Short abstract
Objective
To investigate the effects of Huang Qin Hua Shi (HQ) decoction on the hypothalamic–pituitary–adrenal (HPA) axis in rats under high-temperature (hT)- and high-humidity (hH)-induced stress.
Methods
Male rats were randomized into four groups: rats without stress; rats induced with hT (35 ± 1°C) and hH (85 ± 5% humidity); rats induced with hT and hH and treated with HQ decoction; and rats induced with hT and hH and treated with mifepristone. After 3 weeks, rats underwent the Morris water maze and open-field test. Rat hypothalami were analyzed pathologically using hematoxylin and eosin staining and glucocorticoid receptor (GR) mRNA expression was evaluated by in situ hybridization. Serum levels of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and corticosteroid (CORT) were assessed by enzyme-linked immunosorbent assay.
Results
The administration of mifepristone or HQ in stressed rats significantly improved their performance in the Morris water maze test and increased the central-to-peripheral ratio and incidence of deep rearing in the open-field test. Mifepristone and HQ also reversed histological changes in the hypothalami of stressed rats. Compared with control rats, GR mRNA expression in the hypothalamus and serum CRH, ACTH, and CORT were significantly elevated in rats stressed with hT and hH, and these changes were attenuated by mifepristone and HQ.
Conclusion
HQ decoction protects against hT- and hH-induced cognitive-behavioral disorder and its therapeutic effect is associated with decreased HPA axis activity.
Keywords: Adrenocorticotropic hormone, corticosteroid, corticotropin-releasing hormone, glucocorticoid receptor, high-temperature and high-humidity stress, Huang Qin Hua Shi decoction, hypothalamic–pituitary–adrenal axis
Introduction
Living environments with appropriate heat and humidity are conducive to optimum mental activity, and exposure of mammals to temperatures and humidity beyond the tolerable zone of comfort represents an environmental stress that may impair mental performance.1–3 Accumulating evidence over recent decades has demonstrated that climatic conditions such as high temperature (hT) and high humidity (hH) may cause substantial harm both directly and indirectly to the mental health of humans.4–6 Generally, an environment with humidity above 60%7 and temperature above 32°C (workplace) or 35°C (home) is believed to be harmful to human health.8,9 Patients with hT- and hH-induced stress typically complain of irritation of the eyes, nose, and respiratory tract, including sneezing and coughing.10–12 Other symptoms including asthma,13–15 poor appetite, loose stools,16,17 anxiety, and faintness are also frequently diagnosed in the clinic. However, few studies to date have explored the impact of hT and hH on the hypothalamic–pituitary–adrenal (HPA) axis.
Previous studies have demonstrated a profound impact of heat stress on brain structure and function, leading to neural circuit modification, neuronal loss, neurological defects, convulsions, heat stroke, and accelerated brain dysfunction.18–21 Notably, at the same effective temperatures, the magnitude of the overall effect on psychological function under humid conditions is considered to be relatively greater than that under dry conditions.1 Recently, a systematic study involving 7.5 million people in Australian clearly demonstrated a negative association between hot weather and mental health, and such outcomes should be taken into account when reforming health-care systems to respond to the challenges of climatic change.20,22 This finding has been substantiated in an animal study that showed significant damage to learning and memory in mice subjected to a combination of hT and hH.21,23
Activation of the HPA axis is believed to represent the first step in stress response by stimulating the secretion of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus followed by adrenocorticotropic hormone (ACTH) from the pituitary gland and, finally, glucocorticoid from the adrenal cortex into the blood circulation,23,24 which is beneficial for maintaining glucose homeostasis in the brain during stress.25,26 However, excessive or prolonged secretion often causes dysfunction and imbalance of the internal environment,27 which has been considered a key pathological factor in cognitive-behavioral disorders.28,29 The glucocorticoid receptor (GR) is abundantly distributed in the HPA axis, particularly in the hypothalamus, and represents a commonly implicated link between stress and psychopathology.30 The binding of excessive glucocorticoid to GR is a vital step in stress responses,31 resulting in the peripheral secretion of corticosteroid (CORT) in the HPA axis as well as effector molecules and potent modulators involved in stress responses and subsequent psychological disorders.24,32 Limited evidence has indicated that environmental humidity-induced stress plays a role in stimulating cutaneous cortisol synthesis;22 however, the impact of hT and hH on the secretion of CRH, ACTH, CORT, and GR remains unknown.
Interestingly, heat- and humidity-associated chronic diseases were first described several millennia ago in ancient traditional Chinese medicine (TCM) texts.33 According to TCM theory, these diseases are caused by the impairment of spleen yang, which leads to distention and fullness in the abdomen, poor appetite, loose stools, and generalized edema.34,35 These symptoms are, to some extent, consistent with modern understanding of heat- and humidity-associated chronic diseases. Many formulas, such as Huang Qin Hua Shi (HQ) decoction, have been developed and widely used in TCM clinics to treat these diseases. The chemical composition of HQ decoction indicates a high proportion of flavonoids from Radix Scutellariae (Scutellaria baicalensis Georgi), which has been recently shown to attenuate chronic mild unpredictable stress-induced depressive-like behavior.36 We therefore sought to investigate whether HQ decoction may exert similar effects on hT- and hH-induced stress in rats.
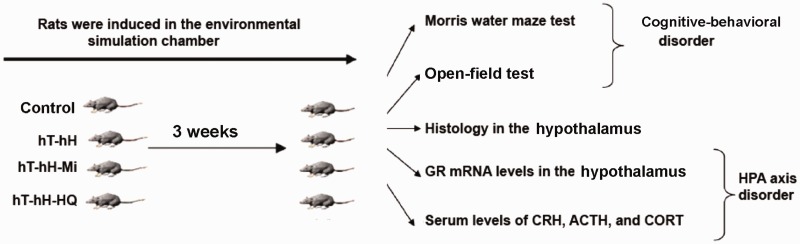
This study was conducted to investigate the effect of HQ decoction on the HPA axis in rats exposed to hH and hT for 3 weeks in comparison with the effect of mifepristone, a GR antagonist and regulator of HPA axis function.37,38 The cognitive ability of stressed rats was evaluated using the Morris water maze test and open-field test. The pathologic phenotype, expression of GR mRNA in rat hypothalami, and circulating secretion of hormones related the HPA axis were also assessed (Figure 1). The aim of the study was to clarify the association between therapeutic effects of HQ decoction and alterations in HPA axis-related hormones in rats stressed by hH and hT.
Figure 1.
Research scheme for stress induction and evaluation of cognitive–behavioral phenotypes and disorders in the HPA axis in rats exposed to hT and hH. Forty SD rats were randomly assigned to four groups: control group; hT-hH group (35 ± 1°C and 85 ± 5% humidity); hT-hH-Mi group (35 ± 1°C and 85 ± 5% humidity and 25 mg/kg mifepristone daily), and hT-hH-HQ group (35 ± 1°C and 85 ± 5% humidity and 1 mL/100 g HQ decoction daily). Control rats and stress-induced rats were subjected to the Morris water maze test, open-field test, pathologic analysis, and assays for detecting the expression of GR mRNA in the hypothalami and serum levels of CRH, CORT, and ACTH 3 weeks after treatment (N = 10 for each group). ACTH, adrenocorticotropic hormone; CORT, corticosteroid; CRH, corticotropin-releasing hormone; GR, glucocorticoid receptor; Mi, mifepristone; hH, high-humidity; HPA, hypothalamic–pituitary–adrenal; HQ, Huang Qin Hua Shi; hT, high-temperature.
Methods
Animals
Forty male Sprague–Dawley (SD) rats weighing 190 to 210 g were obtained from Chengdu Dashuo Laboratory Animal Co., Ltd. (Chengdu, China; production license SCXK (CHUAN) 2008-24). Prior to being exposed to hH and hT, the rats were housed in a specific-pathogen-free environment with (50 ± 5)% humidity and 24 ± 1°C ambient temperature with a 12-hour light–dark cycle and free access to food and water. Animal welfare was ensured according to relevant international experimental animal rules and guidelines. All procedures involving experimental animals were approved by the University Committee on the Use and Care of Animals at Chengdu University of Traditional Chinese Medicine.
Equipment and materials
An environmental simulation chamber was obtained from Chongqing Inborn Experiment Instrument Co., Ltd. (Chongqing, China). An ultrasonic humidifier (YC-D209) was purchased from Beijing Yadu Indoor Environmental Protection Sci & Tech Co., Ltd. (Beijing, China). A Morris water maze system was obtained from Shanghai Mobile Datum Science & Technology Co., Ltd. (Shanghai, China). An open-field analysis system was purchased from Chengdu Techman Software Co., Ltd. (Chengdu, China). Enzyme-linked immunosorbent assay (ELISA) kits for detecting rat CRH, ACTH, and CORT were purchased from Shanghai Xinle Sci & Tech Co., Ltd. (Shanghai, China). A GR in situ hybridization kit was purchased from Boster Biotech Co., Ltd. (Wuhan, China). A BA400 digital camera microscope system and image analysis software, Image-Pro Plus 6.0, were donated by Motic China Group Co., Ltd. (Chengdu, China).
Drugs
HQ decoction comprises the water extract of seven traditional drugs: Radix Scutellariae (Scutellaria baicalensis Georgi), 9 g; talcum [Mg3(Si4O10)(OH)2)], 9 g, Poria [Poria cocos (Schw.) Wolf], 9 g; Polyporus [Polyporus umbellatus (Pers.) Fries], 9 g; Pericarpium Arecae (Areca catechu L.), 6 g, Fructus Amomi Rotundus (Amomum kravanh Pierre ex Gagnep), 3 g; and Medulla Tetrapanacis [Tetrapanax papyriferus (Hook.). Koch)] 3 g. The drugs were obtained from Derentang Pharmacy Co., Ltd. (Chengdu, China) and authenticated by Professor HU Yonghe, Department of TCM, Rheumatology Center of Integrated Medicine, the General Hospital of Western Theater Command PLA. The quality of voucher specimens was verified using two-dimensional liquid chromatography, and the drugs were subsequently stored at Chengdu University of Traditional Chinese Medicine. As described previously,39 the HQ decoction was prepared and concentrated in the routine dosage ratio. Briefly, HQ decoction materials were boiled in distilled water for approximately 1 hour to produce a concentrated solution. Next, the water extract was filtered through Whatman No.1 filter paper, resulting in a yield of 1 mL of concentrated solution containing 0.8 g herbal extracts which was stored at 4°C before being administered to rats. Under ultraperformance liquid chromatography (UPLC), the chemical composition of the extract was shown to consist mainly of flavonoids from Radix Scutellariae (Scutellaria baicalensis Georgi), including catechin and epicatechin (C15H14O6), chrysin 6-C-β-glucoside 8-C-α-arabinoside (C26H28O13), Scutellarin (C21H18O12), 4'-hydroxywogonin-7-O-glucuronide (C22H20O12), baicalin (C21H18O11), dihydrobaicalin (C21H20O11), apigenin-7-O-glucuronide (C21H18O11), baicalein-5-O-glucoside (C21H18O11), 4'-hydroxywogonin-5-O-glucuronide (C22H20O12), wogonoside (C22H20O11), 4'-hydroxywogonin-4-O-glucuronide (C22H20O12), scutellarin-5-O-glucuronide (C22H20O11), 4'-methoxy baicalein-7-O-glucuronide (C23H22O12), baicalein (C15H10O5), wogonin (C16H12O5) and skullcapflavone-II (C19H18O8). Mifepristone tablets (batch no. 43170108; donated by CR Zizhu Pharmaceutical Co., Ltd., Beijing, China) were dissolved in pure water to 2.5 mg/mL and stored at 4°C until use.
Groups and treatments
All rats were randomly assigned into 4 groups (10 rats per group): rats without stress (control group); rats induced with hT and hH (hT-hH group); rats induced with hT and hH and treated with HQ decoction (hT-hH-HQ group); and rats induced with hT and hH and treated with mifepristone (C29H35NO2) (hT-hH-Mi group). Mifepristone, a GR antagonist and a regulator of the function of the HPA axis,37,38 was used as a control in the hT-hH-Mi group. Treatments and humidity and temperature conditions were as follows. (1) In the control group, rats were maintained at 24°C with 50% humidity and without environmental stress. (2) In the hT-hH group, rats were stressed with exposure to hT of 35 ± 1°C and hH of 85 ± 5% humidity. (3) In the hT-hH-Mi group, rats were housed under the same environmental conditions as those in the hT-hH group, and were administered mifepristone (25 mg/kg daily) by gastric lavage. (4) In the hT-hH-HQ group, rats were housed under the same environmental conditions as those in the hT-hH and hT-hH-Mi groups, and were administered HQ decoction (1 mL/100 g daily) by gastric lavage. As described previously,39 HQ decoction and mifepristone was prepared and concentrated in the routine dosage ratio (conversion ratio from a 70-kg man to a 200-g rat = 0.018).
Stress induction
An environmental simulation chamber was used to expose the rats to heat and humidity. Prior to stimulation, a complete, standard, and customized environmental–temperature–humidity cycle was programmed using a temperature–humidity controller as previously described.40 In each cycle, the program was initiated for 1 second, followed by step 1 with +24°C and 50% humidity for 4 hours (control group). Steps 2, 3, and 4 were conducted under the same conditions, with +35°C and +85% humidity for 4 hours (for the hT-hH, hT-hH-Mi, and hT-hH-HQ groups). The ramp time between steps was 30 minutes. Finally, the program remained on hold or idle for the next round. The chamber maintained the humidity ranges of ±5% and temperature ranges of ±1°C. Rats in each group received one round of stress from a crankcase heater (SHH250GS; Chongqing Inborn Experiment Instrument Co., Ltd.) and the ultrasonic humidifier every day for 3 weeks. All rats were closely monitored for possible adverse events during the use of the environmental simulation chamber. After 3 weeks, all rats were subjected to the Morris water maze test and open-field test to assess cognitive and behavioral stress-induced effects.
Morris water maze test
The Morris water maze system was used to evaluate hippocampal-dependent learning and memory among the four groups. The system consisted of a circular tank, 1 m in diameter, which was divided into four quadrants. The tank was filled with opaque water and maintained at a controlled temperature between 21.5°C and 22.5°C to minimize stress. The water level was at least 1 cm above the upper surface of the platform. The rats were trained in different quadrants for 3 consecutive days. Each rat was given 2 minutes to find the way to the platform. If the rat could not locate the platform within 2 minutes, the rat would be gently guided to the platform. During the test, the total swimming distance, escape latency, and number of times that the platform was reached were recorded using a video tracking device.
Open-field test
The open-field analysis system consisted of a closed-wall chamber measuring 100 cm (length) × 100 cm (width) × 35 cm (height), constructed out of white high-density nonporous plastic. The floor was textured for traction during ambulation, while the maze walls were smooth. Illumination was provided by a 40-W incandescent lamp that was raised 2.8 m above the center of the chamber. A smart video tracking system was used to record and evaluate movement. As previously described,41 the chamber was sanitized with 95% ethanol prior to initial use and before subsequent tests to remove any scent clues left by the previous rat subject. The rats were allowed no fewer than 30 minutes to acclimatize to the procedure room prior to the test. Each rat underwent one test, during which time the rat was placed facing the wall into one of the four corner squares and allowed to freely explore the environment for 3 minutes. In the open-field test session, durations of rest or movement, central or peripheral stay, and deep rearing were automatically monitored and recorded.
Histological examination of hypothalamus
Three weeks after the start of stress induction, all rats were sacrificed and the brains were separated from the skulls. Brain specimens were fixed in 4% phosphate-buffered formaldehyde for 8 hours, dehydrated in graded series ethanol, cleared with dimethylbenzene, and embedded in paraffin blocks. Next, the specimens were coronally cut into a series of slices of 4-µm thickness, splayed on the water surface, and mounted on microscope slides. Routine hematoxylin and eosin (H&E) staining was performed as described in the following steps. (1) The sections were deparaffinized in xylene I, II, and III for 10 minutes each, rehydrated in a graded series of ethanol for 3 minutes each, and rinsed in distilled water for 3 minutes. (2) The sections were stained with hematoxylin for 20 minutes, rinsed with running tap water for 3 minutes, decolorized with acid alcohol for 5 seconds, rinsed well with tap water for 3 minutes, and immersed in water at 50°C until stained blue. This was followed by 3 minutes of rinsing with tap water and 5 minutes of immersion in 85% ethanol. (3) The sections were counterstained in eosin for 5 minutes and again dehydrated in a graded series of ethanol for 3 minutes each. Subsequently, the sections were cleared in xylene I and II for 5 minutes each and mounted with neutral balsam.
Measurement of GR expression
In situ hybridization was used to detect the expression of GR mRNA in cryosections after 3 weeks. Oligonucleotide probes were 5’-AGGTT TCTGC GTCTT CACCC TCACT GGCTG-3’, 5’-AAGAG CAGTG GAAGG ACAGC ACAAT TACCT-3’, and 5’-AGCTG AAATC ATCAC CAATC AGATA CCAAA-3’. The cryosections were stained with hematoxylin for 20 minutes and fixed with 4% paraformaldehyde containing 1% diethyl pyrocarbonate/0.1M phosphate-buffered saline at room temperature for 30 minutes. The cryosections were immersed in hydrogen peroxide for 30 minutes and washed with distilled water for 5 minutes three times. The cryosections were digested with pepsin at room temperature for 60 seconds, followed by post-fixing with 1% paraformaldehyde containing 1% diethyl pyrocarbonate/0.1M phosphate-buffered saline at room temperature for 10 minutes. The sections were washed again with distilled water, followed by prehybridization at 40°C overnight and hybridization at 42°C for 3 hourd. They were washed using a standard saline citrate solution followed by confining liquid at 37°C for 30 minutes. Biotinylated rat anti-digoxin was added at 37°C for 60 minutes. The streptavidin–biotin complex was added at room temperature for 30 minutes. Biotinylated peroxidase was added at 37°C for 20 minutes. After color development using 3,3'-diaminobenzidine, dehydration with alcohol, and clearing with dimethylbenzene, the sections were mounted onto slides. GR mRNA expression was observed under a light microscope at a magnification of 10 × 40. Images were obtained using a BA200Digital digital camera microscope system (Motic China Group Co., Ltd.). The images were digitized using a frame grabber and displayed on an RGB 17-inch screen with a resolution of 760 × 560 pixels per microscopic field. As previously described, the results were indicated as means of integrated optical density (MIOD).42 The entire spectrum of optical density ranged between 0 (complete brightness) and 250 (complete darkness). A single pixel in the image measured 0.14407625911 µm2 by spatial calibration in the actual ground coverage area.
Enzyme-linked immunosorbent assay for serum CRH, ACTH, and CORT
To prevent butylone-related bias during the experiments, blood was sampled from eight randomly selected normal SD rats prior to and 20 minutes after anesthesia to detect the serum levels of CRH, ACTH and CORT. No significant difference was observed in these hormones before and after anesthesia. Therefore, all experimental rats were anesthetized using butylone (3 mg/100 g body weight for at least 20 minutes) after 3 weeks, and non-anticoagulated blood samples were obtained via the abdominal aorta. The blood samples were centrifuged at 5000×g for 15 minutes at room temperature. The resulting supernatant was collected and stored at −20°C until use. ELISA was performed as follows: first, 40 µL of sample; 10 µL of detection antibody of CRH, or ACTH, or CORT; and 50 µL of horseradish peroxidase-conjugated streptavidin were added to the appropriate well of the assay plate. Next, the plate was sealed and incubated at 37°C for 60 minutes, followed by five washings with washing buffer containing 10 mM phosphate buffer pH 7.4, 150 mM NaCl, and 0.05% Tween 20. Second, 50 µL of color developing agent A and 50 µL of color developing agent B were added to each well and the plate was sealed and vibrated slightly at 37°C for 10 minutes. Finally, the reaction was stopped with 50 µL of 0.5M H2SO4. A Multiskan Mk3 microplate reader (Thermo Fisher Instrument Co., Ltd., Shanghai, China) was used to measure the absorbance at 450 nm.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPPS Inc., Chicago, IL, USA). The count variables were frequency and percentage and the measurement variables were statistically described using mean ± standard deviation (SD). The Kolmogorov–Smirnov test was used to examine the normal distribution of the tested variables. Where normal distribution was observed, comparisons among multiple groups were performed using one-way analysis of variance, and the LSD (least significant difference)-t was used for comparisons between two groups. Where variables did not follow a normal distribution, the medians of multiple groups were compared using the Kruskal–Wallis test, the two groups were compared using the Mann–Whitney U test, and the P value was calibrated using the Bonferroni method. Values of P value ≤ 0.05 were considered statistically significant.
Results
General phenotypes of stressed rats following exposure to hT and hH
Simple morphological and behavioral characteristics were compared by observation in rats with and without exposure to hT and hH to confirm the establishment of the animal model. Three weeks after stress induction, all rats exhibited symptoms such as loss of appetite, greasy or watery stool, weight loss, secretions around the eyes, constant scratching, scabs in hair, hair loss, reduced water intake, and easy alarming. These phenotypes appeared to be more frequently observed in stressed rats compared with rats in the control group, indicating a possible effect of hT and hH on the general health status of rats. The Morris water maze test and open-field test were subsequently conducted to determine out the therapeutic effects of mifepristone and HQ on mental health.
Mifepristone or HQ reversed the compromised ability of stressed rats in the Morris water maze test
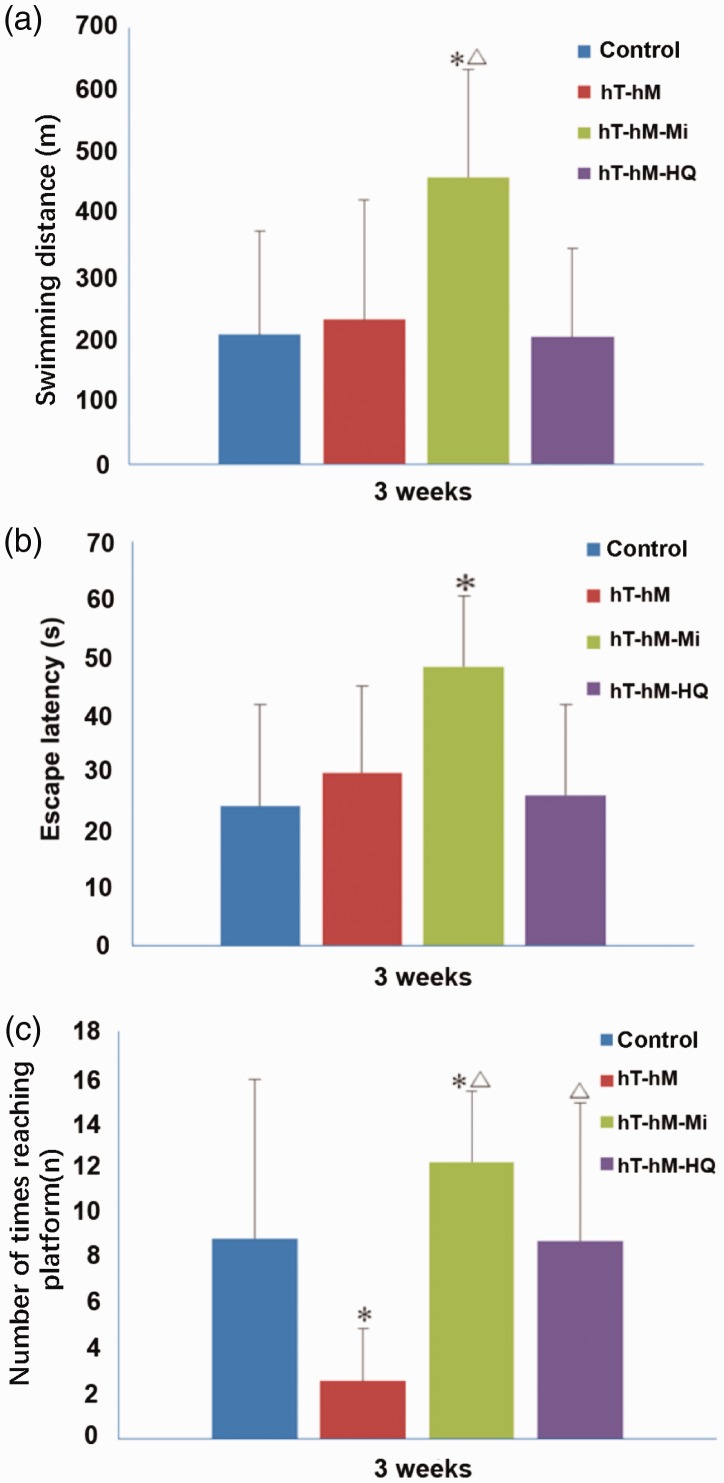
All rats underwent the Morris water maze test 3 weeks after stress induction and parameters including swimming distance, escape latency, and number of times reaching the platform were recorded. As shown in Figure 2a, no significant differences in swimming distance were observed among the control, hT-hH, and hT-hH-HQ groups. However, rats that received mifepristone in the hT-hH-Mi group covered a significantly greater distance compared with the other groups. Similar trends were observed for escape latency, with only rats in the hT-hH-Mi group showing a significantly longer escape latency compared with the control group (Figure 2b). Notably, a significant difference was observed in the number of times that the platform was reached between rats in the control (8.86 ± 6.98) and hT-hH (2.57 ± 2.32) groups. A single administration of either mifepristone or HQ significantly enhanced the number of times that the platform was reached in both the hT-hH-HQ and hT-hH-Mi groups (Figure 2c). These data indicate that hT- and hH-induced stress may compromise the ability of rats to reach the platform, and that this effect could be reversed by either mifepristone or HQ administration.
Figure 2.
Results of the Morris water maze test in control and stressed rats 3 weeks after treatment. Parameters including (a) swimming distance, (b) escape latency, and (c) number of times reaching the platform are shown. *P < 0.05, compared with the control group; △P < 0.05, compared with the hT-hH group. hH, high-humidity; hT, high-temperature.
Mifepristone or HQ significantly increased the central-to-peripheral ratio and incidence of deep rearing in the open-field test
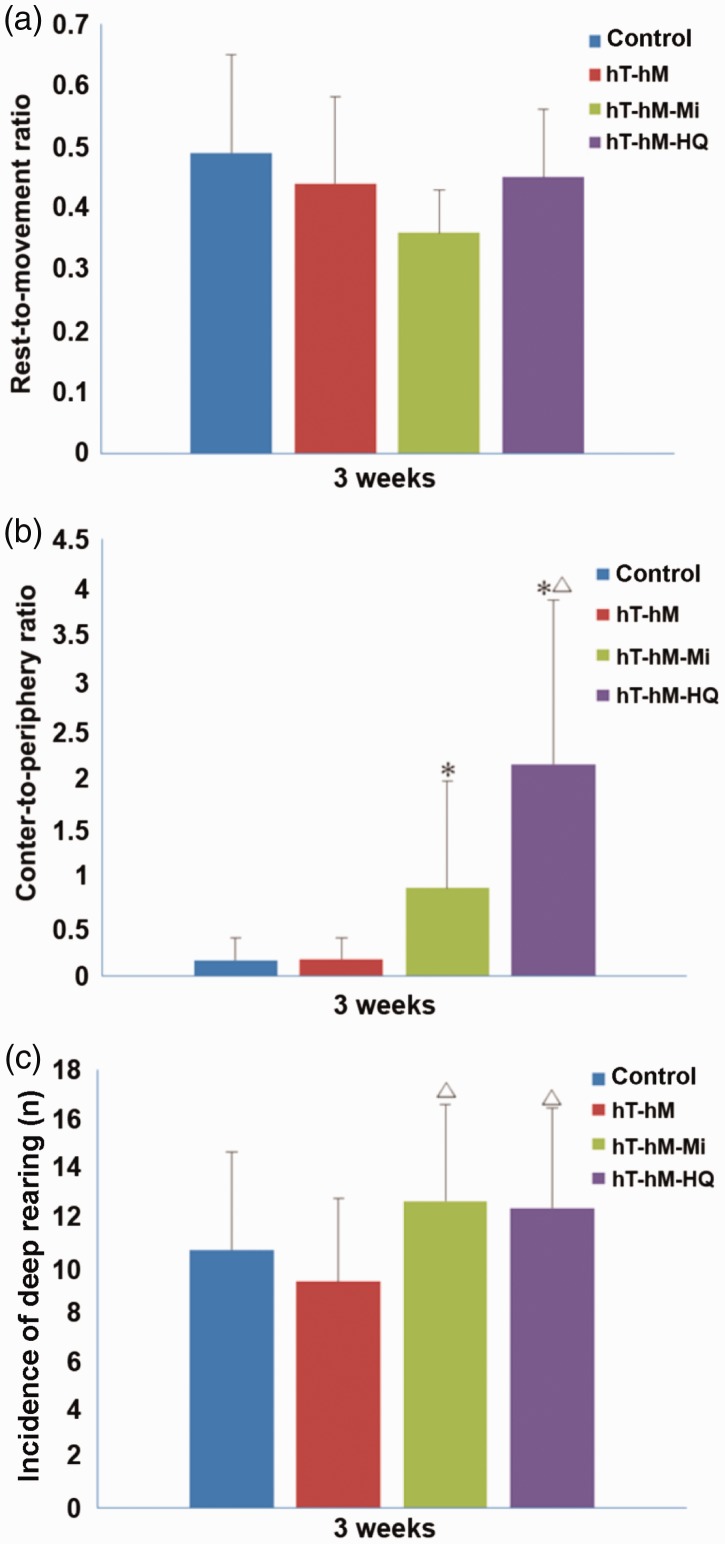
In the open-field test, the rest-to-movement ratio and the central-to-peripheral ratio were calculated for each rat during observation periods. Furthermore, the incidence of deep rearing was recorded and compared. Exposure to hT and hH appear to exert a minor effect on the performance of rats in the open-field test 3 weeks after stress induction, as there were no significant differences between the control and hT-hH groups in terms of the rest-to-movement ratio (Figure 3a), central-to-peripheral ratio (Figure 3b), and incidence of deep rearing (Figure 3c). However, mifepristone or HQ significantly improved cognitive ability, as demonstrated by the increased central-to-peripheral ratio (Figure 3b) and incidence of deep rearing (Figure 3c) in the hT-hH-HQ and hT-hH-Mi groups.
Figure 3.
Results of the open-field test in control and stressed rats 3 weeks after treatment. Parameters including (a) rest-to-movement ratio, (b) central-to-peripheral ratio, and (c) incidence of deep rearing are shown. *P < 0.05, compared with the control group; △P < 0.05, compared with the hT-hH group. hH, high-humidity; hT, high-temperature.
Mifepristone or HQ reversed histological changes in the hypothalami of stressed rats
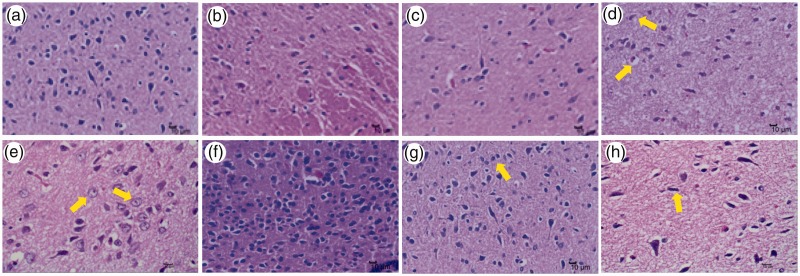
Rat hypothalami were stained with H&E to investigate the cellular mechanism by which mifepristone and HQ may mitigate the compromised cognitive ability observed in stressed rats. As expected, limited pathological changes were observed in rat hypothalami among the control (Figure 4a), hT-hH-Mi (Figure 4b), and hT-hH-HQ (Figure 4c) groups in terms of neural degeneration and accumulation and basophilic enhancement of nerve cells 3 weeks after treatment. In contrast, vacuolar degeneration (Figure 4d), ballooning degeneration (Figure 4e), and accumulation and basophilic enhancement of nerve cells (Figure 4f) were clearly apparent in rat hypothalami of the hT-hH group. In addition, glial cell hyperplasia (Figure 4g) and nerve cell atrophy (Figure 4h) were observed in the hT-hH group.
Figure 4.
Histological changes in hypothalami of hT- and hH-stressed rats 3 weeks after treatment. Representative images of H&E staining of rat hypothalami are shown at × 400 magnification. Pathological changes in nerve cells in the hT-hH group are indicated with yellow arrows. Limited pathological changes were observed in rat hypothalami in the control (a), hT-hH-Mi (b), and hT-hH-HQ (c) groups, while in the hT-hH group, vacuolar degeneration (d), ballooning degeneration (e), and accumulation and basophilic enhancement of nerve cells (f) were observed. Glial cell hyperplasia (g) and nerve cell atrophy (h) were also observed in the hT-hH group. hH, high-humidity; hT, high-temperature; HQ, Huang Qin Hua Shi; H&E, hematoxylin and eosin; Mi, mifepristone.
Mifepristone or HQ significantly reduced GR mRNA expression in the hypothalami of stressed rats
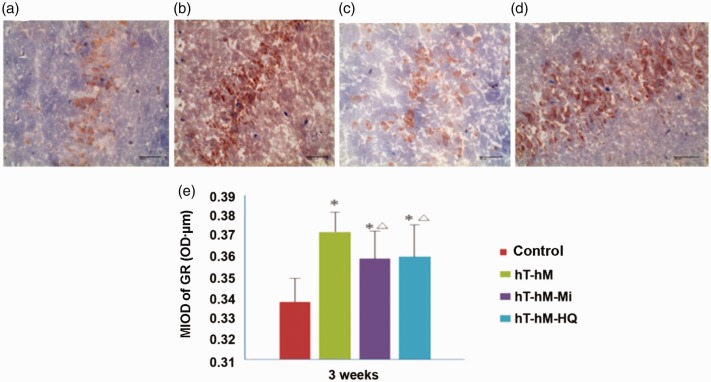
The expression of GR mRNA was evaluated in rat brains using in situ hybridization to determine whether mifepristone and HQ could restore internal homeostasis by regulating the HPA axis. Under light microscopy, blue-stained areas were considered negative for hybridization while the yellow- or brown-stained areas were considered positive. As shown in Figure 5, brain tissues showed fewer positively stained regions in the control, hT-hH-Mi, and hT-hH-HQ groups. However, the stressed group (hT-hH) showed more positively stained regions (Figure 5b). The positive expression of GR was generally distributed in the cytoplasm of brain cells. MIOD in the hT-hH groups was 0.3722 ± 0.0096 OD ⋅ µm, which was significantly higher than that in the control group (P < 0.05). Significant differences were also observed between the drug-treated groups (hT-hH-Mi and hT-hH-HQ) and the stressed groups (hT-hH). MIOD in the hT-hH-Mi group (0.3591 ± 0.0135 OD ⋅ µm) and the hT-hH-HQ group (0.3601 ± 0.0156 OD ⋅ µm) indicated a significant decrease compared with that in the hT-hH group (P < 0.05, Figure 5e).
Figure 5.
Expression of GR mRNA in hypothalami of hT- and hH-stressed rats 3 weeks after treatment. (a–d) Representative images showing the results of in situ hybridization of GR mRNA in the (a) control group, (b) hT-hH group, (c) hT-hH-Mi group, and (d) hT-hH-HQ group. Scale bar, 40 µm. (e) Summary of MIOD of GR mRNA in all groups. *P < 0.05, compared with the control group; △P < 0.05, compared with the hT-hH group. GR, glucocorticoid receptor; hH, high-humidity; HQ, Huang Qin Hua Shi; hT, high-temperature; Mi, mifepristone; MIOD, means of integrated optical density.
Mifepristone or HQ significantly reduced serum CRH, ACTH, and CORT in stressed rats
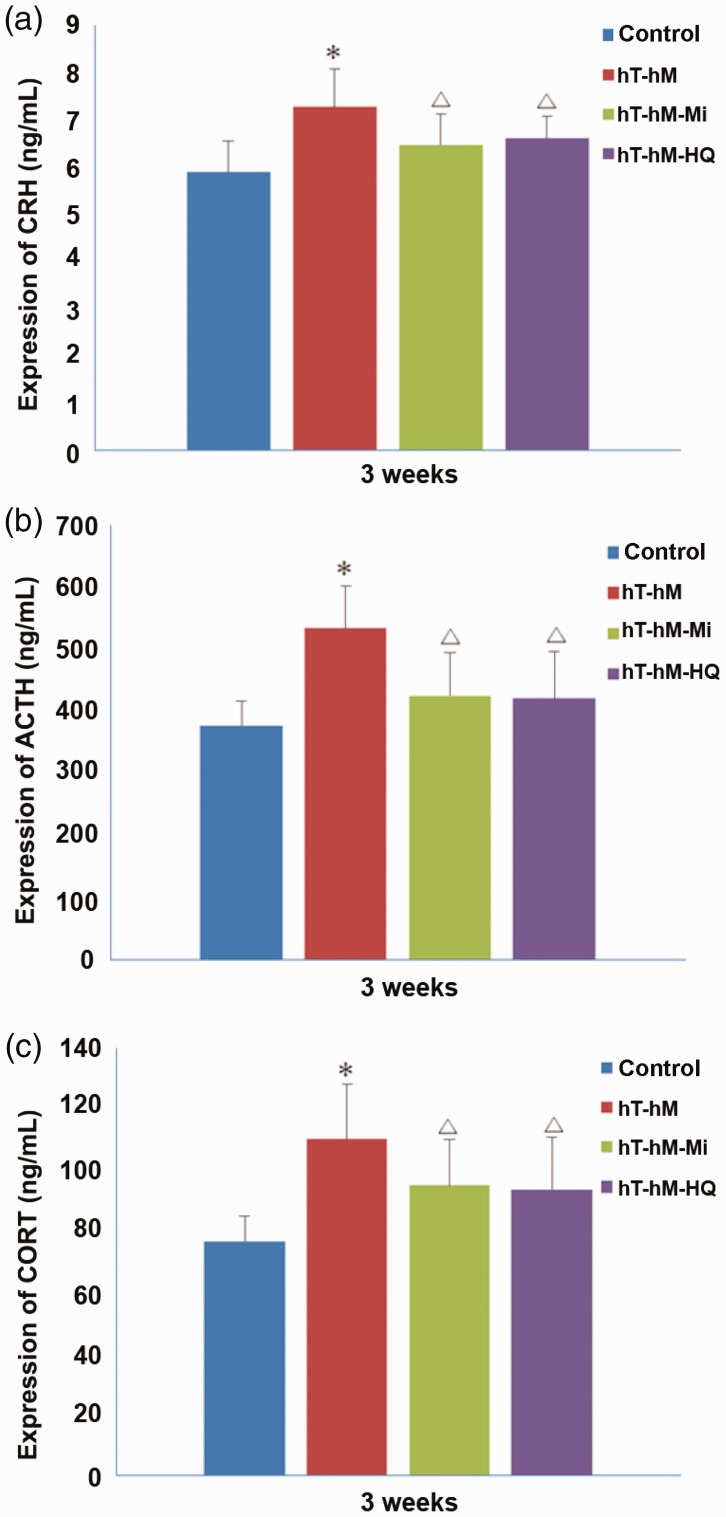
The levels of HPA axis–related hormones CRH, ACTH, and CORT were evaluated in rat serum by ELISA. The serum levels of CRH (Figure 6a), ACTH (Figure 6b), and CORT (Figure 6c) in the hT-hH group were 7.27 ± 0.80 ng/L, 533.15 ± 67.40 ng/L, and 110.09 ± 18.12 ng/L, respectively, and were significantly higher than the levels in the control group (P < 0.05). The expression level of CRH (6.60 ± 0.47 ng/L), ACTH (420.75 ± 75.36 ng/L), and CORT (93.63 ± 17.01 ng/L) showed a significant decrease in the hT-hH-HQ group compared with the hT-hH group (P < 0.05). Similarly, the serum levels of these hormones were significantly decreased in the hT-hH-Mi group compared with the hT-hH group (Figure 6c). Therefore, both mifepristone and HQ significantly reduced the release of HPA axis–related hormones into the serum of rats stressed by hT and hH.
Figure 6.
Serum levels of CRH, ACTH, and CORT in hT- and hH-stressed rats 3 weeks after treatment. Serum levels of (a) CRH, (b) ACTH, and (c) CORT were measured by ELISA. *P < 0.05, compared with the control group; △P < 0.05, compared with the hT-hH group. ACTH, adrenocorticotropic hormone; CORT, corticosteroid; CRH, corticotropin-releasing hormone; ELISA, enzyme-linked immunosorbent assay; hH, high-humidity; hT, high-temperature.
Discussion
Cognitive impairment induced by long-term humidity and heat exposure has been well described, but the mechanisms of action of drugs capable of mitigating this effect remain unclear. In the present study, the potential therapeutic effects of HQ decoction on the HPA axis were explored in SD rats stressed by hT and hH. We found that, under hT- and hH, all stressed rats tended to show more evident morphological and behavioral changes compared with control, indicating a potential stress response.
To confirm this finding, rats were subjected to the Morris water maze test and the open-field test to explore cognitive–behavioral changes after 3 weeks of exposure to stress. We found that the number of times of reaching the platform, central-to-peripheral ratio, and incidence of deep rearing in both the hT-hH-HQ and hT-hH-Mi groups were significantly increased, while rats in the stressed group reached the platform significantly fewer times and showed higher escape latency compared with the control group. This finding indicates that stress induced by environmental factors such as hT (35 ± 1°C) and hH (85 ± 5%) can lead to cognitive–behavioral changes that could be reversed by either mifepristone or HQ. However, in the Morris water maze test, only the number of times of reaching the platform was significantly different in both the hT-hH-HQ and hT-hH-Mi groups compared with the hT-hH group, while no difference in swimming distance or escape latency was observed. Furthermore, the number of times of reaching the platform, swimming distance, and escape latency were significantly different only in the hT-hH-Mi group, and not in the hT-hH-HQ group, compared with the control group. This finding may be indicative of experimental bias, meaning that further animal experiments are required.
To further clarify the cellular mechanism by which mifepristone and HQ may mitigate cognitive ability in stressed rats, pathological observation and analysis were performed. Pathologic changes such as the accumulation and basophilic enhancement of nerve cells and neural degeneration were observed only in the stressed group, indicating that histological changes in the hypothalami of stressed rats might be reversible using mifepristone or HQ.
Activation of the HPA axis is typically the first response to stress, and results in the secretion of CRH from the paraventricular nucleus, ACTH from the pituitary gland, and circulating glucocorticoid to maintain internal homeostasis.25,26 Glucocorticoid subsequently binds to its receptor to mediate peripheral secretion of CRH and CORT. To determine whether mifepristone and HQ could mitigate for these effects by regulating the HPA axis, we first examined the expression of GR mRNA in the hypothalami and the level of serum ACTH in rats. Significant differences were observed between the drug-treated groups (hT-hH-Mi and hT-hH-HQ) and the stress-treated groups (hT-hH), indicating that excessive circulating levels of ACTH and GR induced by hT and hH could be reduced using mifepristone or HQ. Next, we explored the serum levels of CRH and CORT in the rat groups. As expected, the expression of CRH and CORT differed significantly in the stressed group compared with the control group. These findings indicate that environmental or indoor humidity may damage the HPA axis in rats under hT- and hH-induced stress, which is associated with cognitive–behavioral disorders, endocrine disorders, and hormone imbalance.
Rats were treated with mifepristone or HQ to further evaluate the effects of these agents stress induced by hT and hH. In the hT-hH-Mi group, 25 mg/kg mifepristone was administered for 3 weeks. Mifepristone, a GR antagonist, was approved by the United States Food and Drug Administration in 2012 for treating patients with hyperglycemia secondary to Cushing’s syndrome37 and has been reported to reduce HPA axis activation and restore weight through adipose tissue recovery.38 In the present study, variables including swimming distance, number of times of reaching the platform, expression of GR mRNA in the hypothalami, and serum levels of CRH, ACTH, and CORT were significantly improved following mifepristone treatment compared with control, which was consistent with previous reports.42 Similar results were found for rats treated with HQ decoction (hT-hH-HQ group). According to TCM theory, the pharmacological properties of Radix Scutellariae (Scutellariabaicalensis Georgi), including the ability to clear heat and eliminate dampness, are indicated for treating damp-heat syndrome.43 HQ decoction is a water extract of seven traditional drugs and contains a mixture of flavonoids. UPLC has shown that most of the flavonoids derive from the herb Radix Scutellariae (Scutellaria baicalensis Georgi), which has recently been shown to attenuate chronic mild unpredictable stress-induced depressive-like behavior.36 Therefore, we sought to determine whether hT- and hH-induced stress in rats could be improved using HQ decoction, with mifepristone as an active comparator. As expected, HQ decoction-treated rats showed a significant improvement in cognitive–behavioral disorders, expression of GR mRNA in the hypothalami, and serum levels of CRH, ACTH, and CORT. Hence, the protective effect of HQ decoction may potentially be associated with the downregulation of GR mRNA and reduction of CRH, ACTH, and CORT. Similar to mifepristone, HQ decoction appears to mitigate the damage to cognitive ability and to significantly attenuate HPA axis signaling in stressed rats, indicating a novel cellular and molecular basis for the clinical application of HQ.
In conclusion, all rats stressed by hT and hH showed various stress responses and dysregulation of the HPA axis, as evaluated using cognitive–behavioral tests, pathological analysis, and detection of the expression of HPA axis-associated molecules. The stress-induced responses and disorders included significant cognitive–behavioral changes; pathological lesions such as vacuolar and ballooning degeneration, glial cell hyperplasia, and nerve cell atrophy in rat hypothalami; and active expression of GR mRNA in the hypothalami and active secretion of CRH, ACTH, and CORT in blood. These effects were improved by treatment with HQ decoction. Similar to mifepristone, HQ decoction demonstrated a protective role against hT- and hH-induced stress in rats which may be associated with the downregulation of GR mRNA, CRH, ACTH, and CORT in the HPA axis. However, the underlying mechanism by which HQ decoction regulates disordered HPA axis signaling requires further investigation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Key Project of the Logistics Research Project of the Whole Army (BWS14J023).
References
- 1.Sharma VM, Pichan G, Panwar MR. Differential effects of hot-humid and hot-dry environments on mental functions. Int Arch Occup Environ Health 1983; 52: 315–327. [DOI] [PubMed] [Google Scholar]
- 2.Fritze JG, Blashki GA, Burke S, et al. Hope, despair and transformation: climate change and the promotion of mental health and wellbeing. Int J Ment Health Syst 2008; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourque F, Willox AC. Climate change: the next challenge for public mental health? Int Rev Psychiatry 2014; 26: 415–422. [DOI] [PubMed] [Google Scholar]
- 4.Berry HL, Bowen K, Kjellstrom T. Climate change and mental health: a causal pathways framework. Int J Public Health 2010; 55: 123–132. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien LV, Berry HL, Coleman C, et al. Drought as a mental health exposure. Environ Res 2014; 131: 181–187. [DOI] [PubMed] [Google Scholar]
- 6.Hayes K, Blashki G, Wiseman J, et al. Climate change and mental health: risks, impacts and priority actions. Int J Ment Health Syst 2018; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soneja S, Chen C, Tielsch JM, et al. Humidity and gravimetric equivalency adjustments for nephelometer-based particulate matter measurements of emissions from solid biomass fuel use in cookstoves. Int J Environ Res Public Health 2014; 11: 6400–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Castro J, Jr, Bolfi F, de Carvalho LR, et al. The temperature and humidity in a low-flow anesthesia workstation with and without a heat and moisture exchanger. Anesth Analg 2011; 113: 534–538. [DOI] [PubMed] [Google Scholar]
- 9.d'Ambrosio Alfano FR, Palella BI, Riccio G. Thermal environment assessment reliability using temperature–humidity indices. Ind Health 2011; 49: 95–106. [DOI] [PubMed] [Google Scholar]
- 10.Valtonen V. Clinical diagnosis of the dampness and mold hypersensitivity syndrome: review of the literature and suggested diagnostic criteria. Front Immunol 2017; 8: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi J, Kim J, Si XA, et al. Hygroscopic aerosol deposition in the human upper respiratory tract under various thermo-humidity conditions. J Environ Sci Health A Tox Hazard Subst Environ Eng 2013; 48: 1790–1805. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs J, Borras-Santos A, Krop E, et al. Dampness, bacterial and fungal components in dust in primary schools and respiratory health in schoolchildren across Europe. Occup Environ Med 2014; 71: 704–712. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Li B, Yu W, et al. Rhinitis symptoms and asthma among parents of preschool children in relation to the home environment in Chongqing, China. PLoS One 2014; 9: e94731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karvala K, Nordman H, Luukkonen R, et al. Asthma related to workplace dampness and impaired work ability. Int Arch Occup Environ Health 2014; 87: 1–11. [DOI] [PubMed] [Google Scholar]
- 15.Voelker R. Asthma forecast: why heat, humidity trigger symptoms. JAMA 2012; 308: 20. [DOI] [PubMed] [Google Scholar]
- 16.Katoch R, Yadav A, Godara R, et al. Prevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J Parasit Dis 2012; 36: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eze JI, Scott EM, Pollock KG, et al. The association of weather and bathing water quality on the incidence of gastrointestinal illness in the west of Scotland. Epidemiol Infect 2014; 142: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HG, Kim TM, Park G, et al. Repeated heat exposure impairs nigrostriatal dopaminergic neurons in mice. Biol Pharm Bull 2013; 36: 1556–1561. [DOI] [PubMed] [Google Scholar]
- 19.Sinha RK. An approach to estimate EEG power spectrum as an index of heat stress using backpropagation artificial neural network. Med Eng Phys 2007; 29: 120–124. [DOI] [PubMed] [Google Scholar]
- 20.Ding N, Berry HL, Bennett CM. The importance of humidity in the relationship between heat and population mental health: evidence from Australia. PLoS One 2016; 11: e0164190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inan SY, Aksu F. Amnesic effects of relative humidity and temperature in mice. Lab Anim (NY) 2002; 31: 40–48. [DOI] [PubMed] [Google Scholar]
- 22.Zhu G, Janjetovic Z, Slominski A. On the role of environmental humidity on cortisol production by epidermal keratinocytes. Exp Dermatol 2014; 23: 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahng JW, Lee JH. Activation of the hypothalamic-pituitary-adrenal axis in lithium-induced conditioned taste aversion learning. Eur J Pharmacol 2015; 768: 182–188. [DOI] [PubMed] [Google Scholar]
- 24.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem 2014; 112: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burford NG, Webster NA, Cruz-Topete D. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int J Mol Sci 2017; 18: pii: E2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo T, McQueen A, Chen TC, et al. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol 2015; 872: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav 2014; 38: 148–159. [DOI] [PubMed] [Google Scholar]
- 28.Gray JD, Kogan JF, Marrocco J, et al. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat Rev Endocrinol 2017; 13: 661–673. [DOI] [PubMed] [Google Scholar]
- 29.Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry 2014; 171: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 30.Thiagarajah AS, Eades LE, Thomas PR, et al. GILZ: glitzing up our understanding of the glucocorticoid receptor in psychopathology. Brain Res 2014; 1574: 60–69. [DOI] [PubMed] [Google Scholar]
- 31.Romanov RA, Alpár A, Hökfelt T, et al. Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus. J Endocrinol 2017; 232: R161–R172. [DOI] [PubMed] [Google Scholar]
- 32.Spijker AT, Giltay EJ, van Rossum EF, et al. Glucocorticoid and mineralocorticoid receptor polymorphisms and clinical characteristics in bipolar disorder patients. Psychoneuroendocrinology 2011; 36: 1460–1469. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Sun BG, Zhang SJ, et al. Observations of TCRVbeta gene expression in rats with dampness syndrome. Evid Based Complement Alternat Med 2014; 2014: 373608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Y, Liu L, He X, et al. The th17/treg immune balance in ulcerative colitis patients with two different chinese syndromes: dampness-heat in large intestine and spleen and kidney yang deficiency syndrome. Evid Based Complement Alternat Med 2015; 2015: 264317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Lin Y, Zhang S, et al. Correlation between pathogenesis of dampness syndrome and interleukin-2, interleukin-8 in rats. J Tradit Chin Med 2013; 33: 114–118. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Guo L, Ji Z, et al. Radix scutellariae attenuates CUMS-induced depressive-like behavior by promoting neurogenesis via cAMP/PKA pathway. Neurochem Res 2018; 43: 2111–2120. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Fang M, Davies H, et al. Mifepristone: a potential clinical agent based on its anti-progesterone and anti-glucocorticoid properties. Gynecol Endocrinol 2014; 30: 169–173. [DOI] [PubMed] [Google Scholar]
- 38.Bou Khalil R, Smayra V, Saliba Y, et al. Mifepristone reduces hypothalamo-pituitary-adrenal axis activation and restores weight loss in rats subjected to dietary restriction and methylphenidate administration. Neurosci Res 2017; 135: 46–53. [DOI] [PubMed] [Google Scholar]
- 39.Yang M, Xiao C, Wu Q, et al. Anti-inflammatory effect of Sanshuibaihu decoction may be associated with nuclear factor-kappa B and p38 MAPK alpha in collagen-induced arthritis in rat. J Ethnopharmacol 2010; 127: 264–273. [DOI] [PubMed] [Google Scholar]
- 40.Even-Tzur N, Zaretsky U, Grinberg O, et al. Climate chamber for environmentally controlled laboratory airflow experiments. Technol Health Care 2010; 18: 157–163. [DOI] [PubMed] [Google Scholar]
- 41.Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 2015; 96: e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang M, Liu C, Niu M, et al. Phage-display library biopanning and bioinformatic analysis yielded a high-affinity peptide to inflamed vascular endothelium both in vitro and in vivo. J Control Release 2014; 174: 72–80. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Chen JX, Li W, et al. Comparative chemical and statistical analysis of cultivated and wild Radix Scutellariae. Am J Chin Med 2011; 39: 1029–1041. [DOI] [PubMed] [Google Scholar]