Short abstract
Objective
This study aimed to determine the association of asymptomatic sexually transmitted infections (STIs), including Ureaplasma urealyticum (UU), Mycoplasma hominis (MH), Mycoplasma genitalium, Chlamydia trachomatis, and herpes simplex virus type 2, with high-risk human papillomavirus (hrHPV) in cervical intraepithelial lesions and neoplasms.
Methods
A total of 320 hrHPV-positive and 160 hrHPV-negative women were divided into high-grade squamous intraepithelial lesion (HSIL) + invasive cervical cancer and low-grade squamous intraepithelial lesion + normal subgroups, respectively, on the basis of pathological cervical lesions. Cervical brush specimens were amplified and hybridized using polymerase chain reaction kits.
Results
MH was associated with hrHPV infection, but not with specific hrHPV genotypes or with single or multiple genotypes. Coinfection of hrHPV and UU serotype 14 (Uup14) showed an increased risk of HSILs and cervical carcinoma (odds ratio [OR]: 12.541, 95% confidence interval [CI]: 3.625–43.390). U. urealyticum biovar (Uuu) and Uup1 infections showed a similar increased risk (OR: 11.646, 95% CI: 1.493–90.850; OR: 7.474, 95% CI: 1.140–49.015, respectively) without hrHPV.
Conclusions
Asymptomatic STIs are widespread. This study shows an association between UU subtypes and cervical cancer, providing new insight into cervical lesion etiology. Screening for MH, Uup14, Uup1, and Uuu is important under different hrHPV statuses.
Keywords: Asymptomatic sexually transmitted infection, cervical intraepithelial neoplasia, high-risk human papillomavirus, lower reproductive tract, uterine cervical neoplasm, lesion, Ureaplasma urealyticum
Introduction
Cervical cancer (CC) is the fourth most common malignant tumor in women worldwide. The incidence of CC has remained high, with approximately 570,000 cases worldwide in 2018. Approximately 311,000 CC-related deaths occur globally each year.1 Although the incidence of CC has decreased in recent decades, a huge burden remains, especially in low Human Development Index countries.
The most accepted cause of CC is persistent infection with the high-risk human papillomavirus (hrHPV), including human papillomavirus (HPV) genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68. Women with cervical neoplastic lesions have HPV rates of 83.7% in China, 86% to 91% in Asia, and even almost 99% when using the most sensitive HPV detection methods.2 Subtypes 16 and 18 are considered the most dangerous, and the HPV-16-positive rate is as high as 66% in all patients with CC and hrHPV. Among women in China, the most predominant genotypes are 16, 58, 18, and 52.3
Precancerous cervical lesions take a long time to progress to carcinoma. Persistent hrHPV infection and other cofactors can lead to cell transformation, development of low-grade squamous intraepithelial lesions (LSILs), progression to high-grade squamous intraepithelial lesions (HSILs), and even invasive cervical cancer (ICC). Multiple sexual partners, early sexual activity, poor sexual hygiene, and lower genital tract infections are important factors that can alter the vaginal microenvironment and accelerate hrHPV infection.4 Asymptomatic infections are easily ignored by patients and doctors. Therefore, pathogens can coexist for a long time and cause chronic injury.5 Ureaplasma urealyticum (UU), Mycoplasma hominis (MH), Mycoplasma genitalium (MG), Chlamydia trachomatis (CT), and herpes simplex virus type 2 (HSV-2) may cause sexually transmitted infections (STIs) without apparent symptoms. The associations between these pathogens, especially UU subtypes, and hrHPV infection are unclear. This study aimed to determine the association between non-hrHPV STIs of the lower reproductive tract and cervical lesions in women in Xi’an, China. We hoped to provide more evidence for preventing and treating precancerous lesions and uterine cervical neoplasms.
Patients and methods
Study participants
From July 2017 to July 2018, participants were randomly recruited from thousands of outpatients, inpatients, and healthy check-up populations at the Department of Gynecology, the First Affiliated Hospital of Xi’an Jiaotong University. Histological biopsies were performed when necessary on the basis of results of a gynecological examination, laboratory testing, and cytology tests. The results were interpreted with a diagnosis as follows.
Results were considered normal as follows: when healthy women had no typical infectious symptoms or signs and no reason for a colposcopy examination; or patients whose biopsy results that were diagnosed by two doctors were “cervicitis” without a squamous intraepithelial lesion (SIL). LSIL was diagnosed when a woman’s biopsy results showed cervical intraepithelial neoplasia (CIN) 2 and p16− or CIN 1. HSIL was diagnosed when a woman’s biopsy results showed CIN 2 and p16 + or CIN 3. Women with cervical squamous cell carcinoma were diagnosed with ICC.
Women were excluded for any of the following reasons: no history of sexual activity or more than one sexual partner; pregnant or having a menstrual period at the time of the test; previous history of cervical surgery or physiotherapy; previous pelvic radiation therapy or chemotherapy; suffering from acquired immune deficiency syndrome or other immune disorders; a previous gynecological examination or sexual activity in the past 3 days; and medication, especially antibiotic use, in the past week.
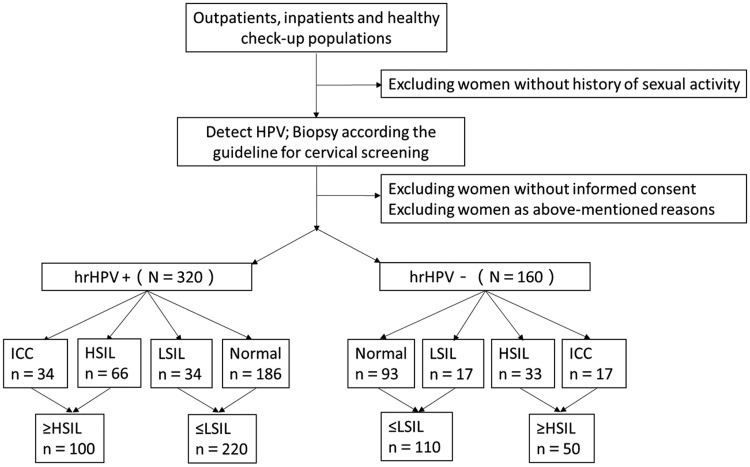
Women were divided into an hrHPV-negative group and an hrHPV-positive group (Figure 1). No participants had apparent clinical symptoms of STIs. Our study was approved by the First Affiliated Hospital of Xi’an Jiaotong University Institutional Review Board. Each participant signed their written informed consent, and case histories were reviewed in detail. All specimens were anonymized.
Figure 1.
Procedure for choosing participants.
Detection of microorganisms
Specimens were sampled by professional gynecologists after standard training. After conventional vulvar disinfection, a sterile speculum without lubricant was used to expose the cervix. Cervical exfoliated cells and secretions were removed from the ectocervix using a cytobrush and preserved in buffer solution specialized for HPV-DNA testing immediately or within 10 days of storage at 4°C.
The 21 HPV GenoArray Diagnostic Kit (HBGA-21PKG; HybriBio, Ltd., Chaozhou, China) with the Rapid Capture System uses an HPV genotyping macroarray for human papillomavirus identification. This kit detects fifteen hrHPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 53, and 66) and six low-risk HPV types (not mentioned in our study). We used this kit by following the manufacturer’s instructions and analyzed the results after cell lysis, DNA extraction, polymerase chain reaction (PCR) amplification, and hybridization.
A nucleic acid test kit (HBRT-STD6; HybriBio Ltd.) was used to simultaneously detect STIs, such as UU, MH, MG, CT, and HSV-2. (This kit can also detect Neisseria gonorrhoeae, but it was not evaluated in our study.) The UU subtypes, including U. parvum serotypes 1, 3, 6, and 14 (Uup1, 3, 6, and 14) and the other biovar U. urealyticum (Uuu), were detected. Using the real-time PCR fluorescent probe method, five microorganisms that are responsible for sexually transmitted diseases were quantitatively detected in a single reaction. This method is applicable to single and mixed infections.
Statistical analysis
Data were analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Normality tests and Student’s t-test were used for statistical evaluations between groups. The prevalence of infection was calculated, and the differences were examined via the chi-squared test, continuity correction, and Fisher’s exact test. Logistic regression was used for multivariate analysis. Statistical tests were considered significant if the P value was ≤0.05.
Results
There were 160 women in the hrHPV-negative group, which included 93 normal cases, 17 LSIL cases, 33 HSIL cases, and 17 ICC cases. There were 320 women in the hrHPV-positive group, which included 186, 34, 66, and 34 cases in the same four cervical lesion stages, respectively (Figure 1). Women in the hrHPV-positive group were aged 21 to 76 years (mean, 44.23 ± 11.492 years) and those in the hrHPV-negative group were aged 20 to 71 years (mean, 42.62 ± 10.731 years). Age was not significantly different between the two groups (P = 0.519).
The overall infection rate (positive for at least one non-hrHPV pathogen) was not significantly different between the groups (Table 1). UU was the most common pathogen. Among the UU subtypes, the rates of U. parvum were 42.8% and 42.5% in the positive and negative groups, respectively, and these rates were significantly higher than those of U. urealyticum at 12.2% and 10.6%, respectively (both P < 0.001). For U. parvum, Uup3 was the most prevalent, followed by Uup6. Only Uup14 and MH infections were significantly different between the hrHPV-positive and hrHPV-negative groups (P = 0.003 and P = 0.005, respectively), which indicated that these pathogens may be related to hrHPV infection. However, further analysis showed that the prevalence of Uup14 and MH infections was not significantly different among the different hrHPV genotype subgroups (the four most common HPV types [16/18/52/58] in Chinese women, as well as the other high-risk subtypes) or in the single and multiple genotype groups (Tables 2 and 3).
Table 1.
General characteristics of each group.
| hrHPV+ (n = 320) |
hrHPV−(n = 160) |
χ² | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Overalla | 203 | 63.4 | 95 | 59.4 | 0.748 | 0.387 |
| UU | 164 | 51.3 | 84 | 52.5 | 0.067 | 0.796 |
| Uuu | 39 | 12.2 | 17 | 10.6 | 0.253 | 0.615 |
| Uup1 | 35 | 10.9 | 17 | 10.6 | 0.011 | 0.917 |
| Uup3 | 58 | 18.1 | 38 | 23.8 | 2.109 | 0.146 |
| Uup6 | 46 | 14.4 | 24 | 15.0 | 0.033 | 0.855 |
| Uup14 | 21 | 6.6 | 24 | 15.0 | 8.938 | 0.003 |
| MH | 77 | 24.1 | 21 | 13.1 | 7.853 | 0.005 |
| MG | 7 | 2.2 | 0 | 0 | 2.193 | 0.139 |
| CT | 16 | 5 | 4 | 2.5 | 1.670 | 0.196 |
| HSV-2 | 3 | 0.9 | 0 | 0 | 0.377 | 0.539 |
aPositive for any STD-causing microorganisms, including multiple infections. hrHPV: high-risk human papillomavirus; UU: Ureaplasma urealyticum; Uuu: Ureaplasma urealyticum biovar; Uup: Ureaplasma parvum serotype; MH: Mycoplasma hominis; MG: Mycoplasma genitalium; CT: Chlamydia trachomatis; HSV-2: herpes simplex virus type 2.
Table 2.
Different infections by hrHPV genotype.
| Overall(n = 320) |
HPV-16(n = 109) |
HPV-18(n = 32) |
HPV-52(n = 49) |
HPV-58(n = 64) |
Other genotypes(n = 99) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | χ² | P* | n | χ² | P* | n | χ² | P* | n | χ² | P* | n | χ² | P* | |
| Uup14 | 21 | 10 | 0.827 | 0.363 | 3 | 0.055 | 0.815 | 2 | 0.124 | 0.725 | 6 | 0.287 | 0.592 | 3 | 1.747 | 0.186 |
| MH | 77 | 25 | 0.057 | 0.811 | 5 | 1.159 | 0.282 | 12 | 0.384 | 0.535 | 15 | 0.011 | 0.915 | 24 | 0.001 | 0.971 |
*Compared with the overall infection rate in different hrHPV genotype groups, adjusted P* = 0.05/5 = 0.01. hrHPV: high-risk human papillomavirus; HPV: human papillomavirus.
Table 3.
Different infections in participants with single and multiple hrHPV genotypes.
| Single hrHPV genotype (n=228) |
Multiple hrHPV genotypes (n=92) |
χ² | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Uup14 | 16 | 7.0 | 5 | 5.4 | 0.268 | 0.605 |
| MH | 58 | 25.4 | 19 | 20.7 | 0.822 | 0.365 |
hrHPV: high-risk human papillomavirus.
To compare differences in infection based on age group, participants were grouped into reproductive age (≤49 years) and non-reproductive age (>49 years) groups (Table 4a). The reproductive age group included 224 cases with hrHPV and 118 cases without hrHPV. Uup14 was negatively associated with hrHPV (P = 0.020), while MH was positively associated with hrHPV (P = 0.001) in the reproductive age group. However, no significant differences in infection were observed in women in the non-reproductive age group.
Table 4a.
STIs between reproductive age and non-reproductive age groups.
| Reproductive age (≤49) |
Non-reproductive age (>50) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
HPV+ (n=224) |
HPV−(n=118) |
χ² | P |
HPV+ (n=96) |
HPV−(n=42) |
χ² | P | |
| n | n | n | n | |||||
| Overall | 159 | 72 | 3.501 | 0.061 | 44 | 23 | 0.932 | 0.334 |
| UU | 134 | 64 | 0.989 | 0.320 | 30 | 20 | 3.388 | 0.066 |
| Uuu | 27 | 14 | 0.003 | 0.959 | 12 | 3 | 0.401 | 0.527 |
| Uup1 | 32 | 14 | 0.389 | 0.533 | 3 | 3 | 0.373 | 0.541 |
| Uup3 | 51 | 30 | 0.302 | 0.583 | 7 | 8 | 3.043 | 0.081 |
| Uup6 | 37 | 16 | 0.517 | 0.472 | 9 | 8 | 2.531 | 0.112 |
| Uup14 | 15 | 17 | 5.417 | 0.020 | 6 | 7 | 2.595 | 0.107 |
| MH | 60 | 14 | 10.149 | 0.001 | 17 | 7 | 0.022 | 0.882 |
| MG | 3 | 0 | 0.426 | 0.514 | 4 | 0 | 0.626 | 0.429 |
| CT | 13 | 3 | 1.843 | 0.175 | 3 | 1 | 0.000 | 1.000 |
| HSV-2 | 1 | 0 | 1.000 | 0.655 | 2 | 0 | – | 1.000 |
STIs: sexually transmitted infections; HPV: human papillomavirus; UU: Ureaplasma urealyticum; Uuu: Ureaplasma urealyticum biovar; Uup: Ureaplasma parvum serotype; MH: Mycoplasma hominis; MG: Mycoplasma genitalium; CT: Chlamydia trachomatis; HSV-2: herpes simplex virus type 2.
Table 4b.
STIs between the age groups of ≤29 and 30 to 49 years.
| ≤29 years |
30 to 49 years |
|||||||
|---|---|---|---|---|---|---|---|---|
|
HPV+ (n=36) |
HPV− (n=25) |
χ² | P |
HPV+ (n=188) |
HPV− (n=93) |
χ² | P | |
| n | n | n | n | |||||
| Overall | 31 | 14 | 6.913 | 0.009 | 128 | 58 | 0.910 | 0.340 |
| UU | 27 | 12 | 4.665 | 0.031 | 107 | 52 | 0.025 | 0.873 |
| Uuu | 4 | 2 | <0.001 | 1.000 | 23 | 12 | 0.026 | 0.873 |
| Uup1 | 7 | 3 | 0.177 | 0.674 | 25 | 11 | 0.120 | 0.729 |
| Uup3 | 11 | 7 | 0.046 | 0.830 | 40 | 23 | 0.427 | 0.514 |
| Uup6 | 5 | 1 | 0.703 | 0.402 | 32 | 15 | 0.036 | 0.850 |
| Uup14 | 2 | 4 | 0.828 | 0.363 | 13 | 13 | 1.764 | 0.184 |
| MH | 8 | 1 | 2.581 | 0.108 | 52 | 13 | 6.550 | 0.010 |
| MG | 1 | 0 | – | 1.000 | 2 | 0 | – | 1.000 |
| CT | 2 | 2 | <0.001 | 1.000 | 11 | 1 | 0.036 | 0.850 |
| HSV-2 | 1 | 0 | – | 1.000 | 0 | 0 | – | 1.000 |
STIs: sexually transmitted infections; HPV: human papillomavirus; UU: Ureaplasma urealyticum; Uuu: Ureaplasma urealyticum biovar; Uup: Ureaplasma parvum serotype; MH: Mycoplasma hominis; MG: Mycoplasma genitalium; CT: Chlamydia trachomatis; HSV-2: herpes simplex virus type 2.
The 2016 American College of Obstetricians and Gynecologists CC screening clinical guidance recommends that women aged younger than 30 years should be tested for only HPV-DNA. Therefore, we further grouped reproductive-aged women into ≤29 and 30 to 49 years (Table 4b). The overall and UU infection rates of women aged ≤29 years were significantly higher in the hrHPV-positive group than in the hrHPV-negative group (P = 0.009 and P = 0.031, respectively). In 30- to 49-year-old participants, only the MH infection rate was significantly different between the hrHPV-positive and hrHPV-negative groups (P = 0.010). There were no significant differences in UU subtype infection between the age groups. Differences in the prevalence of MG, CT, and HSV-2 were considered meaningless because of the low infection rates.
In the hrHPV-positive and hrHPV-negative groups, summing the frequencies of normal + LSIL and HSIL + ICC improved statistical accuracy and showed more obvious differences of STIs for benign diseases (≤LSIL) and cervical neoplastic lesions (≥HSIL). Table 5a shows that in the hrHPV-positive group, only women with Uup14 showed an increased risk for HSIL + ICC (odds ratio [OR]: 12.541, 95% confidence interval [CI]: 3.625–43.390, P<0.001) after adjustment by the potential confounder of age. None of the other infections (including P = 0.050 for CT) were significantly different between the ≤LSIL and ≥HSIL subgroups. In the hrHPV-negative group (Table 5b), there was an increased risk for HSIL + ICC in women with Uuu (OR: 11.646, 95% CI: 1.493–90.850, P = 0.019) and Uup1 (OR: 7.474, 95% CI: 1.140–49.015, P = 0.036) between the two subgroups. However, the other pathogens were not significantly different between the subgroups after being adjusted by age. No patient was infected with MG or HSV-2 in the hrHPV-negative group.
Table 5a.
Different microorganism infections between cervical lesions in hrHPV-positive women.
|
≤LSIL (n=220) |
≥HSIL (n=100) |
P | OR | 95% CI | |
|---|---|---|---|---|---|
| n | n | ||||
| Age | – | – | 0.017 | 1.030 | 1.005–1.056 |
| Overall | 135 | 68 | 0.705 | 0.798 | 0.248–2.571 |
| UU | 108 | 56 | 0.823 | 1.200 | 0.243–5.915 |
| Uuu | 24 | 15 | 0.408 | 1.702 | 0.483–5.996 |
| Uup1 | 22 | 13 | 0.864 | 1.131 | 0.278–4.608 |
| Uup3 | 40 | 18 | 0.586 | 0.698 | 0.191–2.547 |
| Uup6 | 32 | 14 | 0.833 | 1.170 | 0.273–5.019 |
| Uup14 | 5 | 16 | <0.001 | 12.541 | 3.625–43.390 |
| MH | 47 | 30 | 0.104 | 1.883 | 0.878–4.037 |
| MG | 3 | 4 | 0.178 | 3.360 | 0.576–19.605 |
| CT | 15 | 1 | 0.050 | 0.116 | 0.013–1.000 |
| HSV-2 | 1 | 2 | 0.126 | 8.185 | 0.553–121.097 |
hrHPV: high-risk human papillomavirus; LSIL; low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; OR: odds ratio; CI: confidence interval; UU: Ureaplasma urealyticum; Uuu: Ureaplasma urealyticum biovar; Uup: Ureaplasma parvum serotype; MH: Mycoplasma hominis; MG: Mycoplasma genitalium; CT: Chlamydia trachomatis; HSV-2: herpes simplex virus type 2.
Table 5b.
Different microorganism infections between cervical lesions in hrHPV-negative women.
|
≤LSIL (n=110) |
≥HSIL (n=50) |
P | OR | 95% CI | |
|---|---|---|---|---|---|
| n | n | ||||
| Age | – | – | 0.047 | 1.037 | 1.000–1.074 |
| Overall | 66 | 29 | 0.331 | 0.343 | 0.040–2.966 |
| UU | 58 | 26 | 0.561 | 0.447 | 0.030–6.734 |
| Uuu | 8 | 9 | 0.019 | 11.646 | 1.493–90.850 |
| Uup1 | 11 | 6 | 0.036 | 7.474 | 1.140–49.015 |
| Uup3 | 28 | 10 | 0.123 | 3.984 | 0.687–23.123 |
| Uup6 | 17 | 7 | 0.137 | 3.615 | 0.664–19.696 |
| Uup14 | 19 | 5 | 0.209 | 0.374 | 0.081–1.731 |
| MH | 13 | 8 | 0.513 | 1.593 | 0.395–6.419 |
| MG | 0 | 0 | – | – | – |
| CT | 2 | 2 | 0.292 | 4.430 | 0.279–70.454 |
| HSV-2 | 0 | 0 | – | – | – |
hrHPV: high-risk human papillomavirus; LSIL; low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; OR: odds ratio; CI: confidence interval; UU: Ureaplasma urealyticum; Uuu: Ureaplasma urealyticum biovar; Uup: Ureaplasma parvum serotype; MH: Mycoplasma hominis; MG: Mycoplasma genitalium; CT: Chlamydia trachomatis; HSV-2: herpes simplex virus type 2.
Discussion
In low Human Development Index regions, CC is still a heavy burden in women.1 Current emphasis on reproductive tract infections and cancer of reproductive organs has increased. Our study investigated the prevalence of female asymptomatic STIs and their association with cervical lesions in women in Xi’an, northwest China. In studying the etiology of CC, we examined lower genital tract infections, which often lead to asymptomatic chronic cervicitis and vaginitis. A previous study showed that cervicitis increased the risk of developing CC.6 Neisseria gonorrhoeae and CT are considered the main pathogens leading to cervicitis. Some scholars believe that MG is the only pathogen related to cervicitis among Mycoplasmas.7 Common apparent infections, such as bacterial infections, Trichomonas, and candidal vaginitis, promote development of CC.8,9 Whether other non-hrHPV sexually transmitted pathogens, such as UU, CT, MH, MG, and HSV-2, are associated with CC is controversial.
Our study showed that the prevalence of overall non-hrHPV infection was as high as 63.4% in the hrHPV-positive group and 59.4% in the hrHPV-negative group. UU, MH, and MG belong to the Mycoplasmataceae family, and their rate of infection has successively declined. UU is an opportunistic pathogen in the female lower reproductive tract and it infects hosts silently in most cases. UU was not previously considered a clinical pathogen, but it causes urethritis, infertility, and preterm delivery. In our study, the rate of UU infection was the highest among non-HPV pathogens, which is similar to data reported by Xu et al. in Shanghai and Beijing, China.10,11 Such high rates indicate that these silent infections require earlier diagnosis and specific treatment to prevent adverse outcomes. Condom use and healthy sexual habits are important to reduce the chance of transmission.
UU can be classified into different biovars and serotypes via PCR. U. parvum (biovar 1), including Uup1, 3, 6, and 14, and biovar 2, also called Uuu, were both detected in our study. Biovar 1 was the most common in UU and Uup3 was the most common in biovar 1. The pathogenicity of these biovars was considered different in previous studies. Domingues et al. showed that biovar 2 was more strongly associated than biovar 1 with the loss of lactobacilli and genital–urinary tract infections.12 Our study showed that Uup14 infection was closely associated with development of hrHPV-positive CC, though it appeared to be negatively associated with hrHPV infection. Moreover, Uuu and Uup1 showed a higher risk for HSIL + ICC than normal + LSIL in the hrHPV-negative group. To the best of our knowledge, our study is the first to describe the association between cervical lesions and the prevalence of UU biovars and serotypes in a population of Chinese women. Consequently, Uup14 may be a risk cofactor for hrHPV-related CC and a biomarker for cervical neoplasia screening. However, upon further analysis, the HPV genotypes (16/18/52/58 and the other subtypes) and the presence of single or multiple genotypes did not affect Uup14. Apart from hrHPV, Uuu and Uup1 may be potential risk factors for promoting carcinogenesis and cancer progression in CC, but this possibility requires further study.
Some serotypes of UU are considered to be associated with cervical lesions. Scholars have suggested that 15% to 30% of women who are ultimately diagnosed with CC have inflammation caused by UU.13 Biernat-Sudolska et al.14 found that more patients with CC had UU than did patients without CC, and HPV was significantly associated with detection of UU. The probability of being infected with Uup and Uuu was reported to be six-fold higher in HPV-positive women than in HPV-negative women (P = 0.0016).15 The pathogenic mechanism of UU infection to cervical lesions is unclear. Zhang et al.16 reported that mycoplasma infection alters gene expression in cervical epithelial cells, which may contribute to cervical oncogenesis and metastasis and enhance invasiveness. Verteramo et al.17 found that asymptomatic chronic cervicitis caused by mycoplasma affects the immune function of host cells and generates free radicals, thus increasing susceptibility to HPV and altering HPV persistence. Inflammation may disrupt homeostasis in the lower genital tract, thus facilitating entry of virions.18 Some studies have shown that only HPV and high-load UU are significantly associated.17,19,20 However, we did not find a similar result because of the indefinite load.
Comparison of the hrHPV-positive and hrHPV-negative groups among women of different ages showed that MH was more prevalent in reproductive-aged women than in non-reproductive-aged women with hrHPV. For women aged 20 to 29 years (<30 years), the chance of being infected with overall pathogens or UU was higher in the presence of hrHPV, as was the chance for MH infection in women aged 30 to 49 years. The reason for these findings may be that more frequent sexual activity and less healthy habits increase the chance of disease transmission. In conclusion, women of childbearing age are more sensitive to interaction of hrHPV with other pathogens. Different screening strategies for women in different stages are good for earlier diagnosis of disease and cost saving for medical resources. In our study, only MH showed a significant positive association with hrHPV, as previously described,21,22 although these previous studies could not rule out reverse causation. We did not find that MH infection was related to cervical carcinogenesis. In contrast to our results, previous studies showed that mixed infections with MH and UU were significantly associated with LSIL.23
The association between MG and hrHPV was insufficient for determining statistical significance. Some studies have suggested that MG is not significantly associated with abnormal cervical cytology,5,20 but UU and MG are associated with an increased risk of hrHPV infection.24
CT is one of the most common sexually transmitted pathogens globally.5 In our study, although the prevalence of CT infection was slightly higher in HPV-DNA-positive cases, no significant association was observed between CT and hrHPV and cervical lesions. In the hrHPV-positive group, CT infection tended to be different between the ≤LSIL and ≥HSIL groups (P = 0.050). The prevalence of CT appeared to be unassociated with a particular age group. Golijow et al.25,26 also concluded that CT positivity was unassociated with a higher risk of CC, but was associated with LSIL and HSIL. Nonetheless, some studies have shown a significant association between CT and HPV infection17,19,27 and intraepithelial lesions.28 A meta-analysis showed that individuals infected with CT had a higher risk of CC.29 HSV-2 was discovered to increase the risk of abnormal cervical cytology when coinfected with HPV by promoting malignant cell transformation, not by participating in synergistic action in progression of cervical lesions.5 Some scholars have suggested that HSV-2 serves as an independent predictor and cofactor for CC.30,31 Unfortunately, from the perspective of analysis, only two participants in our study were infected with HSV-2, and thus we did not obtain a significant result. Cross-sectional measurement of infection cannot determine natural causation.
In conclusion, there are some significant differences in asymptomatic STIs and cervical lesions among women in Xi’an, China. Uup14 appears to cooperate with hrHPV infection to increase the risk of cancer progression. Uuu and Uup1 are associated with HSIL and neoplasms, and thus may be potential risk factors in CC. The widespread prevalence of MH is strongly positively associated with hrHPV, but unassociated with development of neoplasms.
Acknowledgments
We are grateful for the support from the participants and Hybribio Biochemistry Co., Ltd. We appreciate the assistance of our study teams and the laboratory staff for conducting the laboratory tests.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol 2016; 27: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao YP, Li N, Smith JS, et al. Human papillomavirus type-distribution in the cervix of Chinese women: a meta-analysis. Int J STD AIDS 2008; 19: 106–111. [DOI] [PubMed] [Google Scholar]
- 4.Shew ML, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med 2006; 160: 151–156. [DOI] [PubMed] [Google Scholar]
- 5.de Abreu AL, Malaguti N, Souza RP, et al. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am J Cancer Res 2016; 6: 1371–1383. [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Wu EQ, Yu XH, et al. Detection of human papillomavirus genotypes associated with mucopurulent cervicitis and cervical cancer in Changchun, China. Int J Gynaecol Obstet 2013; 120: 124–126. [DOI] [PubMed] [Google Scholar]
- 7.Lusk MJ, Konecny P, Naing ZW, et al. Mycoplasma genitalium is associated with cervicitis and HIV infection in an urban Australian STI clinic population. Sex Transm Infect 2011; 87: 107–109. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Li T, Chen L, et al. Epidemiological investigation of the relationship between common lower genital tract infections and high-risk human papillomavirus infections among women in Beijing, China. Plos One 2017; 12: e178033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke MA, Rodriguez AC, Gage JC, et al. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis 2012; 12: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu WH, Chen JJ, Sun Q, et al. Chlamydia trachomatis, Ureaplasma urealyticum and Neisseria gonorrhoeae among Chinese women with urinary tract infections in Shanghai: a community-based cross-sectional study. J Obstet Gynaecol Res 2018; 44: 495–502. [DOI] [PubMed] [Google Scholar]
- 11.Liang YY, Zhai HY, Li ZJ, et al. Prevalence of Ureaplasma urealyticum, Chlamydia trachomatis, Neisseria gonorrhoeae and herpes simplex virus in Beijing, China. Epidemiol Infect 2018; 3: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingues D, Tavira LT, Duarte A, et al. Ureaplasma urealyticum biovar determination in women attending a family planning clinic in Guine-Bissau, using polymerase chain reaction of the multiple-banded antigen gene. J Clin Lab Anal 2002; 16: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak K, Jiang R, Wang JW, et al. Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta human papillomavirus types. Plos One 2014; 9: e97232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biernat-Sudolska M, Szostek S, Rojek-Zakrzewska D, et al. Concomitant infections with human papillomavirus and various mycoplasma and ureaplasma species in women with abnormal cervical cytology. Adv Med Sci 2011; 56: 299–303. [DOI] [PubMed] [Google Scholar]
- 15.Camporiondo MP, Farchi F, Ciccozzi M, et al. Detection of HPV and co-infecting pathogens in healthy Italian women by multiplex real-time PCR. Infez Med 2016; 24: 12–17. [PubMed] [Google Scholar]
- 16.Zhang S, Wear DJ, Lo S. Mycoplasmal infections alter gene expression in cultured human prostatic and cervical epithelial cells. FEMS Immunol Med Microbiol 2000; 27: 43–50. [DOI] [PubMed] [Google Scholar]
- 17.Verteramo R, Pierangeli A, Mancini E, et al. Human papillomaviruses and genital co-infections in gynecological outpatients. BMC Infect Dis 2009; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magana-Contreras M, Contreras-Paredes A, Chavez-Blanco A, et al. Prevalence of sexually transmitted pathogens associated with HPV infection in cervical samples in a Mexican population. J Med Virol 2015; 87: 2098–2105. [DOI] [PubMed] [Google Scholar]
- 19.Zheng MY, Zhao HL, Di JP, et al. Association of human papillomavirus infection with other microbial pathogens in gynecology. Zhonghua Fu Chan Ke Za Zhi 2010; 45: 424–428. [PubMed] [Google Scholar]
- 20.Kim SI, Yoon JH, Park DC, et al. Co-infection of Ureaplasma urealyticum and human papilloma virus in asymptomatic sexually active individuals. Int J Med Sci 2018; 15: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adebamowo SN, Ma B, Zella D, et al. Mycoplasma hominis and Mycoplasma genitalium in the vaginal microbiota and persistent high-risk human papillomavirus infection. Front Public Health 2017; 5: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikas I, Hapfelmeier A, Mollenhauer M, et al. Integrated morphologic and molecular analysis of Trichomonas vaginalis, Mycoplasma hominis, and human papillomavirus using cytologic smear preparations. Parasitol Res 2018; 117: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 23.Friedek D, Ekiel A, Chełmicki Z, et al. HPV, Chlamydia trachomatis and genital mycoplasmas infections in women with low-grade squamous intraepithelial lesions (LSIL). Ginekol Pol 2004; 75: 457–463. [PubMed] [Google Scholar]
- 24.Ye H, Song T, Zeng X, et al. Association between genital mycoplasmas infection and human papillomavirus infection, abnormal cervical cytopathology, and cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 2018; 297: 1377–1387. [DOI] [PubMed] [Google Scholar]
- 25.Golijow CD, Abba MC, Mouron SA, et al. Chlamydia trachomatis and human papillomavirus infections in cervical disease in Argentine women. Gynecol Oncol 2005; 96: 181–186. [DOI] [PubMed] [Google Scholar]
- 26.de Abreu AL, Nogara PR, Souza RP, et al. Molecular detection of HPV and Chlamydia trachomatis infections in Brazilian women with abnormal cervical cytology. Am J Trop Med Hyg 2012; 87: 1149–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza L, Mongelos P, Paez M, et al. Human papillomavirus and other genital infections in indigenous women from Paraguay: a cross-sectional analytical study. BMC Infect Dis 2013; 13: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlmeister D, Vianna DR, Helfer VE, et al. Association of human papillomavirus and Chlamydia trachomatis with intraepithelial alterations in cervix samples. Mem Inst Oswaldo Cruz 2016; 111: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Shen Z, Luo H, et al. Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine (Baltimore) 2016; 95: e3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Wen X. Seropositivity to herpes simplex virus type 2, but not type 1 is associated with cervical cancer: NHANES (1999-2014). BMC Cancer 2017; 17: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosch FX, andde Sanjose S.. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers 2007; 23: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]