Abstract
Critical Issue: Chronic nonhealing wounds of the lower extremities resulting in major amputations are a major health problem worldwide.
Significance: Diabetes and ischemia are two major etiologies of nonhealing wounds of the lower extremities. Hyperglycemia from diabetes and oxidative stress from ischemia activate polyadenosine diphosphate (ADP)-ribose polymerase-1 (PARP-1), which is a nuclear enzyme that is best known for its role in DNA repair. However, the exact function of PARP-1 in ischemic/diabetic wound healing has not been well studied.
Recent Advances: Poly-ADP-ribose (PAR) polymer has been detected in the wound bed and many of the PARylation-related reactions (oxidative stress response, expression of inflammatory cytokines and chemokines, cell proliferation, and migration) are important in the wound healing process. However, the role of PARP-1 in wound healing and the potential of targeting PARP-1 therapeutically in wounds are only recently being elucidated, with much still unknown. This review summarizes the recent advances in this field, highlighting some of the mechanisms through which PARP-1 may affect normal wound closure.
Future Directions: The review also presents a perspective on some of the downstream targets of PARP-1 that may be explored for their role in wound healing and discusses about the therapeutic potential of PARP inhibitors for wound healing.
Keywords: poly(ADP-ribose) polymerase, PARP, PARylation, wound healing
Jaideep Banerjee, PhD.

Scope and Significance
Chronic wounds of the lower extremities present a substantial economic burden to the health care system and significantly reduce the quality of life. In the United States, chronic wounds affect ∼5.7 million people (∼2% of population) and incur an annual cost of $25 billion.1,2 This problem is further exacerbated by diabetes mellitus because it causes a 10–15% increase in foot ulceration. Approximately 40% of diabetic foot ulcers also suffer from inadequate perfusion because of arterial occlusive disease.3,4 Although revascularization remains the best approach to improve perfusion, as many as 30% of patients with arterial occlusive disease are not candidates for revascularization.5 Therefore, medical therapy to enhance angiogenesis is urgently needed to promote wound healing and prevent major amputations.
Translational Relevance
Arterial occlusive disease associated with diabetes mellitus increases the risk of limb loss. Surgical revascularization is still the most effective way for limb salvage but not everyone is a candidate. Therefore, new therapeutic targets for medical therapy of angiogenesis are urgently needed.
Clinical Relevance
Polyadenosine diphosphate (ADP)-ribose polymerase-1 (PARP-1) is a nuclear enzyme that is hyperactivated in ischemic/diabetic wounds and regulates wound healing by many different mechanisms. A better understanding of the function of PARP-1 in wound healing could introduce PARP-1-targeted therapeutic strategies to the clinics and repurpose several Food and Drug Administration (FDA)-approved PARP-1 inhibitors for wound healing.
Background: Structure, Mechanism of Action, and Regulation and Functions of PARP-1
Structure of PARP-1
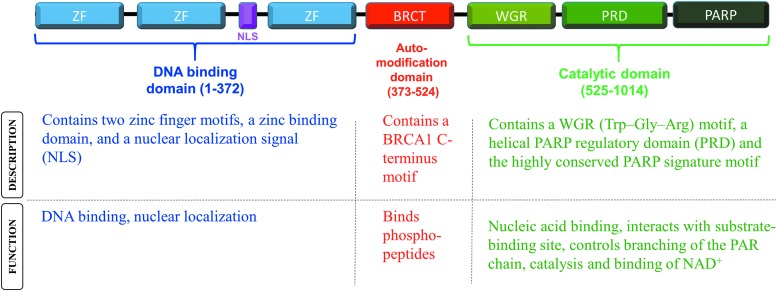
PARPs are a family of enzymes that catalyze the transfer of poly-ADP-ribose (PARs) to target proteins through a process called poly-ADP-ribosylation or PARylation.6,7 PARP-1 is the most well characterized among 18 identified members of the human PARP family. Almost 85–90% of the enzymatic activity is exerted by PARP-1 alone.8 PARP-1 is a 116 kDa nuclear chromatin-associated protein that is well known for its role in DNA repair and regulation of gene transcription.7,9,10 There are three major functional domains in its structure: (1) the DNA binding domain at the N-terminus that includes two zinc finger subdomains, a zinc binding subdomain and a nuclear localization signal, (2) the automodification domain in the middle, and (3) the catalytic domain at the C-terminus that contains a nucleic acid binding WGR motif, a helical PARP regulatory subdomain, and the highly conserved ADP-ribosyl transferase subdomain6,10,11 (Fig. 1).
Figure 1.
Description and functions of the domains of PARP-1. PARP-1, polyadenosine diphosphate (ADP)-ribose polymerase-1
Role of PARP-1 and mechanism of action
PARP-1 is best known for its role in DNA repair, maintenance of genomic integrity, and regulation of telomerase activity.12 It is also well known for its catalytic function where NAD+ is used as the substrate to generate PAR before attaching these large branched polymers to suitable protein acceptors (a process called PARylation7) and alters their functions.
However, in addition to its role in post-translational modification of targeted proteins, PARP-1 also regulates gene transcription through several mechanisms. It can either stimulate or inhibit gene transcription by binding to the regulatory elements in the promotor regions of target genes13–16 through the double zinc finger DNA-binding domain. PARP-1 can enhance gene transcription by forming the RNA polymerase-II preinitiation complex17 or acting as a scaffold protein and recruit coregulator complexes to the promoters of target genes.9,10 In contrast, PARylation of transcription factors prevents binding to their specific promoter target sites18–23 and inhibits gene transcription.
At the epigenetic level, PARP-1 regulates gene expression by modulating chromatin structure.24–27 PARylation of the core histones results in electrostatic repulsion and reduces histone–DNA affinity,28 decondenses the chromatin, creates a more relaxed structure, and allowed the DNA to be more accessible to the transcriptional machineries.24,28,29 In addition, PARP-1 regulates gene transcription by inhibiting the expression and activity of DNA methyltransferase Dnmt1,30,31 and altering chromatin structure through changing the global levels of H3K27me3.27
Regulation of PARP-1 induction
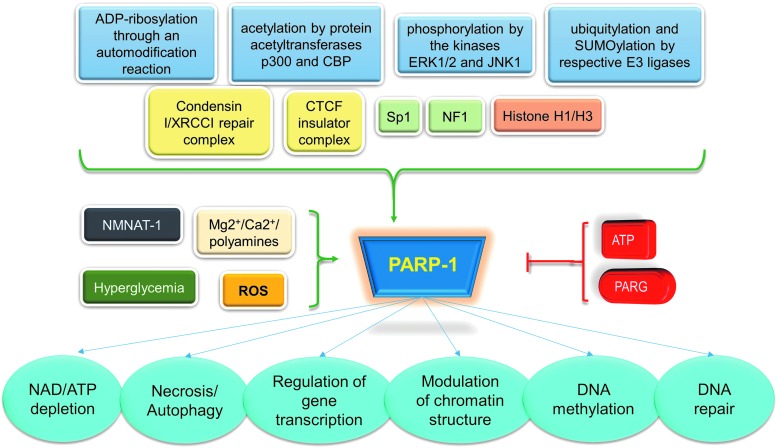
In addition to understanding the downstream targets of PARP-1, it is also important to mention the different factors that lead to PARP-1 activation that includes transcriptional regulation and post-translational modification of PARP-1. PARP-1 is regulated at the transcriptional level by DNA and protein binding partners such as nucleosomes, histones H1 and H3, the TLE (transducin-like enhancer of split) corepressor complex, a condensin I/XRCC1 repair complex, a CTCF insulator complex, and regulation by transcription factors Sp1 and NFI.10,11,32,33
Post-translational modifications that regulate PARP-1 include (1) ADP-ribosylation (through an auto-modification reaction), (2) ubiquitylation and SUMOylation (by E3 ligases), (3) phosphorylation (by ERK1/2 and JNK1), and (4) acetylation (by p300 and CBP).10,11,34
The earliest known regulator of PARP-1 enzymatic activity has been damaged DNA35 and DNA hairpins, cruciform, and stably unpaired regions.36 Other recently identified regulators of PARP-1 activity are as follows (included in Fig. 2):
-
(1)
Phosphorylation state of nicotinamide mononucleotide adenylyltransferase-1 (NMNAT-1), which is the central enzyme in NAD+ biosynthesis. PARP-1 uses this NAD+ to generate PAR and PAR-ylate target proteins to modify their functions.37–39
-
(2)
PARG or poly(ADP-ribose) glycohydrolase that degrades PAR, which is synthesized by PARP-1 and thus reverses the enzymatic effects of PARP-1.40,41
-
(3)
Oxidative-nitrosative stress induces PARP-1.42
- (4)
-
(5)
Physiologic cellular concentrations of ATP inhibit PARP-1.33
-
(6)
Mg2+, Ca2+, and polyamines allosterically activate PARP-1.33
Figure 2.
Regulation and function of PARP-1. PARG, poly(ADP-ribose) glycohydrolase; ROS, reactive oxygen species.
Discussion: Role of PARP in Different Stages of Wound Healing
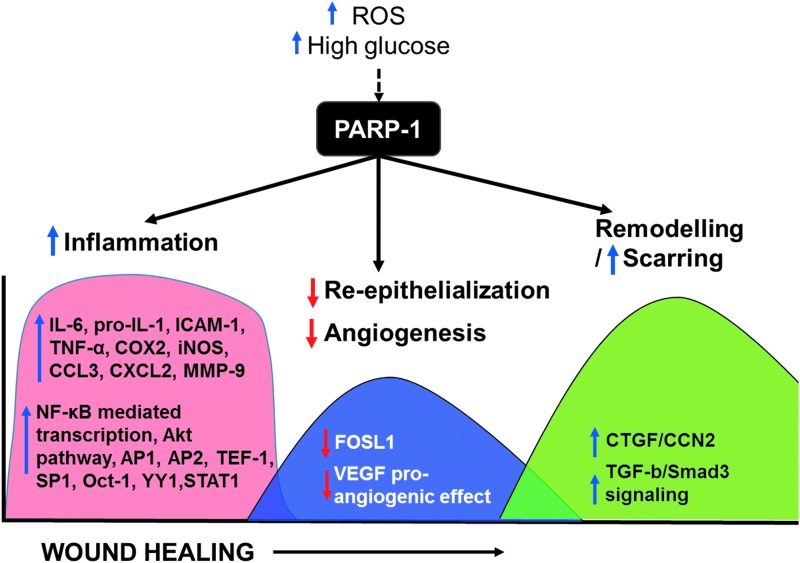
Wound healing is a well-orchestrated process that proceeds through a cascade of events involving hemostasis, inflammation, proliferation, and remodeling. Ischemia and diabetes affect the healing process in all these stages, but their effect is most prominent in the proliferative phase where angiogenesis and granulation tissue formation occur.2,45–50 Even though it is well known that both diabetes mellitus and ischemia induce PARP-1 hyperactivity,51–55 and many of the PARylation-related reactions (oxidative stress response, expression of inflammatory cytokines and chemokines, cell proliferation, and migration)14,56 are very important in the wound healing process, the role of PARP-1 in ischemic diabetes wound healing has not been well elucidated. This review summarizes the recent advances in this field, highlighting some of the mechanisms through which PARP-1 regulates the healing process of cutaneous wounds (Fig. 3).
Figure 3.
Role of PARP-1 in the different phases of wound healing. Blue arrows indicate upregulation and red arrows indicate downregulation. The arrow on the X-axis indicates the progression of wound healing through the different phases (inflammation, followed by re-epithelialization, and angiogenesis followed by remodeling). VEGF, vascular endothelial growth factor.
Inflammatory phase: targeting PARP to reduce wound inflammation
Although the initiation of wound healing starts with an inflammatory process, normal healing also requires the resolution of inflammation. Yet, in the chronic nonhealing wounds, the inflammation never resolves, leading to overproduction of reactive oxygen species (ROS), which can drive the hyperactivity of PARP-1.45,57 Subsequently, PARP-1 depletes cellular ATP supply due to excessive consumption of NAD+, resulting in cellular necrosis. PARP-1 contributes to the production of a variety of proinflammatory mediators.11,58,59 PARP-1 acts as a coactivator in NF-κB-mediated transcription, leading to induction of proinflammatory mediators such as IL-6, pro-IL-1, ICAM-1, TNF-α, COX2, iNOS, CCL3, and CXCL2,58,60,61 and acts as a stimulator of the Akt pathway and can contribute to the activation of a variety of inflammation-related transcription factors, including AP1, AP2, TEF-1, SP1, Oct-1, YY1, and STAT1.62 Pharmacological inhibition of PARP-1 can attenuate the production of various proinflammatory mediators such as IL-6, pro-IL-1, ICAM-1, TNF-α, COX2, iNOS, CCL3, and CXCL2,60 many of which are widely implicated in wound healing. Inhibition of PARP-1 by 3-aminobenzamide (3-AB) has also been shown to attenuate oxidative/nitrosative stress response and accelerate healing.63
Taken together, evidence suggests that reduction of oxidative stress and inflammation may be two potential mechanisms by which PARP-1 accelerates wound healing.63
One potential risk of targeting wound inflammation by PARP-1 may be an increased favorability for wound infection. Any such treatment approach that reduces inflammation, therefore, should be applied after sufficient debridement and preparation of the wound bed with antimicrobials as per standard of care.
Proliferation phase: targeting PARP to improve angiogenesis and granulation tissue formation
This phase is marked by the formation of granulation tissue that is composed of proliferation and migration of keratinocytes, new connective tissue, and microscopic blood vessels that are formed by angiogenesis at the base of the wounds.64
Keratinocytes proliferate and migrate faster (as assayed by a scratch wound healing assay) in the presence of PARP inhibitors 3-AB or PJ34.56 Although the role of PARP-1/PARylation in cell proliferation is controversial,65–67 its role in cell migration has not yet been systematically investigated.
PARP-1 also has a major role to play in wound angiogenesis. There are some conflicting data on the role of PARP in angiogenesis between in vitro experiments and uninjured tissue vs in vivo injured tissue, especially in diabetic ischemic wounds. Although in uninjured tissue, PARP inhibition may block angiogenesis-related endothelial properties leading to the inhibition of new blood vessel formation,68 in a wound environment, PARP-1 has been shown as an antiangiogenic agent by suppression of multiple proangiogenic factors such as HIF-2α, angiopoietin-like 4, erythropoietin, SIRT-1, Smad3/4, and VEGFR2.69,70 Indeed, a PARP-1 inhibitor 3-AB and an FDA-approved PARP-1 inhibitor olaparib both accelerate wound healing in mouse model by stimulating angiogenesis through upregulating the expression of urokinase type plasminogen activator and matrix metalloproteinase 2 (MMP-2) in transformed endothelial cells GM7373 and stimulating tubulogenic activity.62,63,71 Similarly, PARP-1 knockout mice exhibited accelerated wound healing by suppressing inflammatory mediators, promoting keratinocyte migration56 and improving angiogenesis in animal models.72 Furthermore, FDA-approved PARP-1 inhibitor olaparib accelerates wound healing in mouse burn injury.62,63 Low doses of 3-AB, a PARP inhibitor, have also been reported to stimulate angiogenesis by regulating expression of urokinase type plasminogen activator and MMP-2 in transformed endothelial cells GM7373 and stimulating tubulogenic activity.71 PAR modification also has a reciprocal effect on the angiogenic effect of vascular endothelial growth factor (VEGF) in chorio allantoic membrane assay.73 In an angiogenesis model under hyperglycemia using zebra fish, angiogenesis was impaired by hyperglycemia and it remained impaired even when the fish return to a euglycemic state.72 However, when PARP-1 inhibitor was added in a hyperglycemic setting, angiogenesis was rescued. Even though PARP-1 has been identified as an underlying cause of diabetic vasculopathy,43 the exact molecular mechanisms by which it controls angiogenesis is still being elucidated.
We have shown that PARP-1 hyperactivity in ischemic/diabetic wounds impairs in vitro endothelial cell migration.74 PARP-1 inhibition by a PARP-1 inhibitor PJ34 enhances both endothelial cell migration and tube formation.74 In a mouse ischemic/diabetic wound model, PARP-1 inhibition increases mobilization of endothelial progenitor cells into the systemic circulation,74 resulting in better perfusion in the wound bed, measured by laser Doppler scanning and higher levels of endothelial cell markers such as VEGFR2 and eNOS.74
Taken together, PARP-1 plays an important role in angiogenesis, and a better understanding of the mechanisms behind this will potentially provide a novel therapeutic target to enhance angiogenesis and promote wound healing.
Remodeling phase: targeting PARP to reduce scarring
The remodeling phase starts at the end of the granulation tissue development. In this phase, fibroblasts differentiate into myofibroblasts and induce healing by some contraction.75,76 Collagen I is the predominant form of the extracellular matrix that rebuilds the tensile strength of the tissue.
Cleavage of PARP-1 facilitates cellular disassembly and induces cells toward apoptosis. Apoptotic mechanisms are necessary for resolution of inflammation and development of the granulation tissue and are, therefore, an important mechanism of wound healing. This phenomenon is performed by neutrophils and macrophages after they infiltrate the wound.77,78 As the wound heals, fibroblast downregulation and apoptosis occur along with decreased vascularity through apoptosis of endothelial cells and myofibroblasts.75,76 Apoptosis also regulates number of fibroblasts and collagenase activity and thus plays a role in remodeling by regulating collagen synthesis and degradation within the wound.79 MMPs are important in the remodeling phase of wound healing, and increased MMP-9 has been suggested to predict poor wound healing in diabetic foot ulcers.80,81 Interestingly, MMP-9 expression is suppressed in PARP-1-deficient mice, suggesting that PARP-1 silencing may enhance the remodeling process and accelerate wound healing. Furthermore, PARP-1 silencing inhibits TGF-β/Smad3 pathway,82 which is widely implicated in scarring. PARP-1 upregulation also promotes transcription of profibrotic gene CCN2/CTGF in tubular epithelial cells,83 and absence of PARP-1 protects peritoneal mesothelial cells from fibrotic responses induced by high glucose.84 These data suggest a potential role of PARP-1 in scarless wound healing. It is interesting to know that PARP-1 may also have a role in fetal scarless wound repair. Cutaneous wounds in E15 fetal mice heal in a scarless manner, whereas similar wounds in E18 mice heal with scar formation. PARP-1 cleavage has been associated with scarless healing (at E15, PARP-1 is increased by greater than twofold), whereas scar-forming healing (E18) only showed a small amount of cleaved PARP.85
Genomic analysis of PARP-1 binding genes that may be implicated in wound healing
One of the mechanisms by which PARP-1 regulates gene expression is by directly binding to the gene near the transcription start site (TSS).9,86,87 Genetic analysis of PARP-1 binding sites and the genes regulated by PARP-1 can further elucidate the role of PARP-1 in wound healing. Although there is currently no reported consensus sequence for PARP-1 binding site, a sequence 5′-GGAAAGG-3′ has been found to be in proximity to PARP-1 binding sites.87 Recent studies using chromatin immunoprecipitation (ChIP)-seq analyses provide some clues to wound healing-related genes regulated by PARP-1.87 Better understanding of these downstream pathways of PARP-1 could reveal novel therapeutic targets to enhance wound healing.
PARP-1 binding to many genes has been reported to silence gene expression.87,88 A ChIP-seq approach was used to study PARP-1 protein interaction with chromatin in HEK293 cells.87 A search for wound healing-related genes among the PARP-1 binding genes revealed from the ChIP-seq data (MGI database, ID: GO: 0042060) at least six genes to have PARP-1 binding near the TSS.
Toll-like receptor 4 (TSS to PARP-1 binding–downstream 285 bp)
Toll-like receptor 4 (TLR4) is a proinflammatory molecule and acts as a proangiogenic factor by stimulating VEGF. TLR4 increases in murine cutaneous wounds at the early stages, and closure of excisional wounds is significantly delayed in TLR4-deficient mice. IL-1β, IL-6, as well as epidermal growth factor production are significantly lower in the wounds of TLR4-deficient mice.89,90 TLR4 protein is also significantly downregulated in diabetic foot ulcer patients as compared with controls and is one of the main contributors implicated in impaired diabetic wound healing.91
TEK (TEK receptor tyrosine kinase)/TIE2 (TSS to PARP-1 binding–downstream 89 bp)
TEK/TIE2 is a regulator of angiogenesis and vessel maturation. Pericyte TIE2 controls sprouting angiogenesis, which is an important aspect of wound healing.92
FOSL1/Fos-related antigen 1 (TSS to PARP-1 binding–downstream 4,838 bp)
FOSL1 controls the assembly of endothelial cells into organized capillary tubes by repressing integrin αvβ3 transcription,93 inducing MMP-2 expression,94 and driving endothelial cell migration.95 The pivotal role of FOSL1 in angiogenesis is evident from the lethality of FOSL1−/− embryos due to vascular defects.93 Data from our laboratory (unpublished) demonstrate that silencing PARP-1 increases FOSL1 expression by multiple folds, but when FOSL1 is also silenced along with PARP-1 in PARP-1-silenced endothelial cells, tube formation was impaired, suggesting that PARP-1 silencing enhances endothelial tube formation through the expression of FOSL1. This novel PARP-1–FOSL1 axis in wound angiogenesis should be further explored. Interestingly, PARP-1 was found to bind to FOSL1 in the mitotic phase,85 suggesting that PARP-1 remains bound to FOSL1 within mitotic chromatin. This suggests “mitotic bookmarking,” where regulatory information is transmitted through the transcriptionally silent mitotic phase,96 leading to delayed reactivation of FOSL1 even after exit from mitosis.97
SERPINB2/plasminogen activator inhibitor 2 (TSS to PARP-1 binding–upstream 215 bp)
SerpinB2/plasminogen activator inhibitor 2 (PAI-2) is induced by inflammation, infection, or injury. It inhibits urokinase and proteolysis and thus protects the integrity and barrier function of the stratum corneum during inflammation assault.98,99 SerpinB2−/− mice show impaired skin barrier function, a defective stratum corneum and increased transepidermal water loss. Protease activity is also important for cell migration, matrix degradation, and cellular adhesion, and thus protease activators can potentially influence wound healing through multiple pathways. The concentration of SERPINB2/PAI-2 has been reported to be significantly lower in nonhealing wounds.100
CXCR1 (TSS to PARP-1 binding–downstream 478 bp)
CXCR1 is a chemokine receptor that is expressed in epidermis and can induce keratinocyte migration and/or proliferation. Blocking of receptor–ligand interactions can delay re-epithelialization and wound closure after skin injury.101
CD24/heat stable antigen (TSS to PARP-1 binding–upstream 987 bp)
CD24 is a heavily glycosylated cell surface protein that plays an important role in the inflammation, proliferation, and migration. Wound healing has been shown to be impaired in CD24 knockout mice.102
PARP inhibitors and their potential in facilitating wound healing
PARP inhibitors have been well studied for their therapeutic efficacy in a variety of diseases,103,104 although most of the clinical trials have been aimed at cancers.105 Topically applied pharmacological inhibitors have been shown to accelerate wound closure in mouse models of excision wounds.56,74 The approval and clinical availability of PARP-1 inhibitors have opened the door for repurposing of these drugs, as well as those that are expected to be approved in the future, for other nononcological indications.
Some of the well-studied PARP inhibitors are highlighted (Table 1).
Table 1.
PARP-1 inhibitors in clinical development that may be repurposed for wound healing
| PARP Inhibitor | Target PARP |
|---|---|
| Talazoparib (BMN-673)—most potent | PARP-1/2 |
| Veliparib (ABT-888) | PARP-1/2 |
| Rucaparib (AG-014699. CO-338) | PARP-1/2 |
| Olaparib (AZD-2281) | PARP-1/2/3 |
| CEP-9722 | PARP-1/2 |
| Niraparib (MK-4827) | PARP-1/2 |
PARP, polyadenosine diphosphate (ADP)-ribose polymerase.
Rucaparib (AG-014,69, CO-338)
Rucaparib has been investigated in epithelial ovarian cancers. The recommended Phase 2 dose is 600 mg twice a day for oral rucaparib.106 Treatment-induced adverse events (AEs) include nausea, vomiting, asthenia/fatigue, anemia, and transient transaminitis, and are mainly lower grade AEs, occur early in treatment, and are transient and easily managed with supportive treatment, dose interruption, or discontinuation.106
Olaparib
Olaparib is a competitive inhibitor of PARP-1. Olaparib can affect PARP activity (PARP-1/2/3) by multiple mechanisms. It competes with the binding of NAD+ to PARP and can also trap PARP on DNA.107 Olaparib maximally inhibits PARP enzyme at a dose of 40 mg,108 whereas rucaparib maximally inhibits PARP enzyme at a dose of 92 mg.109
Talazoparib (BMN-673)
Talazoparib has a significantly greater potency in vitro and lowest clinical dose efficacy, relative to other PARP inhibitors in clinical development.110 It is the most specific PARP inhibitor in clinical development111 and has lower off-target cell toxicity.112
Niraparib (MK4827)
Niraparib is an oral PARP inhibitor that is currently approved for the maintenance treatment of women with recurrent ovarian cancer. Niraparib inhibits PARP enzymatic activity as well as increases formation of PARP–DNA complexes through “trapping” the PARP enzyme on damaged DNA. Phase 1 testing established the maximally tolerated dose of 300 mg daily administered orally. Toxicities include hematologic (thrombocytopenia, anemia, neutropenia, and leukopenia), gastrointestinal, fatigue, and cardiovascular (hypertension, tachycardia, and palpitations).113
Puerarin
Puerarin [8-β-d-glucopyranosyl-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] is a major bioactive ingredient extracted from the root of the Pueraria lobata.114–116 Puerarin has been reported to inhibit PARP-1 expression and prevent CCl4-induced liver fibrosis in mice.117 Puerarin fits tightly into the active pocket of PARP-1 and has structure similar to the structures of well-known PARP-1 inhibitors such as olaparib.118,119 Inhibition of PARP-1 by puerarin can provide protection by dramatic suppression of NF-κB, ROS, and effective maintaining of mitochondrial homeostasis.
3-Aminobenzamide
Benzamides, particularly the 3-AB, inhibit/deactivate PARP-1 by interfering with the binding of NAD to the enzyme's active site, and by binding to DNA and preventing the recognition of DNA breaks by the enzyme.120 Application of 3-AB has been reported to restore cellular energy, decrease inflammation, and reduce oxidative/nitrosative stress. 3-AB inhibits PARP-1 around 1 mM concentration.
Nicotinamide
Nicotinamide is a water-soluble amide active form of vitamin B3 or niacin. This was identified as the first inhibitor of PARP. Nicotinamide inhibits PARP activity at concentrations starting at 0.5 mM.35
Summary
PARP-1 is hyperactivated in ischemic diabetic wounds and this review clarifies some of the mechanisms by which elevated PARP-1 may regulate the process of wound healing, implicating that PARP-1 may be an important therapeutic target for improving ischemic diabetic wound healing.
Take-Home Messages.
PARP-1 is a nuclear enzyme that is upregulated in ischemic diabetic wounds and impairs wound healing by multiple mechanisms.
PARP inhibitors attenuate wound inflammation, accelerate keratinocyte migration, and improve angiogenesis in ischemic diabetic wounds.
FDA-approved PARP inhibitors for the treatment of cancers can be repurposed to treat ischemic diabetic wound healing.
Acknowledgments and Funding Sources
This study was supported by start-up funds from George Washington University to B-N.N., American Surgical Association Foundation to B-N.N., and NIH1K08HL129072-01A1 to B-N.N.
Abbreviations and Acronyms
- 3-AB
3-aminobenzamide
- AEs
adverse events
- ChIP
chromatin immunoprecipitation
- FDA
Food and Drug Administration
- MMP
matrix metalloproteinase
- PAI-2
plasminogen activator inhibitor 2
- PAR
poly-ADP-ribose
- PARP
polyadenosine diphosphate (ADP)-ribose polymerase
- ROS
reactive oxygen species
- TLR4
toll-like receptor 4
- TSS
transcription start site
- VEGF
vascular endothelial growth factor
Author Disclosure and Ghost Writing
No competing financial interests exist. The authors expressly wrote the content of this article. No ghostwriters were used to write this article.
About the Authors
Jaideep Banerjee, PhD is a postdoctoral scientist at George Washington University conducting research on the role of PARP-1 in diabetic ischemic wounds. He has >9 years of experience in wound healing research and has contributed to understanding the cellular and molecular biology aspects of microRNAs and redox biology in wound healing as well in wound infection. Niraj Lodhi, PhD is a senior research scientist and has diverse research experience in epigenetics and how epigenetic changes can affect gene expression. He has >5 years of experience on clinical research using PARP-1 inhibitors. Bao-Ngoc Nguyen, MD is board certified in both vascular surgery and general surgery and is an associate professor of Surgery at the George Washington University School of Medicine & Health Sciences. Her study is focused on the angiogenesis/vasculogenesis in ischemic diabetic wound healing.
References
- 1. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4:560–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bondor CI, Veresiu IA, Florea B, Vinik EJ, Vinik AI, Gavan NA. Epidemiology of diabetic foot ulcers and amputations in Romania: results of a cross-sectional quality of life questionnaire based survey. J Diabetes Res 2016;2016:5439521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157–162 [DOI] [PubMed] [Google Scholar]
- 5. Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg 2011;53:445–453 [DOI] [PubMed] [Google Scholar]
- 6. Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays News Rev Mol Cell Dev Biol 2004;26:882–893 [DOI] [PubMed] [Google Scholar]
- 7. D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J 1999;342:249–268 [PMC free article] [PubMed] [Google Scholar]
- 8. Szanto M, Brunyanszki A, Kiss B, et al. : Poly(ADP-ribose) polymerase-2: emerging transcriptional roles of a DNA-repair protein. Cell Mol Life Sci 2012;69:4079–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol 2008;20:294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 2010;39:8–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev 2012;26:417–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatterjee S, Berger SJ, Berger NA. Poly(ADP-ribose) polymerase: a guardian of the genome that facilitates DNA repair by protecting against DNA recombination. Mol Cell Biochem 1999;193:23–30 [PubMed] [Google Scholar]
- 13. Izhar L, Adamson B, Ciccia A, et al. A systematic analysis of factors localized to damaged chromatin reveals PARP-dependent recruitment of transcription factors. Cell Rep 2015;11:1486–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nirodi C, NagDas S, Gygi SP, Olson G, Aebersold R, Richmond A. A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J Biol Chem 2001;276:9366–9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plaza S, Aumercier M, Bailly M, Dozier C, Saule S. Involvement of poly (ADP-ribose)-polymerase in the Pax-6 gene regulation in neuroretina. Oncogene 1999;18:1041–1051 [DOI] [PubMed] [Google Scholar]
- 16. Butler AJ, Ordahl CP. Poly(ADP-ribose) polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol Cell Biol 1999;19:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meisterernst M, Stelzer G, Roeder RG. Poly(ADP-ribose) polymerase enhances activator-dependent transcription in vitro. Proc Natl Acad Sci U S A 1997;94:2261–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oei SL, Griesenbeck J, Schweiger M, Babich V, Kropotov A, Tomilin N. Interaction of the transcription factor YY1 with human poly(ADP-ribosyl) transferase. Biochem Biophys Res Commun 1997;240:108–111 [DOI] [PubMed] [Google Scholar]
- 19. Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem 1999;380:953–959 [DOI] [PubMed] [Google Scholar]
- 20. Rawling JM, Alvarez-Gonzalez R. TFIIF, a basal eukaryotic transcription factor, is a substrate for poly(ADP-ribosyl)ation. Biochem J 1997;324:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nie J, Sakamoto S, Song D, Qu Z, Ota K, Taniguchi T. Interaction of Oct-1 and automodification domain of poly(ADP-ribose) synthetase. FEBS Lett 1998;424:27–32 [DOI] [PubMed] [Google Scholar]
- 22. Kannan P, Yu Y, Wankhade S, Tainsky MA. PolyADP-ribose polymerase is a coactivator for AP-2-mediated transcriptional activation. Nucleic Acids Res 1999;27:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oei SL, Griesenbeck J, Schweiger M, Ziegler M. Regulation of RNA polymerase II-dependent transcription by poly(ADP-ribosyl)ation of transcription factors. J Biol Chem 1998;273:31644–31647 [DOI] [PubMed] [Google Scholar]
- 24. Poirier GG, de Murcia G, Jongstra-Bilen J, Niedergang C, Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci USA 1982;79:3423–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huletsky A, de Murcia G, Muller S, et al. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. J Biol Chem 1989;264:8878–8886 [PubMed] [Google Scholar]
- 26. Kraus WL, Lis JT. PARP goes transcription. Cell 2003;113:677–683 [DOI] [PubMed] [Google Scholar]
- 27. Martin KA, Cesaroni M, Denny MF, Lupey LN, Tempera I. Global transcriptome analysis reveals that poly(ADP-ribose) polymerase 1 regulates gene expression through EZH2. Mol Cell Biol 2015;35:3934–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathis G, Althaus FR. Release of core DNA from nucleosomal core particles following (ADP-ribose)n-modification in vitro. Biochem Biophys Res Commun 1987;143:1049–1054 [DOI] [PubMed] [Google Scholar]
- 29. Tulin A, Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science 2003;299:560–562 [DOI] [PubMed] [Google Scholar]
- 30. Reale A, Matteis GD, Galleazzi G, Zampieri M, Caiafa P. Modulation of DNMT1 activity by ADP-ribose polymers. Oncogene 2005;24:13–19 [DOI] [PubMed] [Google Scholar]
- 31. Zampieri M, Passananti C, Calabrese R, et al. Parp1 localizes within the Dnmt1 promoter and protects its unmethylated state by its enzymatic activity. PLoS One 2009;4:e4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark NJ, Kramer M, Muthurajan UM, Luger K. Alternative modes of binding of poly(ADP-ribose) polymerase 1 to free DNA and nucleosomes. J Biol Chem 2012;287:32430–32439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kun E, Kirsten E, Mendeleyev J, Ordahl CP. Regulation of the enzymatic catalysis of poly(ADP-ribose) polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3, and ATP. Biochemistry 2004;43:210–216 [DOI] [PubMed] [Google Scholar]
- 34. Kauppinen TM, Chan WY, Suh SW, Wiggins AK, Huang EJ, Swanson RA. Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci U S A 2006;103:7136–7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dominguez-Gomez G, Diaz-Chavez J, Chavez-Blanco A, et al. Nicotinamide sensitizes human breast cancer cells to the cytotoxic effects of radiation and cisplatin. Oncol Rep 2015;33:721–728 [DOI] [PubMed] [Google Scholar]
- 36. Lonskaya I, Potaman VN, Shlyakhtenko LS, Oussatcheva EA, Lyubchenko YL, Soldatenkov VA. Regulation of poly(ADP-ribose) polymerase-1 by DNA structure-specific binding. J Biol Chem 2005;280:17076–17083 [DOI] [PubMed] [Google Scholar]
- 37. Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev 2005;19:1951–1967 [DOI] [PubMed] [Google Scholar]
- 38. Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta 2010;1804:1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang T, Berrocal JG, Yao J, et al. Regulation of poly(ADP-ribose) polymerase-1-dependent gene expression through promoter-directed recruitment of a nuclear NAD+ synthase. J Biol Chem 2012;287:12405–12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gagne JP, Hendzel MJ, Droit A, Poirier GG. The expanding role of poly(ADP-ribose) metabolism: current challenges and new perspectives. Curr Opin Cell Biol 2006;18:145–151 [DOI] [PubMed] [Google Scholar]
- 41. Frizzell KM, Gamble MJ, Berrocal JG, et al. Global analysis of transcriptional regulation by poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase in MCF-7 human breast cancer cells. J Biol Chem 2009;284:33926–33938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez-Vargas JM, Ruiz-Magana MJ, Ruiz-Ruiz C, et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res 2012;22:1181–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia Soriano F, Virag L, Jagtap P, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med 2001;7:108–113 [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Wu Y, Wang B, Yuan X, Fang B. High levels of glucose induced the caspase-3/PARP signaling pathway, leading to apoptosis in human periodontal ligament fibroblasts. Cell Biochem Biophys 2013;66:229–237 [DOI] [PubMed] [Google Scholar]
- 45. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci 2017;18:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Endara M, Masden D, Goldstein J, Gondek S, Steinberg J, Attinger C. The role of chronic and perioperative glucose management in high-risk surgical closures: a case for tighter glycemic control. Plast Reconstr Surg 2013;132:996–1004 [DOI] [PubMed] [Google Scholar]
- 48. Lan CC, Wu CS, Huang SM, Wu IH, Chen GS. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes 2013;62:2530–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci 2016;84:121–127 [DOI] [PubMed] [Google Scholar]
- 50. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165–171 [DOI] [PubMed] [Google Scholar]
- 51. Mohammad G, Siddiquei MM, Abu El-Asrar AM. Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy. Mediat Inflamm 2013;2013:510451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Long CA, Boulom V, Albadawi H, et al. Poly-ADP-ribose-polymerase inhibition ameliorates hind limb ischemia reperfusion injury in a murine model of type 2 diabetes. Ann Surg 2013;258:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kassan M, Choi SK, Galan M, et al. Enhanced NF-kappaB activity impairs vascular function through PARP-1-, SP-1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes 2013;62:2078–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li B, Li R, Zhang C, et al. MicroRNA-7a/b protects against cardiac myocyte injury in ischemia/reperfusion by targeting poly(ADP-ribose) polymerase. PLoS One 2014;9:e90096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tas Hekimoglu A, Toprak G, Akkoc H, et al. Protective effect of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) polymerase in distant liver injury induced by renal ischemia-reperfusion in rats. Eur Rev Med Pharmacol Sci 2014;18:34–38 [PubMed] [Google Scholar]
- 56. El-Hamoly T, Hegedus C, Lakatos P, et al. Activation of poly(ADP-ribose) polymerase-1 delays wound healing by regulating keratinocyte migration and production of inflammatory mediators. Mol Med 2014;20:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)—a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater 2012;24:249–265 [DOI] [PubMed] [Google Scholar]
- 58. Olah G, Finnerty CC, Sbrana E, et al. Increased poly(ADP-ribosyl)ation in skeletal muscle tissue of pediatric patients with severe burn injury: prevention by propranolol treatment. Shock 2011;36:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jagtap PG, Baloglu E, Southan GJ, et al. Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J Med Chem 2005;48:5100–5103 [DOI] [PubMed] [Google Scholar]
- 60. Rosado MM, Bennici E, Novelli F, Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology 2013;139:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 2005;4:421–440 [DOI] [PubMed] [Google Scholar]
- 62. Ahmad A, Olah G, Herndon DN, Szabo C. The clinically used PARP inhibitor olaparib improves organ function, suppresses inflammatory responses and accelerates wound healing in a murine model of third-degree burn injury. Br J Pharmacol 2018;175:232–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. El-Hamoly T, El-Denshary ES, Saad SM, El-Ghazaly MA. 3-aminobenzamide, a poly (ADP ribose) polymerase inhibitor, enhances wound healing in whole body gamma irradiated model. Wound Repair Regen 2015;23:672–684 [DOI] [PubMed] [Google Scholar]
- 64. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3:445–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simbulan-Rosenthal CM, Rosenthal DS, Hilz H, et al. The expression of poly(ADP-ribose) polymerase during differentiation-linked DNA replication reveals that it is a component of the multiprotein DNA replication complex. Biochemistry 1996;35:11622–11633 [DOI] [PubMed] [Google Scholar]
- 66. Eki T. Poly (ADP-ribose) polymerase inhibits DNA replication by human replicative DNA polymerase alpha, delta and epsilon in vitro. FEBS Lett 1994;356:261–266 [DOI] [PubMed] [Google Scholar]
- 67. Pagano A, Metrailler-Ruchonnet I, Aurrand-Lions M, Lucattelli M, Donati Y, Argiroffo CB. Poly(ADP-ribose) polymerase-1 (PARP-1) controls lung cell proliferation and repair after hyperoxia-induced lung damage. Am J Physiol Lung Cell Mol Physiol 2007;293:L619–L629 [DOI] [PubMed] [Google Scholar]
- 68. Pyriochou A, Olah G, Deitch EA, Szabo C, Papapetropoulos A. Inhibition of angiogenesis by the poly(ADP-ribose) polymerase inhibitor PJ-34. Int J Mol Med 2008;22:113–118 [PubMed] [Google Scholar]
- 69. Gonzalez-Flores A, Aguilar-Quesada R, Siles E, et al. Interaction between PARP-1 and HIF-2alpha in the hypoxic response. Oncogene 2014;33:891–898 [DOI] [PubMed] [Google Scholar]
- 70. Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol 2008;28:711–717 [DOI] [PubMed] [Google Scholar]
- 71. Caldini R, Fanti E, Magnelli L, et al. Low doses of 3-aminobenzamide, a poly(ADP-ribose) polymerase inhibitor, stimulate angiogenesis by regulating expression of urokinase type plasminogen activator and matrix metalloprotease 2. Vasc Cell 2011;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sarras MP, Jr., Mason S, McAllister G, Intine RV. Inhibition of poly-ADP ribose polymerase enzyme activity prevents hyperglycemia-induced impairment of angiogenesis during wound healing. Wound Repair Regen 2014;22:666–670 [DOI] [PubMed] [Google Scholar]
- 73. Binu S, Soumya SJ, Kumar VB, Sudhakaran PR. Poly-ADP-ribosylation of vascular endothelial growth factor and its implications on angiogenesis. Adv Exp Med Biol 2012;749:269–278 [DOI] [PubMed] [Google Scholar]
- 74. Zhou X, Patel D, Sen S, et al. Poly-ADP-ribose polymerase inhibition enhances ischemic and diabetic wound healing by promoting angiogenesis. J Vasc Surg 2017;65:1161–1169 [DOI] [PubMed] [Google Scholar]
- 75. Compton CC, Gill JM, Bradford DA, Regauer S, Gallico GG, O'Connor NE. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest J Tech Methods Pathol 1989;60:600–612 [PubMed] [Google Scholar]
- 76. Green DR. A Myc-induced apoptosis pathway surfaces. Science 1997;278:1246–1247 [DOI] [PubMed] [Google Scholar]
- 77. Rai NK, Tripathi K, Sharma D, Shukla VK. Apoptosis: a basic physiologic process in wound healing. Int J Low Extrem Wounds 2005;4:138–144 [DOI] [PubMed] [Google Scholar]
- 78. Tidball JG, St Pierre BA. Apoptosis of macrophages during the resulution of muscle inflammation. J Leukoc Biol 1996;59:380–388 [DOI] [PubMed] [Google Scholar]
- 79. Rizzi N, Denegri M, Chiodi I, et al. Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock. Mol Biol Cell 2004;15:543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008;40:1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers: response to Muller et al. Diabetes Care 2009;32:e137. [DOI] [PubMed] [Google Scholar]
- 82. Wang Y, Wang L, Zhang F, et al. Inhibition of PARP prevents angiotensin II-induced aortic fibrosis in rats. Int J Cardiol 2013;167:2285–2293 [DOI] [PubMed] [Google Scholar]
- 83. Okada H, Inoue T, Kikuta T, et al. Poly(ADP-ribose) polymerase-1 enhances transcription of the profibrotic CCN2 gene. J Am Soc Nephrol 2008;19:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lei P, Jiang Z, Zhu H, Li X, Su N, Yu X. Poly(ADP-ribose) polymerase-1 in high glucose-induced epithelial-mesenchymal transition during peritoneal fibrosis. Int J Mol Med 2012;29:472–478 [DOI] [PubMed] [Google Scholar]
- 85. Carter R, Sykes V, Lanning D. Scarless fetal mouse wound healing may initiate apoptosis through caspase 7 and cleavage of PARP. J Surg Res 2009;156:74–79 [DOI] [PubMed] [Google Scholar]
- 86. Yu W, Ginjala V, Pant V, et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet 2004;36:1105–1110 [DOI] [PubMed] [Google Scholar]
- 87. Lodhi N, Kossenkov AV, Tulin AV. Bookmarking promoters in mitotic chromatin: poly(ADP-ribose)polymerase-1 as an epigenetic mark. Nucleic Acids Res 2014;42:7028–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sultan M, Schulz MH, Richard H, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science 2008;321:956–960 [DOI] [PubMed] [Google Scholar]
- 89. Suga H, Sugaya M, Fujita H, et al. TLR4, rather than TLR2, regulates wound healing through TGF-beta and CCL5 expression. J Dermatol Sci 2014;73:117–124 [DOI] [PubMed] [Google Scholar]
- 90. Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 2013;133:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh K, Singh VK, Agrawal NK, Gupta SK, Singh K. Association of Toll-like receptor 4 polymorphisms with diabetic foot ulcers and application of artificial neural network in DFU risk assessment in type 2 diabetes patients. BioMed Res Int 2013;2013:318686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Teichert M, Milde L, Holm A, et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun 2017;8:16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Evellin S, Galvagni F, Zippo A, et al. FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of alphav and beta3 integrin transcription. Mol Cell Biol 2013;33:1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Singh NK, Quyen DV, Kundumani-Sridharan V, Brooks PC, Rao GN. AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis. J Biol Chem 2010;285:16830–16843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Galvagni F, Orlandini M, Oliviero S. Role of the AP-1 transcription factor FOSL1 in endothelial cells adhesion and migration. Cell Adh Migr 2013;7:408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenet Chromatin 2013;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kadauke S, Udugama MI, Pawlicki JM, et al. Tissue-specific mitotic bookmarking by hematopoietic transcription factor GATA1. Cell 2012;150:725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schroder WA, Major L, Suhrbier A. The role of SerpinB2 in immunity. Crit Rev Immunol 2011;31:15–30 [DOI] [PubMed] [Google Scholar]
- 99. Lee JA, Cochran BJ, Lobov S, Ranson M. Forty years later and the role of plasminogen activator inhibitor type 2/SERPINB2 is still an enigma. Semin Thromb Hemost 2011;37:395–407 [DOI] [PubMed] [Google Scholar]
- 100. Stacey MC, Mata SD. Lower levels of PAI-2 may contribute to impaired healing in venous ulcers - a preliminary study. Cardiovasc Surg 2000;8:381–385 [DOI] [PubMed] [Google Scholar]
- 101. Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, Waaijman T, Scheper RJ, Gibbs S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol 2012;132:216–225 [DOI] [PubMed] [Google Scholar]
- 102. Shapira S, Ben-Amotz O, Sher O, et al. Delayed wound healing in heat stable antigen (HSA/CD24)-deficient mice. PLoS One 2015;10:e0139787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer 2010;10:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol 2011;22:268–279 [DOI] [PubMed] [Google Scholar]
- 105. Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol 2012;13:411–424 [DOI] [PubMed] [Google Scholar]
- 106. Colombo I, Lheureux S, Oza AM. Rucaparib: a novel PARP inhibitor for BRCA advanced ovarian cancer. Drug Des Dev Ther 2018;12:605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bixel K, Hays JL. Olaparib in the management of ovarian cancer. Pharmacogenomics Pers Med 2015;8:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–134 [DOI] [PubMed] [Google Scholar]
- 109. Drew Y, Ledermann J, Hall G, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer 2016;114:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shen Y, Rehman FL, Feng Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res 2013;19:5003–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wahlberg E, Karlberg T, Kouznetsova E, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol 2012;30:283–288 [DOI] [PubMed] [Google Scholar]
- 112. Murai J, Huang SY, Renaud A, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 2014;13:433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol 2018;149:214–220 [DOI] [PubMed] [Google Scholar]
- 114. Zhu X, Wang K, Zhang K, Lin X, Zhu L, Zhou F. Puerarin protects human neuroblastoma SH-SY5Y cells against glutamate-induced oxidative stress and mitochondrial dysfunction. J Biochem Mol Toxicol 2016;30:22–28 [DOI] [PubMed] [Google Scholar]
- 115. Zhu X, Xie M, Wang K, et al. The effect of puerarin against IL-1beta-mediated leukostasis and apoptosis in retinal capillary endothelial cells (TR-iBRB2). Mol Vis 2014;20:1815–1823 [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang Z, Lam TN, Zuo Z. Radix Puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2013;53:787–811 [DOI] [PubMed] [Google Scholar]
- 117. Wang S, Shi XL, Feng M, et al. Puerarin protects against CCl4-induced liver fibrosis in mice: possible role of PARP-1 inhibition. Int Immunopharmacol 2016;38:238–245 [DOI] [PubMed] [Google Scholar]
- 118. Maeda J, Roybal EJ, Brents CA, Uesaka M, Aizawa Y, Kato TA. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol Rep 2014;31:551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhou P, Huang J, Tian F. Specific noncovalent interactions at protein-ligand interface: implications for rational drug design. Curr Med Chem 2012;19:226–238 [DOI] [PubMed] [Google Scholar]
- 120. Southan GJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors. Curr Med Chem 2003;10:321–340 [DOI] [PubMed] [Google Scholar]