ABSTRACT
BACKGROUND
Intervention studies suggest that incorporating walnuts into the diet may improve blood lipids without promoting weight gain.
OBJECTIVE
We conducted a systematic review and meta-analysis of controlled trials evaluating the effects of walnut consumption on blood lipids and other cardiovascular risk factors.
Design
We conducted a comprehensive search of PubMed and EMBASE databases (from database inception to January 2018) of clinical trials comparing walnut-enriched diets with control diets. We performed random-effects meta-analyses comparing walnut-enriched and control diets for changes in pre-post intervention in blood lipids (mmol/L), apolipoproteins (mg/dL), body weight (kg), and blood pressure (mm Hg).
RESULTS
Twenty-six clinical trials with a total of 1059 participants were included. The following weighted mean differences (WMDs) in reductions were obtained for walnut-enriched diets compared with control groups: −6.99 mg/dL (95% CI: −9.39, −4.58 mg/dL; P < 0.001) (3.25% greater reduction) for total blood cholesterol (TC) and −5.51 mg/dL (95% CI: −7.72, −3.29 mg/dL; P < 0.001) (3.73% greater reduction) for low-density lipoprotein (LDL) cholesterol. Triglyceride concentrations were also reduced in walnut-enriched diets compared with control [WMD = −4.69 (95% CI: −8.93, −0.45); P = 0.03; 5.52% greater reduction]. More pronounced reductions in blood lipids were observed when walnut interventions were compared with American and Western diets [WMD for TC = −12.30 (95% CI: −23.17, −1.43) and for LDL = −8.28 (95% CI: −13.04, −3.51); P < 0.001]. Apolipoprotein B (mg/dL) was also reduced significantly more on walnut-enriched diets compared with control groups [WMD = −3.74 (95% CI: −6.51, −0.97); P = 0.008] and a trend towards a reduction was observed for apolipoprotein A [WMD = −2.91 (95% CI: −5.98, 0.08); P = 0.057]. Walnut-enriched diets did not lead to significant differences in weight change (kg) compared with control diets [WMD = −0.12 (95% CI: −2.12, 1.88); P = 0.90], systolic blood pressure (mm Hg) [WMD = −0.72 (95% CI: −2.75, 1.30); P = 0.48], or diastolic blood pressure (mm Hg) [WMD = −0.10 (95% CI: −1.49, 1.30); P = 0.88].
Conclusions
Incorporating walnuts into the diet improved blood lipid profile without adversely affecting body weight or blood pressure.
Keywords: walnuts, blood lipids, apolipoprotein, body weight, meta-analysis
INTRODUCTION
Nuts are a key component of several healthy dietary patterns and recommendations. The literature has consistently shown that frequent nut consumption is associated with improved cardiovascular risk factors (1–3) and lower risk of cardiovascular disease (CVD) (4, 5). Inverse associations between nut intake and CVD risk have been observed in large prospective cohort studies (5). In addition, several clinical trials have reported beneficial effects of nut consumption on blood lipids (2), inflammatory parameters, insulin resistance (6, 7), and blood pressure (8). Of note, in a pooled analysis of 25 trials including 583 participants with normolipidemia and hypercholesterolemia, a reduction of total cholesterol (TC), LDL cholesterol, the ratio of LDL to HDL cholesterol, and the ratio of TC to HDL cholesterol was observed when incorporating nuts into the diet (mean daily consumption of 67 g of nuts) (2). In a more recent meta-analysis of 61 clinical trials, of which 21 included walnut intervention groups, walnut consumption was associated with significant reductions in several blood lipid parameters including TC, LDL cholesterol, and apolipoproteins (apo) A and B (3).
Nuts are a good source of unsaturated fatty acids, and are rich in fiber, minerals (potassium, calcium, and magnesium), vitamins (folate and E), phytosterols, and polyphenols (9). The fatty acid composition across types of nuts varies widely. Whereas almonds, hazelnuts, pistachios, cashews, and peanuts are rich in MUFAs, walnuts are rich in PUFAs (10). Walnuts are especially rich in α-linolenic acid (18:3n–3) and linoleic acid (18:2n–6) (10). α-Linolenic acid is an essential precursor of ω-3 PUFAs found in fish oils, which have been associated with anti-inflammatory and anti-atherogenic properties (11). It has been postulated that the unique fatty acid composition of walnuts may provide beneficial effects of lowering blood cholesterol and triglyceride (TG) concentrations (12).
Although many clinical trials have evaluated the effects of walnut consumption on blood lipids (12), there are discrepancies in the results for specific blood lipids and apolipoproteins, possibly due to the limitations of small sample sizes and differences in intervention duration, design, or population.
In 2009, we published a meta-analysis on this topic that included 13 clinical trials representing 365 participants (12). The results indicated that diets supplemented with walnuts significantly decreased total and LDL cholesterol, beyond that of the comparator diets. Since then, more than 10 clinical trials with larger samples sizes and longer follow-ups have been published. Therefore, we sought to conduct an updated systematic review and meta-analysis of controlled trials evaluating the effects of walnut-enriched diets compared with control diets on blood lipids and apo profiles. Additionally, we aimed to evaluate whether incorporating walnuts into the diet led to changes in body weight. We also evaluated other cardiovascular risk factors that were reported in the trials identified in our updated literature review, including blood pressure. Finally, we aimed to investigate heterogeneity between studies, and if present, to evaluate the potential sources, such as study duration, baseline comorbidities, populations, and type of control diets.
METHODS
Search strategy
We conducted systematic literature searches in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (http://www.embase.com) for controlled trials describing the effects of walnut consumption compared with a control diet on blood lipids from the inception date of each database through January 2018. The search strategy was as follows: (juglans[MeSH] OR walnuts[MeSH] OR walnut) AND (humans[Mesh]) AND (English[lang]) (See Online Supplemental Material). In addition, we scanned the bibliographies of all retrieved relevant articles and reviews. We restricted the search to clinical trials in adult humans, and to articles published in English.
Selection criteria
We included controlled trials that evaluated a walnut-enriched diet compared with a control diet. Trials that compared only the effect of other nuts or walnuts as part of mixed nuts were excluded. Accepted studies were required to report baseline and follow-up outcome values, the mean change from baseline for each intervention group, or the mean change difference between interventions groups for at least one lipid variable (TC, LDL cholesterol, HDL cholesterol, TGs, or apolipoproteins). We included trials that specifically tested walnut-based interventions and clearly stated the amount and frequency of walnuts given or instructed in the diet. We excluded acute intervention studies evaluating only postprandial effects because this was beyond the scope of the present review. We screened the titles and abstracts of the identified manuscripts, and the full texts of potentially relevant articles were reviewed independently by two investigators (MG-F and JL). Any disagreement was resolved by consensus or consultation with the other coauthors.
Data extraction and quality assessment
We extracted information on study characteristics (citation, study name, authors, publication year), study inclusion and exclusion criteria, participant characteristics (location, number of participants and attrition, mean age or age range, sex, mean BMI or body weight, presence of comorbidities), study design (randomized, crossover, or parallel), intervention and control diets (specific amount of walnuts in percentage of energy from fat or grams per day; control intervention details), study duration, analysis strategy (intention-to-treat analysis or per-protocol analysis), use of run-in or wash-out periods, funding sources, and methods for assessment of compliance. We extracted blood lipid concentrations at baseline and follow-up for total population or by intervention group [mean ± SD, mean ± SE, mean (95% CI or IQR)]. We extracted means and SDs/SEs of changes from baseline to follow-up (for intervention and control groups from all trials). SDs were derived from SEs, CIs, or IQRs, when necessary, assuming a normal distribution of lipid parameters. We also extracted other nonlipid endpoints including weight change, blood pressure, markers of inflammation, oxidative stress, endothelial function, and antioxidant capacity.
We used the Jadad score to assess study quality (13). Trials scored 1 point for each item addressed in the study design, including random assignment, blinding, description of withdrawals and dropouts, methods of random assignment, and double-blinding status. The Jadad score ranges from 0 to 5 points, with higher scores representing the quality of a given study. We considered a Jadad score of ≥3 as high quality because double-blinding was not possible in most of the trials. Another method used for quality assessment was monitoring of the participants’ compliance.
Statistical analysis: data synthesis and meta-analysis
Our primary outcome of interest was the difference in pre- to postintervention changes in blood lipids and apolipoproteins, comparing intervention (walnut-enriched diets) with control groups. Secondary outcomes included body weight, BMI, and blood pressure. We converted TC, LDL cholesterol and HDL cholesterol to mg/dL (conversion factor for cholesterol: mmol/L multiplied by 38.67; conversion factor for TGs: mmol/L multiplied by 88.57). We used mg/dL for apolipoproteins A and B, kg for body weight, kg/m2 for BMI, and mm Hg for blood pressure, making the appropriate conversions when necessary.
We extracted pre- and postintervention means, SDs, change values, and the SD of the change values from the studies when available. If changes were not reported, we calculated the mean and SD of changes from baseline to follow-up for each intervention and control group and used Openmeta[Analyst] (http://www.cebm.brown.edu/openmeta/) to estimate the differences between groups. For manuscripts reporting only the mean difference between the walnut and the control group, the control group's mean change was set as 0 and the walnut group's mean was set as the reported mean difference (14–16). We excluded those data points from the calculation of the percentage change. Consistent with a previous meta-analysis from our group (12), if more than one time point for follow-up was reported, we included the value closest to the time point used in the other trials for our primary analysis. For clinical trials with more than one comparison group, we included the control diet most similar to the walnut diet after the exclusion of walnuts or other nuts.
Random-effects meta-analyses with inverse-variance weighting were performed to pool the effect estimates from each study. We reported the results as the weighted mean difference (WMD). We derived the percentage change from the WMD for the intervention and control groups, dividing by the weighted mean level at baseline, and multiplying by 100. We estimated heterogeneity between studies with the use of Cochran’s Q test and the I2 statistic. An I2 statistic >30% was considered moderate heterogeneity (http://handbook.cochrane.org). We considered heterogeneity to be statistically significant at P < 0.10, a conservative standard for meta-analysis. Primary results were presented based on the random-effects model because it incorporates both within-study and between-studies components of variance. Fixed-effects models were evaluated secondarily. We examined potential sources of heterogeneity with the following subgroup analyses: walnut intervention dose (comparing <1 serving/d (28g) with ≥1 serving/d; or walnuts per day accounting for 5–10% of total energy with walnuts per day accounting for 10–25% of total energy); duration of intervention period (<8 wk compared with ≥8 wk); participants with normolipidemia compared with hyperlipidemia at baseline; type of control diet (comparing usual diet with Mediterranean diet, low-fat diet, average American diet, and meals provided by the study investigators); mean age of study population (<50 y compared with ≥50 y); baseline BMI (mean BMI <25 kg/m2 compared with ≥25 kg/m2); walnut company as a funding source (yes compared with no); study quality (Jadad score ≥3 compared with <3). The effect modification by the stratifying variables was evaluated with the use of random-effects meta-regressions, in which categoric effect modifiers of each study (i.e., age, BMI, study duration, etc.) were the independent variables, and the mean changes in each lipid outcomes were the dependent variables.
We performed a dose-response meta-analysis to quantify the changes in lipid outcomes according to the dose of walnuts given in the intervention studies. Cubic spline regressions were fitted through (restricted) maximum likelihood with the use of random-effects models, and mean differences were used to approximate the (co)variance matrix of the outcome. The analyses were performed with the dosresmeta package in R version 3.1.1 (R Development Core Team, 2011; http://cran.r-project.org).
Publication bias was assessed by visual inspection of funnel plots, looking for a skewed (nonsymmetrical) distribution of standard errors around the study-level effect estimates, and Egger's and Begg's tests, taking a significance level of P < 0.05 to indicate significant asymmetry (17, 18). Analyses were performed with STATA version 12.0 (StataCorp LP) with a 2-tailed α < 0.05 considered statistically significant.
RESULTS
Literature search
The literature search yielded 3696 unique abstracts (Supplemental Figure 1). After independent review by 2 investigators, 3472 abstracts were excluded. Among the 121 full-text articles reviewed thereafter, 97 were excluded based on our study selection criteria (acute trials, no control group, not reporting blood lipids, interventions with mixed nuts) or because they were unrelated to the scope of the present review (Supplemental Figure 1). After final exclusions, and adding 2 references identified by screening article citations, 26 publications met the inclusion criteria and were included in the present systematic review.
Characteristics of the controlled trials
The study characteristics of the 26 clinical trials representing a total of 1059 participants are shown in Table 1. Of the 26 trials, 6 were randomized parallel trials and 20 had a crossover design. The sample size ranged from 16 to 194 participants, with a mean age ranging from 22 to 75 y. The mean baseline BMI ranged from 20.7 to 36 kg/m2. Nine trials were restricted to participants with normal cholesterol concentrations; 9 trials included only participants with moderate hypercholesterolemia (Table 1). The remaining trials included participants with type 2 diabetes, overweight, obesity, or with metabolic syndrome. Baseline lipid concentrations are presented in Table 1.
TABLE 1.
Study characteristics of clinical trials on walnut consumption and blood lipids1
| Study | n | Participant characteristics | Age, y | BMI, kg/m2 (body weight, kg) | Baseline lipid concentration, mg/dL | Males, % | Study design | Diet duration, wk | Walnut diet | Control diet | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bamberger 2017 (16) | 194 | Healthy nonsmoking participants >50 y | 63 (7) | 25.1 (4.0) | Total population—TC: 232 (37); LDL:146(32); HDL: 69 (17); TG: 101 (55) | 31 | Randomized crossover | 8 | Walnut-enriched diet (43 g shelled walnuts/d). Replace either 70 g carbohydrates, 30 g saturated fat with walnuts, or 35 g carbohydrates and 15 g fat | Nut-free control diet | 3 |
| Wu 2014 (24) | 40 | Healthy Caucasian men and postmenopausal women (>50 y) | 60 (6.3) | 24.09 (3.79) | Total population—TC: 220 (31); LDL:133(31); HDL: 71 (12); TG: 88 (38) | 25 | Randomized crossover | 8 | Replace 30 g saturated fat with walnuts (providing 43 g walnuts/d) | Western-type control | 2 |
| Burns-Whitmore 2014 (38) | 20 | Free-living healthy adults2 | 38 (3) | 23 (1) | Walnut—TC: 184.9 (10.3); LDL: 108.5 (13.2). Control—TC: 184.9 (9.9); LDL: 106.1 (13.2) | 25 | Randomized crossover | 8 | Habitual diet supplemented with 28.4 g of nuts 6/wk | Habbitual diet supplemented with a standard egg 6/wk | 3 |
| Katz 2012 (39) | 40 | Overweight adults with elevated waist circumference and ≥1 metabolic syndrome features | 57.4 (11.9) | 33.2 (4.4) | Total population—TC: 205.1 (29.1); HDL: 52.7 (15.1); LDL: 121.4 (24.5); TG: 157.0 (60.3) | 40 | Randomized crossover | 8 | Ad libitum + 56 g of walnuts provided | Ad libitum2 | 3 |
| Din 2011 (25) | 30 | Healthy males | 23 (3) | 24.5 (2.5) | Total population—TC: 176.4 (40.9); LDL: 104.2 (35.5); HDL: 50.9 (9.2); TG: 107.9 (51.3) | 100 | Randomized crossover | 4 | 15 g of walnuts in addition to the habitual diet, no other dietary advice | Habitual diet2 | 3 |
| Pérez-Martínez 2011 (26) | 20 | Healthy, no lipid-altering medication | 22 (18) | BMI: 24.5 (2.7) | NR | 100 | Randomized crossover | 4 | 5% E from walnuts, low-fat (<30% E from fat) meals provided | Mediterranean meals provided | 2 |
| Wu 2010 (21) | 122 | Participants with 3 metabolic syndrome criteria | Walnuts: 48.2 (8.4); control: 48.6 (8.0) | Walnuts: 25.7 (2.9): control: 25.4 (2.4) | Walnuts—TC: 223.9 (54.0); LDL: 158.3 (46.3); HDL: 50.2 (15.4); TG: 176.1(134.3). Control—TC: 235.5 (65.6); LDL: 166.0 (54.05); HDL: 54.0 (19.5); TG: 171.7 (139.3) | 55 | Randomized parallel | 12 | Lifestyle counseling based on AHA guidelines + 100 g isocaloric bread (30 g of walnuts) | Lifestyle counseling based on AHA guidelines | 2 |
| Ma 2010 (27) | 22 | Type 2 diabetic patients, no insulin therapy | 58.1 (9.2) | BMI: 32.5 (5) | Total population—TC: 183.4 (38.9); HDL: 56.0 (14.7); LDL: 102.9 (32.4); TG: 123.6 (67.0) | 42 | Randomized crossover | 8 | Ad libitum diet + 56 g of walnuts (provided) | Ad libitum2 | 3 |
| Torabian 2010 (50) | 87 | Normal to moderate high plasma cholesterol | 54 (10.2) | BMI: 26.5 (3.3) | Total population—TC: 220.8 (32.1); LDL: 129.3 (33.9); HDL: 59.0 (15.0); TG: 123.1 (68.1) | 44 | Randomized crossover | 6 mo | Habitual diet supplemented with walnuts (12% E) (28–64 g/d) | Habitual diet | 2 |
| Damasceno 2010 (36) | 18 | Moderate hypercholesterolemia | 56 (13) | BMI: 25.7 (2.3) | Total population—TC: 271.4 (31.7); LDL: 196.1 (17.7); HDL: 62.9 (14.6); TG: 120.3 (68.8) | 50 | Randomized crossover | 4 | Mediterranean diet supplemented with walnuts (40–65 g) | Mediterranean diet supplemented with virgin olive oil | 3 |
| Tapsell 2009 (28) | 35 | Type 2 diabetic patients, no insulin therapy | 54 (8.7) | BMI—control: 33 (4); walnut: 33.2 (4.4) | Walnut—TC: 189.2 (34.7); LDL: 104.2 (19.3); HDL: 54.0 (15.4); TG: 176.9 (88.5). Control—TC: 193.0 (27.0); LDL: 100.4 (34.7); HDL: 50.2 (19.3); TG: 176.9 (88.5) | NR but no significant differences between genders at baseline | Randomized parallel | 1 y | Low-fat isocaloric diet (supplemented with 30 g walnuts/d—30% E from fat) | Low-fat diet, 30% E from fat | 4 |
| Rajaram 2009 (51) | 25 | Normal to mildly hyperlipidemic adults | 23–65 | BMI: 24.8 (18.7–36.6) | Total population—TC: 208.9(203.9); LDL: 136.3 (179.6); TG: 110.6 (286.2) | 56 | Randomized crossover | 4 | 25–35% E from fat, 10% E from SFAs; walnut diet included 42.5 g of walnuts 4 d/wk | 25–35% E from fat, 10% E from SFAs; control diet excluding nuts and fatty fish | 2 |
| Spaccarotella 2008 (33) | 21 | Healthy, nonsmoking, participants had total PSA ≥2.0 ng/mL (2.0 μg/L), but they did not have clinically diagnosed prostate cancer, no lipid-altering medications | 65.9 (55–75) | 84.8 kg (13.3) | Total population—TC: 193.4 (28.3); LDL: 120.1 (26.5); HDL: 52.6 (10.6) | 100 | Randomized crossover | 8 | 24% E from walnuts (75 g/d) | Average American or usual diet | 2 |
| Canales 2007 (20) | 22 | Overweight or obese with additional CHD risk factor, TC 220 mg/dL, smoker, hypertension | 54.8 (8.3) | BMI: 29.6 (3.4) | Total population—TC: 218.1 (43.3) | 60 | Randomized crossover | 5 | 13% E from walnuts; walnut-enriched meat provided | Control: meat provided without walnut paste | 1 |
| Mukkudem-Petersen 2007 (37) | 43 | Metabolic syndrome: lipid-altering medications allowed if consistently used | 45 (10) | BMI—walnut: 36.0 (33.3–38.7); control: 35.1 (32.8–37.4 | Walnut—TC: 185.3 (18.3): LDL: 115.4 (18.8); HDL: 36.3 (9.3); TG: 168.1 (81.6). Control—TC: 189.2 (32.3); LDL: 123.9 (28.6; HDL: 32.8 (6.96); TG: 164.6 (60.8) | 45 | Randomized parallel | 8 | 20% E from walnuts (63–108 g/d); meals provided | Control:3 meals provided with % E intake from protein:carbohydrate: fat = 20:47:33 | 3 |
| Pérez-Martínez 2007 (23) | 16 | Healthy,2 no lipid-altering medication | NR | NR | NR—normal according to the author's criteria | 100 | Randomized crossover | 4 | 5% E from walnuts, low-fat (<30% E from fat) meals provided | Mediterranean meals provided | 1 |
| Schutte 2006 (30) | 41 | Patients with metabolic syndrome | Walnut: 45.5 (40.3–50.7); control: 44.4 (40.2–48.6) | BMI—walnut: 35.9 (33.1–38.7); control: 35.5 (33.1–37.8) | Walnut—HDL: 35.5 (9.1); TG: 174.3 (78.47). Control—HDL: 32.8 (6.7); TG: 161.1 (60.2) | 50 | Randomized parallel | 8 | 20% E from walnuts (63–108 g/d) | Control diet with 32.6% of fat, meals provided | 2 |
| Ros 2004 (35) | 20 | Hypercholesterolemia LDL >130 mg/dL, TG >250 mg/dL, no lipid-altering medication | 55 (26–75) | Body weight: 70.6 (10.3) | Total population—TC: 267.5 (27.0); LDL: 183.4 (23.9; HDL: 62.2 (15.8); TG: 123.1 (52.2) | 40 | Randomized crossover | 4 | 18% E from walnuts (40–65 g/d) Mediterranean | Mediterranean | 2 |
| Tapsell 2004 (52) | 37 | Type 2 diabetic patients, no insulin therapy | Walnut: 57.2 (9.0); control: 59.3 (7.1) | BMI—walnut: 30.7 (3.8); control: 30.2 (4.5) | Walnut—TC: 158.9 (31.3); LDL: 83.9 (50.7); HDL: 42.5 (9.3); TG: 168.3 (65.5). Control—TC: 176.8 (33.9); LDL: 99.6 (50.2); HDL: 42.8 (9.3); TG: 155.7 (72.5) | Walnut: 65; control: 50 | Randomized parallel | 12, 24 | 10% E from walnuts (30 g/d); modified low-fat diet (<30% E from “good” fat) | Modified low-fat (<30% E from “good” fat) | 2 |
| Zhao 2004 (19) | 23 | Hypercholesterolemia—TC: 200–240 mg/dL; LDL: 40th–90th percentile; no lipid-altering medications | 49.8 (7.7) | BMI: 28.1 (3.4) | Total population–TC: 225.8 (22.2); LDL: 153.6 (20.3); HDL: 44.8 (7.3); TG: 136.3 (67.2) | 87 | Randomized crossover | 6 | 18% E from walnuts (37 g/d) and walnut oil (15 g/d); high PUFA | Average American | 1 |
| Iwamoto—men 2002 (15) | 20 | Healthy2 | 23.8 (3.1) | BMI: 22.2 (2.2) | TC: 183.4 (32.9); TG: 226.5 (122.6) | 100 | Randomized crossover | 4 | 12.5% E from walnuts (52 g/d); traditional Japanese meals provided | Traditional Japanese meals provided | 2 |
| Iwamoto—women 2002 (15) | 20 | Healthy2 | 23.6 (4.9) | BMI: 20.7 (2.2) | TC: 174.9 (27.6); TG: 33.6 (166.2) | 0 | Randomized crossover | 4 | 12.5% E from walnuts (52 g/d); traditional Japanese meals provided | Traditional Japanese meals provided | 2 |
| Morgan 2002 (22) | 42 | Hypercholesterolemia: TC >200 mg/dL; no CVD | 55.7 (11.8) | BMI: 27.7 (5.8) | Total population—TC: 231.6 (30.9); LDL: 154.4 (23.2); HDL: 57.9 (15.4); TG: 59.3 (106.2) | 40 | Randomized crossover | 6 | 20% E from walnuts (64 g/d) low-fat/cholesterol (<30% E from fat, <200 mg cholesterol) | Low-fat/cholesterol (<30% E from fat, <200 mg cholesterol) | 2 |
| Almario 2001 (53) | 18 | Hypercholesterolemia: TC >200 mg/dL; LDL cholesterol >130 mg/dL; no lipid-altering medications | 60(8) | BMI: 29.0 (1.2) | Total population habitual diet—TC: 230.5 (47.5); LDL cholesterol:137.8 (45.6); HDL cholesterol: 49.0 (11.2); TG: 218.6 (82.3) | 28 | Nonrandomized consecutive | 6 | 16.5% E from walnuts (48 g/d); low fat (<20% E from fat) | Low-fat diet (<20% fat) | NA |
| Zambón 2000 (54) | 49 | Polygenic hypercholesterolemia2 (LDL >130 mg/dL, TG <250 mg/dL); no lipid-altering medications | 56 (11) | 27.0(3.1) | Total population—TC: 278 (33); LDL: 196 (30); HDL: 56 (13); TG: 136 (43) | 51 | Randomized crossover | 6 | 18% E from walnuts (41–56 g/d) (35% of total fat) Mediterranean | Mediterranean | 3 |
| Chisholm 1998 (29) | 16 | Moderate hypercholesterolemia (TC: 212–290 mg/dL) | 45 (6.8) | BMI: 28.4 (4.3) | Total population—TC: 240.9 (23.5); LDL: 166.0 (23.9); HDL: 40.9 (12.7); TG: 92.9 (66.3) | 100 | Randomized crossover | 4 | 20% E from walnuts (78 g/d) low fat (<30% E from fat) | Low fat (<30% E from fat) | 1 |
| Sabaté 1993 Med (14) | 18 | Healthy; no lipid-altering medication | 30 (21–43) | BMI: 23.8 (18.7–30.6) | Total population—TC: 198; TG: 117 | 100 | Randomized crossover | 4 | 20% E from walnuts (84 g/d); meals provided | Cholesterol-lowering meals provided | 2 |
1Values are means; SDs or ranges in parentheses. CHD, coronary heart disease; E, total energy; NA, not applicable; NR, not reported; PSA, prostate-specific antigen; TC, total cholesterol; TG, triglyceride.
2Stated by author.
The intervention periods of the trials ranged from 4 wk to 1 y, with a mean duration of 8 wk. The amount of walnuts ranged from 15 to 108 g/d (daily mean) and represented 5–24% of the total energy in the prescribed interventions. Most of the trials provided whole walnuts to be incorporated in the intervention diet (i.e., habitual or usual diet, lifestyle counseling, Mediterranean diet, or low-fat diets). One intervention allowed consumption of 15 g of walnut oil on top of 37 g walnuts/d (19). Two other trials incorporated walnuts into meat products (20) and bread (21). Control diets were diverse: ad libitum control diet (n = 7); Mediterranean diet (n = 5); background diet of the population (2 American diets and Japanese diet) (n = 3); low-fat diets (<30% of total fat) (n = 5); fat lowering diet as per the American Heart Association guidelines (n = 1); control meals were provided by the study investigators (n = 4).
Trial quality assessment
We used the Jadad score to evaluate study quality, and 9 clinical trials were deemed of high quality (Jadad score ≥3) (Table 1). All trials described methods for evaluating or verifying participant compliance. Dietary records and food-frequency questionnaires were used in all but one study (26). Some trials additionally weighed foods or counted returned walnut packages. Changes in serum fatty acids, especially α-linolenic acid, as well as tocopherol were used as biomarkers of walnut intake in all but 8 trials (15, 16, 22–27). Most of the trials reported good to excellent compliance with the intervention, except one where the authors noted that neutral results may be due to lack of adherence to the walnut intervention (25), and another one where adherence decreased after 3 mo of the intervention (28). The number of dropouts during the interventions were reported in all except in 8 manuscripts (15, 19, 20, 23, 25, 26, 29, 30). Most of the manuscripts reported that these withdrawals were not due to the intervention per se but for other reasons such as personal reasons or illnesses. However, 5 trials indicated that some dropouts were related to walnut consumption (22, 28, 33, 53, 54).
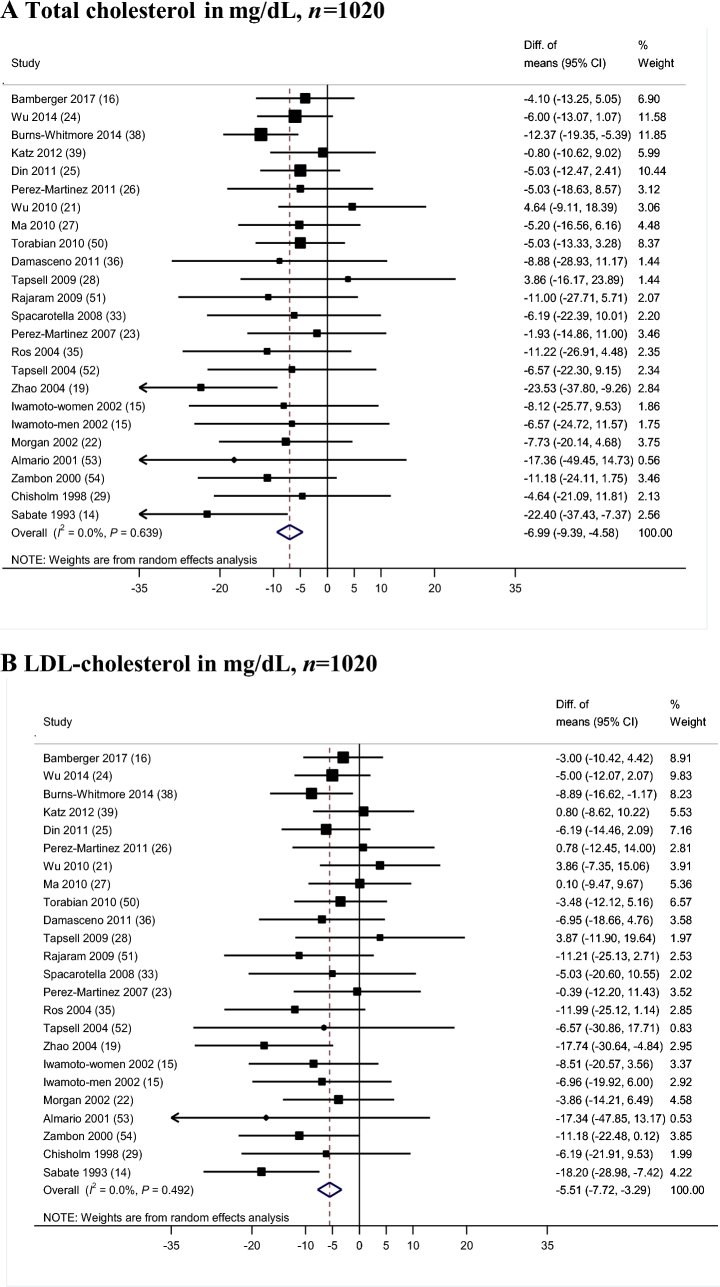
Meta-analysis of blood lipids
Results for mean differences in TC and LDL cholesterol between the walnut intervention and the control groups were reported in 24 controlled trials (Figure 1A, B, respectively) including 1020 participants. The meta-analyzed WMD showed a significantly greater reduction in TC with the walnut-enriched diets than with the control diets (WMD = −6.99 mg/dL; 95% CI: −9.39, −4.58 mg/dL; P < 0.01), with no significant heterogeneity (I2 = 0%; P-heterogeneity = 0.64). This difference represents a 3.25% greater decrease in TC concentration in walnut-enriched diets compared with control diets. We observed a greater reduction in LDL cholesterol concentrations in walnut diets compared with controls (WMD = −5.51 mg/dL; 95% CI: −7.72, −3.29 mg/dL; P < 0.001) without apparent heterogeneity (I2 = 0.0%; P-heterogeneity = 0.49). In percentage terms, there was a 3.73% significantly greater decrease in LDL cholesterol concentration with the walnut-intervention diets than with the control diets.
FIGURE 1.
Results for primary meta-analysis of controlled trials for the effects of walnut-enriched compared with control diets on blood lipids. Randomeffects model meta-analysis for changes in total cholesterol (A), LDL cholesterol (B), HDL cholesterol (C), and triglyceride concentrations (B) in mg/dL. Conversion factors from mmol/L to mg/dL: total cholesterol, 38.67; total triglycerides, 88.57. Closed rectangles and horizontal bars represent the overall estimates (difference of means) and 95% CIs for individual studies. Diamonds represent the overall estimate combining all the studies.
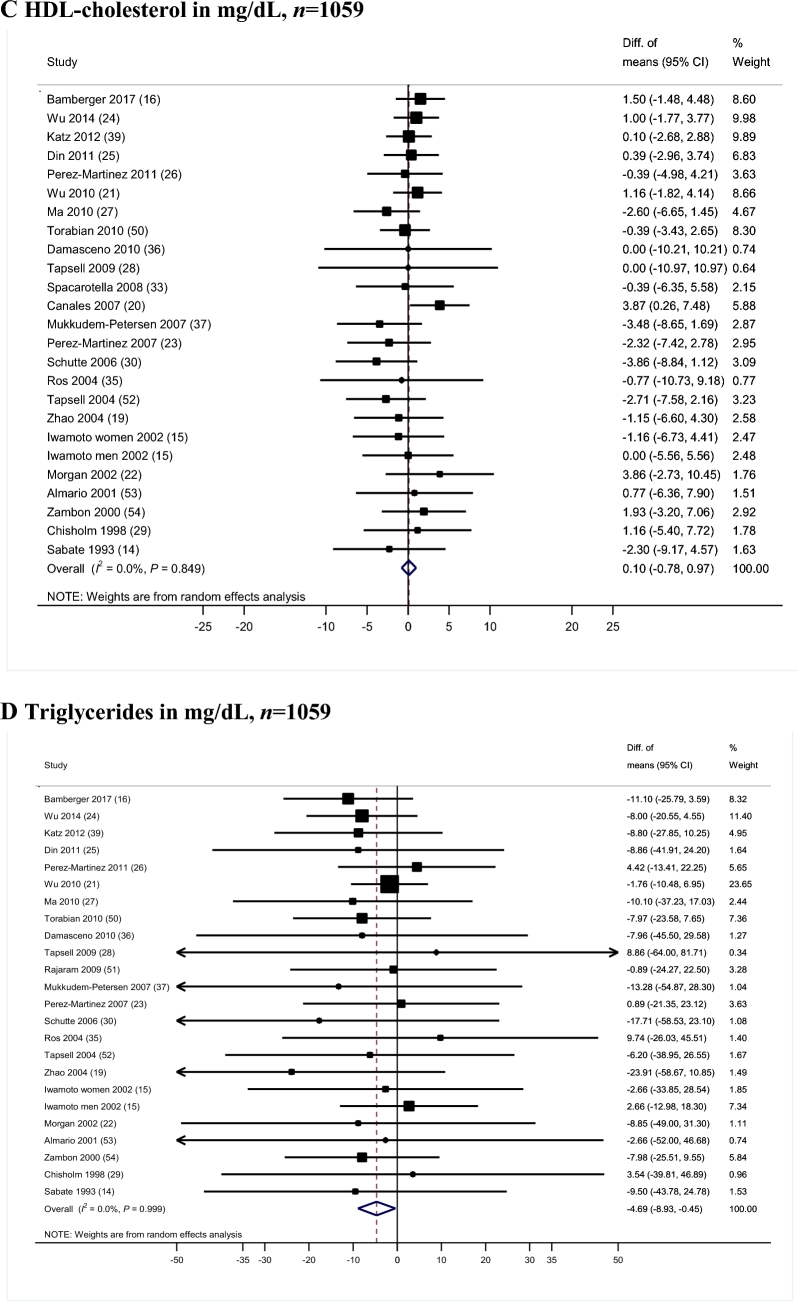
Twenty-four controlled trials including 1059 participants reported results for HDL cholesterol (Figure 1C). There was no significant difference for changes in HDL cholesterol concentrations between the walnut-enriched and the control diets (WMD = 0.10 mg/dL; 95% CI: −0.78, 0.97 mg/dL; P = 0.83), with no heterogeneity (I2 = 0.0%; P-heterogeneity = 0.85). Compared with control diets, walnut-enriched diets showed a greater reduction in the ratio of LDL cholesterol to HDL cholesterol (WMD = −0.14 mg/dL; 95% CI: −0.25, −0.03 mg/dL; P = 0.01; I2 = 0.0%; P-heterogeneity = 0.89) (data for this analysis come from 253 participants from 7 trials that reported LDL:HDL ratios). No significant differences were observed for the change in the TC:HDL cholesterol ratio (WMD = −0.05; 95% CI: −0.20, 0.11; P = 0.54; I2 = 0.0%; P-heterogeneity = 0.98). Very-low-density lipoprotein concentrations were reported in 4 trials and we did not observe a significant effect of walnut compared with control diets (WMD = −1.17 mg/dL; 95% CI: −3.56, 1.22 mg/dL; P = 0.34; I2 = 0.0%; P-heterogeneity = 0.94). Non-HDL concentrations were reported in only 2 trials and no significant effect of walnut interventions compared with control was observed (WMD = −4.49 mg/dL; 95% CI: −9.77, 0.78 mg/dL; P = 0.09; I2 = 6.27%; P-heterogeneity = 0.30).
Twenty-four clinical trials with 1059 participants reported TG concentrations (Figure 1D). We observed a greater reduction in TG concentrations in participants consuming a walnut-enriched diet compared with those in the control groups (WMD = −4.69 mg/dL; 95% CI: −8.93, −0.45 mg/dL; P = 0.03). No heterogeneity was apparent (I2 = 0%; P = 0.99). This difference represents a 5.52% greater decrease in TG concentrations caused by consuming walnut-enriched diets.
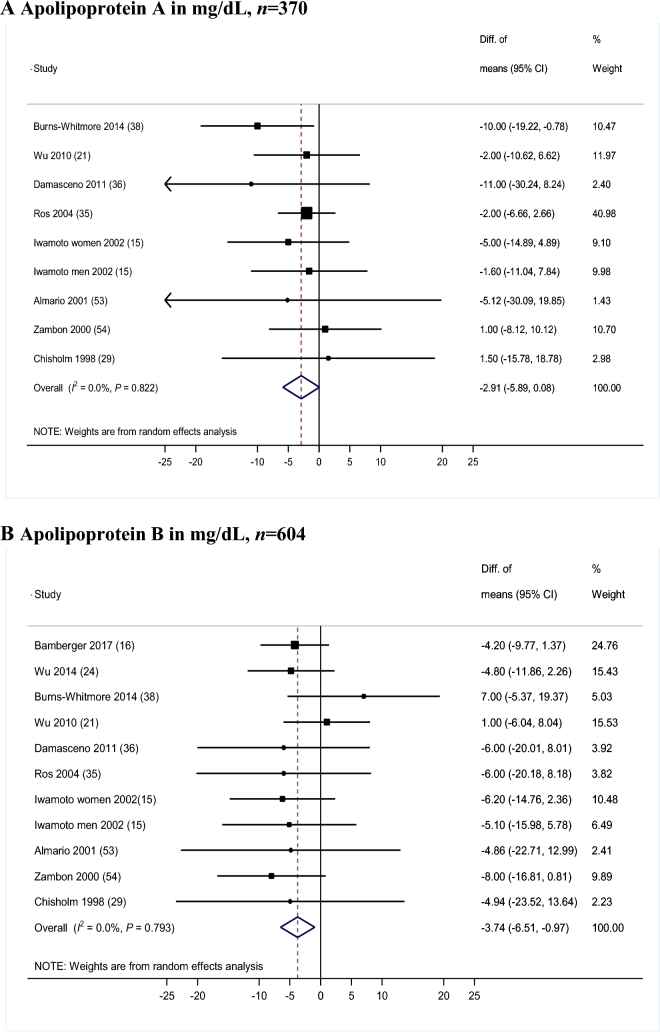
We observed a greater reduction in apoB for the walnut-enriched diet groups than for the control groups (Figure 2B). The WMD between walnut-enriched diets and controls was −3.74 mg/dL (95% CI: −6.51, −0.97 mg/dL; P = 0.008). A trend towards a reduction was also observed for apoA (WMD = −2.91 mg/dL; 95% CI: −5.89, 0.08 mg/dL; P = 0.057) (Figure 2A) with no evidence of heterogeneity (I2 = 0; P-heterogeneity > 0.79). These results correspond to a 1.10% greater decrease in apoA and 4.19% greater decrease in apoB for the walnut diets groups compared with the control diet groups.
FIGURE 2.
Results for meta-analysis of controlled trials for the effects of walnut-enriched compared with control diets on apolipoproteins. Random-effects model meta-analysis for changes in apolipoprotein A (A) and apolipoprotein B (B) in mg/dL. Closed rectangles and horizontal bars represent the overall estimates (difference of means) and 95% CIs for individual studies. Diamonds represent the overall estimate combining all the studies.
Supplemental Figure 2 shows the splines for the continuous dose-response meta-analysis of the effects of walnut intake (g/d) compared with control diet on mean change in blood lipids. The curve estimates refer to the mean change difference in blood lipid outcomes (in mg/dL) per each additional gram of walnut intake, compared with no walnut intake. A linear dose-response relationship between walnut intake and TC was observed [curve (estimate)] −0.345 mg/dL; 95% CI: −0687, −0.002 mg/dL; P = 0.04] as well as a nonsignificant trend between walnut intake with lower LDL cholesterol [curve (estimate): −0.288 mg/dL; 95% CI: −0.603, 0.025; P = 0.07].
Subgroup and sensitivity analysis
Findings for subgroup analysis were generally consistent with the primary analysis, as would be expected given the minimal between-study heterogeneity (Supplemental Table 1).
Tests for effect modification based on meta-regressions indicated no statistically significant differences between subgroups (P-heterogeneity > 0.05). However, we did observe a significantly greater effect of walnut-enriched diets compared with control diets on changes in TC (P-heterogeneity = 0.03) and LDL cholesterol (P-heterogeneity = 0.04) when the walnut intervention accounted for 10–25% of total energy per day (TC WMD = −7.35 mg/dL; LDL cholesterol WMD = −5.82 mg/dL) compared with the subgroup of trials with walnuts, which accounted for 5–10% of total energy per day (TC WMD = −4.42 mg/dL; LDL cholesterol WMD = −2.17 mg/dL). The reductions in TC and LDL cholesterol were modestly greater in those trials in which walnut-enriched diets were compared against average American and Western diets (TC: WMD = −12.30 mg/dL, P < 0.01; LDL cholesterol: WMD = −10.83 mg/dL, P < 0.01) (P values of the meta-regressions were <0.05). We did not observe significant differential effects of walnuts compared with control diets on HDL cholesterol and TGs in the subgroup analyses. (Supplemental Table 1) (All P values of the meta-regressions >0.05). We conducted sensitivity analysis with the use of a fixed-effect inverse variance approach and the results were consistent with the primary meta-analysis. In addition, results were similar when we removed trials that only reported the between-group mean change, in which the change in control group was set to 0 (14–16).
Meta-analysis on BMI and body weight
Body weight and BMI changes were reported in 10 and 6 trials, respectively. Individual trials did not show appreciable effects on body weight or BMI after following a walnut-enriched diet and there were no significant differences from the control diets (body weight WMD = −0.12 kg; 95% CI: −2.12, 1.88 kg; P = 0.90; BMI WMD = −0.11 kg/m2; 95% CI: −1.15, 0.92 kg/m2; P = 0.82) (Supplemental Figure 3).
Other cardiovascular risk markers
Eight of the included clinical trials accounting for 363 participants reported results for systolic and diastolic blood pressure. The WMD for change in systolic blood pressure was −0.72 mm Hg (95% CI: −2.75, 1.30 mm Hg; P = 0.48), and for diastolic blood pressure −0.10 mm Hg (95% CI: −1.49, 1.30 mm Hg; P = 0.88), with no apparent heterogeneity (Supplemental Figure 4).
Other cardiovascular risk factors, including markers of oxidative stress, antioxidant capacity, inflammation, and endothelial function, were sparsely reported the trials included in this study.
Markers of oxidative stress, including LDL cholesterol–conjugated diene formation, oxidized LDL, malondialdehyde, lipid peroxidation, and uric acid, were measured in 7 trials (15, 20, 34–37, 54). There was no significant effect of walnut compared with control diets on these markers. Inflammation parameters, such as C-reactive protein, nuclear transcription factor κB, and fibrinogen, were reported in 6 trials (19, 22, 23, 30, 35, 37). A trend towards a reduction in C-reactive protein after walnut-enriched diet was observed in 4 trials, but results did not reach statistical significance (19, 30, 35, 37). Endothelial function was evaluated in 7 trials (19, 23, 25, 30, 35, 36, 39) with vascular adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1), TNF α, IL-6, E-selectin, and hyperemic flow. Walnut-enriched diets led to reductions in VCAM-1 and ICAM-1 compared with control diets in most of these trials, suggesting beneficial effects of walnuts on endothelial function. Finally, glucose, insulin, or HOMA-IR values were reported in 6 clinical trials (21, 26, 28, 30, 37, 40) with mixed results. Improvements in fasting insulin, glucose concentration, and HbA1c were observed in one trial after a walnut diet in 50 overweight adults with non-insulin–treated diabetes (28). Reductions in fasting glucose concentrations were also observed in another trial with lifestyle counseling and walnut enrichment diet (21). Other controlled trials did not report beneficial effects of walnuts on markers of glycemia and insulinemia.
Publication bias
The Egger and Begg's tests did not indicate the presence of publication bias (P values for Begg and Egger tests >0.05 for all outcomes). Visual inspections of the funnel plots agreed with the statistical tests, with no apparent asymmetry (Supplemental Figure 5).
DISCUSSION
Our meta-analysis of 26 controlled trials and 1059 participants provides consistent evidence that incorporating walnuts into a diet leads to significantly lower TC, LDL cholesterol, TGs, and apoB concentrations compared with control diet interventions. Importantly, we did not observe adverse effects of walnut consumption on weight gain, BMI gain, or blood pressure levels. The cholesterol-lowering effects were more pronounced among trials comparing a walnut diet with an average American or Western diet. Briefly, Western diets are usually characterized for being high in red and processed meats, high-fat dairy products, processed and artificially sweetened foods, and with minimal intake of fruits, vegetables, fish, legumes, or whole grains. This systematic review provides the most updated and comprehensive estimates of the effects of walnut consumption on blood lipids and other cardiovascular risk factors.
Consistent with an earlier meta-analysis by our group based on 13 trials (12), diets rich in walnuts resulted in a WMD of −6.9 mg/dL (3.2% greater reduction from baseline) in TC, and −5.5 mg/dL (3.7% greater reduction) in LDL cholesterol compared with control diets. In our previous meta-analysis (12), a trend towards a greater decrease in TG concentrations favored by walnut-enriched diets was observed. Including 13 additional trials and more than a doubling of participants, we now observe a significant reduction of TGs following the consumption of walnut-enriched diets. We now also include estimates for the effects of the walnut compared with control interventions on the outcomes of apolipoproteins, cholesterol ratios, and blood pressure. This study indicates that walnut-enriched diets lead to greater reductions in apoB and a trend towards a reduction for apoA. These findings are consistent with a recent meta-analysis of 61 trials that reported that among the 21 trials that included walnut intake, walnuts significantly lowered TC, LDL cholesterol, and apolipoproteins A and B (3).
Our subgroup analyses demonstrated more pronounced effects on lowering TC and LDL cholesterol concentrations when walnuts accounted for 10–25% of total energy compared with a lower intervention dose of <10% of total energy. In addition, we observed a significant continuous dose-response relationship between walnut intake and TC. Thus, dose may be a relevant factor in incorporating walnuts to improve cardiometabolic risk factors. We did not observe differences in effects for trials enrolling subjects with hypercholesterolemia compared with normocholesterolemic trial populations. However, in a previous publication reporting pooled estimates of 25 clinical trials, total nuts favored TG reduction only among the trials specifically enrolling hyperglyceridemia patients (2). In that review, the effects of nut consumption were significantly modified by baseline LDL cholesterol, BMI, and diet type: the lipid-lowering effects of nut consumption were greatest among subjects with high baseline LDL cholesterol and with low BMI and among those consuming Western diets (2). This last finding is consistent with our analyses that showed that walnut diets were more effective in improving blood lipids when background diets were consistent with the Western dietary pattern, compared with Mediterranean or low-fat background diets. This supports dietary guidelines emphasizing adherence to overall healthful dietary patterns and the importance of food substitution (40): when foods high in unsaturated fats, such as walnuts, replace foods in the diet that are high in saturated fat and cholesterol, such as red and processed meat, they may be particularly effective in improving lipid profiles, compared with diets in which walnuts replace olive oil or other nuts.
Indeed, the strongest evidence to support nut consumption for CVD prevention comes from the PREDIMED trial of adhering to a Mediterranean diet supplemented with mixed nuts (7.5 g hazelnuts, 7.5 g almonds, and 15 g walnuts per day) compared with a control diet that did not contain nuts. The Mediterranean diet and nut intervention had beneficial effects on lipid profiles and plasma apo levels (41), and reduced incident CVD risk by 28% (HR: 0.72; 95% CI: 0.54, 0.96) (42). In particular, walnut consumption was associated with 47% (HR: 0.53; 95% CI: 0.29, 0.98) lower risk of CVD mortality in a secondary observational analysis of the PREDIMED trial (43). Also, in a recent analysis from the Nurses’ Health Study I and II, and Health Professionals’ Follow-up Study cohorts that included 210,836 participants, consuming ≥1 servings of walnuts per week was associated with a 19% lower risk of incident CVD and 23% lower risk of incident coronary heart disease (44).
Importantly, we did not observe an effect of walnut-enriched diets on weight gain. The pooled estimates from our meta-analysis confirm that the beneficial effects on blood lipids are independent of changes in body weight, and recommendations to include walnuts in the diet are feasible without adverse weight gain. This is consistent with observational studies suggesting that nut consumption is associated with less weight gain and a lower risk of obesity, and can be considered a healthy snack (45–47). Additionally, no significant effects of walnut intake were observed on blood pressure. A recent meta-analysis of randomized controlled trials on this topic reported a lack of effect of total nuts and walnut intake on blood pressure systolic blood pressure, consistent with our results presented here (8).
Walnuts are rich in plant sterols, which are natural compounds that might contribute to cholesterol-lowering effects by interfering with cholesterol absorption. In addition, cell culture experiments have shown that LDL enrichment with α-linolenic acid following a walnut diet facilitates receptor-mediated LDL clearance (32). Nuts are rich in polyphenols and tocopherol, which have recognized antioxidant and anti-inflammatory properties. Although our review of the literature indicated inconsistent findings, walnut consumption has been demonstrated to have beneficial effects on some peripheral inflammatory and oxidation parameters (48). Because walnuts are usually consumed raw they have the highest antioxidant efficacy compared with nuts that are typically consumed roasted (49). The antioxidant capacity of walnuts has been observed in several clinical trials (15, 20, 32, 34–37). In addition, walnut consumption also improved markers of endothelial function such as VCAM-1 and ICAM-1 (19, 23, 35, 36).
There are some limitations to this review and meta-analysis. First, most of the trials identified in our review had relatively small sample size, which may limit our statistical power to detect significant effects, even when pooled across trials. Second, although the compliance with the interventions was reportedly good, maintaining a high consumption of walnuts (≥28 g/d) may be difficult in the long-term for free-living individuals. However, our subgroup analysis showed that lower amounts of walnuts (<28 g/d) also led to a significant reduction in TC and LDL cholesterol, suggesting that more tolerable lower walnut doses may lead to benefits in CVD risk factor profiles. Third, for practical reasons, none of the trial interventions used a double-blinded design, which would ensure minimal bias and may explain why only 9 trials were of high quality according to the Jadad rating scale. The strengths of the present review and meta-analysis include our systematic protocol and comprehensive literature review approach, thus minimizing the possibility that any major published report was missed. The inclusion of manuscripts reporting controlled trials, with all but one being randomized, minimizes bias due to confounding at baseline, and enhances the causal interpretation of these findings. The updated meta-analysis of walnut interventions on several blood lipids, apolipoproteins, body weight, and blood pressure provide the best available evidence regarding the effects of walnut-enriched diets on cardiovascular risk factors. The results of our meta-analysis were consistent regardless of whether random-effects or fixed-effect models were used, due to minimal between-study heterogeneity.
The present meta-analysis of controlled trials provides robust evidence for the benefits of walnut consumption on blood lipids without adversely affecting body weight or blood pressure and supports the results of epidemiologic studies showing inverse associations between walnut consumption and CVD risk. Despite walnuts being energy-dense, the consumption of walnuts does not promote weight gain and thus they can be incorporated into an overall healthy dietary pattern to enhance health benefits.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—MG-F and FBH: designed the research; MG-F, JL, JSS, FBH, and DKT: conducted research; MG-F, JL, and FBH: performed systematic review and analyzed the data; MG-F and DKT: drafted the manuscript; MG-F, JL, JSS, FBH, and DKT: made critical revisions to the manuscript for important intellectual content; MG-F and DKT: had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript.
Notes
Supported by NIH grant P30 DK46200 and the California Walnut Commission. MG-F was supported by a fellowship granted by the Private Foundation Daniel Bravo (Spain). JL was supported by an American Diabetes Association-Pfizer New England Cardiovascular-Metabolic Fellowship Award (9-17-CMF-011).
Author disclosures: FBH has received grant support from the California Walnut Commission. JS-S has received research funding and is a nonpaid member of the scientific advisory committee of the International Nut Council. MG-F, JL, and DKT, no conflicts of interest.
The funding sources played no role in the design, collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Supplemental Methods, Supplemental Table 1, and Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- apo
apolipoprotein
- CVD
cardiovascular disease
- ICAM-1
intracellular adhesion molecule 1
- TC
total cholesterol
- TG
triglyceride
- VCAM-1
vascular adhesion molecule 1
- WMD
weighted mean difference
- LDL
include abbreviation here
- apo
apolipoproteins
REFERENCES
- 1. Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89:S1649–56. [DOI] [PubMed] [Google Scholar]
- 2. Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7. [DOI] [PubMed] [Google Scholar]
- 3. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr 2015;102:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:256–69. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casas-Agustench P, Bullo M, Salas-Salvado J. Nuts, inflammation and insulin resistance. Asia Pac J Clin Nutr 2010;19:124–30. [PubMed] [Google Scholar]
- 7. Ros E, Tapsell LC, Sabate J. Nuts and berries for heart health. Curr Atheroscler Rep 2010;12:397–406. [DOI] [PubMed] [Google Scholar]
- 8. Mohammadifard N, Salehi-Abargouei A, Salas-Salvadó J, Guasch-Ferré M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr 2015;101:966–82. [DOI] [PubMed] [Google Scholar]
- 9. Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation 2013;128:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Tsao R, Yang R, Kramer JKG, Hernandez M. Fatty acid profiles, tocopherol contents, and antioxidant activities of heartnut (Juglans ailanthifolia Var. cordiformis) and Persian walnut (Juglans regia L.). J Agric Food Chem 2007;55:1164–9. [DOI] [PubMed] [Google Scholar]
- 11. Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: a comprehensive update. J Clin Lipidol 6:216–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993;328:603–7. [DOI] [PubMed] [Google Scholar]
- 15. Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, Kono M. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr 2002;56:629–37. [DOI] [PubMed] [Google Scholar]
- 16. Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, Altenhofer J, Henze K, Parhofer KG. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients 2017;9:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 19. Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 2004;134:2991–7. [DOI] [PubMed] [Google Scholar]
- 20. Canales A, Sánchez-Muniz FJ, Bastida S, Librelotto J, Nus M, Corella D, Guillen M, Benedi J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur J Clin Nutr 2011;65:703–10. [DOI] [PubMed] [Google Scholar]
- 21. Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, Yu D, Zong G, Zhou Y et al., Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr 2010;140:1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan JM, Horton K, Reese D, Carey C, Walker K, Capuzzi DM. Effects of walnut consumption as part of a low-fat, low-cholesterol diet on serum cardiovascular risk factors. Int J Vitam Nutr Res 2002;72:341–7. [DOI] [PubMed] [Google Scholar]
- 23. Perez-Martinez P, Lopez-Miranda J, Blanco-Colio L, Bellido C, Jimenez Y, Moreno JA, Delgado-Lista J, Egido J, Perez-Jimenez F. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor κB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis 2007;194:e141–6. [DOI] [PubMed] [Google Scholar]
- 24. Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, Koletzko B, Stark RG, Nagel JM et al., Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. 2014;63(3):382–91. [DOI] [PubMed] [Google Scholar]
- 25. Din JN, Aftab SM, Jubb AW, Carnegy FH, Lyall K, Sarma J, Newby DE, Flapan AD. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. Eur J Clin Nutr 2011;65:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez-Martinez P, Ordovas JM, Garcia-Rios A, Delgado-Lista J, Delgado-Casado N, Cruz-Teno C, Camargo A, Yubero-Serrano EM, Rodriguez F, Perez-Jimenez F. Consumption of diets with different type of fat influences triacylglycerols-rich lipoproteins particle number and size during the postprandial state. Nutr Metab Cardiovasc Dis 2011;21:39–45. [DOI] [PubMed] [Google Scholar]
- 27. Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care 2010;33:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009;63:1008–15. [DOI] [PubMed] [Google Scholar]
- 29. Chisholm A, Mann J, Skeaff M, Frampton C, Sutherland W, Duncan A, Tiszavari S. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr 1998;52:12–6. [DOI] [PubMed] [Google Scholar]
- 30. Schutte AE, Van Rooyen JM, Huisman HW, Mukuddem-Petersen J, Oosthuizen W, Hanekom SM, Jerling JC. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am J Hypertens 2006;19:629–36. [DOI] [PubMed] [Google Scholar]
- 31. Kalgaonkar S, Almario RU, Gurusinghe D, Garamendi EM, Buchan W, Kim K, Karakas SE. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr 2011;65:386–93. [DOI] [PubMed] [Google Scholar]
- 32. Muñoz S, Merlos M, Zambón D, Rodríguez C, Sabaté J, Ros E, Laguna JC. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. J Lipid Res 2001;42:2069–76. [PubMed] [Google Scholar]
- 33. Spaccarotella KJ, Kris-Etherton PM, Stone WL, Bagshaw DM, Fishell VK, West SG, Lawrence FR, Hartman TJ. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J 2008;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis L, Stonehouse W, Loots du T, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, Jerling JC, Loots DT, Mukuddem-Petersen J et al., The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr 2007;46:155–64. [DOI] [PubMed] [Google Scholar]
- 35. Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109:1609–14. [DOI] [PubMed] [Google Scholar]
- 36. Damasceno NRT, Pérez-Heras A, Serra M, Cofán M, Sala-Vila A, Salas-Salvadó J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis 2011;21:S14–20. [DOI] [PubMed] [Google Scholar]
- 37. Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr 2007;97:1144–53. [DOI] [PubMed] [Google Scholar]
- 38. Burns-Whitmore B, Haddad E, Sabaté J, Rajaram S. Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study. Nutr J 2014;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr 2012;31:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Department of Agriculture, US Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (DC): US Department of Health and Human Services; 2015. [cited 2017 April 4]. Available from: https://health.gov/dietaryguidelines/2015-scientific-report. [Google Scholar]
- 41. Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, Frances F, Cabezas C, Lopez-Sabater Mdel C et al., Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–203. [DOI] [PubMed] [Google Scholar]
- 42. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M et al., Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 43. Guasch-Ferre M, Bullo M, Martinez-Gonzalez MA, Ros E, Corella D, Estruch R, Fito M, Aros F, Warnberg J et al., Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med 2013;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guasch-Ferré M, Liu X, Malik VS, Sun QQ, Willett WCWC, Manson JEJE, Rexrode KMKM, Li Y, Hu FBFB, Bhupathiraju SNSN. Nut consumption and risk of cardiovascular disease. J Am Coll Cardiol 2017;70:2519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr 2005;94:859–64. [DOI] [PubMed] [Google Scholar]
- 46. Bes-Rastrollo M, Sabate J, Gomez-Gracia E, Alonso A, Martinez JA, Martinez-Gonzalez MA. Nut consumption and weight gain in a Mediterranean cohort: the SUN study. Obesity (Silver Spring) 2007;15:107–16. [DOI] [PubMed] [Google Scholar]
- 47. Bes-Rastrollo M, Wedick NM, Martinez-Gonzalez MA, Li TY, Sampson L, Hu FB. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am J Clin Nutr 2009;89:1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kris-Etherton PM. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. J Nutr 2014;144:547S–54S. [DOI] [PubMed] [Google Scholar]
- 49. Vinson JA, Cai Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct 2012;3:134–40. [DOI] [PubMed] [Google Scholar]
- 50. Torabian S, Haddad E, Cordero-MacIntyre Z, Tanzman J, Fernandez ML, Sabate J. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur J Clin Nutr 2010;64:274–9. [DOI] [PubMed] [Google Scholar]
- 51. Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89:1657S–63S. [DOI] [PubMed] [Google Scholar]
- 52. Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27:2777–83. [DOI] [PubMed] [Google Scholar]
- 53. Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr 2001;74:72–9. [DOI] [PubMed] [Google Scholar]
- 54. Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.