ABSTRACT
Background
Bed rest studies document that a lower dietary acid load is associated with lower bone resorption.
Objective
We tested the effect of dietary acid load on bone metabolism during spaceflight.
Design
Controlled 4-d diets with a high or low animal protein–to-potassium (APro:K) ratio (High and Low diets, respectively) were given to 17 astronauts before and during spaceflight. Each astronaut had 1 High and 1 Low diet session before flight and 2 High and 2 Low sessions during flight, in addition to a 4-d session around flight day 30 (FD30), when crew members were to consume their typical in-flight intake. At the end of each session, blood and urine samples were collected. Calcium, total protein, energy, and sodium were maintained in each crew member's preflight and in-flight controlled diets.
Results
Relative to preflight values, N-telopeptide (NTX) and urinary calcium were higher during flight, and bone-specific alkaline phosphatase (BSAP) was higher toward the end of flight. The High and Low diets did not affect NTX, BSAP, or urinary calcium. Dietary sulfur and age were significantly associated with changes in NTX. Dietary sodium and flight day were significantly associated with urinary calcium during flight. The net endogenous acid production (NEAP) estimated from the typical dietary intake at FD30 was associated with loss of bone mineral content in the lumbar spine after the mission. The results were compared with data from a 70-d bed rest study, in which control (but not exercising) subjects’ APro:K was associated with higher NTX during bed rest.
Conclusions
Long-term lowering of NEAP by increasing vegetable and fruit intake may protect against changes in loss of bone mineral content during spaceflight when adequate calcium is consumed, particularly if resistive exercise is not being performed. This trial was registered at clinicaltrials.gov as NCT01713634.
Keywords: animal protein, bone mineral content, bone resorption, bone-specific alkaline phosphatase, dietary acid, dietary sulfur, N-telopeptide, net endogenous acid production, resistive exercise, weightlessness
INTRODUCTION
Though the mechanism of bone mineral loss associated with spaceflight is not completely understood, it is clearly multifactorial. With adequate nutritional support, resistive exercise can mitigate decrements in bone mineral density (BMD) during spaceflight (1, 2), and it is likely that diet can be optimized to further protect bone (3, 4). Although pharmacologic agents provide an alternative in the event of exercise device failure (5), avoiding these agents in otherwise healthy, relatively young individuals seems prudent. Dietary effects on bone are well established, and have virtually no risk of side effects.
Ground-based evidence supports the hypothesis that a suboptimal diet is associated with lower bone mineral status and higher osteoporosis and fracture risk in healthy subjects (6). Mediterranean diets rich in fruit and vegetables are generally beneficial to bone (7–9), possibly because low endogenous acid production is associated with fruit and vegetable intake. Endogenous acids include sulfuric acid produced from sulfur-containing proteins and amino acids. Generally, foods containing animal protein are higher in sulfur-containing amino acids than are plant protein sources, and plants have a higher content of alkaline precursors. Phosphoric acid is another nonvolatile acid that can be either ingested in the diet or produced endogenously. Anions, including conjugate bases of organic acids, make up the majority of dietary base precursors that the body metabolizes to bicarbonate. Potassium is the predominant intracellular inorganic cation that balances the charge of organic anions; therefore, dietary potassium intake can be used to estimate the content of base precursors in the diet. Frassetto and colleagues (10) developed a model for estimating net endogenous acid production (NEAP) on the basis of the acid and base precursors in the diet. According to this model, renal net acid excretion can be predicted from 2 dietary components: total protein and potassium.
In bed rest, an analog of spaceflight with regard to effects on bone, we showed that a higher ratio of dietary animal protein to potassium (APro:K) was associated with more excretion of both calcium and markers of bone resorption (i.e., collagen crosslinks), but had no association with markers of bone formation, such as bone-specific alkaline phosphatase (BSAP) (11, 12). Although this observation was clear after 2–3 wk of bed rest, it was not so pronounced before bed rest when the subjects were ambulatory. This suggests that the impact of diet on bone is more pronounced in nonexercising individuals, specifically those whose bone is in a resorptive state, as is the case during bed rest. In a separate study in which bed rest subjects were supplemented with essential amino acids, including methionine (a sulfur-containing amino acid), urinary pH was lower and the concentration of markers of bone resorption was higher in subjects receiving the supplement, whereas bone formation was not affected (13).
On the basis of these findings, we proposed that the APro:K ratio in the diet would be associated with bone metabolism during spaceflight. We report here results from a controlled diet study in which we investigated acute effects of controlled diets on biochemical markers of bone metabolism. For comparison, similar data from a 70-d ground-based bed rest study evaluating exercise effects on bone and other systems are also presented.
METHODS
Seventeen International Space Station (ISS) astronauts [13 men and 4 women; mean ± SD age at the time of launch: 47 ± 6 y; mean ± SD BMI (in kg/m2): 24.6 ± 3.0; mission durations of 160 ± 20 d], participated in the study. The protocol was reviewed and approved by the National Aeronautics and Space Administration (NASA) Johnson Space Center (JSC) Institutional Review Board, the Japanese Aerospace Exploration Agency, and the European Space Agency Medical Boards. Written informed consent was obtained from all crew members before their participation. Although we have published other bone and related data from astronauts on ISS missions, none of the data reported here have been previously published, and none of the data from the astronauts included here were included in those other publications.
Dietary treatments, recording, and analysis
The experimental goal was to test 2 diets: 1 with a high APro:K ratio (High; 1.0–1.3 g animal protein/mEq potassium) and 1 with a low APro:K ratio (Low; 0.3–0.6 g/mEq). The ranges were selected to represent the high and low ends of the linear relation between dietary APro:K and N-telopeptide (NTX) from a bed rest study (11). Four-day menus for each diet were developed from available space foods, and foods for each crew member were developed from the same lot for both the preflight and in-flight controlled-diet sessions. The High and Low 4-d menus for each crew member were designed to provide similar (within 5% of each other) intakes of total energy [based on WHO calculations for that crew member (14)], total protein, sodium, and calcium intakes within a crew member. A research dietitian met with each crew member in advance to plan acceptable High and Low menus given the available space foods. Identical menus were provided in a semi-randomized crossover fashion, so that each crew member had 1 High and 1 Low diet session before flight and 2 High and 2 Low sessions during flight. The randomization was stunted to yield (roughly) equal numbers of High and Low diet sessions at each of the designated time points during flight. The preflight sessions occurred at ∼6 mo and 3 mo before the mission, and the in-flight sessions occurred around flight day (FD) 15, FD60, FD120, and FD180. All crew members completed ≥3 controlled diet sessions, but not all crew members completed all 4 controlled diet intake sessions during flight due to the shorter durations of some missions. Note that, although 7, 8, or 9 subjects participated in each of the High or Low sessions, because of randomization, the participants were not the same individuals from one session to the next.
In addition to the controlled dietary intake sessions during flight, there was a 4-d session at FD30 when crew members had no dietary restrictions but were instead asked to consume and record their typical intake. This session was intended to provide an understanding of the crew members’ typical intake during the mission. Crew members completed similar 4-d monitored intake sessions after flight (return plus 30, 180, and 365 d) (data not reported here). A fasting blood sample and a 24-h urine collection were also obtained between ∼10 and 45 d before flight with no diet monitoring (and no diet restrictions).
Dietary intake data were collected and analyzed using Nutrition Data System for Research software versions 2007, 2010, 2012, and 2014, developed by the Nutrition Coordinating Center, University of Minnesota. Space foods are analyzed in the Water & Food Analytical Laboratory at NASA JSC for macronutrient and mineral content, including moisture, fat (total, saturated, and unsaturated), fiber, protein, carbohydrate, calories, calcium, magnesium, sodium, potassium, phosphorus, copper, iron, manganese, zinc, chloride, iodine, fatty acid profile, and cholesterol. Recipes and nutrients analyzed by the laboratory for each food item were submitted to the Nutrition Coordinating Center and the content of other nutrients was imputed according to the analyzed data and recipes. Dietary sulfate was determined using an equation from Remer and Manz (15). Diets were designed for each individual crew member according to their estimated energy requirements (14) and study constraints. In some cases, however, a crew member simply could not consume the prescribed amount of food, and in other cases crew members reported being hungry at the end of the day when the allotted food items had been eaten. In the former cases, attempts were made to adjust menus for subsequent sessions to maintain study constraints at lower energy intakes. In the latter cases, nonprotein-containing food items were provided to increase the caloric intake. Actual intakes are reported in the results.
All crew members were provided with vitamin D supplements (800 IU/d) during flight. They were asked to refrain from taking any other supplements during the 4-d controlled dietary intake sessions.
In addition to using the APro:K ratio to approximate the NEAP, it was also estimated by a calculation from the dietary components that incorporates the potential renal acid load of the diet and the diet-independent organic acid excretion estimated from the surface area of the body (15).
Biological sample collections and biochemical analyses
At the end of each 4-d session, 24-h urine samples and fasting blood samples were collected, with the closing urine void collected at around the same time as the fasting blood draw. We have previously reported the details of the techniques and equipment used for in-flight biological sample collections and processing (1, 16).
Blood samples were analyzed for bone biochemical markers using standard techniques, as described previously (1, 2). Urine samples were analyzed for collagen crosslinks, including NTX, C-telopeptide, and helical peptide (HP), with commercially available kits (Osteomark, Ostex International; Osteometer BioTech; and Quidel, respectively). Urinary sulfate was measured as previously described (17). Urinary pH was measured in 24-h urine collections with a standard pH meter. Urinary pH was determined during flight with a paper strip technique, but we found that the lighting on board the ISS did not show the color gradations on the strip clearly enough to allow precise resolution of the pH.
Exercise
All crew members regularly performed resistive exercise on the ISS Advanced Resistive Exercise Device (ARED) (2, 18). The types of exercise performed on the ARED include squats, heel raises, deadlift, shoulder press, abdominals, and bent-over row. All of the exercises performed on the ARED were included in the reported totals. The amount of exercise was determined by multiplying the amount of weight used by the number of repetitions and sets for each exercise performed on the device. The daily total pounds lifted in the 3 wk before the blood and urine collection associated with each dietary session was then calculated. If less than 3 wk of data were available (i.e., for the FD15 session), then only the available data were used.
Bone densitometry
Bone mineral content (BMC) and BMD were assessed ∼3 mo before and within 1 mo after each flight by dual-energy X-ray absorptiometry (DXA) with a fan beam densitometer (Hologic Discovery, Hologic, Inc.), as described previously (2).
Environmental data
ISS cabin CO2 was determined by the ISS Cabin Gas Analyzer and by the Major Constituent Analyzer. The mean of the 2 measurements in the 24-h day of each blood draw was used in the analyses.
Bed rest
Comparison data are reported here from a 70-d bed rest study evaluating exercise as a countermeasure to physiologic effects of simulated microgravity (19). These data are from one of a series of bed rest studies conducted at the University of Texas Medical Branch (UTMB) in Galveston, TX (20). The protocols were reviewed by the NASA JSC and UTMB institutional review boards, and written informed consent was obtained before participation.
Although we and others have reported data from this (19) and similar (21, 22) bed rest studies, to our knowledge, none of the data reported here have been published previously (and certainly no data pertaining to dietary APro:K effects on bone).
Subjects (n = 11, 10 men, 1 woman; age 38 ± 7 y; BMI 25.2 ± 2.2) participated in a 70-d 6° head-down–tilt bed rest study. Five subjects completed daily supine exercise, which started on the first day of bed rest, and 6 subjects served as nonexercising controls. The exercise consisted of aerobic and resistance exercise (e.g., squat, heel raise, leg press, cycling, and treadmill), designed to be similar to the exercise profiles being tested on the ISS.
Subjects were housed in the Institute for Translational Sciences-Clinical Research Center at UTMB for ≥13 d before the start of bed rest until 6 d after reambulation following the 70-d bed rest phase of the study. Food was weighed and dietary intake was recorded daily by metabolic kitchen staff, as described for similar bed rest studies in the Institute for Translational Sciences-Clinical Research Center (23). These diets were controlled, but not with respect to the APro:K ratio. For this report, the dietary intake data were analyzed for the 7 d before the urine collection and the mean APro:K was determined. Urine was collected in 24-h pools 4 times before bed rest (collected 10, 9, 3, and 2 d before bed rest) and monthly during bed rest. For this report, the pre-bed rest NTX mean represents the mean NTX of the 4 days before bed rest and the bed rest NTX is the mean of the last 2 days of bed rest (bed rest days 69 and 70).
Statistical analyses
To test for differences in demographic, in-flight exercise, environmental, and reported dietary intake measures between astronauts assigned to the High and Low APro:K diet at FD15, FD60, FD120, and FD180, we used 2-sided t tests and Fisher's exact tests for continuous and discrete data, respectively. Additionally, we used 2-sided, paired t tests to investigate differences between astronauts’ mean in-flight exercise, environmental, and dietary intake measures while they were on the monitored diet session at FD30 and the same measures while they were on the High or Low APro:K diet at FD15, FD60, FD120, and FD180. To control the false discovery rate, we adjusted the α-level of the tests using the adaptive Benjamini, Krieger, and Yekutieli procedure (24): we restricted the false discovery rate to 0.05, which means that we would expect 5% of the rejected null hypotheses to be false (incorrect rejection).
Two mixed-effects linear regression models were fitted using the lme4 package (25) in R (26) to assess differences in the changes in urinary NTX, BSAP, and daily urinary calcium excretion from baseline across assigned diets (High, Low, monitored) for measures collected before and during flight (first model), and for in-flight measures only (second model). All dependent variables (urinary NTX, BSAP, urinary calcium) were normalized by subtracting astronauts’ respective baseline measures collected 10 d before flight (L–10), when they were not on a controlled (or monitored) diet. We incorporated a random intercept term in each model, which accommodated random heterogeneity in astronauts’ changes in NTX, BSAP, and urinary calcium from baseline that persisted throughout the study. Diet was treated as a 3-level categoric covariate and modeled using indicator variables, with the monitored diet session serving as the reference category. Each model controlled for fixed effects of age, body mass (kilograms), sex (male as reference), energy (kilocalories per day), total protein (grams per day), dietary sulfur (milliequivalents per day), dietary potassium (milligrams per day), dietary calcium (milligrams per day), dietary sodium (milligrams per day), NEAP (milliequivalents per day), and flight day. For the first (preflight and in-flight) model, we incorporated a main effect for in-flight status (using preflight data as reference) and an interaction between in-flight status and relative day from launch when significant. For the in-flight–only models, we additionally controlled for cumulative 3 wk of exercise (expressed as lb) and mean CO2 (expressed as mm Hg). P values for significance of the regression coefficient were obtained using the Kenward-Roger approximation with the R package pbkrtest (27). Significance was determined at the 0.05 α-level.
FD30/DXA analysis
We assessed whether changes in BMC and BMD after spaceflight were associated with dietary protein using simple linear regression models. Thereafter, we fitted multiple regression models to investigate whether dietary sulfur confounded these relations. Additionally, we took a similar approach to assess whether changes in total lumbar spine BMC were associated with NEAP at FD30, controlling for age and sex. Significance was determined at the 0.05 α-level.
Bed rest analyses
Pearson correlation coefficients were determined for the control and exercise groups for the 7-d mean APro:K (7 d before urine collections) and the mean pre-bed rest NTX as well as the mean NTX from bed rest days 69 and 70.
RESULTS
All crew members completed all planned sessions, although 3 had truncated mission durations so could not complete an FD180 session. Demographic, in-flight exercise and CO2, and summary dietary intake data are shown in Table 1. An extended set of intake data is provided in Supplemental Table 1. It is important to recall that crew members did not follow the same pattern of diets, thus the subjects in the High or Low groups were not the same individuals from one session to the next. This was accounted for in the analysis.
TABLE 1.
In-flight metabolism-related and dietary intake data for ISS crew members during 4-d controlled-intake and monitored-intake sessions1
| Pre (L–180) | Pre (L–45) | FD15 | FD30 | FD60 | FD120 | FD1802 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | Monitored | High | Low | High | Low | High | Low | |
| (n = 8) | (n = 9) | (n = 8) | (n = 8) | (n = 8) | (n = 9) | (n = 17) | (n = 8) | (n = 9) | (n = 9) | (n = 8) | (n = 7) | (n = 7) | |
| Body mass, kg | 79 ± 12 | 81 ± 16 | 82 ± 14 | 79 ± 12 | 72 ± 9a | 86 ± 14 | 79 ± 13 | 79 ± 14 | 80 ± 13 | 88 ± 9a | 70 ± 11 | 81 ± 16 | 84 ± 9 |
| Female, n (%) | 2 (25) | 2 (22) | 1 (13) | 2 (25) | 3 (38) | 1 (11) | 4 (24) | 1 (13) | 3 (33) | 1 (11) | 3 (38) | 2 (29) | 1 (14) |
| Cumulative 3-wk exercise (1000 lb lifted) | — | — | — | — | 327 ± 147b | 210 ± 137b | 528 ± 302 | 703 ± 412b | 618 ± 273 | 840 ± 445b | 501 ± 174 | 644 ± 295b | 966 ± 557b |
| Mean CO2, mm Hg | — | — | — | — | 3.38 ± 0.44 | 3.08 ± 0.38 | 3.13 ± 0.43 | 3.25 ± 0.41 | 2.94 ± 0.54 | 2.69 ± 0.89 | 2.61 ± 0.58b | 2.89 ± 0.47 | 2.56 ± 0.70 |
| Dietary intake | |||||||||||||
| Energy, kcal/d | 2622 ± 454b | 2856 ± 631b | 2943 ± 682b | 2701 ± 457b | 2661 ± 507b | 2938 ± 750b | 2199 ± 648 | 2829 ± 691b | 2675 ± 594b | 2992 ± 698b | 2435 ± 378b | 2399 ± 471a,b | 3303 ± 651b |
| Energy/body mass, kcal · d−1 · kg−1 | 34 ± 7 | 36 ± 6 | 36 ± 7 | 35 ± 7 | 37 ± 5 | 35 ± 8 | 28 ± 7 | 36 ± 7 | 34 ± 8 | 34 ± 9 | 35 ± 6 | 31 ± 8 | 39 ± 6 |
| Total protein, g/d | 116 ± 11 | 115 ± 26 | 128 ± 23 | 111 ± 12 | 109 ± 16 | 124 ± 25 | 98 ± 35 | 126 ± 25 | 107 ± 17 | 130 ± 20 | 100 ± 14 | 110 ± 15 | 131 ± 25 |
| Total protein/body mass, kcal · d−1 · kg−1 | 1.49 ± 0.21 | 1.45 ± 0.25 | 1.58 ± 0.25 | 1.43 ± 0.22 | 1.51 ± 0.16 | 1.47 ± 0.28 | 1.23 ± 0.39 | 1.61 ± 0.28 | 1.35 ± 0.18 | 1.48 ± 0.24 | 1.45 ± 0.23 | 1.38 ± 0.22 | 1.56 ± 0.24 |
| Animal protein, g/d | 84 ± 8 | 56 ± 15 | 95 ± 19 | 54 ± 8 | 81 ± 12 | 61 ± 14 | 67 ± 29 | 94 ± 19 | 52 ± 9 | 94 ± 17 | 48 ± 6 | 81 ± 12 | 63 ± 13 |
| Dietary potassium, mg/d | 2895 ± 300 | 4723 ± 810 | 3249 ± 549 | 4612 ± 535 | 2835 ± 374 | 5094 ± 785 | 3499 ± 1036 | 3134 ± 642 | 4469 ± 523 | 3217 ± 568 | 4179 ± 394 | 2810 ± 529 | 5046 ± 933 |
| APro:K, g · mEq−1 · d−1 | 1.13 ± 0.04 | 0.47 ± 0.08 | 1.13 ± 0.08 | 0.47 ± 0.04 | 1.13 ± 0.04 | 0.47 ± 0.08 | 0.70 ± 0.20 | 1.17 ± 0.12 | 0.47 ± 0.08 | 1.13 ± 0.08 | 0.47 ± 0.08 | 1.13 ± 0.12 | 0.51 ± 0.08 |
| NEAP, mEq/d | 86 ± 11a,b | 44 ± 11b | 91 ± 14a,b | 42 ± 3b | 84 ± 10a,b | 46 ± 10b | 57 ± 14 | 94 ± 14a,b | 46 ± 11 | 96 ± 13a,b | 44 ± 9 | 88 ± 10a,b | 54 ± 9b |
| Dietary sulfur, mEq/d | 64 ± 7b | 55 ± 12 | 71 ± 12a,b | 54 ± 6 | 61 ± 10b | 59 ± 12 | 51 ± 19 | 70 ± 13a,b | 52 ± 8 | 72 ± 11a,b | 48 ± 7b | 61 ± 8b | 63 ± 11 |
| Dietary calcium, mg/d | 1357 ± 192 | 1369 ± 178 | 1516 ± 289 | 1427 ± 203 | 1172 ± 167 | 1315 ± 242 | 1118 ± 397 | 1252 ± 169 | 1162 ± 217 | 1218 ± 240 | 1135 ± 176 | 1117 ± 177 | 1240 ± 198 |
| Dietary sodium, mg/d | 4644 ± 1289 | 4203 ± 642 | 4525 ± 586 | 4358 ± 1224 | 4375 ± 921 | 4479 ± 805 | 3726 ± 1145 | 4631 ± 591 | 4065 ± 909 | 4251 ± 995 | 4399 ± 384 | 4506 ± 575 | 3991 ± 943 |
| Dietary fiber, g/d | 22 ± 2a,b | 42 ± 7b | 25 ± 4a | 42 ± 6b | 21 ± 3a | 45 ± 8b | 28 ± 7 | 24 ± 5a,b | 40 ± 6b | 25 ± 4a,b | 38 ± 5b | 21 ± 3a | 48 ± 8b |
1All values are means ± SDs unless otherwise indicated. See the Statistical analyses section for full details. Measures in the first column were included in linear mixed-effects models comparing controlled preflight and in-flight sessions with the FD30 monitored session. A separate model using only the in-flight data controlled for exercise and CO2, as these data were not available before flight. To test for differences in demographic, in-flight exercise, environmental, and reported dietary intake measures between astronauts assigned to High and Low APro:K diets at FD15, FD60, FD120, and FD180, 2-sided t tests and Fisher's exact tests for continuous and discrete data, respectively, were used. Additionally, we used 2-sided, paired t tests to investigate differences between astronauts’ mean in-flight exercise, environmental, and dietary intake measures while they were on the monitored diet session at FD30 and the same measures while they were on the High or Low diet at FD15, FD60, FD120, and FD180. The false discovery rate was controlled using the adaptive Benjamini, Krieger, and Yekutieli procedure (24): the false discovery rate was restricted to 0.05. aSignificantly different from Low for the same FD; bsignificantly different from the FD30 monitored session. FD, flight day; High, high animal protein:potassium ratio diet; ISS, International Space Station; Low, low animal protein:potassium ratio diet; NEAP, net endogenous acid production; Pre (L–X), X days before launch.
2Not all astronauts were measured at FD180 because of flight duration, as described in Methods.
Energy intake was generally maintained during High and Low APro:K diet sessions, but small adjustments were made when crew members felt that they could not consume the food that met the estimated energy requirements or when crew members requested additional food because they were hungry. Energy intake during the controlled sessions was higher than in the FD30 monitored intake session (Table 1). As designed, dietary total protein, calcium, and sodium were maintained during crew members’ controlled dietary intake sessions. NEAP was different between the 2 diets, significantly higher with the High menus than with the Low menus, as expected (Table 1).
The ISS cabin CO2 concentration was generally constant between sessions (Table 1). With a mean close to 3 mm Hg, the CO2 concentration on the ISS was much higher than terrestrial CO2 at standard pressure (0.3 mm Hg).
We evaluated 3 key bone markers: urinary NTX, urinary calcium, and serum BSAP. Summaries of the measures collected are presented in Table 2, and the results of the linear mixed-effects model for preflight and in-flight measures as well as the model with only in-flight measures are shown in Tables 3–5.
TABLE 2.
Bone markers in ISS crew members before and during flight, by 4-d controlled-intake dietary session1
| Pre (L–180) | Pre (L–45) | FD15 | FD60 | FD120 | FD180 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre (L–10) | FD30 | |||||||||||||
| High | Low | High | Low | Uncontrolled | High | Low | Monitored | High | Low | High | Low | High | Low) | |
| (n = 8) | (n = 9) | (n = 8) | (n = 8) | (n = 17) | (n = 8) | (n = 9) | (n = 17) | (n = 8) | (n = 9) | (n = 9) | (n = 8) | (n = 7) | (n = 7) | |
| Urine NTX | 408 ± 207 | 458 ± 135 | 376 ± 169 | 407 ± 166 | 433 ± 198 | 766 ± 301 | 827 ± 251 | 851 ± 379 | 803 ± 343 | 850 ± 243 | 832 ± 231 | 716 ± 277 | 730 ± 227 | 945 ± 275 |
| nmol/d | −44 ± 220 | 41 ± 96 | −56 ± 71 | −45 ± 172 | — | 341 ± 161 | 386 ± 197 | 417 ± 314 | 433 ± 233 | 360 ± 232 | 314 ± 179 | 374 ± 141 | 252 ± 130 | 458 ± 178 |
| Δ | 1 ± 32 | 13 ± 24 | −14 ± 18 | 0 ± 27 | — | 85 ± 35 | 111 ± 76 | 108 ± 74 | 130 ± 70 | 91 ± 57 | 71 ± 45 | 124 ± 68 | 77 ± 71 | 100 ± 36 |
| % Δ | ||||||||||||||
| Serum BSAP | 22 ± 7 | 26 ± 7 | 25 ± 7 | 25 ± 7 | 28 ± 6 | 24 ± 6 | 29 ± 5 | 28 ± 5 | 39 ± 5 | 31 ± 5 | 38 ± 10 | 43 ± 8 | 41 ± 11 | 38 ± 13 |
| U/L | −4.0 ± 5.1 | −2.8 ± 6.4 | −3.9 ± 6.4 | −1.9 ± 4.4 | — | −0.8 ± 1.7 | −0.6 ± 5.2 | 0.6 ± 4.4 | 8.2 ± 7.0 | 6.4 ± 3.7 | 10.0 ± 8.7 | 14.4 ± 6.7 | 14.4 ± 8.6 | 8.3 ± 9.3 |
| Δ | −15 ± 19 | −9 ± 23 | −13 ± 22 | −8 ± 17 | — | −3 ± 7 | −1 ± 15 | 3 ± 14 | 29 ± 27 | 28 ± 16 | 37 ± 30 | 51 ± 25 | 54 ± 28 | 29 ± 28 |
| % Δ | ||||||||||||||
| Urine calcium | 5 ± 2 | 3 ± 2 | 5 ± 2 | 4 ± 1 | 5 ± 3 | 8 ± 4 | 7 ± 2 | 9 ± 3 | 9 ± 2 | 6 ± 3 | 8 ± 2 | 5 ± 2 | 5 ± 1 | 6 ± 2 |
| mmol/d | 0.0 ± 2.8 | −2.1 ± 2.5 | −1.2 ± 2.2 | −1.2 ± 2.0 | — | 3.7 ± 2.5 | 1.9 ± 3.4 | 3.5 ± 2.8 | 3.4 ± 3.3 | 0.8 ± 2.4 | 1.3 ± 2.6 | 0.7 ± 2.2 | 1.2 ± 2.2 | −0.5 ± 2.9 |
| Δ | 24 ± 83 | −32 ± 25 | −12 ± 24 | −13 ± 52 | — | 83 ± 40 | 77 ± 100 | 97 ± 88 | 99 ± 112 | 29 ± 50 | 39 ± 60 | 28 ± 61 | 58 ± 72 | 1 ± 29 |
| % Δ | ||||||||||||||
1Data are expressed as actual amount or concentration, as change from the Pre (L–10) (i.e., baseline) session when crew members were not on a controlled diet, and as percentage change from the Pre (L–10) session. All data values are means ± SDs. BSAP, bone-specific alkaline phosphatase; FD, flight day; High, high animal protein:potassium ratio diet; ISS, International Space Station; Low, low animal protein:potassium ratio diet; NTX, N-telopeptide; Pre (L–X), X days before launch; Δ, change.
TABLE 3.
Results of 3 linear mixed-effects models investigating the relation between changes in astronaut urinary NTX excretion and HPK and LPK diets relative to a monitored diet1
| Pre- and in-flight model | In-flight model | In-flight model without outlier | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t | P value | Estimate | SE | t | P value | Estimate | SE | t | P value | |
| Measure | ||||||||||||
| Intercept | −1231 | 348 | −3.5 | <0.01 | −1106 | 397 | −2.8 | 0.01 | −641 | 374 | −1.7 | 0.09 |
| HPK diet | −147 | 75 | −2.0 | 0.06 | −218 | 80 | −2.7 | 0.01 | −120 | 78 | −1.5 | 0.13 |
| LPK diet | −61 | 69 | −0.9 | 0.39 | −24 | 71 | −0.3 | 0.74 | 32 | 67 | 0.5 | 0.64 |
| Body weight, kg | 0.2 | 2.5 | 0.1 | 0.93 | 3 | 3 | 1.2 | 0.26 | 1 | 3 | 0.5 | 0.61 |
| Female | 80 | 84 | 1.0 | 0.35 | 56 | 95 | 0.6 | 0.56 | −18 | 86 | −0.2 | 0.83 |
| Age, y | 18 | 6 | 3.1 | <0.01 | 17 | 7 | 2.5 | 0.02 | 14 | 6 | 2.4 | 0.02 |
| Energy, kcal/d | 0.00 | 0.00 | 0.3 | 0.80 | 0.04 | 0.07 | 0.6 | 0.56 | −0.01 | 0.06 | −0.1 | 0.89 |
| NEAP, mEq/d | −2 | 2 | −1.2 | 0.25 | −1 | 2 | −0.3 | 0.74 | −1 | 2 | −0.4 | 0.66 |
| Dietary sulfur, mEq/d | 8 | 3 | 2.3 | 0.03 | 6 | 3 | 1.8 | 0.08 | 5 | 3 | 1.5 | 0.15 |
| Dietary sodium, mg/d | 0.02 | 0.03 | 0.9 | 0.38 | 0.04 | 0.03 | 1.3 | 0.19 | 0.03 | 0.02 | 1.2 | 0.25 |
| Dietary fiber, g/d | −2 | 5 | −0.5 | 0.65 | −5 | 4 | −1.0 | 0.31 | −3 | 4 | −0.8 | 0.42 |
| Flight day, d | −0.2 | 0.2 | −1.2 | 0.22 | 0.04 | 0.39 | 0.1 | 0.92 | −0.26 | 0.38 | −0.7 | 0.50 |
| In-flight status | 453 | 67 | 6.7 | <0.0001 | — | — | — | — | — | — | — | — |
| Exercise, lb lifted | — | — | — | — | 0.00 | 0.00 | −0.7 | 0.47 | 0.00 | 0.00 | 0.1 | 0.94 |
| Mean CO2, mm Hg | — | — | — | — | 43 | 29 | 1.5 | 0.15 | 27 | 27 | 1.0 | 0.32 |
| Random effects | ||||||||||||
| σastronaut | 86 | 100 | 87 | |||||||||
| σ | 134 | 115 | 105 | |||||||||
1Changes and percentage changes in urinary NTX excretion (Table 2) were measured between each 4-d controlled-diet session and the Pre (L–10) session, when crew members were not on a controlled diet. The preflight and in-flight model controlled for body weight; sex; age; energy; NEAP; dietary sulfur, sodium, and fiber; flight day; and in-flight status. The in-flight model controlled for exercise (cumulative resistive exercise for 3 wk before data collection) and mean CO2. The data include all subjects (n = 17), but 1 subject (outlier) was removed from the analyses because of an extremely high response during the FD30 session relative to the preflight baseline (as discussed in Results and shown in Supplemental Figure 1). FD, flight day; HPK, high animal protein:potassium ratio; LPK, low animal protein:potassium ratio; NEAP, net endogenous acid production; NTX, N-telopeptide.
TABLE 5.
Results of 2 linear mixed-effects models investigating the relation between changes in astronaut urinary calcium and HPK and LPK diets relative to a monitored diet1
| Pre- and in-flight model | In-flight model | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t | P value | Estimate | SE | t | P value | |
| Measure | ||||||||
| Intercept | −4.86 | 6.23 | −0.8 | 0.44 | 2.04 | 6.56 | 0.3 | 0.76 |
| HPK diet | −0.29 | 1.00 | −0.3 | 0.77 | −1.08 | 0.83 | −1.3 | 0.21 |
| LPK diet | 0.96 | 0.71 | 1.4 | 0.19 | −1.33 | 0.75 | −1.8 | 0.08 |
| Body weight, kg | −0.05 | 0.05 | −1.1 | 0.27 | −0.02 | 0.05 | −0.3 | 0.76 |
| Female | −0.63 | 1.54 | −0.4 | 0.68 | −0.71 | 1.66 | −0.4 | 0.67 |
| Age, y | 0.08 | 0.10 | 0.8 | 0.45 | 0.06 | 0.11 | 0.6 | 0.58 |
| Energy, kcal/d | 0.00 | 0.00 | 0.2 | 0.85 | 0.00 | 0.00 | 0.5 | 0.66 |
| NEAP, mEq/d | −0.01 | 0.02 | −0.6 | 0.57 | 0.00 | 0.02 | −0.1 | 0.94 |
| Dietary sulfur, mEq/d | 0.01 | 0.03 | 0.3 | 0.78 | −0.02 | 0.03 | −0.5 | 0.65 |
| Dietary sodium, mg/d | 0.001 | 0.00 | 2.5 | 0.02 | 0.001 | 0.00 | 2.8 | 0.01 |
| Dietary fiber, g/d | −0.07 | 0.04 | −1.6 | 0.12 | −0.08 | 0.04 | −1.8 | 0.09 |
| Flight day | 0.02 | 0.00 | 4.2 | <0.001 | −0.02 | 0.00 | −3.8 | <0.01 |
| In-flight status | 3.86 | 0.61 | 6.4 | <0.0001 | — | — | — | — |
| Flight day × in-flight status | −0.02 | 0.00 | −5.6 | <0.0001 | — | — | — | — |
| Exercise, lb lifted | — | — | — | — | 0.00 | 0.00 | −1.0 | 0.31 |
| Mean CO2, mm Hg | — | — | — | — | 0.08 | 0.29 | 0.3 | 0.78 |
| Random effects | ||||||||
| σastronaut | 2.0 | 2.1 | ||||||
| σ | 1.2 | 1.1 | ||||||
1Changes and percentage changes in urinary calcium concentration (Table 2) were measured between each 4-d controlled-diet session and the Pre (L–10) session, when crew members were not on a controlled diet. The preflight and in-flight model controlled for body weight; sex; age; energy; NEAP; dietary sulfur, sodium, and fiber; flight day; and in-flight status. The in-flight model controlled for exercise (cumulative resistive exercise for 3 wk before data collection) and mean CO2. n = 17. HPK, high animal protein:potassium ratio; LPK, low animal protein:potassium ratio; NEAP, net endogenous acid production; Pre (L–X), X days before launch.
As expected, NTX increased during flight (raw and change data are presented in Table 2; statistical models are presented in Table 3). In the preflight and in-flight model (Table 3), the High (P = 0.06) and Low (P = 0.39) diets were not significantly associated with changes in NTX (from L–10) relative to the monitored diet on FD30. However, age (P < 0.01) and dietary sulfur (P = 0.03) were significantly associated with changes in NTX from baseline (L–10). Specifically, in looking at the estimate numbers in Table 3, for every 1-y increase in age and 10 mEq/d increase in dietary sulfur, we would expect 18 and 80 nmol/d increases in excretion of NTX, respectively, for the typical astronaut, holding all else constant. When we investigated the potential confounding effects of exercise and CO2 exposure (looking at in-flight data only), the High diet was associated with a decrease in NTX. However, further investigation revealed that this relation was potentially driven by one individual who had a sharp increase in NTX from the baseline (L–10) at FD30, when crew members were not on a controlled diet (see Supplemental Figure 1). Refitting the model with the individual removed negated the significant difference (P = 0.130, Table 3). For the in-flight–only model, only age remained significantly associated with changes in NTX from baseline (P = 0.02, Table 3). However, the magnitudes of the effects of age on NTX were relatively consistent between the preflight and in-flight model and the in-flight–only model. The same model was applied to urinary CTX and helical peptide, 2 additional markers of bone resorption, and the results were generally similar to what was observed for NTX (data not shown).
The data did not provide enough evidence to suggest that either the High or the Low controlled diet was significantly associated with differences between the change in serum BSAP during flight (from L–10) relative to the FD30 monitored session in either model (Table 4). BSAP increased during flight (Table 2), and increased further with increased duration of flight (P < 0.0001 for flight day in the pre- and in-flight model). For the in-flight–only model, flight day remained significant (P < 0.001) and CO2 also had a small but significant (P = 0.01) association with BSAP (Table 4). For every 10-d increase in days of flight, we would expect a 1-U/L increase in BSAP for the typical astronaut, holding all else constant. Also, for every 1-mm Hg increase in mean CO2 during flight, we would expect a 3-U/L increase in BSAP for the typical astronaut, holding all else constant.
TABLE 4.
Results of 2 linear mixed-effects models investigating the relation between changes in astronaut serum BSAP and HPK and LPK diets relative to a monitored diet1
| Pre- and in-flight model | In-flight model | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t | P value | Estimate | SE | t | P value | |
| Measure | ||||||||
| Intercept | −3.7 | 12.9 | −0.3 | 0.77 | −9.0 | 12.3 | −0.7 | 0.47 |
| HPK diet | −2.7 | 3.8 | −0.7 | 0.48 | 4.1 | 2.9 | 1.4 | 0.17 |
| LPK diet | −5.0 | 2.6 | −1.9 | 0.06 | 1.9 | 2.6 | 0.7 | 0.48 |
| Body weight, kg | −0.1 | 0.1 | −1.1 | 0.28 | −0.1 | 0.1 | −0.9 | 0.40 |
| Female | −3.9 | 2.9 | −1.4 | 0.17 | −2.5 | 2.9 | −0.9 | 0.38 |
| Age, y | 0.3 | 0.2 | 1.5 | 0.15 | 0.1 | 0.2 | 0.7 | 0.49 |
| Energy, kcal/d | 0.0 | 0.0 | −0.8 | 0.41 | 0.0 | 0.0 | −1.1 | 0.30 |
| NEAP, mEq/d | −0.1 | 0.1 | −1.8 | 0.08 | 0.0 | 0.1 | −0.5 | 0.61 |
| Dietary sulfur, mEq/d | 0.1 | 0.1 | 0.8 | 0.45 | 0.0 | 0.1 | −0.2 | 0.82 |
| Dietary sodium, mg/d | 0.0 | 0.0 | −0.2 | 0.83 | 0.0 | 0.0 | 1.3 | 0.19 |
| Dietary fiber, g/d | −0.1 | 0.2 | −0.5 | 0.61 | 0.0 | 0.2 | 0.1 | 0.94 |
| Flight day | −0.1 | 0.0 | −6.3 | <0.0001 | 0.1 | 0.0 | 7.1 | <0.001 |
| In-flight status | 5.4 | 2.4 | 2.3 | 0.02 | — | — | — | — |
| Flight day × in-flight status | 0.1 | 0.0 | 8.0 | <0.0001 | — | — | — | — |
| Exercise, lb lifted | — | — | — | — | 0.0 | 0.0 | 0.6 | 0.54 |
| Mean CO2, mm Hg | — | — | — | — | 3.0 | 1.1 | 2.7 | 0.01 |
| Random effects | ||||||||
| σastronaut | 2.9 | 2.6 | ||||||
| σ | 4.6 | 4.3 | ||||||
1Changes and percentage changes in BSAP concentration (Table 2) were measured between each 4-d controlled-diet session and the Pre (L–10) session, when crew members were not on a controlled diet. The preflight and in-flight model controlled for body weight; sex; age; energy; NEAP; dietary sulfur, sodium, and fiber; flight day; and in-flight status. The in-flight model controlled for exercise (cumulative resistive exercise for 3 wk before data collection) and CO2. n = 17. BSAP, bone-specific alkaline phosphatase; HPK, high animal protein:potassium ration; LPK, low animal protein:potassium ration; NEAP, net endogenous acid production; Pre (L–X), X days before launch.
The urinary calcium data also did not provide enough evidence to suggest that either the High or the Low controlled diet was significantly associated with differences between urinary calcium excretion change (from L–10) during flight and urinary calcium during the FD30 monitored session (Table 5); however, higher dietary sodium was associated with higher urinary calcium (P < 0.05) in both of the mixed-effects models. Urinary calcium was significantly higher during flight than before flight (P < 0.0001), but there was a significant interaction between flight day and in-flight status (P < 0.0001). Thus, as time in flight (flight day) increased, urinary calcium increased at a slower rate (change and percentage change decreased, Table 2). When looking at the in-flight–only model (Table 5), which controlled for CO2 and exercise, there was still no statistically significant difference for urinary calcium between the High or the Low diet and a monitored diet. Dietary sodium and flight day remained significant during flight.
Dietary intake from the FD30 monitored session (Table 1), used as a representation for typical in-flight dietary intake, revealed that a higher acid load (estimated by NEAP) was associated with a greater decrease in BMC of the lumbar spine (Figure 1, P < 0.01, Pearson r = −0.66). This relation held when the FD30 NEAP was multiplied by the flight duration (Pearson r = −0.68), and after adjusting for age and sex.
FIGURE 1.
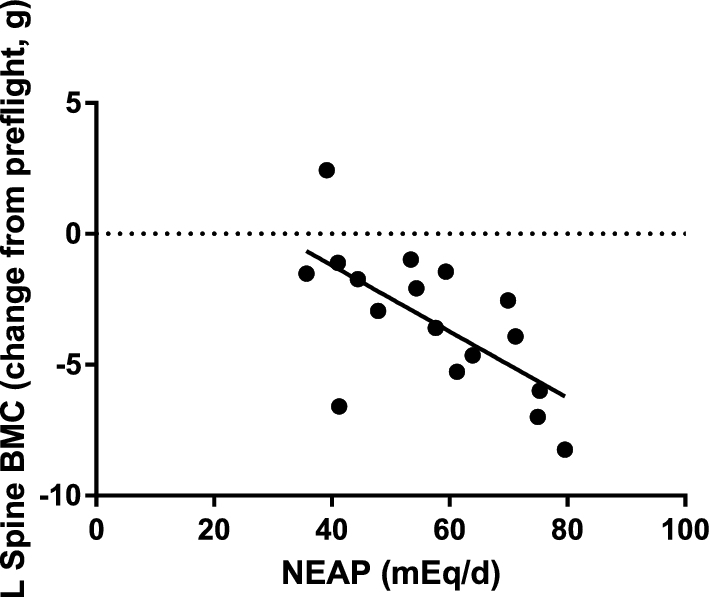
The dietary NEAP at the FD30 session (an average of 4 d) was related by Pearson correlation to the change in lumbar spine BMC after flight (r = 0.66, P < 0.01). Each point represents a single crew member (n = 17). Data from the FD30 time point, estimated from crew members’ diet for 4 d, was used as a proxy for crew members’ typical intake throughout their mission. BMC, bone mineral content; FD, flight day; L, lumbar; NEAP, net endogenous acid production.
In the bed rest study, APro:K was not correlated with urinary NTX before bed rest, but was correlated with NTX in the control (no exercise) subjects during bed rest (r = 0.82, P < 0.05) (Figure 2). This effect was not observed in exercising subjects, and during bed rest those subjects showed a trend for a negative correlation (r = −0.84, P = 0.07). The bed rest dietary intake data are presented in Table 6.
FIGURE 2.
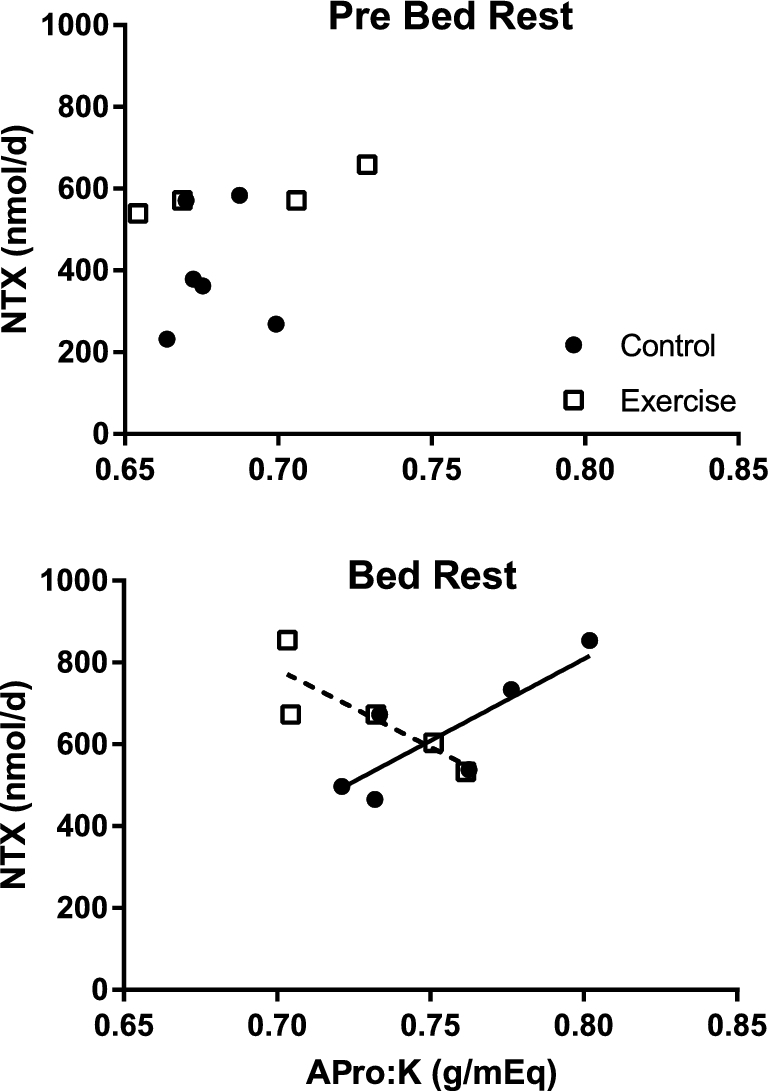
Relation of NTX to dietary APro:K ratio before (Pre Bed Rest) and during bed rest in sedentary (Control, n = 6) and exercising subjects (n = 5). During bed rest the Pearson correlation between NTX and APro:K was significant for the Control (r = 0.82, P < 0.05) but not for the Exercise group (r = −0.84, P = 0.07). APro:K, animal protein:potassium; NTX, N-telopeptide.
TABLE 6.
Dietary intake data before and at the end of the 70-d bed rest study1
| Pre-bed rest | Bed rest | |
|---|---|---|
| APro:K, g/mEq | ||
| Control | 0.68 ± 0.01 | 0.75 ± 0.03 |
| Exercise | 0.68 ± 0.04 | 0.73 ± 0.03 |
| Energy, kcal/kg | ||
| Control | 34.2 ± 1.5 | 31.4 ± 2.9 |
| Exercise | 36.6 ± 1.7 | 37.3 ± 2.2 |
| Total protein, g/kg | ||
| Control | 1.31 ± 0.05 | 1.18 ± 0.12 |
| Exercise | 1.40 ± 0.07 | 1.43 ± 0.09 |
| Potassium, mg/d | ||
| Control | 3553 ± 307 | 3142 ± 446 |
| Exercise | 3834 ± 327 | 3839 ± 468 |
| Calcium, mg/d | ||
| Control | 1746 ± 247 | 1531 ± 277 |
| Exercise | 1884 ± 104 | 1785 ± 114 |
| Sodium, mg/d | ||
| Control | 3698 ± 385 | 3212 ± 478 |
| Exercise | 3946 ± 316 | 3835 ± 398 |
1The data are 7-d means ± SDs before bed rest (pre-bed rest) and 7-d means ± SDs during the last week of bed rest (bed rest). Control, n = 6; exercise, n = 5. APro:K, animal protein:potassium ratio.
DISCUSSION
The literature on the effects of protein (type and amount) on bone are mixed, with some studies demonstrating that diets providing a high protein intake are detrimental to bone (28–30); conversely, many studies report high protein intake having a protective effect on bone (31, 32). In light of our previous bed rest data showing that the ratio of dietary APro:K was positively associated with urinary NTX excretion (11), we hypothesized that a higher APro:K diet during spaceflight would yield a greater change in urinary NTX excretion than a diet with lower APro:K, and we hypothesized that diet would have no effect before flight. Contrary to our hypothesis, we did not observe any differences in the effect on bone turnover markers when crew members were on a High or a Low ratio controlled diet for 4-d periods during flight. Rather than concluding that our findings support the argument that there is no effect or a positive effect of protein on bone, we maintain that these results help to clarify the discord in the literature.
The majority of the studies reporting detrimental effects of protein on bone had less than optimal calcium intake, and the studies reporting beneficial effects on bone had calcium intakes adequate to support bone formation (33–35). de Jonge and colleagues (36) found that dietary acid load may be associated with negative bone outcomes only in subgroups of individuals. They found that in individuals with higher intakes of dietary fiber, a higher NEAP was more detrimental to bone; this result was hypothesized to be caused by inhibited intestinal absorption of calcium (36). In our study, dietary fiber was twice as high in the Low menus as in the High menus. We included dietary fiber in the regression models, but it was not statistically significant for any of the outcomes studied. It is also important to point out that crew members in this flight study were consuming high-protein diets overall, relative to the current RDA of 0.8 g protein/kg. The mean protein intake during the controlled diet sessions was 1.5 ± 0.2 g/kg body weight (1.2 ± 0.4 g/kg body weight for the FD30 session). The mean calcium intake was 1275 ± 229 mg/d (1118 ± 397 mg/d during the FD30 session), which is more than sufficient to support bone formation. High protein intake is often argued to be detrimental to bone because it is often associated with increased urinary calcium excretion. For every 10-g increase in dietary protein, urinary calcium increases by 16 mg (37), and because excess dietary protein is catabolized to ammonium ion and sulfates from the sulfur-containing amino acids, it has been hypothesized that bone is resorbed to neutralize the dietary acid load. A key consideration is that increased dietary protein caused an increase in calcium absorption in some studies (38–40). In our study, when comparing dietary effects on NTX before and during flight, we found that a higher dietary sulfur intake was associated with an increase in urinary NTX excretion, but it was not associated with urinary calcium. Dietary sodium had a small but significant positive association with urinary calcium. Higher dietary sodium was not, however, associated with a greater change in NTX, so it is possible that the calcium could come from increased intestinal absorption instead of being released from bone. To more fully investigate the effects on calcium, balance studies would need to be conducted. We previously documented that calcium absorption decreased during spaceflight, but that was in crew members who did not have access to resistance exercise (41, 42). Interestingly, Thorpe and colleagues (43) found a positive association of lumbar spine BMD with dietary protein in postmenopausal women consuming adequate calories, but the association was suppressed by a negative association with protein sulfur. Sulfur intakes were ∼30 mEq/d in that study, which was about half of the sulfur intake of the crew members in this study. We attempted to reproduce these findings with the monitored intake data at FD30, but the total protein and sulfur were highly correlated (Pearson's r = 0.9968), and therefore we could not effectively distinguish between the effects of sulfur and protein.
Exercise, or even degree of ambulation, is a major factor in these studies. Exercise is an operational countermeasure on the ISS, meaning that all crew members on the ISS are required to exercise—using both aerobic and resistive exercise devices before and throughout their missions (44, 45). In this study, all crew members had access to heavy resistance exercise, which has been shown to mitigate bone loss during flight (2, 42) and in bed rest (46). Our previous bed rest studies showed a positive relation between APro:K and NTX. This relation was more pronounced in control subjects than in exercising subjects (11), and more pronounced in men (11) than women (12). The exercise in those studies was a unique protocol combining aerobic (treadmill) exercise with lower-body negative pressure. In the bed rest study reported here, diet had no effect on bone resorption during the ambulatory pre-bed rest phase. During bed rest, there was a significant association of diet and bone resorption in sedentary subjects, which was absent in exercising subjects.
Because the High and Low diets lasted for only 4 d and were not representative of the entire mission intake, we could not look at those diets with respect to overall changes in BMC. The FD30 monitored dietary intake session is the closest estimate we have to a snapshot of the typical intake of the crew members during the other ∼160 d of the mission when their diets were not controlled for this study. For the 4 d of that monitored session, crew members were asked to record all fluid and food intake, with no restrictions on what they could have. The mean NEAP from FD30, estimated from acid and base components of the diet, was negatively associated with the change in lumbar spine BMC. We specifically looked at lumbar spine BMC because in previous studies it was the region with the highest percentage change from preflight values for crew members using the ARED (2). Total BMD and BMC were not related to NEAP, and it is possible that the diet effects on these whole-body measurements were swamped by the protective effect of exercise. Furthermore, Remer and colleagues (47) have suggested that the potential renal acid load of the diet may be more related to the cortical area of the bone (bone size), as assessed by peripheral quantitative computed tomography (pQCT), and not to areal BMD, as assessed by DXA. This hypothesis is based on previous pQCT findings that dietary potential renal acid load was associated with cortical area and BMC but not with volumetric cortical BMD (48). Remer et al. (47) argue that true volumetric density, bone thickness, and bone size are integrated into a single number with DXA, and the acid load of the diet may be more closely related to bone size and bone mass than to the BMD measurement. We unfortunately do not have any pQCT data for the crew members in this study, but the relation between dietary acid load and impact on bone is worth testing in the future.
A potential limitation and confounding factor of the present study could be that the 4-d controlled dietary sessions were simply not long enough to observe an effect on bone turnover markers. Studies have documented rapid (i.e., within days) increases in bone resorption response to bed rest (49) and response to dietary changes during bed rest (50, 51), but response to dietary changes with 4-d menus has not been evaluated during spaceflight. In light of the observation that FD30 NEAP was associated with BMC loss, and assuming that intake during the FD30 session accurately represented the typical intake for the entire mission, it is possible that we simply missed this relation with the biochemical markers after 4 d of controlled diets, and that 4 d was not long enough to observe the effect on bone biochemistry, or even that the NEAP of the diet should have been the driving factor behind the High and Low diets instead of APro:K. Shea and colleagues (52) showed that grains and fruit had an impact on net acid excretion whereas protein did not, which could explain why the NEAP difference between the diets was not as high as expected at some time points. Now that detailed, daily dietary intake logging has been initiated on the ISS (as opposed to weekly questionnaires), more detailed analyses regarding the effect of overall mission dietary intake on bone and other systems will be possible.
An abundance of literature exists on both sides of the protein-bone question. We have long maintained that spaceflight and flight analog research highlights the fact that neither side is “right,” and that the effect of protein on bone depends on many factors—key among them being adequacy of other dietary components (calcium, energy, etc.) and ambulation (53, 54). Although we had hoped that this study would yield a dietary countermeasure for spaceflight-induced bone loss and expected the microgravity environment to offer a better model of bone loss than bed rest, it seems the effects of exercise in protecting BMD during flight (2) swamp the dietary effects on bone. Regardless, the association between NEAP and BMD, along with the relation between sulfur and NTX, are key findings. Although these results further document the complexity of this relation, our basic tenet remains that providing a long-term diet rich in fruit and vegetables will have a positive impact on bone on extended-duration missions. Such a diet would also be beneficial for other biological systems.
Supplementary Material
Acknowledgements
Spaceflight research requires tremendous support and coordination from a team of individuals, and this study extended that further than most. We thank the astronauts for their time, effort, and participation. We thank Vickie Kloeris and the JSC Food Systems Laboratory for their efforts in preparing, packing, and manifesting the controlled menu containers. Bed rest studies are almost as complex as flight studies, and require extensive support and coordination. We thank the test subjects for their time and participation, and the UTMB General Clinical Research Center Flight Analog Research Unit personnel for their support of all study logistics, planning, and execution. We thank the NASA JSC Nutritional Biochemistry Laboratory personnel for coordination of both of these projects, and their outstanding support of data collection sessions, sample management and analysis, and data management. We are also grateful to Natalie Baecker, University of Bonn, Germany, for her excellent support in sample and data management. We thank the NASA Human Research Program's Human Health Countermeasures Element, ISS Medical Project, and Flight Analogs Project for their support of this research. Finally, we thank Jane Krauhs for her editorial assistance in preparing the manuscript.
The authors’ responsibilities were as follows—SMS, SRZ, MH, and LCS: designed research; SMS and SRZ: oversaw data collection and management; BLR and HD: designed menus; MK: performed statistical analysis; all authors: were involved in interpreting the data and preparing the manuscript; SMS: had primary responsibility for final content; and all authors: read and approved the final manuscript. All authors declare that they have no potential conflicts of interest.
Notes
This study was supported by the Human Health Countermeasures Element of the NASA Human Research Program and by the Institute for Translational Sciences at the University of Texas Medical Branch, and in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health. The research was also funded in part by the German Aerospace Center DLR, Space Program, Grant 50WB1231.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
BLR is currently retired.
Abbreviations used: APro:K, animal protein:potassium; ARED, Advanced Resistive Exercise Device; BMC, bone mineral content; BMD, bone mineral density; BSAP, bone-specific alkaline phosphatase; DXA, dual-energy X-ray absorptiometry; FD, flight day; High, diet with a high animal protein:potassium ratio; HP, helical peptide; ISS, International Space Station; JSC, Johnson Space Center; Low, diet with a low animal protein:potassium ratio; L–, days before flight; NASA, National Aeronautics and Space Administration; NEAP, net endogenous acid production; NTX, N-telopeptide; pQCT, peripheral quantitative computed tomography.
References
- 1. Smith SM, Heer M, Shackelford LC, Sibonga JD, Spatz J, Pietrzyk RA, Hudson EK, Zwart SR. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015;81:712–20. [DOI] [PubMed] [Google Scholar]
- 2. Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res 2012;27:1896–906. [DOI] [PubMed] [Google Scholar]
- 3. Smith SM, Heer M, Zwart SR. Nutrition and bone health in space. In: Holick M, Nieves J, editors. Nutrition and bone health. 2nd ed New York: Springer; 2015. p. 687–705. [Google Scholar]
- 4. Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM, Shapses SA, Sinaki M, Watts NB, Sibonga JD. Skeletal health in long-duration astronauts: nature, assessment and management recommendations from the NASA bone summit. J Bone Miner Res 2013;28:1243–55. [DOI] [PubMed] [Google Scholar]
- 5. Leblanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, Evans H, Spector E, Ploutz-Snyder R et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int 2013;24:2105–14. [DOI] [PubMed] [Google Scholar]
- 6. Movassagh EZ, Vatanparast H. Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byberg L, Bellavia A, Larsson SC, Orsini N, Wolk A, Michaelsson K. Mediterranean diet and hip fracture in swedish men and women. J Bone Miner Res 2016;31:2098–105. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez-Real JM, Bullo M, Moreno-Navarrete JM, Ricart W, Ros E, Estruch R, Salas-Salvado J. A mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J Clin Endocrinol Metab 2012;97:3792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, Feriche B, Lorenzo ML, Olea F. Mediterranean diet and bone mineral density in two age groups of women. Int J Food Sci Nutr 2013;64:155–61. [DOI] [PubMed] [Google Scholar]
- 10. Frassetto LA, Todd KM, Morris RC Jr., Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 1998;68:576–83. [DOI] [PubMed] [Google Scholar]
- 11. Zwart SR, Hargens AR, Smith SM. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr 2004;80:1058–65. [DOI] [PubMed] [Google Scholar]
- 12. Zwart SR, Hargens AR, Lee SM, Macias BR, Watenpaugh DE, Tse K, Smith SM. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 2007;40:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM. Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol 2005;99:134–40. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: World Health Organization; 1985. [PubMed] [Google Scholar]
- 15. Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 1995;95:791–7. [DOI] [PubMed] [Google Scholar]
- 16. Smith SM, Heer M, Wang Z, Huntoon CL, Zwart SR. Long-duration space flight and bed rest effects on testosterone and other steroids. J Clin Endocrinol Metab 2012;97:270–8. Erratum JCEM 97:3390. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan JL, Heer M, Hargens AR, Macias BR, Hudson EK, Shackelford LC, Zwart SR, Smith SM. Sex-specific responses of bone metabolism and renal stone risk during bed rest. Physiol Rep 2014;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loehr JA, Lee SM, English KL, Sibonga J, Smith SM, Spiering BA, Hagan RD. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med Sci Sports Exerc 2011;43:146–56. [DOI] [PubMed] [Google Scholar]
- 19. Taibbi G, Cromwell RL, Zanello SB, Yarbough PO, Ploutz-Snyder RJ, Godley BF, Vizzeri G. Ophthalmological evaluation of integrated resistance and aerobic training during 70-day bed rest. Aerosp Med Hum Perform 2017;88:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meck JV, Dreyer SA, Warren LE. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med 2009;80:A1–A8. [DOI] [PubMed] [Google Scholar]
- 21. Spector ER, Smith SM, Sibonga JD. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med 2009;80:A23–28. [DOI] [PubMed] [Google Scholar]
- 22. Zwart SR, Oliver SAM, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 2009;80:A15–22. [DOI] [PubMed] [Google Scholar]
- 23. Inniss AM, Rice BL, Smith SM. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med 2009;80:A9–14. Erratum in Aviat Space Environ Med. 2014;85(7):768. [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y, Krieger A, Yekutieli D. Adaptive linear set-up procedures that control the false discovery rate. Biometrica 2006;93:491–507. [Google Scholar]
- 25. Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P, Bolker MB. Package ‘lme4’. Vienna, Austria: R Foundation for Statistical Computing; 2012. p. 12. [Google Scholar]
- 26. R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 27. Halekoh U, Højsgaard S. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models—the R package pbkrtest. J Stat Softw 2014;59:1–30.26917999 [Google Scholar]
- 28. Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol 1996;143:472–9. [DOI] [PubMed] [Google Scholar]
- 29. Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 2001;73:118–22. [DOI] [PubMed] [Google Scholar]
- 30. Heer M, Baecker N, Frings-Meuthen P, Graf S, Zwart SR, Biolo G, Smith SM. Effects of high-protein intake on bone turnover in long-term bed rest in women. Appl Physiol Nutr Metab 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 31. Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res 2011;26:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Hooven EH, Ambrosini GL, Huang RC, Mountain J, Straker L, Walsh JP, Zhu K, Oddy WH. Identification of a dietary pattern prospectively associated with bone mass in Australian young adults. Am J Clin Nutr 2015;102:1035–43. [DOI] [PubMed] [Google Scholar]
- 33. Mangano KM, Sahni S, Kerstetter JE. Dietary protein is beneficial to bone health under conditions of adequate calcium intake: an update on clinical research. Curr Opin Clin Nutr Metab Care 2014;17:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicoll R, McLaren Howard J. The acid-ash hypothesis revisited: a reassessment of the impact of dietary acidity on bone. J Bone Miner Metab 2014;32:469–75. [DOI] [PubMed] [Google Scholar]
- 35. Dawson-Hughes B. Interaction of dietary calcium and protein in bone health in humans. J Nutr 2003;133:852S–4S. [DOI] [PubMed] [Google Scholar]
- 36. de Jonge EA Koromani F, Hofman A, Uitterlinden AG, Franco OH, Rivadeneira F, Kiefte-de Jong JC. Dietary acid load, trabecular bone integrity, and mineral density in an ageing population: the Rotterdam study. Osteoporos Int 2017;28:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Massey LK. Dietary animal and plant protein and human bone health: a whole foods approach. J Nutr 2003;133:862S–5S. [DOI] [PubMed] [Google Scholar]
- 38. Cao JJ, Johnson LK, Hunt JR. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. J Nutr 2011;141:391–7. [DOI] [PubMed] [Google Scholar]
- 39. Hunt JR, Johnson LK, Fariba Roughead ZK. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr 2009;89:1357–65. [DOI] [PubMed] [Google Scholar]
- 40. Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr 2002;75:773–9. [DOI] [PubMed] [Google Scholar]
- 41. Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE et al. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol 1999;277:R1–10. [DOI] [PubMed] [Google Scholar]
- 42. Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J Bone Miner Res 2005;20:208–18. [DOI] [PubMed] [Google Scholar]
- 43. Thorpe M, Mojtahedi MC, Chapman-Novakofski K, McAuley E, Evans EM. A positive association of lumbar spine bone mineral density with dietary protein is suppressed by a negative association with protein sulfur. J Nutr 2008;138:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayes J. The first decade of ISS exercise: lessons learned on Expeditions 1–25. Aerosp Med Hum Perform 2015;86:1–6. [DOI] [PubMed] [Google Scholar]
- 45. Loerch LH. Exercise countermeasures on ISS: summary and future directions. Aerosp Med Hum Perform 2015;86:92–4. [DOI] [PubMed] [Google Scholar]
- 46. Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 2004;97:119–29. [DOI] [PubMed] [Google Scholar]
- 47. Remer T, Shi L, Alexy U. Potential renal acid load may more strongly affect bone size and mass than volumetric bone mineral density. Bone 2011;48:414–5. [DOI] [PubMed] [Google Scholar]
- 48. Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr 2005;82:1107–14. [DOI] [PubMed] [Google Scholar]
- 49. Baecker N, Tomic A, Mika C, Gotzmann A, Platen P, Gerzer R, Heer M. Bone resorption is induced on the second day of bed rest: results of a controlled crossover trial. J Appl Physiol 2003;95:977–82. [DOI] [PubMed] [Google Scholar]
- 50. Buehlmeier J, Frings-Meuthen P, Remer T, Maser-Gluth C, Stehle P, Biolo G, Heer M. Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: results of a randomized controlled trial. J Clin Endocrinol Metab 2012;97:4789–97. [DOI] [PubMed] [Google Scholar]
- 51. Frings-Meuthen P, Baecker N, Heer M. Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J Bone Miner Res 2008;23:517–24. [DOI] [PubMed] [Google Scholar]
- 52. Shea MK, Gilhooly CH, Dawson-Hughes B. Food groups associated with measured net acid excretion in community-dwelling older adults. Eur J Clin Nutr 2017;71:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zwart SR, Smith SM. The impact of space flight on the human skeletal system and potential nutritional countermeasures. Intl SportMed J 2005;6:199–214. [Google Scholar]
- 54. Smith SM, Abrams SA, Davis-Street JE, Heer M, O'Brien KO, Wastney ME, Zwart SR. Fifty years of human space travel: implications for bone and calcium research. Annu Rev Nutr 2014;34:377–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


