Short abstract
Telomeres and telomerase play important roles in the occurrence and development of hypertension. This review was performed to clarify the factors that influence telomere length and telomerase activity in older patients and elucidate the association of these factors with hypertension. A PubMed search and critical review of studies assessing the risk factors underlying the association of hypertension with telomere length and telomerase activity was performed. Telomere length and telomerase activity were found to be associated with hypertension. The factors that influence telomere length and telomerase activity in older patients with hypertension include genetics, demographics, social and environmental factors, chronic disease, psychological factors, and antihypertensive drug treatment. A better understanding of the molecular mechanisms underlying the association of hypertension with telomere length and telomerase activity may help to reduce the incidence of hypertension.
Keywords: Telomere, telomerase, hypertension, cardiovascular disease, oxidative stress, older adults
Introduction
Telomeres are chromosomal structures at the ends of linear chromosomes that maintain genomic stability by protecting DNA sequence loss, blocking end-to-end fusion, and distinguishing the linear ends of chromosomes from DNA breaks.1 In mammals, telomeres are formed from a highly conserved, hexameric tandem repeat DNA sequence (TTAGGG). The telomere length shortens with each cell division, and telomere attrition is related to the replicative capacity in vitro and the aging process in vivo.2 Hence, the telomere length is widely considered a marker of biological aging. Telomerase is an RNA-dependent DNA polymerase, and telomere biosynthesis is dependent on telomerase.3 Hypertension, the major factor leading to mortality, is a chronic disease with a high incidence in older adults.4 Hypertension affects 30% of adults and is an important risk factor for cardiovascular and cerebrovascular diseases, peripheral vascular disease, and chronic kidney disease, and it may have a negative impact on the quality of life of older adults to some extent. Uncontrolled hypertension leads to serious cardiovascular events. Current knowledge indicates that age-dependent telomere dysfunction may be involved in the pathogenesis of hypertension.5,6 Both telomere length and telomerase activity have been consistently associated with hypertension in previous research.5
The telomere length decreases with increasing age in humans.7 Telomere shortening is associated with inflammation, exposure to telomere inhibitory factors, and other types of damage. Replication stress due to severe DNA damage or structural obstacles is the main cause of telomere shortening; however, weakened oxidative stress repair mechanisms and an impaired DNA damage response are also associated with telomere shortening.8 Many studies have shown no difference in telomere length between males and females.9 However, at least one study has identified significantly longer telomeres in women than men.9 Because telomerase is activated mainly in cancer cells, limited studies have focused on its activity in the setting of normal leukocytes.
In our previous review, we reported that telomeres and telomerase activity have played an important role in the occurrence and development of hypertension in both animal and human studies.10 Short leukocyte telomere length (LTL) and low telomerase activity are associated with plaque instability, leading to senescent cell accumulation and finally contributing to heart failure.11 Another study of patients with hypertension showed that a higher apnea–hypopnea index in polysomnographic examinations was associated with lower telomerase activity.12 However, one study showed that telomerase activator 65, a compound with lifespan-extending abilities, improved key markers of cardiovascular disease risk (plasma lipids and inflammatory cytokines).13 In the present study, we reviewed factors that influence telomere length and telomerase activity in the white blood cells of older adults and evaluated their association with hypertension.
Telomeres and telomerase
Telomere length
Telomeres
Telomeres are genetic structures that play an important role in disease and mortality. Telomere length is a marker of cellular aging and senescence and is measured in leukocytes. Telomere shortening is generally caused by DNA replication and cell division and is therefore associated with tissue renewal. With the development of emerging telomere measurement methods and genome-wide genotyping platforms, telomere molecular epidemiology has emerged as a new field for studying the role of telomere biology in humans.14 Shorter LTLs have been found in patients with cardiovascular disease than in normal controls.15,16
Telomere length and oxidative stress
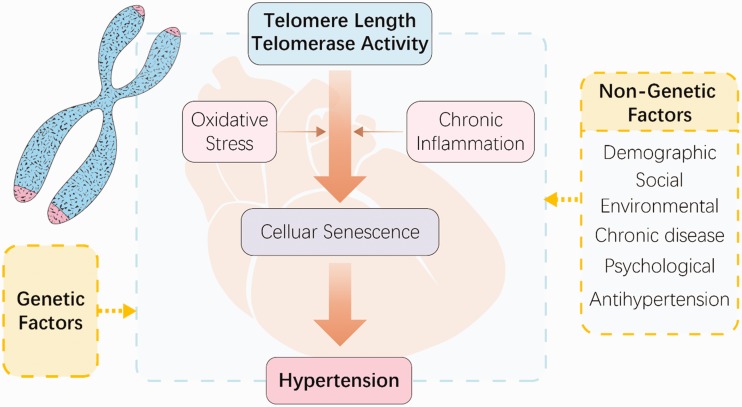
Telomeres are maintained by two main independent mechanisms: a telomerase-dependent mechanism involving de novo synthesis of telomeric DNA by telomerase and a telomerase-independent mechanism termed alternative lengthening of telomeres, which involves homologous recombination-mediated DNA replication.17 Oxidative stress and chronic inflammation might explain the underlying biology of the association between telomere length and hypertension (Figure 1). Oxidative stress is correlated with accelerated telomere shortening and dysfunction. Telomeres induce telomere loss and dysfunction because of their high sensitivity to DNA damage. However, the process by which oxidative lesions impact telomere maintenance, cellular function, and the organism’s health and aging remain unknown.18
Figure 1.
Schematic overview of telomere length and hypertension.
Telomerase
Telomerase is expressed in approximately 90% of cancer cells and tumor tissues.19 Telomerase is an enzyme responsible for the synthesis of telomeres. During genome replication, telomerase adds repeats to the ends of chromosomes to balance the loss of telomeric DNA. As a ribonucleoprotein, telomerase is composed of both RNA and proteins and consists of two molecules each of telomerase reverse transcriptase (TERT), telomere RNA, and dyskerin.20 TERT limits telomerase activity and telomere elongation. However, telomerase activity, which serves as a proliferation marker, is not detectable in most somatic cells with the exception of embryonic tissues, stem cells, and reproductive organs21; this suggests that telomerase-based therapeutic approaches with an emphasis on the role of telomerase in cancer stem cell biology have a promising future. However, some stem cells weakly express telomerase to delay telomere shortening.
Factors associated with telomere length in older adults with hypertension
Genetic factors
Numerous genome-wide association studies have identified variants in telomere biology genes as being associated with diseases. For example, SNPs in the TERT-CLPTM1L locus on chromosome 5p15.33 are associated with cancer,22 but these variants do not specifically encode deleterious coding alleles in TERT.23 The identification of TREX1 has provided an important screening and treatment target.24 Another study indicated that telomeres undergo rapid elongation followed by an acute breakdown, finally leading to chromosomal instability after disrupting CTC1.25 Cells were unable to replicate efficiently after knockout of POLD3, another gene responsible for genomic stability.26 Telomere fusion results in massive rearrangements of genes on chromosomes as well as localized hypermutations.27 Animal models of hypertension have shown that differences in telomere lengths between BPH/2J and BPN/3J mice occur after the development of hypertension and do not cause hypertension in BPH/2J mice.28 However, NAF1 genetic variation has been shown to be associated with both longer telomeres and a higher risk of atherosclerotic cardiovascular disease.29,30
Non-genetic factors
Demographic factors
Telomeres shorten with age, with the LTL declining from 11 kbp at birth to less than 4 kbp in older adults31; hence, telomere shortening is frequently used to indicate cellular senescence.32 One study showed that LTL was positively associated with diastolic blood pressure in patients with hypertension but negatively associated with systolic blood pressure and pulse pressure in the same patient groups.33 In the women of that study, changes in the LTL were an indicator of obesity-related complications.33 Telomeres are longer in women because of higher estrogen levels, which increase telomerase activity and exert antioxidant effects.31 The inverse association between LTL and age exclusively in women supports the previously reported decline in the attrition rate in men with increasing age; this indicates potential sex dimorphism in LTL regulation and implicates the potential for sex-adjusted health-preventive therapies.34
Social and environmental factors
There is a growing understanding of the association between multiple social and environmental factors and telomere length.31 Smoking, body mass index, reduced exercise, and multiple vascular risk factors are negatively associated with LTL.35,36 These same risk factors are also closely associated with the development of chronic diseases such as hypertension, coronary heart disease, stroke, and diabetes. A study of twins revealed that spare time and the level of sports participation are related to telomere length in peripheral white blood cells.37 The level of physical activity was positively associated with telomere length, and this association was still significant after adjustment for age, sex, body mass index, smoking, and social, and economic status. Regular physical activity has also been associated with decreased levels of oxidative stress and inflammation.36 This suggests that a sedentary lifestyle can negatively affect telomere length and accelerate the aging process. Obesity and smoking are important risk factors for age-related diseases35 because they increase the level of oxidative stress and inflammation and hasten telomere shortening. Telomere length in individuals with obesity is shorter than that in controls of normal weight.38 Tobacco consumption is the main environmental modifying factor and is associated with telomere length and frailty in older adults.39 Obesity, smoking, and aging act in concert to accelerate telomere loss.
Chronic diseases
Age-related LTL shortening is augmented by the cumulative burden of oxidative stress and inflammation that follow replicative stress.40 The main age-related cardiovascular dysfunctions, namely coronary atherosclerosis, arterial stiffness, carotid intima-media thickness, and clinical cardiovascular events such as myocardial infarction and stroke, are all associated with LTL shortening.41–43 The Framingham Heart Study has identified an association between hypertension and reduced LTL.43 This association was enhanced in the presence of insulin resistance, which is also associated with hypertension.44
Individuals with reduced LTL have a higher incidence of cardiovascular events associated with atherosclerotic lesions and atherosclerosis.45 Shorter LTL has been reported in hypertensive men with coronary plaques than in hypertensive men free of plaques.41 The multivariate analysis revealed that in addition to age, the telomere length is an important predictor of coronary artery plaque formation. These studies indicate that the major risk factor for atherosclerosis is hypertension and that leukocyte telomere shortening is associated with an increased occurrence of coronary atherosclerosis. Moreover, telomere dysfunction and cardiovascular risk factors influence telomerase activity and telomere length and contribute to atherosclerosis.46
Telomere dysfunction can induce cardiac hypertrophic disorders and contribute to heart failure. Furthermore, LTL is positively correlated with the left ventricular mass and wall thickness, especially in patients with hypertension. These data suggest that increased LTL may be a marker of left ventricular hypertrophy.47 The association between LTL and left ventricular mass also provides a biological rationale for using LTL to estimate the myocardial cell size and replication potential.48 Arteriosclerosis, left ventricular hypertrophy, and diastolic filling occur in aging patients regardless of whether they are normotensive or hypertensive. The effect of environmental factors on the rate of telomere shortening plays an important role in regulating telomere length in adults.46 Oxidative stress can promote the progression of left ventricular hypertrophy to heart failure and accelerate telomere attrition.49
Psychological factors
Telomere maintenance is also determined by life stressors and lifestyle, highlighting the causal and potentiating roles of telomere attrition in human diseases.50 Social psychological factors can also influence telomere length. LTL is associated with socioeconomic status and life pressures. Furthermore, a low socioeconomic status is associated with reduced life expectancy.51 An analysis that excluded factors such as income, smoking, weight, and exercise, all of which accelerate aging, revealed that telomere length was significantly shorter in economically disadvantaged people than in wealthy individuals. This reduction in telomere length was equivalent to 7 years of aging. People living in poorer economic situations may experience increased mental stress, which promotes increased biological damage and accelerates the natural aging process. People experiencing high levels of psychological stress possess telomeres that are much shorter than equivalent individuals experiencing lower stress levels. Moreover, oxidative stress is considerably higher in these individuals. Psychological stress is significantly associated with higher oxidative stress, lower telomerase activity, and shorter telomere lengths.52 A recent study indicated that socioeconomic factors also influence telomere length as shown by the association of lower neighborhood socioeconomic status and lower individual-level socioeconomic status with shorter telomere lengths.53 Therefore, long-term stress can lead to increased oxidative stress, reduced telomerase activity, and telomere shortening in the peripheral blood mononuclear cells.
Other factors
Recent studies have shown that the availability of B and D vitamins and the concentrations of serum folate and homocysteine are correlated with the LTL.54–56 Telomere length is positively correlated with the levels of high-density lipoprotein, albumin, creatinine, and hemoglobin and the red blood cell count and negatively correlated with immunoglobulin levels.57 Furthermore, in women, although no relationship between age and LTL has been reported, the serum vitamin D level is associated with the LTL,58 while accumulating evidence links the vitamin D level and the hypertension risk.59
Effect of antihypertensive drug therapy on telomere length in older adults with hypertension
Most studies evaluating the association between hypertension and telomere function have been limited to basic research. Information on telomere length and telomerase activity, particularly in patients with senile hypertension receiving clinical drug treatment, is relatively scant. One study showed higher telomerase activity in the lymphocytes of patients with untreated hypertension than in patients taking regular medication for hypertension, suggesting that regular antihypertensive treatment may reduce telomerase activity.60 Studies have consistently shown that lymphocyte telomerase activity in patients with hypertension significantly decreases following treatment with felodipine and benazepril.61 These findings demonstrate the beneficial effects of early antihypertensive treatment. However, the two above-mentioned studies evaluated the telomerase activity in lymphocytes, not leukocytes. Therefore, large-cohort clinical studies in patients with hypertension are required to assess whether telomerase activity can be used to monitor the onset, development, prognosis, and treatment of hypertension-associated disease.
Conclusion
Telomeres and telomerase are associated with hypertension. The factors underlying the association of hypertension with telomere length and telomerase activity include genetic factors and important non-genetic determinants such as demographics; social, environmental, and psychological factors; chronic diseases, and antihypertensive drug therapy. However, most studies are associative, and the cause of hypertension via telomere shortening as well as the underlying mechanisms by which this occurs remain unclear. One study showed that the telomere length attrition rate may be a more sensitive indicator of the health status, including the development of chronic disease, than the baseline telomere length,33 indicating the need for further research on the dynamic changes in telomere length. However, a recent study showed that a short telomere length, but not the telomere attrition rate, was associated with carotid atherosclerosis during the 9.5-year follow-up period, suggesting that the heightened telomere attrition during adult life might not explain the short telomeres.62 Methodological considerations that may affect data interpretation should also be highlighted. Although oxidative stress and chronic inflammation might explain the pathogenic mechanisms underlying the association between telomere length and risk factors such as obesity, atherosclerosis, and coronary heart disease (Figure 1), no direct evidence is available to date. Targeting telomeres could provide a novel therapeutic strategy, as indicated by a recent review reporting on the advent of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated system, which has led to the performance of a wide array of targeted genetic studies to modify telomeres and telomerase and the genes that affect them.27 However, telomerase-targeted therapies may be associated with a risk of malignancy because overexpression of telomerase promotes cancer cell formation and growth.63,64 Therefore, basic research, longitudinal studies, and well-designed clinical trials are imperative to expand our understanding of the molecular mechanisms underlying the association of hypertension with telomere length and telomerase activity. This could help to reduce the incidence of hypertension and assist in preventing hypertensive complications, further improving the quality of life of patients with hypertension.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors, and neither informed consent nor ethics committee approval was therefore required.
Funding
This work was supported by the National Key R&D Program of China (2018YFC2002101), National Natural Science Foundation of China (81600927), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201706), and Milstein Medical Asian American Partnership Foundation Project Award in Geriatrics.
References
- 1.de Lange T. How telomeres solve the end-protection problem. Science 2009; 326: 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner KJ, Vasu V, Griffin DK. Telomere biology and human phenotype. Cells 2019; 8: pii: E73. doi: 10.3390/cells8010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay JW. Telomeres and aging. Curr Opin Cell Biol 2018; 52: 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Takami Y, Rakugi H. Treatment of hypertension in the elderly. Nihon Rinsho 2015; 73: 701–707. [PubMed] [Google Scholar]
- 5.Bhupatiraju C, Saini D, Patkar S, et al. Association of shorter telomere length with essential hypertension in Indian population. Am J Hum Biol 2012; 24: 573–578. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci 2014; 7: 161–167. [DOI] [PubMed] [Google Scholar]
- 7.Anderson R, Lagnado A, Maggiorani D, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 2019; 38: pii: e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynaud CM, Jang SJ, Nuciforo P, et al. Telomere shortening is correlated with the DNA damage response and telomeric protein down-regulation in colorectal preneoplastic lesions. Ann Oncol 2008; 19: 1875–1881. [DOI] [PubMed] [Google Scholar]
- 9.Cherif H, Tarry JL, Ozanne SE, et al. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucl Acids Res 2003; 31: 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Li Y, Wang J. Telomeres and essential hypertension. Clin Biochem 2015; 48: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 11.Yeh JK, Wang CY. Telomeres and telomerase in cardiovascular diseases. Genes (Basel) 2016; 7: pii: E58. doi: 10.3390/genes7090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martynowicz H, Kornafel-Flak O, Urbanik D, et al. Coexistence of obstructive sleep apnea and telomerase activity, concentration of selected adipose tissue hormones and vascular endothelial function in patients with arterial hypertension. Respir Med 2019; 153: 20–25. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez ML, Thomas MS, Lemos BS, et al. TA-65, A telomerase activator improves cardiovascular markers in patients with metabolic syndrome. Curr Pharm Des 2018; 24: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 14.Bodelon C, Savage SA, Gadalla SM. Telomeres in molecular epidemiology studies. Prog Mol Biol Transl Sci 2014; 125: 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haycock PC, Heydon EE, Kaptoge S, et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014; 349: g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Mello MJ, Ross SA, Briel M, et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet 2015; 8: 82–90. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JM, Yadav T, Ouyang J, et al. Alternative lengthening of telomeres through two distinct break-induced replication pathways. Cell Rep 2019; 26: 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 2019; 177: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancers. Science 1994; 266: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen THD, Tam J, Wu RA, et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 2018; 557: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skvortsov DA, Zvereva ME, Shpanchenko OV, et al. Assays for detection of telomerase activity. Acta Naturae 2011; 3: 48–68. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Zhu B, Zhang M, et al. Imputation and subset-based association analysis across different cancer types identifies multiple independent risk loci in the TERT-CLPTM1L region on chromosome 5p15.33. Hum Mol Genet 2014; 23: 6616–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Jia J, Makowski M, et al. Functional characterization of a multi-cancer risk locus on chr5p15.33 reveals regulation of TERT by ZNF148. Nat Commun 2017; 8: 15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brane AC, Tollefsbol TO. Targeting telomeres and telomerase: studies in aging and disease utilizing CRISPR/Cas9 technology. Cells 2019; 8: pii: E186. doi: 10.3390/cells8020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu P, Jia S, Takasugi T, et al. CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length. Aging Cell 2018; 17: e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, Wang L, Ge F, et al. Pold3 is required for genomic stability and telomere integrity in embryonic stem cells and meiosis. Nucleic Acids Res 2018; 46: 3468–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maciejowski J, Li Y, Bosco N, et al. Chromothripsis and kataegis induced by telomere crisis. Cell 2015; 163: 1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu CL, Hearn NL, Paine D, et al. Does telomere shortening precede the onset of hypertension in spontaneously hypertensive mice? Twin Res Hum Genet 2016; 19: 422–429. [DOI] [PubMed] [Google Scholar]
- 29.Stanley SE, Gable DL, Wagner CL, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med 2016; 8: 351ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Said MA, Eppinga RN, Hagemeijer Y, et al. Telomere length and risk of cardiovascular disease and cancer. J Am Coll Cardiol 2017; 70: 506–507. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann M, Pusceddu I, März W, et al. Telomere biology and age-related diseases. Clin Chem Lab Med 2018; 56: 1210–1222. [DOI] [PubMed] [Google Scholar]
- 32.Bernadotte A, Mikhelson VM, Spivak IM, et al. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016; 8: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojcicki JM, Elwan D, Lin J, et al. Chronic obesity and incident hypertension in Latina women are associated with accelerated telomere length loss over a 1-year period. Metab Syndr Relat Disord 2018; 16: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opstad TB, Kalstad AA, Pettersen AA, et al. Novel biomolecules of ageing, sex differences and potential underlying mechanisms of telomere shortening in coronary artery disease. Exp Gerontol 2019; 119: 53–60. [DOI] [PubMed] [Google Scholar]
- 35.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. The Lancet 2005; 366: 662–664. [DOI] [PubMed] [Google Scholar]
- 36.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008; 9: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 37.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 2008; 168: 154–158. [DOI] [PubMed] [Google Scholar]
- 38.Zannolli R, Mohn A, Buoni S, et al. Telomere length and obesity. Acta Paediatr 2008; 97: 952–954. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Ramírez M, Sánchez-García S, García-Dela Torre P, et al. Telomere shortening and frailty in Mexican older adults. Geriatr Gerontol Int 2018; 18: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 40.Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci 2018; 373: pii: 20160436. doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 2004; 43: 182–185. [DOI] [PubMed] [Google Scholar]
- 42.Wang XB, Cui NH, Zhang S, et al. Leukocyte telomere length, mitochondrial DNA copy number, and coronary artery disease risk and severity: a two-stage case-control study of 3064 Chinese subjects. Atherosclerosis 2019; 284: 165–172. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell CJ, Demissie S, Kimura M, et al. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2008; 28: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006; 5: 325–330. [DOI] [PubMed] [Google Scholar]
- 45.Huber M, Treszl A, Wehland M, et al. Genetic variants implicated in telomere length associated with left ventricular function in patients with hypertension and cardiac organ damage. J Mol Med 2012; 90: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 46.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res 2006; 99: 1167–1180. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran SV, Serkalem D, Masayuki K, et al. Association of leukocyte telomere length with echocardiographic left ventricular mass: the Framingham Heart Study. Circulation 2009; 120: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatiana K, Veryan C, Scott B, et al. Association between left ventricular mass and telomere length in a population study. Am J Epidemiol 2010; 172: 440–450. [DOI] [PubMed] [Google Scholar]
- 49.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007; 49: 241–248. [DOI] [PubMed] [Google Scholar]
- 50.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015; 350: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 51.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell 2006; 5: 361–365. [DOI] [PubMed] [Google Scholar]
- 52.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 2004; 101: 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexeeff S, Schaefer C, Kvale M, et al. Telomere length and socioeconomic status at neighborhood and individual levels among 80,000 adults in the Genetic Epidemiology Research on Adult Health and Aging cohort. Environmental Epidemiology 2019; 3: e049 https://journals.lww.com/environepidem/FullText/2019/05000/Telomere_length_and_socioeconomic_status_at.4.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pusceddu I, Herrmann M, Kirsch SH, et al. One-carbon metabolites and telomere length in a prospective and randomized study of B- and/or D-vitamin supplementation. Eur J Nutr 2017; 56: 1887–1898. [DOI] [PubMed] [Google Scholar]
- 55.Paul L, Jacques PF, Aviv A, et al. High plasma folate is negatively associated with leukocyte telomere length in the Framingham offspring cohort. Eur J Nutr 2015; 54: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pusceddu I, Herrmann M, Kirsch SH, et al. Prospective study of telomere length and LINE-1 methylation in peripheral blood cells: the role of B vitamins supplementation. Eur J Nutr 2016; 55: 1863–1873. [DOI] [PubMed] [Google Scholar]
- 57.Maeda T, Oyama JI, Sasaki M, et al. The correlation between the clinical laboratory data and the telomere length in peripheral blood leukocytes of Japanese female patients with hypertension. J Nutr Health Aging 2011; 15: 240–244. [DOI] [PubMed] [Google Scholar]
- 58.Richards JB, Valdes AM, Gardner JP, et al. High serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clinical Nutrition 2007; 86: 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ke L, Mason RS, Kariuki M, et al. Vitamin D status and hypertension: a review. Integr Blood Press Control 2015; 8: 13–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan H, Li D, Huang M. The telomerase activity of lymphocytes in patients with essential hypertension after antihypertensive treatment. Practical Preventive Medicine 2004; 11: 667–669. [Google Scholar]
- 61.Ouyang Y, Lu Y, Hu G. Telomerase activity of peripheral blood of patient with essential hypertension and its levels before and after plendil and lotensin treatment. Laboratory Medicine 2004; 19: 324–326. [Google Scholar]
- 62.Toupance S, Labat C, Temmar M, et al. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension 2017; 70: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang S, Madu C, Lu Y. Telomere and its role in diseases. Oncomedicine 2019; 4: 1–9. [Google Scholar]
- 64.Hong J, Yun CO. Telomere gene therapy: polarizing therapeutic goals for treatment of various diseases. Cells 2019; 8: pii: E392. doi: 10.3390/cells8050392. [DOI] [PMC free article] [PubMed] [Google Scholar]