Short abstract
Objective
Traditional Chinese medicine (TCM) may be beneficial for vascular dementia (VaD). We evaluated the efficacy of Pushen capsule, a compound containing several TCM components, for treating vascular mild cognitive impairment (VaMCI).
Methods
Seventy outpatients with VaMCI were randomized to Pushen capsule or control treatment with Ginkgo biloba. Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Subjective Memory Loss Rating Scale scores; and lipid, lipoprotein, and haemorheological parameters were collected at baseline, week 4, and week 12 of treatment.
Results
MMSE score at week 12 was significantly higher in the treatment group compared with baseline (t = −2.352) but was not significantly different from week 12 in the control group. The MoCA score at week 12 was higher than that at baseline for both the treatment and control groups (t = −2.619 and −2.582, respectively), as was the “delayed recall” item score. Subjective memory loss score and the cognitive function “forgetting acquaintance's name” were significantly higher in the treatment group at week 12 than at baseline (t = −2.621 and χ2 = 4.419, respectively). Lipid, lipoprotein, and haemorheological parameters were significantly different after treatment in both groups.
Conclusion
The benefits of Pushen capsule on cognitive function in VaMCI were comparable with that of Ginkgo biloba.
Keywords: Vascular mild cognitive impairment, Pushen capsule, Ginkgo biloba, randomized controlled trial, Mini Mental State Examination, cognitive function
Introduction
With increasing life expectancies, dementia has become a growing social health burden. According to a World Health Organization (WHO) 2019 report, 50 million people live with dementia globally.1 In China, the number of people with dementia reached 9.19 million in 2010, and the prevalence ranged from 2.6% in people aged 65 to 69 years to 60.5% in people aged 95 to 99 years.2 The dementia epidemic and the financial burden associated with family care costs remain two of the biggest challenges for global public health systems.
Vascular dementia (VaD), the second most common form of dementia following Alzheimer’s disease (AD), accounts for at least 25% of cases of dementia.1 VaD has been accepted as the latest stage of vascular cognitive impairment (VCI), which refers to all forms of cognitive deficits related to cerebrovascular disease or relevant risk factors. Incidence rates of VaD are age related, with the prevalence doubling every 5.3 years.3 Stroke is the most common cause of VaD, with 10% of patients with first stroke living with VaD and more than one third of recurring stroke cases accompanied by VaD.4 Multiple efforts to investigate treatment options for VaD have been made in recent decades, including clinical trials of cholinesterase inhibitors and nonpharmacological treatments; however, valid therapies to prevent the associated progressive cognitive and functional decline are not yet available.5
In contrast to the irreversible progression of VaD, vascular mild cognitive impairment (VaMCI) can be considered a stage of VCI in which intervention is feasible. VaMCI is the early stage of VCI between normal ageing and VaD, in which the severity of cognitive decline does not meet the criteria for dementia.6 VaMCI can be considered an overlap of VCI with mild cognitive impairment (MCI). The prevalence of VaMCI is estimated to be twice that of VaD.7 Moreover, just as MCI is likely to progress to AD, VaMCI is the greatest risk factor for VaD.8 Hence, effective treatment of VaMCI may protect patients from developing VaD, thus decreasing its incidence.
Previous studies have indicated that traditional Chinese medicine (TCM) is effective in the treatment of VaD by regulating cerebral circulation and cellular metabolism.9 Ginkgo biloba is widely used in China to improve the symptoms of cerebral ischaemia because of its antioxidative and protective effects on neuronal cells.10,11 Existing evidence suggests that Ginkgo biloba extract (EGb 761) can enhance the integrity of the cerebral microvasculature and strengthen the ability of cerebral microvascular endothelial cells to resist damage resulting from hypoxia, hyperglycaemia, and amyloid-beta (Aβ) protein.12,13 Additionally, various clinical trials have reported the efficiency of EGb 761 in ameliorating mild-to-moderate age-related cognitive impairments induced by AD, VaD, and mixed dementia.14–16 Furthermore, EGb 761 has been shown to improve the cognitive performance of patients with MCI, including those with VaMCI.17–19
Pushen capsule is a compound preparation of various TCM drugs, and primarily consists of Polygonum multiflorum, Pollen Typhae, Salvia miltiorrhiza, Ligusticum chuanxiong, and paeoniflorin. Several registered pharmaceutical products containing Salvia miltiorrhiza extracts, such as Danshen dripping pill and water-soluble Danshensu, are widely used in clinical settings in China and produce effects that are beneficial to the cardiovascular system including microcirculation improvement, protection against myocardial ischaemia/reperfusion injury, and antiplatelet aggregation.20,21 Polygonum multiflorum and Ligusticum chuanxiong have been reported to improve clinical cognitive impairments induced by occlusion of cerebral blood flow.22,23 Recently, Pushen capsule was reported by the China Food and Drug Administration to regulate hyperlipemia, a risk factor for cognitive impairment, in China.24 However, the efficacy of Pushen capsule for the treatment of VaMCI has not yet been investigated.
In the present study, we evaluated the clinical efficacy of Pushen capsule for treating VaMCI and compared it with that of Ginkgo biloba. Furthermore, blood serum lipid, lipoprotein, and hemorheological parameters of patients with VaMCI were investigated.
Methods
Trial design and population
A randomized hospital-based study with blinded outcome evaluation was performed. Seventy patients with VaMCI were enrolled from the outpatient neurology department at Zhongda Hospital affiliated with Southeast University between April 2016 and April 2018. The groups were assigned randomly to one of two groups as follows: patients labelled with even numbers were given Pushen capsule for 12 weeks and those with an odd number were assigned to the control group and given Ginkgo biloba for 12 weeks. Neither the participants nor the administering clinicians were blinded to the intervention. Written informed consent, which clarified the uncertainty effect of the cognitive improvement and the probable adverse effects of both interventions, was obtained from each participant. The research was conducted according to the World Medical Association Declaration of Helsinki. This experimental protocol was approved by the Ethics Committee of Nanjing University of Chinese Medicine and Southeast University (approval number: 2013NL-026-02). The diagnosis of VaMCI was based on the criteria of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which was updated by the American Psychiatric Association (APA) in 2013, while referencing the statement from the American Heart Association/American Stroke Association.3 Patients were eligible for inclusion in the trial if they were 18 years of age or older, presented with subjective cognitive impairment that interfered in the performance of activities instrumental for daily living, and met the criteria for MCI with cognitive testing.25 In addition, patients were required to have an Mini Mental State Examination (MMSE) score >24 and Montreal Cognitive Assessment (MoCA) score >22 to be diagnosed with MCI and not VaD. We limited the enrolment to patients who had been diagnosed with stroke or lacunar infarction at least 6 months prior to enrolment to reduce the effects of confounding factors and time bias. Ischaemic stroke was diagnosed as an acute focal neurological deficit lasting more than 24 hours and an infarct lesion with a high hyperintense signal on diffusion-weighted magnetic resonance imaging (MRI). Haemorrhagic stroke was confirmed as a new lesion with a high hyperintense signal on a brain computed tomography (CT) scan. For included patients, cognitive impairment should follow stroke. Patients were excluded if they had depression or other mental disorders, traumatic brain injuries, epilepsy, encephalitis, Parkinson's disease, tumour, hypothyroidism, severe anaemia, infections, or other endocrine diseases. Patients were also excluded if there was a clear contraindication for either Pushen capsule or Ginkgo biloba.
Interventions
Patients who were assessed as having VaMCI in the treatment group were orally administered Pushen capsule from Jiangsu Suzhong Haixin Pharmaceutical Company (Jiangsu Sheng, China) at a dose of 1.8 mg, three times a day without any change in other treatment. In the control group, patients were orally administered Ginkgo biloba tablets from the Yangtze River Pharmaceutical Group (Taizhou, China) at a dose of 19.2 mg, three times a day. Patients were asked to adhere to the assigned treatment for 12 weeks unless an adverse reaction was experienced.
Cognitive function assessment
Using an assessment scale approach, we performed a primary analysis to exclude the effect of depression and anxiety using the Hamilton Depression Scale (HAMD-17) and Hamilton Anxiety Scale (HAMA). The MMSE and MoCA were used to assess cognitive function at baseline and at weeks 3 and 12 of treatment. A previous study showed that a short memory questionnaire was useful when screening for MCI.26 We further expanded on the memory questions, resulting in the Subjective Memory Loss Rating Scale (Box 1) to detect the effect of treatment by assessing answers to specific questions targeting problems often encountered in daily life.4,27
Box 1.
Supplementary Subjective Memory Loss Rating Scale.
| Q1: If the score of your best memory state is ten, how many points do you give to your current memory? |
| (The maximal score is ten, and the answer score should be the range from zero to ten in integers.) |
| Q2: Which of the following aspects of memory do you experience? |
| (The answer could be more than one) |
|
Data collection
Demographic data were collected at the first assessment (baseline), while clinical data, including the MRI brain scan and the MMSE, MoCA, and Subjective Memory Loss Rating Scale scores, were recorded at all three time points (baseline, week 4, and week 12). Levels of fasting blood lipids and lipoproteins including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apoA1, apoB, and lipoprotein α, as well as haemorheological parameters consisting of blood viscosity at high, medium, and low shear rate; capillary plasma viscosity; and haematocrit were recorded. Follow-up data were collected at week 3 and week 12 of treatment. To detect adverse effects, items reflecting liver and kidney function and creatine kinase were examined. The statisticians who analysed the data were blinded to the intervention.
Statistical analysis
We estimated that at least 35 patients with VaMCI would need to be assigned to each treatment group. This calculation was based on conservative estimates of a 15% participant dropout rate. All quantitative data are presented as the mean ± standard deviation. For data of equal variance and normal distribution, statistical comparisons between groups were performed using Student’s t-test for between-group comparisons or one- or two-way analysis of variance (ANOVA) for comparisons among the three time points. Dunnett’s t-test was used for multiple comparisons. Categorical data are shown as percentages and were assessed using the chi-squared test. Data that failed tests for normality and equal variance were assessed using a non-parametric test. All reported P values are two-sided, and no adjustment was made for multiple comparisons. All analyses were performed using SPSS software, version 19 (IBM Corp., Armonk, NY, USA) at an alpha level of 0.05.
Results
Characteristics of the study population
From 1 April 2016, to 28 April 2018, 70 patients were screened and 62 eligible patients were randomly assigned to Pushen capsule (n = 30) and or to the control group treated with Ginkgo biloba tablets (n = 32). The loss-to-follow-up rate at week 12 was 11%, resulting from a loss of contact and hospitalization. The baseline characteristics of patients are summarized in Table 1. There was no difference in baseline scores of the scales between the two groups. No significant differences were found in the distribution of demographics, medical history, or laboratory data at baseline, apart from HDL, apoA1, and haematocrit values.
Table 1.
Baseline characteristics of patients with vascular mild cognitive impairment.
| Treatment group (n=30) | Control group (n=32) | t or χ2 value* | P value | |
|---|---|---|---|---|
| Age, years | 64.07 ± 9.18 | 65.71 ± 8.21 | 0.507 | 0.479 |
| Male sex, no. (%) | 15 (50.0) | 20 (62.5) | 0.984 | 0.321 |
| Educational background, no. (%) | 0.946 | 0.623 | ||
| Illiteracy | 1 (3.3) | 2 (6.3) | ||
| Primary education | 5 (16.7) | 3 (9.4) | ||
| Secondary education or above | 24 (80.0) | 27 (84.4) | ||
| Body mass index | 23.86 ± 3.33 | 24.35 ± 2.77 | 0.372 | 0.544 |
| Medical history, no. (%) | ||||
| Tobacco use | 10 (33.3) | 14 (43.8) | 3.050 | 0.218 |
| Alcohol use | 19 (63.3) | 19 (59.4) | 1.938 | 0.380 |
| Hypertension | 12 (40.0) | 17 (53.1) | 3.600 | 0.165 |
| Atrial fibrillation | 2 (6.7) | 3 (9.4) | 2.156 | 0.340 |
| Diabetes mellitus | 8 (26.7) | 15 (46.9) | 5.396 | 0.067 |
| Other disease | 20 (66.7) | 23 (71.9) | 0.198 | 0.657 |
| Genetic history | 1 (3.3) | 1 (3.1) | 0.002 | 0.963 |
| Stroke, no. (%) | 1.552 | 0.213 | ||
| Lacunar infarction | 15 (50.0) | 21 (65.6) | ||
| Other type | 15 (50.0) | 11 (34.4) | ||
| Lipids and lipoprotein | ||||
| TC (mmol/L) | 5.02 ± 1.44 | 4.93 ± 1.09 | 0.263 | 0.794 |
| TG (mmol/L) | 1.46 ± 1.04 | 1.73 ± 0.90 | −1.027 | 0.309 |
| HDL-C (mmol/L) | 1.25 ± 0.35 | 1.06 ± 0.17 | 2.519 | 0.015 |
| LDL-C (mmol/L) | 2.96 ± 1.05 | 3.06 ± 0.82 | −0.404 | 0.688 |
| apoA1 (g/L) | 1.32 ± 0.45 | 1.10 ± 0.23 | 2.331 | 0.023 |
| apoB (g/L) | 0.86 ± 0.26 | 0.84 ± 0.23 | −0.583 | 0.562 |
| Lipoprotein-α (mg/L) | 336.69 ± 209.65 | 294.75 ± 222.1 | 0.733 | 0.467 |
| Blood viscosity | ||||
| High shear rate§ | 4.56 ± 0.43 | 4.60 ± 0.58 | −0.244 | 0.808 |
| Medium shear rate§ | 5.13 ± 0.47 | 5.12 ± 0.60 | 0.082 | 0.935 |
| Low shear rate§ | 8.88 ± 1.04 | 9.31 ± 1.38 | −1.352 | 0.182 |
| Capillary plasma viscosity | 1.27 ± 0.12 | 1.25 ± 0.10 | 0.710 | 0.481 |
| Haematocrit (%) | 43.03 ± 3.64 | 45.20 ± 4.35 | −2.053 | 0.045 |
| Median HAMD-17 score (IQR†) | 2 (0–4) | 0.5 (0–3.75) | 0.534 | 0.596 |
| Median HAMA score (IQR†) | 3 (2–4.5) | 2 (0–3.75) | 1.971 | 0.054 |
| Cognitive function | ||||
| MMSE score | 27.38 ± 2.04 | 27.86 ± 1.96 | −0.901 | 0.371 |
| MoCA score | 24.76 ± 2.95 | 24.07 ± 3.13 | 0.854 | 0.397 |
| Subjective memory loss Rating scale score | 6.17 ± 1.73 | 6.43 ± 1.48 | −0.600 | 0.551 |
TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; HAMD-17, Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment.
Note: *t orχ2 value: Student’s t-test was used to compare quantitative data, including age, body mass index, lipids and lipoprotein, blood viscosity, and HAMD-17 and HAMA scores, while other categorical data were compared by chi-squared test,
Units of blood viscosity at high, medium, and low shear rates are (mPa.s)|150(1/s), (mPa.s)|60(1/s), and (mPa.s)|10(1/s).
IQR denotes interquartile range.
Effect on cognitive function
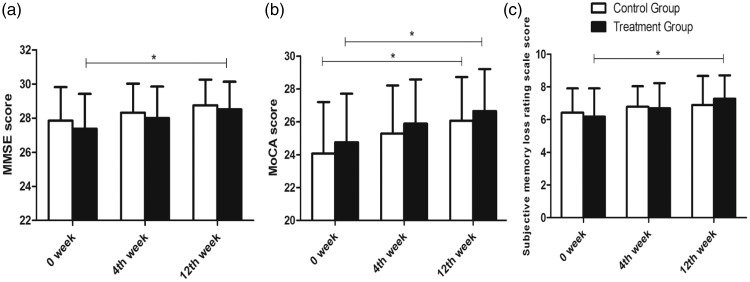
The MMSE and MoCA were used to investigate the effect of Pushen capsule and Ginkgo biloba tablets on cognitive function, in addition to the Subjective Memory Loss Rating Scale. Compared with the MMSE score at baseline, the mean MMSE score for the treatment group was significantly improved at week 12 after treatment with Pushen capsule (t = −2.352, P = 0.022) but was not significantly different from the MMSE score in the control group at the same time point (t = −1.913) (Figure 1a). Specific items of the MMSE were also studied in the two groups, but the scores for these specific items did not change after treatment (Table 4). Furthermore, there were no significant differences between the two groups when comparing the scores at week 0 and week 12.
Figure 1.
Effect of Pushen capsule and Ginkgo biloba tablets on cognitive function as assessed by MMSE (a), MoCA (b), and Subjective Memory Loss Rating Scale (c).
Table 4.
Score on specific items of MMSE in treatment and control groups (x ± s).
|
Treatment group |
Control group |
t value* | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 0 | Week 4 | Week 12 | |||
| Orientation | 9.66 ± 0.61 | 9.83 ± 0.47 | 9.86 ± 0.44 | 9.64 ± 0.73 | 9.71 ± 0.60 | 9.93 ± 0.26 | −0.513 | 0.610 |
| Registration | 2.86 ± 0.44 | 2.93 ± 0.26 | 3.00 ± 0.00 | 2.93 ± 0.26 | 3.00 ± 0.00 | 3.00 ± 0.00 | 0.689 | 0.494 |
| Attention and calculation | 4.10 ± 1.14 | 4.28 ± 1.03 | 4.38 ± 0.90 | 4.43 ± 0.84 | 4.50 ± 0.79 | 4.61 ± 0.79 | 0.683 | 0.498 |
| recall | 1.79 ± 1.05 | 2.00 ± 0.76 | 2.31 ± 0.89 | 2.04 ± 0.88 | 2.21 ± 0.88 | 2.32 ± 0.77 | 1.297 | 0.200 |
| Language and praxis | 8.97 ± 0.19 | 8.97 ± 0.19 | 8.97 ± 0.19 | 8.82 ± 0.55 | 8.89 ± 0.42 | 8.89 ± 0.42 | −1.467 | 0.148 |
Note: *t value and P value were calculated by t-test comparing the score in week 0 and week 12 between the two groups.
The mean MoCA score at the first assessment was 24.76 ± 2.95 and 24.07 ± 3.13 in the treatment group and control group, respectively, and were lower than the MMSE scores, which were 27.38 ± 2.04 and 27.86 ± 1.96, respectively. Our results were consistent with those from a previous study, which showed that MoCA is a concise cognitive screening tool with high sensitivity and specificity for detecting MCI in patients performing in the normal range on the MMSE.28,29 Although the score in week 4 was not significantly different from baseline in both the treatment and control groups (t = −1.539 and −1.500, respectively), it was higher in week 12 (t = −2.619 and −2.582; P = 0.011 and 0.013, respectively) (Figure 1b), and the score of the “delayed recall” item increased significantly with treatment (P = 0.018 and 0.026, respectively) (Table 2). Interestingly, although neither group showed improvements in the “Visuospatial/executive function” score over time, the control group performed better than the treatment group on this item (Z = −3.649, P = 0.00) (Table 2).
Table 2.
Scores for specific items of the Montreal Cognitive Assessment in treatment and control groups (x ± s).
|
Treatment group |
Control group |
t or Z value* | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 0 | Week 4 | Week 12 | |||
| Visuospatial/executive function | 4.38 ± 0.73 | 4.48 ± 0.79 | 4.55 ± 0.69 | 4.11 ± 0.99 | 4.50 ± 0.75 | 4.61 ± 0.63 | −3.649# | 0.000# |
| Naming | 2.97 ± 0.19 | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.96 ± 0.19 | 2.96 ± 0.19 | 3.00 ± 0.00 | −0.025 | 0.980 |
| Attention | 5.45 ± 1.15 | 5.55 ± 1.09 | 5.59 ± 0.98 | 5.64 ± 0.68 | 5.64 ± 0.73 | 5.61 ± 0.74 | 1.380 | 0.173 |
| Language | 2.17 ± 0.66 | 2.28 ± 0.65 | 2.31 ± 0.71 | 2.07 ± 0.77 | 2.29 ± 0.76 | 2.32 ± 0.82 | −0.631 | 0.531 |
| Abstraction | 1.03 ± 0.82 | 1.28 ± 0.75 | 1.48 ± 0.63 | 1.32 ± 0.82 | 1.36 ± 0.73 | 1.46 ± 0.69 | 1.884 | 0.065 |
| Delayed recall | 2.34 ± 1.68 | 2.97 ± 1.48 | 3.38 ± 1.29¥ | 1.82 ± 1.63 | 2.36 ± 1.34 | 2.79 ± 1.32¥ | 0.263 | 0.794 |
| Orientation | 5.93 ± 0.26 | 5.93 ± 0.26 | 5.93 ± 0.26 | 5.89 ± 0.42 | 5.93 ± 0.26 | 6.00 ± 0.00 | −1.160 | 0.251 |
Note: *t value and P value were calculated by t-test comparing the score at week 0 and week 12 between the two groups.
Wilcoxon signed rank test used to calculate the Z value by comparing the score for visuospatial/executive function in week 0 with week 12 because of the heterogeneity of variance.
Score compared with that at week 0 by t-test, resulting in P < 0.05.
We have experienced that post-stroke patients frequently complain of hypomnesis in clinical practice. Hence, we further explored the subjective memory loss that may occur in daily life. In the treatment group, the score on the Subjective Memory Loss Rating Scale was significantly improved at week 12 (t = −2.621, P = 0.011) (Figure 1c). In contrast, the score in the control group remained unchanged with treatment (t = −1.066). Half of the participants reported that they could not remember where they had placed an important item such as their wallet or keys in daily life (Table 3). In our study, Pushen capsule did not ameliorate this impairment but did have a positive effect on the score for “forgetting acquaintance’s name” (χ2 = 4.419, P = 0.036). The treatment group performed better than the control group for this item (χ2 = 6.257, P = 0.044) (Table 3).
Table 3.
Scores for specific items of Subjective Memory Loss Rating Scale in treatment and control groups (no. [%]).
| Item forgotten |
Treatment group |
Control group |
χ2 value* | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 0 | Week 4 | Week 12 | |||
| Acquaintance’s name | 18 (62.1%) | 9 (31.0%) | 10 (34.5%)¥ | 15 (53.6%) | 17 (60.7%) | 16 (57.1%) | 6.257 | 0.044 |
| What I want to do | 17 (58.6%) | 14 (48.3%) | 14 (48.3%) | 12 (42.9%) | 13 (46.4%) | 9 (32.1%) | 0.004 | 0.998 |
| Where the key/wallet is | 20 (69.0%) | 19 (65.5%) | 17 (58.6%) | 16 (57.1%) | 14 (50.0%) | 15 (53.6%) | 2.167 | 0.338 |
| Leave something behind | 8 (27.6%) | 10 (34.5%) | 8 (27.6%) | 4 (14.3%) | 8 (28.6%) | 6 (21.4%) | 2.126 | 0.345 |
| Lost the way | 3 (10.3%) | 2 (6.9%) | 2 (6.9%) | 2 (7.1%) | 0 (0%) | 1 (3.6%) | 1.486 | 0.476 |
| What I have read just now | 17 (58.6%) | 12 (41.4%) | 10 (34.5%) | 16 (57.1%) | 10 (35.7%) | 11 (39.3%) | 1.083 | 0.582 |
| Expressing an idea | 10 (34.5%) | 4 (13.8%) | 4 (13.8%) | 11 (39.3%) | 8 (28.6%) | 5 (17.9%) | 2.879 | 0.237 |
| Recalling what happened | 8 (27.6%) | 7 (24.1%) | 7 (24.1%) | 10 (35.7%) | 12 (42.9%) | 5 (17.9%) | 1.221 | 0.543 |
| Phone number | 5 (17.2%) | 3 (10.3%) | 3 (10.3%) | 7 (25.0%) | 3 (10.7%) | 4 (14.3%) | 1.164 | 0.559 |
Note: *χ2 value and P value were calculated by the chi-squared test compared with week 0 and week 12 between the two groups.
Values compared with week 0 in the chi-squared test, resulting in P < 0.05.
Effect on blood lipids and lipoproteins
Given the reported efficacy of Pushen capsule in improving hyperlipaemia,24 we examined the changes in blood lipids and lipoproteins in the two groups. TC and LDL-C levels in the treatment group differed among the three time points tested (F = 6.126 and 3.976; P = 0.003 and 0.022, respectively). Compared with the levels at baseline, the TC and LDL-C levels were lower at week 12 (P = 0.001 and 0.012, respectively) (Table 5). However, only the TC level in the control group differed among the three time points (F = 3.369; P = 0.039), and further comparison showed that Ginkgo biloba tablets reduced the TC level at week 12 (P = 0.023) (Table 5).
Table 5.
Blood lipids and lipoproteins in treatment and control groups.
|
Treatment group |
Control group |
t value* | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 0 | Week 4 | Week 12 | |||
| TC (mmol/L) | 5.02 ± 1.44 | 4.43 ± 1.02 | 3.92 ± 1.07¥ | 4.93 ± 1.09 | 4.45 ± 1.17 | 4.16 ± 1.08¥ | 0.963 | 0.340 |
| TG (mmol/L) | 1.46 ± 1.04 | 1.19 ± 0.68 | 1.28 ± 0.88 | 1.73 ± 0.90 | 1.48 ± 0.84 | 1.29 ± 0.50 | 1.713 | 0.092 |
| HDL-C (mmol/L) | 1.25 ± 0.35 | 1.31 ± 0.27 | 1.35 ± 0.44 | 1.06 ± 0.17 | 1.13 ± 0.21 | 1.16 ± 0.22 | 0.461 | 0.647 |
| LDL-C (mmol/L) | 2.96 ± 1.05 | 2.65 ± 0.87 | 2.26 ± 0.91¥ | 3.06 ± 0.82 | 2.74 ± 0.96 | 2.52 ± 0.87 | 0.589 | 0.558 |
| apoA1 (g/L) | 1.32 ± 0.45 | 1.31 ± 0.34 | 1.26 ± 0.41 | 1.10 ± 0.23 | 1.11 ± 0.23 | 1.14 ± 0.21 | 0.412 | 0.682 |
| ApoB (g/L) | 0.86 ± 0.26 | 0.86 ± 0.33 | 0.72 ± 0.26 | 0.90 ± 0.23 | 0.84 ± 0.23 | 0.78 ± 0.22 | 1.071 | 0.289 |
| Lipoprotein α (mg/L) | 336.69 ± 209.65 | 326.28 ± 218.64 | 301.97 ± 201.61 | 294.75 ± 222.17 | 326.64 ± 229.05 | 288.57 ± 228.09 | −0.385 | 0.702 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Note: *t value and P value were calculated by t-test comparing the score in week 0 and week 12 between the two groups.
Score compared with that at week 0 in ANOVA, resulting in P < 0.05.
Effect on haemorheology
In both the treatment and control groups, blood viscosity at low, medium, and high shear rates was significantly different among the three time points tested (F = 6.311, 6.078, and 4.489; and 4.158, 6.051, and 3.566, respectively; P = 0.003, 0.003, and 0.014; and 0.019, 0.004, and 0.033, respectively). In comparison with blood viscosity at week 0, blood viscosity at low, medium, and high shear rates in both groups was significantly lower at week 12 (P = 0.001, 0.002, 0.007 in the treatment group and 0.014, 0.002, and 0.017 in the control group, respectively). Notably, Ginkgo biloba tablets effectively improved haematocrit (F = 6.71, P = 0.02), which was reduced to 41.77 ± 3.53 at week 12 compared with 45.20 ± 4.35 at baseline (P = 0.02) (Table 6).
Table 6.
Hemorheological parameters in the treatment and control groups.
| Blood viscosity at |
Treatment group |
Control group |
t value* | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 12 | Week 0 | Week 4 | Week 12 | |||
| Low shear rate | 8.88 ± 1.04 | 8.38 ± 0.99 | 7.96 ± 0.95¥ | 9.31 ± 1.38 | 8.56 ± 1.42 | 8.35 ± 1.07¥ | −0.648 | 0.520 |
| Medium shear rate | 5.13 ± 0.47 | 4.93 ± 0.48 | 4.70 ± 0.47¥ | 5.12 ± 0.60 | 4.80 ± 0.54 | 4.60 ± 0.56¥ | 1.293 | 0.202 |
| High shear rate | 4.56 ± 0.43 | 4.39 ± 0.44 | 4.21 ± 0.48¥ | 4.60 ± 0.58 | 4.36 ± 0.63 | 4.15 ± 0.65¥ | 1.522 | 0.134 |
| Capillary plasma viscosity | 1.27 ± 0.12 | 1.29 ± 0.13 | 1.25 ± 0.11 | 1.25 ± 0.10 | 1.25 ± 0.11 | 1.24 ± 0.12 | 1.017 | 0.314 |
| Haematocrit (%) | 43.03 ± 3.64 | 42.47 ± 3.86 | 42.33 ± 3.17 | 45.20 ± 4.35 | 42.14 ± 3.62 | 41.77 ± 3.53¥ | 2.893 | 0.005 |
Note: Units of blood viscosity at low, medium, and high shear rate are (mPa.s)|10(1/s), (mPa.s)|60(1/s), and (mPa.s)|150(1/s).
*t value and P value were calculated by t-test comparing the score in week 0 and week 12 between the two groups.
Score compared with that at week 0 in ANOVA, resulting in P < 0.05.
Adverse effects
The items reflecting liver and kidney function and creatine kinase function were all normal in both groups throughout the trial. In the treatment group, three participants complained of mild nausea on the days 12, 14, and 38. However, all three participants agreed to continue with study treatment.
Discussion
The present study focused on the effect of a TCM preparation in improving cognitive impairment, using Ginkgo biloba, the effects of which have been studied widely in recent years, as a positive control. Our results indicate that Pushen capsule improved cognitive function in patients with VaMCI, as demonstrated by increases in MMSE and MoCA scores as well as improvements in the “delayed recall” item. In addition, Pushen capsule ameliorated daily amnesia among patients, especially with respect to the item “forgetting acquaintance's name”, whereas Ginkgo biloba did not have an effect on this item. These results suggest that Pushen capsule may represent a novel agent for the treatment of VaMCI.
A previous study reported that the MoCA is superior to the MMSE in discriminating between individuals with MCI and normal cognitive function.30 In our study, Ginkgo biloba tablets had little effect on the MMSE score but improved the MoCA score, consistent with previous results.30 Impaired function exhibited by impairments in the “delayed recalled” item occurs at a high rate in VaMCI patients, and our study indicated that Pushen capsule enhanced this item to a greater extent than it did other cognitive functions, which was in line with the effect of Pushen capsule on the item “forgetting acquaintance's name” when examining subjective memory loss encountered in daily life.
Multiple risk factors for cerebrovascular disease can lead to abnormal vascular endothelial structure and function, resulting in disordered blood haemorheology and subsequent activation of inflammation and oxidative stress, giving rise to advanced destruction of neurological as well as cognitive function. Lipid metabolism disorders and increased blood viscosity are also important in VCI6 and can accelerate the formation of atherosclerosis and thrombosis and promote the transformation of VaMCI into VaD. Our results showed that Pushen capsule treatment reduced the levels of TC and LDL in addition to reducing blood viscosity. Furthermore, Ginkgo biloba decreased the level of haematocrit. Whether Pushen capsule and Ginkgo biloba affect cognitive function by improving lipid metabolism and blood viscosity requires further study.
Pushen capsule consists of various components of TCM including Polygonum multiflorum, which has been shown to enhance the activity of cyclooxygenase and the fluidity of the mitochondrial membrane in the hippocampus of AD rats.31 Moreover, Salvia miltiorrhiza has been shown to reduce the deposition of Aβ by inhibiting BACE1 activity.32 In addition, a herbal extracts of Salvia miltiorrhiza (tanshinone IIA) has been demonstrated to improve memory impairment in a chronic cerebral ischaemia rat model by regulating the levels of glutamate and γ-aminobutyric acid (GABA) and protecting against free radical insults.33 However, the mechanism by which Pushen capsule might improve cognitive function has not been investigated to date. Similar to studies on the molecular mechanism of Ginkgo (EGb 761),12,13 our group is currently exploring the molecular mechanism of Pushen capsule using cell and animal experiments. We aim to identify proteins regulated by Pushen capsule and the precise mechanism by which it exerts its effects in future studies.
Cholinesterase inhibitors34 such as donepezil hydrochloride play an important role in rapidly improving cognitive function, while TCM can exert its effects in cells or organs over prolonged periods.9 The combination of cholinesterase inhibitors and TCM may potentially result in a synergistic effect on cognitive function. Furthermore, non-pharmacological treatments for VaMCI, such as acupuncture,35,36 may be beneficial and cost-effective when combined with Pushen capsule treatment. Further studies are needed to explore these treatment strategies.
VaMCI, a preliminary stage of VaD, is caused by small vessel disease and the accumulation of lacunar infarcts and white matter changes that are responsible for the gradual progression of cognitive impairment.37 Previous studies have indicated that VaMCI can be accompanied by other diseases, such as autoimmune disorders and depression, and that cognitive insult can be resolved by treating these disorders with or without specific treatment for VaMCI.7,38 Moreover, some studies have shown that VaMCI is common among patients with stroke, and improves with stroke recovery.39 Although our study was conducted in patients with MRI-confirmed stroke and showed that Pushen capsule and Ginkgo biloba could improve cognitive function, we cannot exclude the possibility of self-healing as not all cases of VaMCI progress to VaD.
This study had some limitations. Pushen capsule showed a beneficial effect in the treatment of VaMCI and improved blood lipid lipoprotein levels and haemorheological parameters. However, the validity of the results may have been affected by the small sample size, open-label study design, short follow-up time, and potential drug–drug interactions. Notably, the blood lipid and lipoprotein levels and haemorheological parameters of participants remained in the normal range throughout the study, despite showing significant changes over the treatment period. The effect of both types of TCM on hyperlipaemia and haemorheologic disorder requires further exploration. Furthermore, the beneficial treatment effects observed in the present study may be attributable to a slowing of functional decline rather than prevention of progression to VaD.40 Therefore, further studies to evaluate the long-term efficacy of Pushen capsule and Ginkgo extract are required.
Conclusion
Our findings indicate that Pushen capsule may exert beneficial effects, comparable to those of Ginkgo biloba, on the cognitive function of patients with VaMCI, particularly the function of “delayed recall” assessed by the MoCA and the subjective cognitive function of “forgetting acquaintance's name”. Moreover, risk factors for cerebrovascular disease such as lipid and lipoprotein levels and haemorheological parameters were significantly reduced following treatment with Pushen capsule and Ginkgo biloba.
Abbreviations
VaD: vascular dementia, AD: Alzheimer’s disease, VCI: vascular cognitive impairment, VaMCI: vascular mild cognitive impairment, MCI: mild cognitive impairment, TCM: traditional Chinese medicine, Aβ: amyloid-beta, HAMD-17: Hamilton Depression Scale, HAMA: Hamilton Anxiety Scale, MMSE: Mini Mental State Examination, MoCA: Montreal Cognitive Assessment, TG: triglyceride, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, GABA: γ-aminobutyric acid
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant no. KYZZ16_0130).
References
- 1.World Health Organization. Dementia – Fact Sheet Detail. World Health Organization, 2019. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dementia. [Last accessed on 2019 May 14].
- 2.Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 2013; 381: 2016–2023. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 5.Smith EE, Cieslak A, Barber P, et al. Therapeutic strategies and drug development for vascular cognitive impairment. JAHA 2017; 6: e5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dichgans M, Leys D. Vascular Cognitive Impairment. Circ Res 2017; 120: 573–591. [DOI] [PubMed] [Google Scholar]
- 7.Consoli A, Pasi M, Pantoni L. Vascular mild cognitive impairment: concept, definition, and directions for future studies. Aging Clin Exp Res 2012; 24: 113–116. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Wentzel C, Hachinski V, et al. Prevalence and outcomes of vascular cognitive impairment. Neurology 2000; 54: 447–451. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Cui G, Tseng HH, et al. Vascular contributions to cognitive impairment and treatments with Traditional Chinese Medicine. Evid Based Complement Alternat Med 2016; 2016: 9627258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridi R, Crossetti FP, Steffen VM, et al. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res 2001; 15: 449–451. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Chen HY, Wu TS, et al. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J Neurosci Res 2002; 68: 636–645. [DOI] [PubMed] [Google Scholar]
- 12.Yan F, Zheng Y, Zhao F. Effects of ginkgo biloba extract EGb761 on expression of RAGE and LRP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathologica 2008; 116: 529–535. [DOI] [PubMed] [Google Scholar]
- 13.Wan WB, Cao L, Liu LM, et al. EGb761 provides a protective effect against Abeta1-42 oligomer-induced cell damage and blood-brain barrier disruption in an in vitro bEnd.3 endothelial model. PLoS One 2014; 9: e113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanowski S, Herrmann WM, Stephan K, et al. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry 1996; 29: 47–56. [DOI] [PubMed] [Google Scholar]
- 15.Napryeyenko O, Sonnik G, Tartakovsky I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. J Neurol Sci 2009; 283: 224–229. [DOI] [PubMed] [Google Scholar]
- 16.Ihl R, Tribanek M, Bachinskaya N. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761(R) in Alzheimer's disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry 2012; 45: 41–46. [DOI] [PubMed] [Google Scholar]
- 17.Iakhno NN, Zakharov VV, Lokshina AB, et al. Tanakan (EGb 761) in the therapy of mild cognitive impairment. Zh Nevrol Psikhiatr Im S S Korsakova 2006; 106: 41–46. [PubMed] [Google Scholar]
- 18.Gavrilova SI, Preuss UW, Wong JW, et al. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry 2014; 29: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 19.Gavrilova SI, Fedorova I, Roshchina IF, et al. Experience in clinical use of tanakan in the treatment of the syndrome of mild cognitive impairment. Zh Nevrol Psikhiatr Im S S Korsakova 2006; 106: 42–46. [PubMed] [Google Scholar]
- 20.Lin Z, Gu J, Xiu J, et al. Traditional Chinese medicine for senile dementia. Evid Based Complement Alternat Med 2012; 2012: 692621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui G, Shan L, Hung M, et al. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol 2013; 168: 1349–1359. [DOI] [PubMed] [Google Scholar]
- 22.Ni JW, Matsumoto K, Watanabe H. Tetramethylpyrazine improves spatial cognitive impairment induced by permanent occlusion of bilateral common carotid arteries or scopolamine in rats. Jpn J Pharmacol 1995; 67: 137–141. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L, Wang YZ, Liu J, et al. Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sci 2005; 78: 413–420. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZL, Wu SX, Gao GD. Clinical assessment on treatment of hyperlipidemia with pushen capsule. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004; 24: 227–229. [PubMed] [Google Scholar]
- 25.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 26.Lam LC, Lui VW, Tam CW, et al. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer's disease. Int J Geriatr Psychiatry 2005; 20: 876–882. [DOI] [PubMed] [Google Scholar]
- 27.Yoon B, Shim YS, Hong Y, et al. Which symptoms can distinguish between subjective cognitive impairment (SCI) and mild cognitive impairment (MCI)? Arch Gerontol Geriatr 2012; 54: 325–329. [DOI] [PubMed] [Google Scholar]
- 28.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry 2007; 52: 329–332. [DOI] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 30.Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr 2018; 14: 1–14. [DOI] [PubMed] [Google Scholar]
- 31.De-ren HYWLX. Effect of polygonum multiflorum on the fluidity of the mitochondria membrane and activity of COX in the hippocampus of rats with Aβ 1-40-induced Alzheimer's disease. J Cent South Univ(Med Sci) 2008; 33: 986–987. [PubMed] [Google Scholar]
- 32.Durairajan S, Chirasani VR, Shetty SG, et al. Decrease in the generation of amyloid-beta due to salvianolic acid B by modulating BACE1 activity. Curr Alzheimer Res 2017; 14: 1229–1237. [DOI] [PubMed] [Google Scholar]
- 33.He Z, Pan Z, Lu W. Neuroprotective effects of tanshinone II A on vascular dementia in rats. Zhongguo Zhong Yao Za Zhi 2010; 35: 1883–1886. [DOI] [PubMed] [Google Scholar]
- 34.Kaduszkiewicz H, Zimmerman T, Beck-Bornholdt HP, et al. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ 2005; 331: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Wang XJ, Zhang ZC, et al. Neuroprotection against vascular dementia after acupuncture combined with donepezil hydrochloride: P300 event related potential. Neural Regen Res 2016; 11: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inchauspe A. Traditional Chinese medicine K1 Yongquan and resuscitation: another kind of “Lazarus phenomenon”. Resuscitation 2010; 81: 505–506. [DOI] [PubMed] [Google Scholar]
- 37.Pantoni L, Poggesi A, Inzitari D. Cognitive decline and dementia related to cerebrovascular diseases: some evidence and concepts. Cerebrovasc Dis 2009; 27: 191–196. [DOI] [PubMed] [Google Scholar]
- 38.Petri M, Naqibuddin M, Carson KA, et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol 2010; 37: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 39.Esiri MM, Matthews F, Brayne C, et al. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001; 357: 169–175. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Q, Wang CW, Shi J, et al. Effects of Ginkgo biloba on dementia: an overview of systematic reviews. J Ethnopharmacol 2017; 195: 1–9. [DOI] [PubMed] [Google Scholar]