Short abstract
Objective
To evaluate changes in knee articular cartilage of novice half-marathon runners using magnetic resonance imaging T2 relaxation time mapping.
Methods
Healthy subjects were recruited from local running clubs who met the following inclusion criteria: (i) age 18–45 years; (ii) body mass index less than 30 kg/m2; (iii) had participated in one half-marathon or less (none within the previous 6 months); (iv) run less than 20 km/week; (v) no previous knee injury or surgery; (vi) no knee pain. T2 signals were measured pre- and post-race to evaluate the biochemical changes in articular cartilage after the subjects run a half-marathon.
Results
A significant increase in the mean ± SD T2 relaxation time was seen in the outer region of the medial tibial plateau (50.1 ± 2.4 versus 54.7 ± 2.6) and there was a significant decrease in T2 relaxation time in the lateral femoral condyle central region (50.2 ± 4.5 versus 45.4 ± 2.9). There were no significant changes in the patella, medial femoral condyle and lateral tibia articular surfaces.
Conclusion
An increase in T2 relaxation time occurs in the medial tibial plateau of novice half-marathon runners. This limited region of increased T2 values, when compared with complete medial compartment involvement seen in studies of marathon runners, may represent an association between distance run and changes seen in articular cartilage T2 values.
Keywords: T2 mapping, knee, articular cartilage, half-marathon runners
Introduction
The increasing popularity of distance running races encompasses 2 million participants in the United States, due in part to the public awareness of the positive impact of physical exercise on cardiac health.1,2 There was an observed 300% increase in individuals completing running events between 2002 and 2015; approximately 20 million each year.3 Half-marathons have become more popular as well, seeing an increase from 300 000 finishers in 1990 to 1.9 million in 2016.2 A large proportion of these half-marathon runners are first timers, with older women being the growing demographic.4 Distance running has emerged as a popular recreational activity with corresponding research interest.
Running involves a repetitive joint contact force transmission increase of up to four- to eight-times compared with normal ambulation across the lower extremity joints5 and is implicated as a risk factor for osteoarthritis.2 The intra-articular cartilage of the lower extremities acts as biological shock absorbers that displace physiological load to the subchondral bone.6 While the degeneration of cartilage with prolonged unloading of cartilage has been elucidated,7 the sequelae of repetitive loading in distance running has been a topic of much research and debate. In 1997, a study examined the effects of life-long exercise on canine articular cartilage models and found no difference when compared with sedentary controls.8 This randomized, longitudinal canine trial was an extension of previous short-term strenuous animal studies.8 Despite negative animal model results, and mixed results regarding the relationship between osteoarthritis and physical activity, a longitudinal radiographic study conducted in 2008 over an average of 18 years comparing a group of runners with age-matched controls, found that the runners demonstrated positive evidence of radiographic osteoarthritis at baseline, but the results did not yield an increased prevalence of radiographic osteoarthritis than the control group at final follow-up.9
Research focusing on the use of magnetic resonance imaging (MRI) to specifically evaluate the effects of long-distance running on lower extremity joints has not shown internal derangement in previously uninjured joints in short-term follow-up studies.10 New MRI cartilage imaging techniques such as delayed gadolinium enhanced MRI of cartilage, T1rho and T2 relaxation time mapping have broadened the scope of available research with the biochemical assay of the cartilage.11 These MRI techniques enable interpretation of the changes in proteoglycan, water content and collagen array that occur in articular cartilage prior to the development of radiographic changes of osteoarthritis. A previous study demonstrated the MRI applicability of these changes to knee cartilage signal abnormalities as precursors of morphological defects with osteoarthritis, which serve as biomarkers to ascertain risk profiles for cartilage degeneration.6 The implications of imaging biomarkers expand treatment intervention to the prevention of early degenerative changes, onset of morphological defects and prediction of osteoarthritis.
T2 relaxation time mapping has been validated as a tool for analyzing changes in articular cartilage water content and collagen array, with elevated values representing increased water content and loss of cartilage anisotropy.12–14 Research has shown that T2 mapping sequences could effectively identify healthy and early-stage osteoarthritis cartilage.13,15 A previous MRI assessment observed elevated knee articular cartilage T2 values of distance runners 48 hours after completion of a marathon, with persistent elevations in the medial femoral condyle T2 values at the 3-month follow-up scan.4 Furthermore, medial knee pain, in 80 symptomatic subjects, was associated with cartilage loss in the medial tibia and medial femur.16 The aforementioned changes in high demand sports such as training and marathon running lead us to consider whether similar changes are seen during comparatively less strenuous distances such as that of a half-marathon. The purpose of this study was to identify biochemical and radiographic changes in knee articular cartilage in novice, asymptomatic half-marathon runners. This current study hypothesized that MRI scans after running a half-marathon would detect cellular changes of knee cartilage compared with MRI scans prior to running.
Subjects and methods
Study design and subjects
This prospective study recruited subjects from local running clubs in the Miami area who met the following inclusion criteria: (i) age 18–45 years; (ii) body mass index (BMI) less than 30 kg/m2; (iii) had participated in one half-marathon or less (none within the previous 6 months); (iv) run less than 20 km/week; (v) no previous knee injury or surgery; (vi) no knee pain. Volunteers were identified and screened with a physical examination of the knee as well as a Western Ontario and McMaster Universities Osteoarthritis Index questionnaire by the University of Miami Sports Medicine Institute, Department of Orthopedic Surgery, University of Miami Miller School of Medicine, Jackson Memorial Hospital, Miami, FL, USA between January 2011 and February 2011. All subjects underwent an MRI of their right knee early during training and again between 2 and 14 days after completing a half-marathon.
Institutional Review Board (IRB #20090715) approval was provided prior to initiation of the study by the University of Miami Miller School of Medicine and written informed consent was obtained from all participants.
Magnetic resonance imaging
Magnetic resonance imaging scans were performed using a 1.5 T MR unit (Signa HDx; GE Healthcare, Piscataway, NJ, USA) and a dedicated quadrature extremity coil (Signa HD Knee 8 phased array elements). A routine knee MRI protocol used at the University of Miami Sports Medicine Institute was performed as follows: sagittal, axial and coronal intermediate-weighted fast spin-echo sequences (4000/38 [repetition time ms/echo time ms]; slice thickness, 3.5 mm, and no intersection gap); sagittal fat suppressed intermediate-weighted fast spin-echo sequence (4000/31; slice thickness, 3.5 mm; and no intersection gap). The following parameters were used for all fast spin-echo sequences: field of view (FOV), 15 cm; NEX, 2; matrix size, 512 x 384, echo train length, 1; receiver bandwidth, 31.25; frequency direction, anterior–posterior.
Transverse relaxation values (T2 RVs) of articular cartilage in the medial, lateral and patellofemoral compartments were determined using T2 cartigrams (GE Healthcare) at different slice locations in the sagittal and axial planes; each set of scans with a unique TE value. The following protocols were used: (i) sagittal 8 echo T2 cartigram sequence through the medial compartment (1000/8.24, 16.48, 24.72, 32.96, 41.2, 49.44, 57.68, and 65.92 [repetition time ms/echo time ms]; slice thickness, 3.6 mm; intersection gap, 0.5 mm; FOV, 16 cm; matrix size, 320 × 256; NEX, 1; ASSET factor, 1.00); (ii) sagittal 8 echo T2 cartigram sequence through the lateral compartment (1000/8.24, 16.48, 24.72, 32.96, 41.2, 49.44, 57.68, and 65.92 [repetition time ms/echo time ms]; slice thickness, 3.6 mm; intersection gap, 0.5 mm; FOV, 16 cm; matrix size, 320 × 256; NEX, 1; ASSET factor, 1.00); and (iii) axial 8 echo T2 cartigram sequence through the patellofemoral compartment (1000/8.4, 16.4, 24.6, 32.8, 41, 49.2, 57.4, and 65.6 [repetition time ms/echo time ms]; slice thickness, 3.6 mm; intersection gap, 0.5 mm; FOV, 16 cm; matrix size, 320 × 256; NEX, 1; ASSET factor, 1.00).
Integrated analysis
Two observers (L.Q. & J.J.) blinded to the clinical history and the routine clinical sequences performed the T2 RV determination. Differences between the observers were resolved by consensus. Sagittal T2 cartigram sequences were obtained at different slice locations within the medial and lateral compartments, each with a unique TE value, resulting in a data set of images that represented different T2 weighting. The acquired data were processed using FuncTool (GE Healthcare) to produce color maps that demonstrated subtle changes in the cartilage structure. The T2 maps were displayed using a red-blue color map, where red corresponded to T2 11–25 msec and blue corresponded to 75–89 msec. T2 RVs were generated using T2 cartigram software by placing regions of interest (ROIs) over the articular cartilage in the patella, medial and lateral femoral condyles, and medial and lateral tibial plateaus (Figures 1 and 2). The ROIs were defined less than 3 mm2 in area. T2 RV measurements included the superficial (tangential and transitional) zones of articular cartilage, by placing the ROIs on the central portion of the cartilage. Small ROIs were used to minimize potential partial volume averaging from nonchondral structures. Care was taken to place the ROIs away from peripheral cartilage near the tibial eminence and margins of the tibial plateau, where the oblique orientation of the collagen matrix has been shown to increase T2-weighted signal due to magic angle artifact.
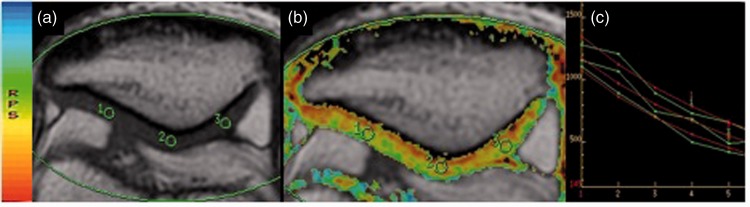
Figure 1.
Representative axial T2 mapping cartigram sequences (a and b) and a transverse relaxation value graph (c) of the patellar articular cartilage. The color version of this figure is available at: http://imr.sagepub.com.
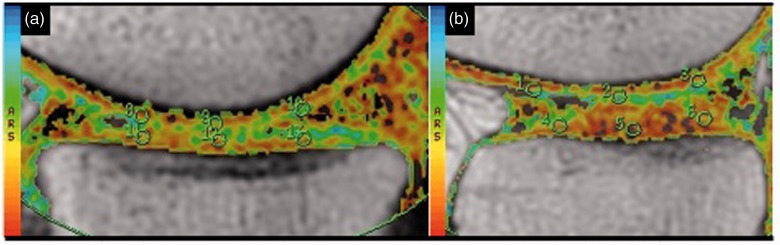
Figure 2.
Representative sagittal T2 mapping cartigram sequences through the medial (a) and lateral (b) compartments demonstrating regions of interest used to calculate transverse relaxation values. The color version of this figure is available at: http://imr.sagepub.com.
The articular surfaces of each medial and lateral femoral condyle as well as the medial and lateral tibial plateau were divided into areas corresponding to the inner, central and outer margins (Figure 3). The patella was divided into lateral, central and medial regions. Patellar, femoral and tibial cartilage was analyzed separately. Using the axial (Figure 1) and sagittal (Figure 2) T2 mapping sequences, ROIs were placed over the anterior, middle and posterior margins of the femoral condyles and tibial plateaus, and the medial, central and lateral margins of the patella. ROIs were defined in articular cartilage over each of these regions. For each ROI, the mean T2 RV and standard deviation (SD) were recorded. Mean T2 values were then calculated for the inner, central and outer regions of the femoral and tibial articular surfaces from the three values obtained in each (anterior, middle and posterior). Patellar mean T2 values for the medial, central and lateral regions were obtained from the mean values of two consecutive axial images. To ensure measurement consistency, the ROIs were placed in the same position for each of the regions between the individual subjects.
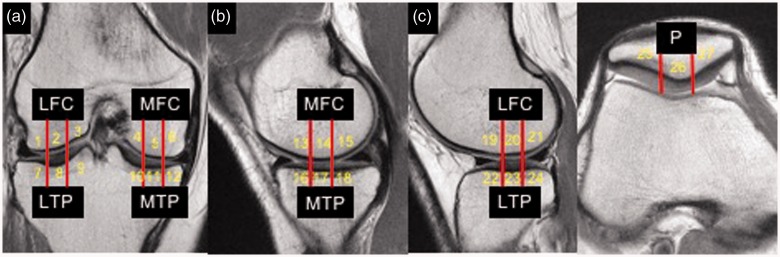
Figure 3.
Representative coronal bicompartment (a), sagittal medial compartment (b), sagittal lateral compartment (c) and axial patellofemoral compartment proton density magnetic resonance images, showing segmentation of articular surfaces all three compartments into 27 quadrants. LFC, lateral femoral condyle; MFC, medial femoral condyle; LTP, lateral tibial plateau; MTP, medial tibial plateau; P, patella. The color version of this figure is available at: http://imr.sagepub.com.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows®. A Wilcoxon Signed Ranks Test was used to compare pre- and post-race T2 relaxation time values. A P-value < 0.05 was considered statistically significant.
Results
This study enrolled six female subjects with a mean age of 33.3 years (range 29–41 years) and a mean BMI of 22.4 kg/m2 (range 19.8–29.9 kg/m2). All subjects had a physical examination of the knee. There was no evidence of effusion, ligamentous instability, joint line tenderness or signs of meniscus pathology. All but one subject was participating in their first half-marathon. Post-race MRIs were obtained a mean of 6 days after the run (range 2.5–14.0 days). All subjects remained asymptomatic throughout the study period.
The MRI results showed that there were no new gross chondral, ligamentous or meniscal lesions seen on the post-race MRIs. Focal regions of low-grade chondral wear remained stable and did not advance to high-grade lesions. Two subjects with pre-existing grade 1 changes to the inner margins of the lateral and medial femoral condyles showed mild increases. One subject with grade 1 changes involving the inner margins of the medial and lateral compartments showed a mild increase involving the medial femoral condyle and no change in the lateral. One subject developed new grade 1 changes in the inner margins of the medial compartment. Two subjects with pre-race grade 2 changes (one lateral femoral condyle, one patellofemoral) remained unchanged on the post-race images.
Pre- and post-race T2 relaxation times for each region are shown in Table 1. A significant increase in mean ± SD T2 values was seen in the outer region of the medial tibia articular surface (50.1 ± 2.4 versus 54.7 ± 2.6, respectively, P = 0.028); however, the adjacent central region did not show a significant increase. A significant decrease in mean ± SD T2 relaxation time was seen in the lateral femoral condyle central region, (50.2 ± 4.5 versus 45.4 ± 2.9, respectively, P = 0.046). There were no significant changes in the patella, medial femoral condyle and lateral tibia articular surfaces.
Table 1.
Comparison of T2 values pre- and post-race by region in healthy female subjects (n = 6) that ran a half-marathon.
| Region | Pre-race T2 value msec | Post-race T2 value msec | Statistical significancea |
|---|---|---|---|
| oLF | 48.4 ± 3.2 | 49.3 ± 3.3 | NS |
| cLF | 50.2 ± 4.5 | 45.4 ± 2.9 | P = 0.046 |
| iLF | 52.5 ± 6.4 | 49.6 ± 4.4 | NS |
| oLT | 48.4 ± 8.5 | 48.3 ± 5.1 | NS |
| cLT | 42.1 ± 7.0 | 42.0 ± 5.4 | NS |
| iLT | 42.7 ± 2.7 | 42.6 ± 4.1 | NS |
| oMF | 53.1 ± 3.3 | 48.7 ± 5.8 | NS |
| cMF | 47.3 ± 5.2 | 50.0 ± 5.1 | NS |
| iMF | 53.6 ± 3.9 | 54.2 ± 4.2 | NS |
| oMT | 50.1 ± 2.4 | 54.7 ± 2.6 | P = 0.028 |
| cMT | 46.8 ± 6.6 | 51.6 ± 6.4 | NS |
| iMT | 48.9 ± 8.7 | 47.8 ± 7.9 | NS |
| lP | 42.6 ± 5.1 | 42.2 ± 7.4 | NS |
| cP | 44.2 ± 7.7 | 44.9 ± 8.9 | NS |
| mP | 38.0 ± 5.3 | 40.2 ± 10.7 | NS |
Data presented as mean ± SD.
aWilcoxon Signed Ranks Test, pre-race versus post-race.
o, outer; c, central; i, inner; M, medial; L, lateral; F, femur; T, tibia; lP, lateral patella; cP, central patella; mP, medial patella; NS, no significant between-group difference (P ≥ 0.05).
Discussion
The results of this current study showed that biochemical changes occur in knee articular cartilage of novice female half-marathon runners. The elevations in T2 values seen in the medial and not in the lateral compartment were consistent with a previous study.4 Though differences in study design are present, the question arises whether the findings in these current half-marathon participants represent a precursor to the changes found in the previous study of marathon runners.4 The observations in the current study are limited to the medial tibia outer region. Furthermore, in contrast to the previous study,4there was no involvement of the medial femoral condyle or patellofemoral joint. The difference in cyclic loading between a half-marathon and a full marathon are not only limited to race mileage, but also accumulated mileage during training. Training programs for novice runners can average 15–25 miles (24–40 km) per week for 14 weeks for a half-marathon and 10–40 miles (10–65 km) per week for 16 weeks for a full marathon.17 Identifying such a dose-dependent effect and the elevated T2 developmental variables in articular cartilage would better elucidate the sequelae of distance running on articular cartilage.
A statistically significant decrease was seen in the post-race T2 values within the central region of the lateral femoral condyle, noting another difference from a non-significant increase as described previously.4 These findings may be explained by a previous study that described decreases in T2 values accounting for deformation of cartilage architecture, extrusion of water content and a relative increase of proteoglycan and collagen content within the cartilage following application of loading similar to walking and standing compared with the supine position.18 We hypothesize that these changes may be the result of a vacuum effect in which adjacent compartments are offloaded as increased forces on the medial compartment accumulate secondary to mechanical alignment. In addition, patients underwent similar unloading periods prior to obtaining both pre- and post-race MRIs, with the T2 mapping being performed as the last of the sequences obtained. The potential of this representing a functional adaptation of cartilage versus random measurement error must be taken into account, therefore further studies with a greater number of participants would be beneficial.
This current study had several limitations. The post-race MRI results were obtained over a range of days (2.5–14.0 days) rather than at a specified time. Although partial recovery after an unloading period has been documented, the amount of time needed for full recovery and if it indeed occurs has yet to be defined. The goal of this current study was to determine if radiographic changes were present after a minimum of 48-h rest period. T2 relaxation time mapping sequences were obtained utilizing the MRI protocol outlined previously.19 However, variation in duration between race completion to MRI acquisition could allow more recovery time for some subjects. Moreover, significant changes were observed at a mean of 6 days after the race. Further studies with long-term MRI follow-up are warranted to determine the duration and clinical relevance of these changes. Though not by design, all the volunteers in the current study were female. Despite sex differences in cartilage metabolism and biomechanics of the knee, a previous study observed no differences in the relaxation properties or the magnitude and spatial dependency of the cartilage T2 maps in healthy male and female volunteers.20
Despite these limitations, this study demonstrated for the first time that biochemical changes occur in knee articular cartilage after a strenuous physical trial such as a half-marathon and are still present at a mean of 6 days after a race. A previous study has attempted to validate ultrasound imaging as a method to evaluate knee cartilage thickness compared with MRI findings.21 However, no study has compared ultrasound versus MRI when evaluating the knees of long-distance runners after a race. Further research is still warranted to determine the relationship between duration and amount of these changes to further developments.
In conclusion, an increase in T2 relaxation time occurs in the medial tibial plateau of novice half-marathon runners. This limited region of increased T2 values, when compared with complete medial compartment involvement seen in studies of marathon runners, may represent an association between distance run and changes seen in articular cartilage T2 values. Further studies to investigate the duration of changes and the effects of distance on these are warranted.
Acknowledgements
The authors acknowledge the invaluable participation of the subjects and the help from the staff of the MRI suite of the University of Miami Hospital and Jackson Memorial Hospital.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med 2012; 366: 130–140. [DOI] [PubMed] [Google Scholar]
- 2.Miller JA. Trends in Running, by the Numbers. The New York Times, https://www.nytimes.com/2019/02/16/well/move/trends-in-running-by-the-numbers.html, 2019.
- 3.Miller TL. Endurance Sports Medicine: A Clinical Guide: Springer International 2016.
- 4.Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med 2010; 38: 2273–2280. [DOI] [PubMed] [Google Scholar]
- 5.van den Bogert AJ, Read L, Nigg BM. An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc 1999; 31: 131–142. [DOI] [PubMed] [Google Scholar]
- 6.Subburaj K, Kumar D, Souza RB, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med 2012; 40: 2134–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanwanseele B, Lucchinetti E, Stussi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage 2002; 10: 408–419. [DOI] [PubMed] [Google Scholar]
- 8.Newton PM, Mow VC, Gardner TR, et al. Winner of the 1996 Cabaud Award. The effect of lifelong exercise on canine articular cartilage. Am J Sports Med 1997; 25: 282–287. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty EF, Hubert HB, Lingala VB, et al. Long distance running and knee osteoarthritis. A prospective study. Am J Prev Med 2008; 35: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohmann E, Wortler K, Imhoff AB. MR imaging of the hip and knee before and after marathon running. Am J Sports Med 2004; 32: 55–59. [DOI] [PubMed] [Google Scholar]
- 11.Hesper T, Miese FR, Hosalkar HS, et al. Quantitative T2(*) assessment of knee joint cartilage after running a marathon. Eur J Radiol 2015; 84: 284–289. [DOI] [PubMed] [Google Scholar]
- 12.Baum T, Joseph GB, Karampinos DC, et al. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage 2013; 21: 1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Wang H, Lu Y, et al. Diagnostic value of T1rho and T2 mapping sequences of 3D fat-suppressed spoiled gradient (FS SPGR-3D) 3.0-T magnetic resonance imaging for osteoarthritis. Medicine (Baltimore) 2019; 98: e13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwaiger BJ, Gersing AS, Mbapte Wamba J, et al. Can signal abnormalities detected with MR imaging in knee articular cartilage be used to predict development of morphologic cartilage defects? 48-month data from the osteoarthritis initiative. Radiology 2016; 281: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami K, Arai Y, Ikoma K, et al. Total resection of any segment of the lateral meniscus may cause early cartilage degeneration: evaluation by magnetic resonance imaging using T2 mapping. Medicine (Baltimore) 2018; 97: e11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaukinen P, Podlipska J, Guermazi A, et al. Associations between MRI-defined structural pathology and generalized and localized knee pain - the Oulu Knee Osteoarthritis study. Osteoarthritis Cartilage 2016; 24: 1565–1576. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich M, Rust CA, Rosemann T, et al. A comparison of anthropometric and training characteristics between female and male half-marathoners and the relationship to race time. Asian J Sports Med 2014; 5: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishii T, Kuroda K, Matsuoka Y, et al. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging 2008; 28: 175–180. [DOI] [PubMed] [Google Scholar]
- 19.Apprich S, Welsch GH, Mamisch TC, et al. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage 2010; 18: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 20.Mosher TJ, Collins CM, Smith HE, et al. Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J Magn Reson Imaging 2004; 19: 323–328. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz RJ, Wang HM, Polprasert DR, et al. Evaluation of knee cartilage thickness: a comparison between ultrasound and magnetic resonance imaging methods. Knee 2017; 24: 217–223. [DOI] [PubMed] [Google Scholar]