The surrounding skin had a low temperature and no redness or ulceration. He complained of mild pain in the bump. The mass was hard to the touch and was relatively immobile. The findings did not indicate metastasis to the superficial lymph nodes of the axillary cavity, neck, or left upper limb. Approximately 45° abduction, 5° adduction, 40° flexion, and 25° external and internal rotation of the left shoulder joint were observed. The patient had good sensation, movement, and blood circulation of the left upper extremity.
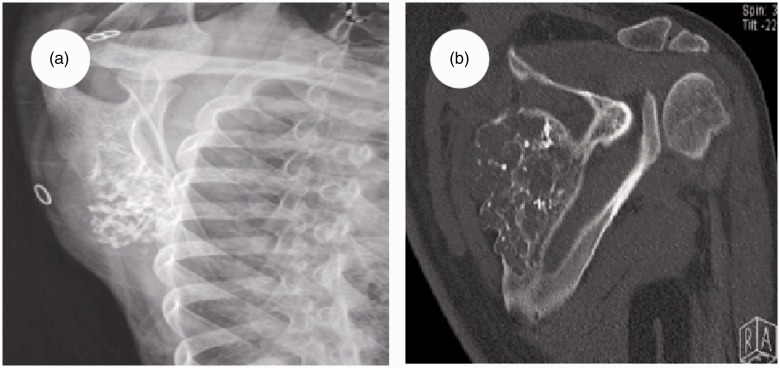
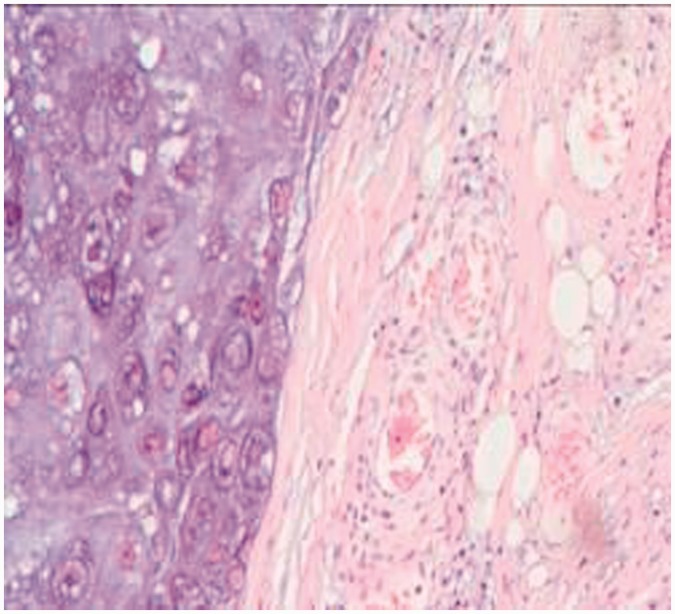
Radiographs of the left shoulder showed an irregular shadow with a bone lesion on the scapula (Figure 1a), located in the S1 region according to Malawer’s classification.12 Neither the acromion nor the glenoid cavity were involved. A computed tomography (CT) scan of his left scapula indicated a moderate low-density bony lesion of approximately 31 × 72 × 78 mm with calcification (Figure 1b). The superior border of the mass extended to 2 cm below the upper margin of the scapula, the left border extended to 4.5 cm from the spinal column, and the inferior border extended to 2 cm above the subscapular angle. No metastases of the lung, mediastinum, upper limb vessels, or abdomen were detected by CT and ultrasonography. A CT scan showed no metastasis to other bone tissues. The needle biopsy suggested grade I chondrosarcoma (Figure 2). In view of the radiologic and histologic findings, the diagnosis was confirmed: left scapular chondrosarcoma, Malawer S1 region, Enneking IA stage,13 grade I. Considering the low-grade malignancy of the tumor, only the S1 region was invaded, and for the greatest likelihood of restoring limb function, the acromion and articular glenoid should be retained. Partial scapula replacement after tumor resection was agreed upon as the treatment protocol.
Figure 1.
Imaging examination findings of the left shoulder. (a) An X-ray showed an irregular shadow with a bone lesion on the scapula, situated in the S1 region. (b) A computed tomography scan revealed a moderate low-density bony lesion approximately 31 × 72 × 78 mm in size with calcification.
Figure 2.
Biopsy suggested a low-grade chondrosarcoma.
Because only part of the scapula would be removed, the prosthesis needed to be customized. The patient agreed to this approach and signed a consent form. The CT scan images of his left shoulder were obtained with 1-mm slices. DICOM files of the images were then uploaded to a software program (Mimics 19.0, Materialise company, Belgium) to acquire a virtual 3D model of his left total scapula by threshold segmentation and region growth processing. After synthesizing two 3D models, including the removed portion of the lesion and the reserved portion, we converted them into STeroLithography (STL) files. The retained portion containing the acromion and glenoid cavity was named S2. The removed portion was called S1. To remove S1 quickly and accurately, an osteotomy navigation template was designed through the software program, which could assign an excision landmark where the tumor would be removed. After resection of the tumor, we were eager to obtain a prosthesis with the same size and shape as the original removed portion without the tumor. Hence, we viewed a mirror model of the scapula from the healthy side as an affected side implant. Consequently, having used mirror imaging technology, we synthesized the 3D image file of the S1 region of the left scapula without tumor and named it N-S1 (that is the prosthesis) according to the right scapula. Finally, the STL files were uploaded to a Tornier 3D printer (SAS, Montbonnot, France) to acquire a solid 3D model consisting of nylon resin material.
First, we attached a navigation template to the left scapula model to excise S1 according to the preoperative scheme (Figure 3a). Second, we carefully matched S2 with the N-S1 prosthesis (Figure 3b). Moreover, we shaped the reconstruction plate and had the two portions securely fastened with 3.5-mm screws (Figure 3c). Subsequently, the file of the N-S1 3D image in STL was transmitted to an orthopedics instrument corporation (NaTon Medical Group, China), and a titanium alloy prosthesis was obtained that was identical to the nylon prosthesis (Figure 3d). The inside of the prosthesis was made of titanium alloy, and was designed to be honeycombed, which could decrease its weight and facilitate the ingrowth of the surrounding tissue. The rotator cuff was of greater concern because of its significant role in shoulder joint stability and function. Hence, many holes were designed at the edge of the prosthesis for soft tissue reconstruction.
Figure 3.
Solid three-dimensional model of the left scapula and preoperative simulation. (a) A navigation template stuck to the removed portion of the scapula (S1). (b,d) The nylon and titanium alloy prostheses (N-S1) were printed using mirror imaging technique. Many holes were designed at the edge of the prosthesis for soft tissue reconstruction. (c) The retained portion of the scapula (S2) and the prosthesis (N-S1) matched well via the reconstruction plate shaped in advance.
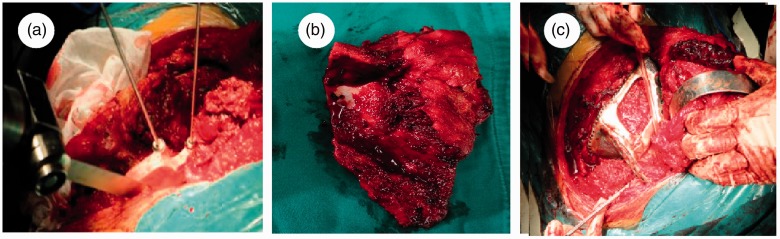
Intraoperatively, the patient was in the lateral position, and a “C”-like incision was taken from the medial border to the glenoid. The soft tissue was dissected and the scapula was exposed. Approximately 3/5 of the scapula and the surrounding soft tissue was eroded by the tumor. While attaining adequate exposure of the tumor tissue, we attached the osteotomy navigation template to the landmark of the scapula and removed the S1 (Figure 4a, 4b). After rinsing the wound repeatedly, we combined the titanium alloy prosthesis (N-S1) with the retained scapula (S2) via a contoured reconstruction plate in advance and 3.5-mm screws. Intraoperative fluoroscopy indicated a good match between N-S1 and S2. Finally, we sutured the surrounding muscle tissue, including the rotator cuff, to the prosthesis through the holes at the edge of the prosthesis (Figure 4c). After reconstructing the rotator cuff, we covered the prosthesis with ambient soft tissue and closed the wound.
Figure 4.
Major intraoperative surgical procedures. (a) The osteotomy navigation template was attached to the scapula landmark to assist with tumor resection. (b) The excised tumor tissue. (c) The remaining rotator cuff was reconstructed with the prosthesis.
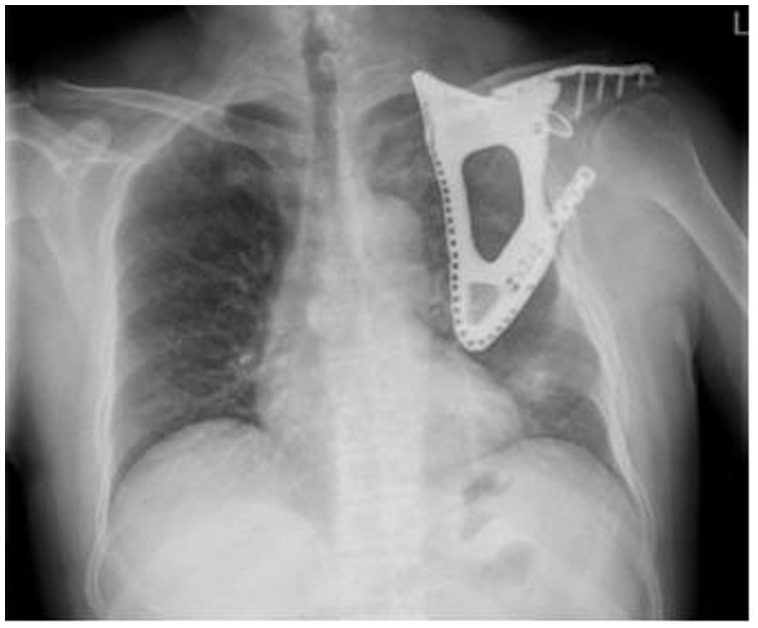
Within 4 weeks after surgery, the upper extremity was placed in the functional position of external support. The patient was instructed to move his hand and elbow joint immediately. The stitches were removed from the incision 2 weeks after surgery. At the 28-month follow-up, an X-ray of the patient’s shoulder showed no tumor recurrence with the prosthesis and the plate in good position (Figure 5). The function of the shoulder joint recovered well (Figure 6). At the final follow-up, the patient’s MSTS (Musculoskeletal Tumor Society) score was 28. Currently, the patient is in good condition, and has no discomfort in his left shoulder.
Figure 5.
No tumor recurrence was found on X-ray, and the prosthesis and plate were in good positions at the 28-month follow-up.
Figure 6.
(a–c) Satisfactory clinical result of the patient’s shoulder function at 32 months.
Discussion
Chondrosarcoma is a malignant tumor that commonly involves the scapula.1 It seriously impairs shoulder joint function and can shorten a patient’s lifespan. The standard treatment for bony tumors is radical resection or curettage.14 In recent years, with the improvement in medical technology, limb salvage therapy has been the mainstream treatment for bone tumors.15 It has been reported that more than 90% of malignant tumors can be treated with limb salvage, with no significant difference in survival rate compared with amputation.16
Generally, limb-sparing resections of scapular tumors mainly include humeral suspension, allograft transplantation, external deactivation replantation, arthrodesis, and prosthesis replacement. Humeral suspension has been mostly performed after total scapulectomy, but with a sinking of the shoulder and limited function of the shoulder joint, such as with poor upper limb weight bearing ability, the humerus cannot actively abduct and lift.6 Similarly, Xu et al.7 reported relatively low MSTS scores after total scapulectomy with humeral suspension. Allograft transplantation and external deactivation replantation may be selected after total or subtotal scapulectomy. Theoretically, the muscles around the scapula can be reattached to the allograft. Unexpectedly, functional scores were satisfactory in the short term, but long-term follow-up showed that allogeneic bone rejection occurred, the infection rate was significantly increased, and a higher rate of secondary fractures occurred.17 Massive allografts also carry a risk of disease transmission. Arthrodesis is no longer in use due to the loss of stability and mobility of the shoulder. Currently, a prosthesis implant is considered to be the most effective method due to better shoulder cosmesis, stability, and function. Min et al.8 reported that reconstruction with a scapular endoprosthesis after resection of a scapular tumor was satisfactory. Furthermore, for scapula tumors, Puchner et al.5 reported that prosthesis implantation after total scapula resection achieved the highest scores compared with humeral suspension or partial and total scapula resection. According to Puchner, compared with total scapula resection, partial excision had higher postoperative MSTS scores. Interestingly, shoulder function with bone and soft tissue reconstruction after total scapulectomy is superior to partial scapulectomy without any reconstruction. Bickels et al.9 further emphasized that satisfactory shoulder function necessitates bone and soft tissue reconstruction after scapulectomy. Conclusively, the high MSTS score of prosthesis implantation closely correlates with bone and soft tissue reconstruction after scapulectomy.
Retrospectively, more scholars advocate the treatment of tumors around the shoulder girdle with artificial prosthesis replacement.18,19 While most of these prostheses do not match the patient's anatomy and may affect shoulder function, the advent of 3D printing, which allows for customization, may help solve this dilemma. Beltram et al.20 presented a customized scapula prosthesis replacement after tumor resection with an MSTS score of 87%. Liu et al.11 reported the application of 3D-printed PolyEtherEtherKetone (PEEK) scapular prosthesis replacement in the treatment of scapular tumors and obtained satisfactory shoulder joint function. 3D printing does have the possibility of accurate reconstruction, improving the operability of surgery and facilitating the early recovery of patients' function. However, all previous reports have applied 3D-printed prostheses for total scapula replacements, and did not involve segmental scapula replacement studies.
Surgeons must take the location and malignancy of the tumor into consideration prior to drafting a surgical tactic. Malawer divided the scapula into five (S1–5) regions12 and proposed that partial scapulectomy can be performed if the tumor only involves the S1 region. In this case, considering the tumor was situated in the S1 region and had a low grade of malignancy, subtotal scapulectomy and segmental prosthesis implantation could be performed to preserve the glenohumeral joint. The retention of the glenohumeral joint plays an important role in the recovery of shoulder function. Nota mentioned that an excellent functional status can be maintained when the glenohumeral joint is preserved.3 We may face the decision of whether the scapula should be reconstructed prior to the tumor being removed. Bickels et al.9 indicated that reconstruction of the bony defect remaining after resection of tumor is aimed at achieving a stable and moveable shoulder. Therefore, with the assistance of 3D printing technique, we accurately excised the S1 region and reconstructed the bone defect with a matching prosthesis. Because muscle reconstruction after scapular endoprosthetic reconstruction is imperative,9 we sutured the retained rotator cuff to the prosthesis.
Intraoperatively, the surgical difficulties may increase as a result of three factors. First, because of normal anatomical structures being eroded by tumors, there were no reliable intraoperative landmarks for removal of the S1 region. Second, the prosthesis must be customized to the patient corresponding to the irregular tumors. Third, the plate must be shaped to make the prosthesis fit in a stable manner to the remaining scapula. To address these difficulties, 3D printing was introduced to reproduce the implant replacing the tumor, perform the preoperative simulation, and synthesize the navigation template to assist the surgeon in completing the operation quickly and accurately.
In this report, we chose segmental scapula prosthesis implantation to reestablish shoulder function after tumor resection, rather than total scapula resection. For osteomas with low malignancy, intraarticular resection is sufficient.9 Schneiderbauer et al.21 suggested that partial scapulectomy for chondrosarcoma in the S1 region is reasonable. To achieve a stable and moveable shoulder while removing the tumor, we suggest the following indications for segmental scapula prosthesis replacement subsequent to partial scapulectomy: (1) The tumor only invades the S1 region of the scapula, and the S2–5 regions are not affected; (2) The glenohumeral joint can be preserved; (3) The prosthesis must be individually designed to match the remaining S1 region of the scapula; (4) The major neurovascular bundle of the upper extremity and chest wall are tumor-free, both of which can be retained during the surgical procedure; (5) The remaining muscles are inadequate for coverage of a scapular endoprosthesis; (6) Preoperative biopsy reveals moderate or low grade malignancy without any metastasis; and (7) The patient consents to the protocol and is in good physical condition to tolerate the surgery.
Owing to 3D printing technology, preoperative simulation was performed, a reconstructed plate was contoured in advance, and a navigation template and a highly individualized prosthesis was designed. Intraoperatively, we replicated the preoperative simulation. Subsequently, fluoroscopy was taken only once and showed a perfect match between the prosthesis and the shoulder joint. The surgical time and the number of fluoroscopic images taken declined significantly. In a word, 3D printing technology offered the possibility of accurate reconstruction, satisfied the need for customized clinical applications, and simplified complex operations.
Our study had several limitations. First, the whole process from the design of the prosthesis to the completion of the operation was time consuming. Second, 3D printers and printing materials are expensive. Third, the follow-up time in our study was relatively short.
Conclusions
Segmental scapula prosthesis prepared by 3D printing technology has not yet been reported clinically. In the past, incomplete matches between the prosthesis and the bone defect due to the tumor resection commonly occurred. In this report, an identically-sized tumor model and a customized prosthesis were produced. Additionally, we marked the osteotomy plane and designed a navigation template with an accurate tumor resection, and shaped the plate in advance and fastened the prosthesis securely. As a result of 3D printing technology, we simplified complicated operations and improved the efficiency of the operation. The 3D printing technology can fulfill the requirements of a highly individualized design, thereby displaying unique advantages in the manufacturing of the implant.
Consent for publication
Written consent for publication of images and necessary data was obtained.
Declaration of conflicting interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of ZhongNan Hospital, WuHan University. Written consent for participation was obtained.
Funding
This study was supported by General Cultivation Project of Zhong Nan Hospital of Wu Han University (znpy2017037).
References
- 1.Chang F, Liu G, Zhang Q, et al. Malawer limb salvage surgery for the treatment of scapular chondrosarcoma. World J Surg Oncol 2014; 12: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist 2011; 16: 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nota SP, Russchen MJ, Raskin KA, et al. Functional and oncological outcome after surgical resection of the scapula and clavicle for primary chondrosarcoma. Musculoskelet Surg 2017; 101: 67–73. [DOI] [PubMed] [Google Scholar]
- 4.Marcove RC, Lewis MM, Huvos AG. En bloc upper humeral interscapulothoracic resection. Clin Orthop 1977; 124: 219–228. [PubMed] [Google Scholar]
- 5.Puchner SE, Panotopoulos J, Puchner R, et al. Primary malignant tumours of the scapula–a review of 29 cases. Int Orthop 2014; 38: 2155–2162. [DOI] [PubMed] [Google Scholar]
- 6.Mayil Vahanan N, Mohanlal P, Bose JC, et al. The functional and oncological results after scapulectomy for scapular tumours: 2-16-year results. Int Orthop 2007; 31: 831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu SF, Yu XC, Xu M, et al. Functional results and emotional acceptance after scapulectomy for malignant shoulder tumors. Orthop Surg 2016; 8: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min L, Zhou Y, Tang F, et al. Reconstruction with scapular hemiarthroplasty endoprosthesis after scapulectomy for malignant tumour. Int Orthop 2017; 41: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 9.Bickels J, Wittig JC, Kollender Y, et al. Limb-sparing resections of the shoulder girdle. J Am Coll Surg 2002; 194: 422–435. [DOI] [PubMed] [Google Scholar]
- 10.Tserovski S, Georgieva S, Simeonov R, et al. Advantages and disadvantages of 3D printing for pre-operative planning of revision hip surgery. J Surg Case Rep 2019; 2019: rjz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D, Fu J, Fan H, et al. Application of 3D-printed PEEK scapula prosthesis in the treatment of scapular benign fibrous histiocytoma: a case report. J Bone Oncol 2018; 12: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malawer MM, Meller I, Dunham WK. A new surgical classification system for shoulder-girdle resections. Analysis of 38 patients. Clin Orthop Relat Res 1991; 267: 33–44. [PubMed] [Google Scholar]
- 13.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980; 153: 106–120. [PubMed] [Google Scholar]
- 14.Dhanda S, Menon S, Gulia A. Atypical giant chondroblastoma mimicking a chondrosarcoma. J Cancer Res Ther 2015; 11: 660. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Otsuka K, Shibata H. Circulating tumor cell count as a biomarker of a specific gastric cancer subgroup characterized by bone metastasis and/or disseminated intravascular coagulation -an early indicator of chemotherapeutic response. Oncol Lett 2016; 11: 1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wodajo FM, Bickels J, Wittig J, et al. Complex reconstruction in the management of extremity sarcomas. Curr Opin Oncol 2003; 15: 304–312. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Duan H, Xiang Z, et al. Surgical technique and clinical results for scapular allograft reconstruction following resection of scapular tumors. J Exp Clin Cancer Res 2009; 28: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savvidou OD, Zampeli F, Georgopoulos G, et al. Total scapulectomy and shoulder reconstruction using a scapular prosthesis and constrained reverse shoulder arthroplasty. Orthopedics 2018; 41: e888–e893. [DOI] [PubMed] [Google Scholar]
- 19.Trouilloud P, Gonzalvez M, Martz P, et al. Duocentric(R) reversed shoulder prosthesis and Personal Fit(R) templates: innovative strategies to optimize prosthesis positioning and prevent scapular notching. Eur J Orthop Surg Traumatol 2014; 24: 483–495. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami G, Ristori G, Scoccianti G, et al. Latissimus dorsi rotational flap combined with a custom-made scapular prosthesis after oncological surgical resection: a report of two patients. BMC Cancer 2018; 18: 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneiderbauer MM, Blanchard C, Gulleru R, et al. Scapular chondrosarcomas have high rates of local recurrence and metastasis. Clin Orthop Relat Res 2004; 426: 232–238. [DOI] [PubMed] [Google Scholar]