Abstract
C1q/tumor necrosis factor (TNF)-related proteins (CTRPs) have been linked to energy homeostasis and vascular health. People with HIV are susceptible to cardiometabolic disease, but the contributions of different CTRPs are unknown. We investigated the associations of HIV and related factors with serum CTRPs, and CTRPs' relationships with cardiometabolic phenotypes. This involved a cross-sectional analysis of participants in the Women's Interagency HIV Study aged ≥35 with (n = 209) and without (n = 92) HIV who underwent carotid ultrasound in 2004–2005 and had stored serum available for measurement of total adiponectin and CTRPs 1, 3, 5, and 9. The Benjamini/Hochberg procedure was used to control the study-wide false-positive rate. HIV-positive women had significantly higher adiponectin than HIV-negative women after adjustment for sociodemographic, behavioral, and clinical variables [beta = 0.29 (95% confidence interval 0.11–0.47)]. Among HIV-positive women, lower CD4 count was associated with higher adiponectin and history of AIDS with higher CTRP9, but these were only nominally significant. There was no relationship between HIV status and CTRP 1, 3, or 5, nor was antiretroviral therapy or viral load associated with any CTRP. In the entire cohort, higher adiponectin was associated with significantly lower fasting glucose and insulin resistance, while higher CTRP5 [beta = -0.02 (−0.033 to −0.007)]—and, at a nominal level, CTRPs 1 and 3—was associated with significantly lower carotid intima-media thickness. In conclusion, in this sample of middle-aged women, HIV serostatus was positively associated with adiponectin, but not CTRPs. In turn, serum adiponectin was inversely associated with glucose dysregulation, whereas CTRP5 was inversely associated with carotid intima-media thickness. Further research is needed to determine CTRPs' role in atherosclerosis.
Keywords: adiponectin, CTRP, HIV, glycemia, insulin resistance, cardiovascular disease
Introduction
The hormone adiponectin has been found in animal models to have insulin-sensitizing, anti-inflammatory, and antiatherogenic properties.1 Corresponding inverse associations with metabolic outcomes have been documented in humans, but the relationship of adiponectin with cardiovascular disease (CVD) is more complex, varying by clinical context. Specifically, high levels of adiponectin have been associated with lower risk of CVD in healthy middle-aged adults, but higher risk of CVD and mortality in individuals with prevalent heart disease, whereas a U-shaped association with CVD or death has been observed in older adults.2,3 The basis for such differences is not fully delineated, but may relate to adiponectin secretion by endothelial cells or muscle in disease states, or upregulation by high-level inflammation.1
Adiponectin belongs to the complement component (C) 1q/tumor necrosis factor (TNF)-related protein (CTRP) family, which comprises more than 15 different CTRPs.4 Mouse knockouts of adiponectin are partially protected against insulin resistance/diabetes, and levels of several CTRPs have been found to be elevated in this context, suggesting that such increases in CTRPs may compensate for the adverse metabolic effects of adiponectin deletion.5 Among CTRPs, four stand out experimentally as being of potential significance to human cardiometabolic disease, namely, CTRPs 1, 3, 5, and 9.5–15 Small studies in people without HIV have shown associations of serum levels of these CTRPs with cardiometabolic measures, but findings have been inconsistent.16
The advent of highly active antiretroviral therapy (HAART) has transformed HIV into a chronic disease characterized by increased susceptibility to metabolic dysregulation and atherosclerosis, among other comorbidities, whose basis remains incompletely defined.17,18 Several factors have been implicated, including chronic immune activation and inflammation, fat redistribution and dysfunction, direct and indirect toxicity of HAART, and increased behavioral and clinical cardiometabolic risk factors.17,18
Women with or at risk for HIV infection represent a particularly vulnerable population,19 coming largely from socioeconomically disadvantaged race/ethnic minority groups that suffer an outsized burden of cardiometabolic disorders.20 Previous studies have evaluated adiponectin in the setting of HIV, demonstrating lower levels with higher visceral and lower subcutaneous adiposity,21–25 but comparisons of adiponectin levels between individuals with and without HIV have yielded inconsistent results.21–23,26,27 Prior investigations of the relationship of circulating adiponectin with glucose dysregulation and vascular disease in people with HIV have been mostly limited to men. The extent to which HIV affects levels of different CTRPs and whether alterations of such CTRPs may underlie cardiometabolic disease in this setting remain unknown.
Here, we leveraged a well-characterized cohort of women with and without HIV drawn from similar sociodemographic backgrounds to investigate these questions. Given the complex influences on, and scarce or conflicting data as related to, adiponectin and its paralogs in human diseases, we chose an agnostic approach as to the directions of the associations under study. Our aim was to determine the prevailing associations of HIV and HIV-related factors with these CTRPs, and the latter's associations with cardiometabolic disease, letting the findings guide future research into this family of molecules as potential targets for therapeutic manipulation.
Materials and Methods
Study population
The Women's Interagency HIV Study (WIHS) is a prospective observational cohort investigation of U.S. women, as detailed elsewhere.28 Briefly, WIHS enrolled women with and without HIV from six U.S. locations in 1994–1995, and again in 2001–2002. HIV-negative participants were recruited from the same clinics as their HIV-positive counterparts. Semiannual visits included an interviewer-administered questionnaire, physical examination, and collection of serum samples and clinical measurements. In 2004–2005, a carotid artery ultrasound substudy was performed in WIHS.29 The present study focused on the Bronx site participants, ages 35 years or older. Of the 347 participants who completed an ultrasound, 301 women had stored serum specimens drawn no more than one semiannual visit removed from the carotid ultrasound examination, and these constituted our study sample.
CTRP measurement
Serum samples were stored at −70°C until the time of CTRP measurement. Total adiponectin was measured by immunoassay (R&D Systems, Minneapolis, MN). Interassay coefficients of variation (CVs) were 4.56%–6.12%. CTRP1 and CTRP9 were measured by enzyme-linked immunosorbent assay (ELISA; Biovendor); interassay CVs were 4.29%–7.12% and 5.32%–13.67%, respectively. CTRP3 and CTRP5 were measured by ELISA (Aviscera, Santa Clara, CA); interassay CVs were 10.06%–38.99% and 5.34%–19.10%, respectively.
Metabolic outcome measures
Fasting specimens for glucose determination were collected in tubes with glycolytic inhibitors, with serum for insulin determination obtained simultaneously, from which homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.30
Carotid ultrasound
Participants underwent high-resolution B-mode carotid artery ultrasound to image the right carotid artery according to previously published procedures.31 A standardized protocol was used and carotid artery outcome measures were obtained at a centralized reading center, as reported previously.2,29,32,33
Covariates
HIV serostatus was assessed by ELISA and confirmed by Western blot. Plasma HIV RNA was measured by commercial assay (lower limit of quantification: 80 copies/mL). CD4+ T cell counts were determined using flow cytometric methods. Anthropometry was determined using standardized approaches. Substance use was assessed by self-report. Hepatitis C virus (HCV) infection was determined by antibody seropositivity and RNA level. Hepatitis B virus (HBV) infection was based on detection of HBV surface antigen. Hypertension was defined by blood pressure ≥140 mm Hg (systolic), ≥90 mm Hg (diastolic), or self-reported use of antihypertensive medication. History of diabetes was based on hemoglobin A1C, fasting glucose, use of antidiabetes medications, or self-report.34 History of myocardial infarction (MI) or stroke was based on self-report. Medication use was obtained by a questionnaire. History of clinical AIDS was defined by prior self-report of AIDS-defining illness. Serum chemistries were measured at a central laboratory, and estimated glomerular filtration rate (eGFR) determined.35
Statistical analysis
In the analysis of HIV status and HIV-related parameters in relation to serum CTRP levels, HIV measures were considered independent variables, and CTRPs dependent variables. Assessment of CTRP levels and cardiometabolic outcomes considered CTRPs as independent variables, and fasting glucose, HOMA-IR, carotid intima-media thickness (CIMT), and carotid distensibility as the dependent variables. All analyses included the entire study sample (n = 301) except for the evaluation of CTRPs in relation to metabolic outcomes, where participants on antidiabetes medications (n = 26) were excluded. Because serum levels of all CTRPs were right skewed, their values were natural log-transformed in linear regression analyses.
We used multiple linear regression to assess relationships of interest, and fit sequential models to account for aggregate confounding, as well as to explore the impact of potential causal intermediates. The impact of potential collinearity was assessed by monitoring for inflation of the 95% confidence interval (CI) upon addition of moderately correlated individual variables; none of the variables included in models resulted in appreciable inflation. Generalized additive model plots of fully adjusted (main) models were assessed for departures from linearity, but no such departures were observed. There was no missingness for variables included in linear regression models.
For analyses of HIV and related factors in relation to CTRPs, initial adjustment included age and race/ethnicity; followed by smoking, alcohol consumption, intravenous drug use, use of crack/cocaine or heroin, HCV, HBV, and eGFR; and thereafter by treatment with beta-blockers, angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, and antidiabetic medications (main model). Additional adjustment for body mass index (BMI), waist circumference, and hip circumference as potential mediators was then undertaken. Further adjustment for self-reported MI or stroke was also explored, as was C-reactive protein (CRP) as an inflammatory mediator.
Analyses of the association of CTRP levels with cardiometabolic outcomes also initially adjusted for the same demographic and behavioral/clinical covariates. However, as anthropometric covariates constitute potential confounders, rather than mediators, in this analysis, we next adjusted additionally for BMI, waist circumference, and hip circumference. Thereafter, we also adjusted for beta-blockers, ACE inhibitors/angiotensin-receptor blockers, and antidiabetes medications (main model). Additional adjustment for self-reported MI or stroke, as well as CRP, was also undertaken. Subsequent models examined the impact of adjusting for other CTRPs simultaneously when appropriate. For the CIMT and carotid distensibility outcomes, additional exploratory models also adjusted for hypertension and HOMA-IR as potential mediators. These same analyses regressing cardiometabolic outcomes on CTRP levels were repeated in the HIV-positive group. In these analyses, the main model was additionally adjusted for HIV-specific factors.
All analyses were performed using R, version 3.3.1 (Vienna, Austria). A nominal significance level of p < .05 was used for analyses among exposures or exposures with covariates. To control for multiple comparisons for exposure/outcome associations, the Benjamini/Hochberg (BH) procedure was used to define statistical significance by keeping the study-wide false-discovery rate no higher than 5%.
Results
Characteristics of study participants are shown in Table 1. Participants were of middle age, predominantly African American and Hispanic, and had a high prevalence of smoking, substance use, clinical cardiovascular risk factors, and history of MI or stroke. More than two-thirds of study participants were HIV positive, of whom almost three-quarters were receiving HAART. Women living with HIV frequently had a history of AIDS, and only a quarter had an undetectable viral load. HIV-positive participants were of comparable age and race/ethnicity as their HIV-negative counterparts. Compared with HIV-negative women, women with HIV had lower BMI and hip circumference, crack/cocaine or heroin use, and high-density lipoprotein (HDL) cholesterol. Women with HIV exhibited higher total adiponectin, CTRP1, and CTRP5 relative to HIV-negative women.
Table 1.
Characteristics of Study Participants
| Variables | Entire cohort (n = 301) | HIV positive (n = 209) | HIV negative (n = 92) | p |
|---|---|---|---|---|
| Demographic and clinical | ||||
| Age, years | 45 ± 6 | 45 ± 6 | 45 ± 8 | .855 |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic white | 8 (2.7) | 6 (2.9) | 2 (2.2) | .361 |
| Non-Hispanic black | 175 (58.1) | 115 (55.0) | 60 (65.2) | |
| Hispanic | 115 (38.2) | 86 (41.1) | 29 (31.5) | |
| BMI, kg/m2 | 29.5 ± 6.7 | 28.7 ± 6.5 | 31.2 ± 7.0 | .005 |
| Waist circumference, cm | 91.2 ± 13.9 | 90.3 ± 13.3 | 93.5 ± 15.2 | .095 |
| Hip circumference, cm | 102.2 ± 13.8 | 100.4 ± 13.4 | 106.1 ± 13.9 | .002 |
| Current smoking, n (%) | 191 (63.5) | 115 (55.0) | 76 (82.6) | <.001 |
| Alcohol use >7 drinks per week, n (%) | 31 (10.3) | 19 (9.1) | 12 (13.0) | .305 |
| History of injection drug use, n (%) | 108 (35.9) | 75 (35.9) | 33 (35.9) | .987 |
| History of crack/cocaine or heroin use, n (%) | 247 (82.1) | 161 (77.0) | 86 (93.5) | <.001 |
| HCV infection, n (%) | 130 (43.2) | 94 (45.0) | 36 (39.1) | .346 |
| HBV infection, n (%) | 5 (1.7) | 4 (1.9) | 1 (1.1) | .999 |
| Hypertension, n (%) | 104 (34.5) | 67 (32.1) | 37 (40.2) | .170 |
| Diabetes, n (%) | 53 (17.6) | 33 (15.8) | 20 (27.1) | .212 |
| Low-density lipoprotein cholesterol, mg/dL | 94 ± 33 | 93 ± 32 | 96 ± 35 | .493 |
| High-density lipoprotein cholesterol, mg/dL | 47 ± 17 | 46 ± 16 | 52 ± 16 | .006 |
| Triglycerides, mg/dL | 119 (80–173) | 125 (86–175) | 105 (73–168) | .458 |
| Antidiabetes medication, n (%) | 26 (8.6) | 16 (7.7) | 10 (10.9) | .361 |
| Beta-blocker, n (%) | 20 (6.6) | 16 (7.7) | 4 (4.3) | .451 |
| ACE inhibitor or ARB, n (%) | 36 (12.0) | 21 (10.0) | 15 (16.3) | .123 |
| History of myocardial infarction or stroke, n (%) | 31 (10.3) | 18 (8.6) | 13 (14.1) | .147 |
| eGFR, mL/min/1.73 m2 | 95.0 ± 21.2 | 94.7 ± 21.4 | 95.8 ± 20.8 | .684 |
| C-reactive protein, mg/L | 2.3 (0.9–5.8) | 2.3 (0.8–5.4) | 2.5 (0.9–6.5) | .234 |
| HIV specific | ||||
| Current HAART, n (%) | NA | 151 (72.2) | NA | NA |
| History of AIDS, n (%) | NA | 113 (54.1) | NA | NA |
| Current CD4+, cells/μL | 496 (261–765) | 363 (222–580) | 1,025 (885–1,280) | NA |
| Current viral load, copies/mL | NA | 210 (80–5,300) | NA | NA |
| CTRPs | ||||
| Total adiponectin, μg/mL | 7.0 (4.6–11.5) | 7.8 (4.9–12.8) | 5.7 (4.3–9.0) | <.001 |
| CTRP1, ng/mL | 485.3 (393.5–637.1) | 504.4 (417.1–652.3) | 437.9 (372.9–575.3) | .022 |
| CTRP3, ng/mL | 31.2 (16.9–48.3) | 30.9 (17.8–47.0) | 34.4 (16.5–52.9) | .163 |
| CTRP5, ng/mL | 9.6 (6.8–13.2) | 10.0 (7.0–14.1) | 9.4 (6.4–11.7) | .030 |
| CTRP9, ng/mL | 0.19 (0.13–1.88) | 0.17 (0.13–1.87) | 0.20 (0.13–2.25) | .120 |
| Cardiometabolic outcome measures | ||||
| Fasting glucose, mg/dL | 95.7 ± 29.8 | 93.2 ± 22.7 | 101.5 ± 4.3 | .103 |
| Homeostasis model assessment of insulin resistancea | 2.4 (1.6–4.2) | 2.5 (1.8–4.3) | 2.4 (1.4–4.1) | .572 |
| Carotid intima-media thickness, mm | 0.76 ± 0.12 | 0.75 ± 0.10 | 0.78 ± 0.14 | .182 |
| Carotid distensibility, 10−6 × N−1 × m2 | 20.9 ± 8.3 | 21.1 ± 8.2 | 20.6 ± 8.6 | .685 |
Among women not treated with antidiabetic medications.
ACE, angiotensin converting enzyme; AIDS, acquired immunodeficiency syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CI, confidence interval; CTRP, complement component 1q/tumor necrosis factor-α-related protein; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus.
Spearman correlations of CTRPs with each other, anthropometric variables, and lipid and inflammatory measures are presented in Supplementary Table S1. Total adiponectin exhibited small-to-moderate positive correlations with CTRP1, CTRP3, and CTRP5, whereas CTRP1 showed small positive correlations with CTRP3 and CTRP5. CTRP3 and CTRP5 exhibited a moderately strong positive correlation, and both CTRP3 and CTRP5 showed small-to-moderate positive correlations with CTRP9.
Findings were consistent overall and among women with HIV. In turn, total adiponectin showed moderate negative correlations with BMI, waist circumference, and hip circumference in the entire sample, whereas CTRP3 and CTRP5 exhibited small negative correlations with these anthropometric variables. Neither CTRP1 nor CTRP9 was correlated with these measures of body size in the overall cohort, but CTRP9 bore a small negative correlation to hip circumference in the HIV-positive group. Turning to lipid and inflammatory measures, total adiponectin was moderately correlated with HDL cholesterol (positively) as well as triglycerides (negatively), and exhibited a small negative correlation with CRP. CTRP1, CTRP3, and CTRP5 showed small negative correlations with low-density lipoprotein cholesterol, small or moderate negative correlations with HDL cholesterol, and, in the case of CTRP1, a small positive correlation with triglycerides. Correlations overall and among women with HIV were mostly comparable.
Associations between HIV status and HIV-specific factors with CTRPs are shown in Table 2. At various levels of adjustment, women with HIV had higher total adiponectin compared with HIV-negative women. In the fully adjusted model (Model 3), there was a 0.29-unit higher log-transformed concentration of total adiponectin, equivalent to 1.34 mg/L (e0.29) higher serum concentration of total adiponectin in women with than without HIV after back-transformation. This was the only main-model association in Table 2 that met BH significance, but there were others that met nominal statistical significance (p < .05). Among HIV-positive women, each standard deviation (SD) increment in CD4 count was associated with a 0.13-unit decrease in the log-transformed concentration of total adiponectin, corresponding to a decrement of 1.14 mg/L (nominally significant). For both HIV status and CD4 count, additional adjustment for CRP or history of MI or stroke did not materially influence the results (not shown). Furthermore, in both cases, additional adjustment for anthropometric variables as putative mediators did not have a meaningful impact (Model 4, Table 2). Neither detectable viral load nor use of HAART, or history of AIDS, exhibited significant associations with total adiponectin.
Table 2.
Associations Between HIV Status or HIV-Related Factors and CTRP Levels
| Log-total adiponectin | Log-CTRP1 | Log-CTRP3 | Log-CTRP5 | Log-CTRP9 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta (95% CI) | P | Beta (95% CI) | p | Beta (95% CI) | p | Beta (95% CI) | p | Beta (95% CI) | p | |
| Entire cohort (n = 301) | ||||||||||
| HIV seropositive status | ||||||||||
| Model 1 | 0.26 (0.09 to 0.43) | .003 | 0.11 (0.01 to 0.20) | .027 | −0.03 (−0.25 to 0.19) | .779 | 0.13 (−0.004 to 0.27) | .057 | −0.10 (−0.59 to 0.38) | .674 |
| Model 2 | 0.29 (0.12 to 0.47) | .001 | 0.09 (−0.002 to 0.19) | .055 | −0.05 (−0.28 to 0.17) | .637 | 0.08 (−0.06 to 0.22) | .262 | −0.32 (−0.84 to 0.21) | .238 |
| Model 3 | 0.29 (0.11 to 0.47) | .001a | 0.09 (−0.006 to 0.18) | .069 | −0.04 (−0.27 to 0.19) | .736 | 0.07 (−0.07 to 0.21) | .343 | −0.34 (−0.87 to 0.19) | .208 |
| Model 4 | 0.31 (0.13 to 0.48) | <.001 | 0.07 (−0.02 to 0.17) | .141 | −0.12 (−0.35 to 0.12) | .337 | 0.03 (−0.11 to 0.18) | .643 | −0.49 (−1.03 to 0.05) | .078 |
| HIV-positive women (n = 209) | ||||||||||
| CD4 count [beta per SD (289 cells/μL) increment] | ||||||||||
| Model 1 | −0.15 (−0.25 to −0.05) | .003 | −0.02 (−0.08 to 0.04) | .487 | −0.06 (−0.19 to 0.06) | .312 | −0.02 (−0.10 to 0.06) | .630 | 0.26 (−0.02 to 0.53) | .071 |
| Model 2 | −0.14 (−0.23 to −0.04) | .006 | −0.03 (−0.08 to 0.03) | .353 | −0.07 (−0.20 to 0.06) | .248 | −0.04 (−0.12 to 0.04) | .332 | 0.25 (−0.04 to 0.54) | .089 |
| Model 3 | −0.13 (−0.23 to −0.04) | .008 | −0.03 (−0.08 to 0.02) | .305 | −0.10 (−0.22 to 0.03) | .134 | −0.04 (−0.12 to 0.04) | .275 | 0.28 (−0.009 to 0.58) | .059 |
| Model 4 | −0.11 (−0.21 to −0.008) | .037 | −0.03 (−0.09 to 0.02) | .254 | −0.11 (−0.25 to 0.03) | .125 | −0.02 (−0.11 to 0.07) | .642 | 0.10 (−0.21 to 0.41) | .514 |
| Presence of detectable viral load | ||||||||||
| Model 1 | 0.11 (−0.09 to 0.31) | .300 | 0.07 (−0.04 to 0.18) | .227 | 0.08 (−0.18 to 0.33) | .542 | 0.05 (−0.11 to 0.21) | .531 | −0.31 (−0.88 to 0.26) | .293 |
| Model 2 | 0.13 (−0.08 to 0.34) | .222 | 0.07 (−0.04 to 0.19) | .210 | 0.10 (−0.17 to 0.37) | .474 | 0.12 (−0.05 to 0.28) | .176 | −0.22 (−0.84 to 0.40) | .488 |
| Model 3 | 0.12 (−0.09 to 0.34) | .256 | 0.06 (−0.05 to 0.17) | .280 | 0.14 (−0.13 to 0.41) | .322 | 0.13 (−0.04 to 0.29) | .146 | −0.23 (−0.86 to 0.39) | .468 |
| Model 4 | 0.07 (−0.13 to 0.27) | .502 | 0.08 (−0.04 to 0.19) | .186 | 0.13 (−0.14 to 0.40) | .341 | 0.12 (−0.05 to 0.29) | .162 | 0.01 (−0.59 to 0.60) | .986 |
| Use of highly active antiretroviral therapy | ||||||||||
| Model 1 | −0.11 (−0.33 to 0.11) | .337 | 0.01 (−0.11 to 0.14) | .858 | 0.07 (−0.21 to 0.35) | .635 | −0.04 (−0.21 to 0.14) | .680 | 0.69 (0.07 to 1.31) | .031 |
| Model 2 | −0.10 (−0.33 to 0.13) | .400 | 0.04 (−0.09 to 0.16) | .560 | 0.07 (−0.22 to 0.37) | .622 | −0.08 (−0.26 to 0.11) | .407 | 0.60 (−0.07 to 1.27) | .082 |
| Model 3 | −0.11 (−0.34 to 0.12) | .348 | 0.05 (−0.07 to 0.17) | .414 | 0.09 (−0.20 to 0.38) | .547 | −0.06 (−0.24 to 0.12) | .516 | 0.57 (−0.10 to 1.25) | .098 |
| Model 4 | −0.08 (−0.30 to 0.13) | .442 | 0.03 (−0.09 to 0.15) | .606 | 0.06 (−0.23 to 0.35) | .702 | −0.08 (−0.26 to 0.10) | .393 | 0.39 (−0.25 to 1.03) | .230 |
| History of AIDS | ||||||||||
| Model 1 | 0.09 (−0.10 to 0.29) | .347 | 0.05 (−0.06 to 0.16) | .363 | 0.26 (0.01 to 0.51) | .041 | −0.06 (−0.22 to 0.10) | .446 | 0.68 (0.13 to 1.24) | .017 |
| Model 2 | 0.03 (−0.17 to 0.23) | .795 | 0.02 (−0.09 to 0.13) | .735 | 0.22 (−0.03 to 0.47) | .093 | −0.11 (−0.27 to 0.05) | .170 | 0.73 (0.15 to 1.31) | .015 |
| Model 3 | −0.03 (−0.17 to 0.23) | .788 | 0.01 (−0.09 to 0.12) | .824 | 0.22 (−0.04 to 0.47) | .101 | −0.10 (−0.26 to 0.06) | .202 | 0.76 (0.18 to 1.34) | .011 |
| Model 4 | 0.08 (−0.11 to 0.27) | .431 | 0.02 (−0.09 to 0.12) | .776 | 0.20 (−0.05 to 0.45) | .127 | −0.08 (−0.24 to 0.08) | .303 | 0.58 (0.02 to 1.14) | .045 |
Model 1: adjusted for age and race/ethnicity. Model 2: Model 1+smoking, alcohol, IV drug use, crack/cocaine/heroin use, HBV, HCV, eGFR. Model 3: Model 2+beta-blocker, ACE inhibitor or ARB, antidiabetic medication (main model). Model 4: Model 3+BMI, and waist and hip circumferences.
Meets Benjamini/Hochberg significance (false discovery rate ≤5%).
IV, intravenous; SD, standard deviation.
As also shown in Table 2, the only significant association between HIV or HIV-related factors and adiponectin paralogs was for CTRP9, but this was only at a nominal level. Specifically, there was a nominally significant relationship between history of AIDS among women with HIV and log-transformed CTRP9, in which such a history was associated with an e0.76 = 2.14 ng/mL higher concentration. This association was not affected meaningfully by further adjustment for CRP or MI or stroke. The association also persisted, although in attenuated form, after additional adjustment for BMI, and waist and hip circumferences as putative mediators.
The associations of CTRPs with fasting glucose and HOMA-IR are presented in Figure 1. Higher total adiponectin was associated with significantly lower fasting glucose and HOMA-IR, associations that satisfied BH significance. After full adjustment for potential confounders (Model 4), each SD increment in log-transformed adiponectin (e0.7 = 2.01 mg/L) was associated with a 3.44 (95% CI 1.22–5.66) mg/dL lower fasting glucose level and a 1.21 (95% CI 0.39–2.03) unit lower HOMA-IR level. Additional adjustment for history of MI or stroke, or CRP, did not have a substantive influence on these results. There were no significant associations between any of the CTRPs and fasting glucose or HOMA-IR.
FIG. 1.
Association between CTRPs and metabolic outcomes. (A) Association between CTRPs and fasting serum glucose. (B) Association between CTRPs and HOMA-IR. SDs of log-transformed CTRPs are as follows: Adiponectin, SD = 0.70 μg/mL; CTRP1, SD = 0.40 ng/mL; CTRP3, SD = 0.89 ng/mL; CTP5, SD = 0.55 ng/mL; CTRP9, SD = 1.97 ng/mL. Model 1: adjusted for age, race/ethnicity. Model 2: Model 1 additionally adjusted for smoking, alcohol consumption, eGFR, IV drug use, crack/cocaine/heroin use, HBV, HCV, and HIV status. Model 3: Model 2 additionally adjusted for BMI, and waist and hip circumferences. Model 4: Model 3 additionally adjusted for use of beta-blocker, angiotensin-receptor blocker/ACE inhibitor, and diabetes medication (main model). *Meets Benjamini/Hochberg significance (false discovery rate ≤5%). ACE, angiotensin converting enzyme; BMI, body mass index; CTRP, C1q/tumor necrosis factor (TNF)-related protein; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; HOMA-IR, homeostasis model assessment of insulin resistance; IV, intravenous; SD, standard deviation.
Findings were generally consistent in women with HIV. There were inverse associations of total adiponectin with fasting glucose and HOMA-IR in this group, but point estimates were more modest and nonsignificant in the fully adjusted model (Supplementary Fig. S1). Results were not appreciably affected by further adjustment for HIV-related factors. No associations were observed for CTRP1, CTRP3, CTRP5, or CTRP9.
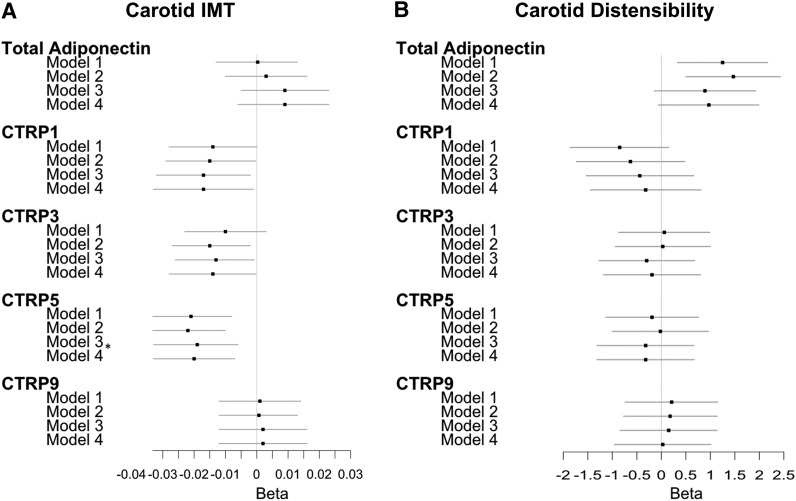
The associations of CTRPs with CIMT and carotid distensibility are depicted in Figure 2. There were no associations between adiponectin or its paralogs with carotid distensibility, either at BH or nominal significance. For CIMT, only one association met BH significance, that for CTRP5. Specifically, CTRP5 showed an inverse association with CIMT [beta = −0.020, 95% CI −0.033 to 0.007, p = .004 (BH significant)], in which, after full adjustment, each SD increment in log-transformed CTRP5 (e0.55 = 1.73 ng/mL) was associated with a 0.020-mm lower CIMT. Both CTRP1 (beta = −0.017, 95% CI −0.033 to −0.001, p = .034) and CTRP3 (beta = −0.014, 95% CI −0.028 to −0.0002, p = .048) also showed inverse associations with CIMT, although at a nominal level of significance. That is, after full adjustment, each SD increment in log-transformed CTRP1 (e0.40 = 1.49 ng/mL) was associated with a 0.017-mm lower CIMT, whereas each SD increment in CTRP3 (e0.89 = 2.44 ng/mL) was associated with a 0.014-mm lower CIMT. Additional adjustment for MI or stroke had no material effect, nor did further adjustment for CRP as a potential confounder and mediator. Further adjustment for hypertension or HOMA-IR as potential mediators likewise did not alter the associations.
FIG. 2.
Association between CTRPs and subclinical CVD outcomes. (A) Association between CTRPs and carotid IMT. (B) Association between CTRPs and carotid distensibility. SDs of log-transformed CTRPs are as follows: Adiponectin, SD = 0.70 μg/mL; CTRP1, SD = 0.40 ng/mL; CTRP3, SD = 0.89 ng/mL; CTP5, SD = 0.55 ng/mL; CTRP9, SD = 1.97 ng/mL. Model 1: adjusted for age, race/ethnnicity. Model 2: Model 1 additionally adjusted for smoking, alcohol consumption, eGFR, IV drug use, crack/cocaine/heroin use, and HBV, HCV, and HIV status. Model 3: Model 2 additionally adjusted for BMI, and waist and hip circumferences. Model 4: Model 3 additionally adjusted for use of beta-blocker, angiotensin-receptor blocker/ACE inhibitor, and diabetes medication (main model). *Meets Benjamini/Hochberg significance (false discovery rate <5%). CVD, cardiovascular disease; IMT, intima-media thickness.
Findings were broadly similar when analyses were restricted to women with HIV (Supplementary Fig. S2). Inverse associations of CTRP1, CTRP3, and CTRP5 with CIMT were attenuated and did not achieve nominal significance. Results were not materially altered by further adjustment for HIV-related factors.
Discussion
Main findings
In this study, we found that women with HIV had a significantly higher serum concentration of total adiponectin compared with those without HIV after adjustment for a range of behavioral, clinical, and laboratory confounders, and even putative anthropometric mediators. Among HIV-positive women, lower CD4 count tended to be associated with higher serum adiponectin, and history of AIDS with higher serum CTRP9 after extensive adjustment, but these associations only met nominal levels of significance. In turn, after similar adjustment, serum adiponectin was significantly associated with lower fasting glucose and HOMA-IR, while CTRP5 was related to lower CIMT, an association that persisted after adjustment for HOMA-IR. CTRP1 and CTRP3 also tended to be associated with lower CIMT, although these associations were only nominally significant.
The present study provides the most detailed evaluation of adiponectin in a cohort of predominantly black and Hispanic women with, or at risk for, HIV infection, and the first assessment of such levels in relation to intermediate cardiometabolic outcomes in this setting. The present study is also the first investigation of key adiponectin paralogs in the context of HIV in any cohort.
Adiponectin findings: context and implications
Previous studies of HIV's relationship with circulating adiponectin have reported conflicting results, with some finding lower,21,22,26 others higher,27 and still others no difference in levels in HIV-positive compared with HIV-negative individuals.23 Such studies, however, largely focused on men, mostly performed unadjusted or partially adjusted comparisons, and frequently relied on healthy volunteers as controls. An analysis evaluating serum adiponectin in relation to bone mineral density in an earlier WIHS ancillary study also found higher adiponectin in HIV-positive than HIV-negative women, but this was an unadjusted comparison.27
Body fat accumulation and distribution are key determinants of adiponectin levels in general cohorts, with higher visceral adiposity bearing a strong association with reduced serum adiponectin.1 The same inverse association between visceral adiposity and serum adiponectin has been observed in individuals with HIV.21–25 In contrast to people without HIV, reduced peripheral subcutaneous adipose tissue among people with HIV is correlated with lower, not higher, serum adiponectin, indicating subcutaneous adipose tissue dysfunction, characteristic of HIV-associated lipoatrophy.21,23 Unlike such prior studies, we did not have imaging-based measures of subcutaneous or visceral adiposity, but did observe numerically stronger negative correlations of waist circumference than BMI or hip circumference with circulating adiponectin.
Prior investigations focusing mostly or exclusively on men have also reported associations in HIV-positive individuals of higher CD4 count, undetectable HIV RNA, and HAART or its duration with lower circulating adiponectin.21,24 Herein, we did not demonstrate any such associations, although this was suggested for CD4 at nominal signifiance. Differences in findings could reflect sex differences, distinct behavioral and clinical risk profiles, variation in type, frequency, and intensity of HAART, or other factors across the studies.
Regardless, the higher adiponectin levels in HIV-positive than HIV-negative women documented here highlight an interesting point. The difference from prior reports showing lower adiponectin in HIV-positive individuals than HIV-negative controls could well relate to the healthier status of such healthy volunteers compared with controls at high cardiometabolic risk recruited in the WIHS. Such high-risk WIHS controls are themselves susceptible to hypoadiponectinemia and its adverse health correlates.
Apart from the foregoing consideration, different explanations can be invoked to account for the higher adiponectin levels seen here in women with HIV. One is that the higher adiponectin levels among HAART-treated women may reflect immune reconstitution and attendant restoration of the adipokine's level in this group. Alternatively, higher adiponectin in HIV-positive than HIV-negative women could be a result of greater immune activation and inflammation in the former group, since higher grade inflammation has been shown to elevate adiponectin levels in disorders such as collagen vascular diseases.1 The suggested inverse association between CD4 count and serum adiponectin would be consistent with this possibility. As we did not have concurrent measures of immune activation and inflammation—apart from CRP, which may have less predictive value in the context of HIV36—the basis for the higher HIV-related adiponectin levels will warrant separate investigation.
Regarding serum adiponectin and cardiometabolic outcome measures, prior studies largely derived from men have documented expected inverse associations with glycemic measures and insulin resistance.24,25 Moreover, in the Multicenter AIDS Cohort Study, lower adiponectin was associated with a greater prevalence of coronary atheroma by coronary computed tomography angiography.21 We observed negative correlations with fasting glucose and HOMA-IR after adjusting for multiple confounders, extending these observations to women with, or at risk for, HIV. We did not detect a significant association with CIMT or carotid distensibility,37 but unfortunately, could not meaningfully evaluate carotid atheroma as this was uncommon in our cohort.
Adiponectin paralogs: context and implications
Unlike adiponectin, prior data on the impact of HIV on adiponectin paralogs or on adiponectin paralogs' association with cardiometabolic outcomes are not available. In this study, HIV and related parameters were not associated with CTRP levels, with the exception of the nominally significant positive association between history of AIDS and CTRP9.
CTRP9 is the paralog with the closest homology to adiponectin,16 but showed no correlation with adiponectin in our sample. In experimental studies, CTRP9 exhibits insulin-sensitizing properties,16 and is a potent inducer of endothelial relaxation.7 Clinical studies of individuals without HIV have shown inconsistent associations of CTRP9 with metabolic traits,38–40 although higher levels of CTRP9 were linked to angiographic coronary artery disease (CAD) and inflammatory marker levels.40 We did not detect any associations of CTRP9 with metabolic or cardiovascular phenotypes, and the observed association of AIDS history with higher CTRP9 is an isolated finding that will require confirmation.
Like CTRP9, none of the remaining CTRPs studied exhibited associations with fasting glucose or HOMA-IR, despite previous experimental or linkage data implicating all three in metabolic regulation.9,10,15,41 Although these adiponectin paralogs did not show associations with carotid distensibility, CTRP5 did exhibit a significant inverse association with CIMT, with similar, but only nominally significant, inverse associations observed for CTRP1 and CTRP3. These results come against a background of conflicting experimental and clinical findings. One study found CTRP5 levels to be higher in patients with, than without, in-stent restenosis, and to have atherogenic effects in human aortic smooth muscle cells.42 However, another study using tissue samples from children showed that although TNF-α upregulated CTRP5 gene expression in adipocytes, it downregulated its expression in endothelial cells, suggesting that higher local concentrations in the endothelium could be protective.41
In turn, CTRP1 has antithrombotic properties43 and was found to prevent vascular smooth muscle proliferation following arterial injury.12 On the contrary, CTRP1 levels have been shown to be elevated in CAD, and the paralog was documented to promote atherogenesis in mice.13 As for CTRP3, the findings accord with its anti-inflammatory properties, although one previous study found higher,44 and another lower, levels in patients with CAD.45
CIMT has been independently associated with a risk of future atherosclerotic CVD.46–48 The present findings suggest that higher levels of CTRP5—and possibly CTRP1 and CTRP3—may bear a salutary relationship with the vasculature specifically in the context of HIV in middle-aged, mostly minority women. Whether CTRP5's emergence as the foremost paralog associated with CIMT, compared with the more questionable associations for CTRP1 and CTRP3, reflects its closer relationship with underlying biologic processes, either as a marker or causal factor, or is instead a consequence of higher assay precision, will require further study. However, the underlying mechanisms driving circulating levels of these paralogs are complex, and substantial additional work will be required to unravel their biology.
Limitations
Among the study's limitations is its cross-sectional design, which does not allow inferences regarding direction or causality. Assessment of fat distribution relied on anthropometry, which is less precise than imaging-based measurements. CTRPs were measured using ELISA, without accounting for the possibility of CTRP multimers, which might have a different biologic effect. In the case of CTRP5 and especially CTRP3, the ELISAs had marked variability, acting to bias the associations toward the null. Given the known stability of peptides to long-term freezing at −70°C,49 the variability in these never-thawed specimens more likely reflects assay imprecision than specimen degradation. That we did detect associations for these peptides with CIMT despite such variability suggests that, at least for CTRP5, the signal is real, and supports the need for additional work to refine measurement of these factors. Last, the data in our study were collected in 2004–2005 and do not necessarily apply to present clinical management.
Conclusions
In summary, in this sample of middle-aged, mostly minority, women with and without HIV, HIV seropositivity was associated with higher levels of adiponectin, with a suggestion that this was also the case for lower CD4 count among women with HIV. Low adiponectin was associated with glucose dysregulation, whereas the adiponectin paralog CTRP5 displayed an inverse association with CIMT. Similar nominal associations with CIMT were observed for CTRP1 and CTRP3. Given the increased susceptibility of women with HIV to metabolic dysregulation and CVD, these findings provide impetus for further investigation to identify the role of CTRPs in these disorders.
Supplementary Material
Acknowledgments
Data in this article were collected by the WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I—WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Presented as an abstract at the Endocrine Society Annual Meeting, Chicago, IL, March 2018.
Author Disclosure Statement
J.R.K. reports stock ownership in Amgen, Bristol Myers Squibb, Gilead Sciences, Johnson & Johnson, Merck, and Pfizer. M.J.G. receives research support for his institution from Gilead Sciences. All other authors have nothing to disclose.
Funding Information
This study was funded by NIH grant number 3U01 AI035004-25S1. J.R.K. was supported by NIH grant number K24 HL135413 and R01 HL132794. D.B.H. was supported by NIH grant number K01 HL137557. R.C.K. was supported by NIH grants R01 HL126543, R01 HL132794, R01 HL095140, and 1R01 HL083760. Q.Q. was supported by K01 HL129892.
Supplementary Material
References
- 1. Kizer JR: Adiponectin, cardiovascular disease, and mortality: Parsing the dual prognostic implications of a complex adipokine. Metabolism 2014;63:1079–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kizer JR, Benkeser D, Arnold AM, et al. : Total and high-molecular-weight adiponectin and risk of coronary heart disease and ischemic stroke in older adults. J Clin Endocrinol Metab 2013;98:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kizer JR, Benkeser D, Arnold AM, et al. : Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: The Cardiovascular Health Study. Circulation 2012;126:2951–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Licinio J, Caglayan S, Ozata M, et al. : Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults Proc Natl Acad Sci U S A 2004;101:4531–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF: Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008;416:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, et al. : Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 2009;23:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng Q, Yuan Y, Yi W, et al. : C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol 2011;31:2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kopp A, Bala M, Buechler C, et al. : C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010;151:5267–5278 [DOI] [PubMed] [Google Scholar]
- 9. Peterson JM, Wei Z, Wong GW: C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 2010;285:39691–39701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han S, Park JS, Lee S, et al. : CTRP1 protects against diet-induced hyperglycemia by enhancing glycolysis and fatty acid oxidation. J Nutr Biochem 2016;27:43–52 [DOI] [PubMed] [Google Scholar]
- 11. Peterson JM, Aja S, Wei Z, Wong GW: CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem 2012;287:1576–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanemura N, Shibata R, Ohashi K, et al. : C1q/TNF-related protein 1 prevents neointimal formation after arterial injury. Atherosclerosis 2017;257:138–145 [DOI] [PubMed] [Google Scholar]
- 13. Lu L, Zhang RY, Wang XQ, et al. : C1q/TNF-related protein-1: An adipokine marking and promoting atherosclerosis. Eur Heart J 2016;37:1762–1771 [DOI] [PubMed] [Google Scholar]
- 14. Wang XQ, Liu ZH, Xue L, et al. : C1q/TNF-related protein 1 links macrophage lipid metabolism to inflammation and atherosclerosis. Atherosclerosis 2016;250:38–45 [DOI] [PubMed] [Google Scholar]
- 15. Lei X, Rodriguez S, Petersen PS, et al. : Loss of CTRP5 improves insulin action and hepatic steatosis. Am J Physiol Endocrinol Metab 2016;310:E1036–E1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seldin MM, Tan SY, Wong GW: Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 2014;15:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan RC, Hanna DB, Kizer JR: Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep 2016;13:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hadigan C, Kattakuzhy S: Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrinol Metab Clin North Am 2014;43:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tillerson K: Explaining racial disparities in HIV/AIDS incidence among women in the U.S.: A systematic review. Stat Med 2008;27:4132–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Nunez AE, An Y, et al. : Burden of cardiovascular disease among multi-racial and ethnic populations in the United States: An update from the National Health Interview Surveys. Front Cardiovasc Med 2014;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ketlogetswe KS, Post WS, Li X, et al. : Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. AIDS 2014;28:901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freitas P, Carvalho D, Santos AC, et al. : Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis 2014;14:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kosmiski LA, Bacchetti P, Kotler DP, et al. : Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab 2008;93:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS: Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab 2003;88:627–636 [DOI] [PubMed] [Google Scholar]
- 25. Tong Q, Sankale JL, Hadigan CM, et al. : Regulation of adiponectin in human immunodeficiency virus-infected patients: Relationship to body composition and metabolic indices. J Clin Endocrinol Metab 2003;88:1559–1564 [DOI] [PubMed] [Google Scholar]
- 26. Dolan SE, Hadigan C, Killilea KM, et al. : Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005;39:44–54 [DOI] [PubMed] [Google Scholar]
- 27. Sharma A, Ma Y, Scherzer R, et al. : Brief report: Association of adipokines with bone mineral density in HIV-infected and HIV-uninfected women. J Acquir Immune Defic Syndr 2016;73:433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bacon MC, von Wyl V, Alden C, et al. : The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan RC, Kingsley LA, Gange SJ, et al. : Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008;22:1615–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 31. Hodis HN, Mack WJ, Lobo RA, et al. : Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;135:939–953 [DOI] [PubMed] [Google Scholar]
- 32. Seaberg EC, Benning L, Sharrett AR, et al. : Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke 2010;41:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN: Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001;154:185–193 [DOI] [PubMed] [Google Scholar]
- 34. Tien PC, Schneider MF, Cox C, et al. : Association of HIV infection with incident diabetes mellitus: Impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr 2012;61:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levey AS, Stevens LA, Schmid CH, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuller LH, Tracy R, Belloso W, et al. : Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huck DM, Hanna DB, Rubin LH, et al. : Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. J Acquir Immune Defic Syndr 2018;78:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolf RM, Steele KE, Peterson LA, et al. : C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J Clin Endocrinol Metab 2016;101:2211–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hwang YC, Woo Oh S, Park SW, Park CY: Association of serum C1q/TNF-related protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int J Obes (Lond) 2014;38:1207–1212 [DOI] [PubMed] [Google Scholar]
- 40. Moradi N, Fadaei R, Emamgholipour S, et al. : Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS One 2018;13:e0192159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartze JT, Landgraf K, Spielau U, et al. : Adipocyte C1QTNF5 expression is BMI-dependently related to early adipose tissue dysfunction and systemic CTRP5 serum levels in obese children. Int J Obes (Lond) 2017;41:955–963 [DOI] [PubMed] [Google Scholar]
- 42. Shen Y, Li C, Zhang RY, et al. : Association of increased serum CTRP5 levels with in-stent restenosis after coronary drug-eluting stent implantation: CTRP5 promoting inflammation, migration and proliferation in vascular smooth muscle cells. Int J Cardiol 2017;228:129–136 [DOI] [PubMed] [Google Scholar]
- 43. Lasser G, Guchhait P, Ellsworth JL, et al. : C1qTNF-related protein-1 (CTRP-1): A vascular wall protein that inhibits collagen-induced platelet aggregation by blocking VWF binding to collagen. Blood 2006;107:423–430 [DOI] [PubMed] [Google Scholar]
- 44. Wang S, Ling Y, Liang W, Shen L: Association of serum C1q/TNF-related protein-3 (CTRP-3) in patients with coronary artery disease. BMC Cardiovasc Disord 2017;17:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fadaei R, Moradi N, Baratchian M, et al. : Association of C1q/TNF-related protein-3 (CTRP3) and CTRP13 serum levels with coronary artery disease in subjects with and without type 2 diabetes mellitus. PLoS One 2016;11:e0168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE: Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997;96:1432–1437 [DOI] [PubMed] [Google Scholar]
- 47. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M: Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007;115:459–467 [DOI] [PubMed] [Google Scholar]
- 48. Eikendal AL, Groenewegen KA, Anderson TJ, et al. : Common carotid intima-media thickness relates to cardiovascular events in adults aged <45 years. Hypertension 2015;65:707–713 [DOI] [PubMed] [Google Scholar]
- 49. Sakkinen P, Abbott RD, Curb JD, Rodriguez BL, Yano K, Tracy RP: C-reactive protein and myocardial infarction. J Clin Epidemiol 2002;55:445–451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.