Abstract
Gait speed declines at a faster rate in persons with HIV (PWH) than in the general population but the risk factors associated with this decline are not well understood. In the AIDS Clinical Trials Group (ACTG) A5322 (HAILO, HIV Infection, Aging, and Immune Function Long-term Observational Study), an observational cohort study of PWH ≥40 years of age, those who developed slow gait during the first 3 years of follow-up were compared with persons who maintained normal speed. Associations with demographic and clinical covariates were assessed using multivariable logistic regression. Of 929 participants, 81% were men, 31% Black, and 20% Hispanic. Median age was 51 years [interquartile range (IQR) = 46–56]. At study entry, 92% had plasma HIV RNA <50 copies/mL with median CD4 count 631 cells/mm3 (IQR = 458–840). At study entry, 7% of participants had slow gait, 16% had neurocognitive impairment (NCI), and 12% had diabetes. Over 3 years, 87% maintained normal gait speed, 3% maintained a slow gait, 6% developed a slow gait, and 4% improved from slow to normal gait speed. In multivariable models, hemoglobin A1C (HbA1C) percentage, per one unit increase [odds ratio (OR) = 1.36; 95% confidence interval (CI) = 1.03–1.81; p = .033], NCI (OR = 3.47; 95% CI = 1.57–7.69 p = .002), and black versus white race (OR = 2.45; 95% CI = 1.08–5.59; p = .032) at entry were significantly associated with development of slow gait compared with those maintaining normal gait speed. The association between baseline HbA1C and development of slow gait speed highlights an intervenable target to prevent progression of physical function limitations.
Keywords: gait speed, hemoglobin A1C, neurocognitive impairment, aging
Introduction
Although routine use of virally suppressive combination antiretroviral therapy (ART) has dramatically extended the life expectancy of persons with HIV (PWH), this population now experiences both earlier onset and higher rates of chronic noninfectious aging-related comorbidities than the general population.1–3 Compared with seronegative controls, PWH exhibit an accelerated decline in physical function and higher than expected rates of frailty.4–6 Such declines in functional status have been associated with poor health outcomes such as disability, falls, and reduced quality of life in this population.7–10
Slow gait speed is a simple, easily obtainable measure in the clinical setting that requires minimal equipment, training, and time.11 Slow gait speed is highly predictive of disability and mortality in older adults,12–14 and we have similarly found that slow gait speed is associated with subsequent poor outcomes including disability, falls, and mortality in middle-aged PWH.15 Schrack et al. previously observed that gait speed declines more rapidly with increasing age in men with HIV compared with demographically similar men without HIV.5 Proposed mechanisms for this accelerated decline in gait speed among PWH include those observed with general aging, such as changes in energy utilization and body composition, decreased aerobic capacity, and worsened biomechanics16–19 and characteristics specific to HIV infection, including exposure to ART, prolonged immune suppression, chronic inflammation and immune activation, and mitochondrial dysfunction.20–23 Multiple studies among PWH have found associations between decreased gait speed and increasing age, socioeconomic factors (such as nonwhite race, less education, public insurance, and social support), obesity, lower physical activity, tobacco or intravenous drug use, certain mitochondrial DNA haplogroups, and medical comorbidities [particularly renal disease, peripheral neuropathy, neurocognitive impairment (NCI), and diabetes].21,24–30 However, many of these studies have been limited to cross-sectional analyses.
The goal of this investigation was to identify baseline characteristics associated with development of slow gait speed among participants currently enrolled in AIDS Clinical Trial Group (ACTG) A5322/HAILO (HIV Infection, Aging, and Immune Function Long-term Observational Study). This cohort of both men and women has well-defined ART exposure and detailed history of immune and clinical health. Through investigation of this diverse and well-characterized cohort, we aim to better understand risk factors for slow gait to guide strategies to screen for and prevent functional impairment among persons aging with HIV.
Methods
Study population
We performed a longitudinal analysis of participants in ACTG A5322/HAILO, an ongoing observational cohort study of older men and women with HIV who initiated ART through an ACTG clinical trial and were previously followed in the observational study ACTG A5001. Between November 2013 and July 2014, a subset of A5001 participants (n = 1,035) ≥40 years old were enrolled in HAILO for continued long-term follow-up. All participants underwent semiannual visits including fasting laboratory tests and annual evaluations of frailty and neurocognitive function. HAILO participants who had more than one gait speed measurement through the first 3 years of follow-up were included in this analysis.
Outcome
Gait speed was measured as part of the Fried frailty assessment. Slow gait was defined by the average of two readings on a 4-m walk: men ≤173 cm and women ≤159 cm in height who required ≥6.22 s or men >173 cm and women >159 cm who required ≥5.33 s to complete the walk met the criteria for slow gait.31
Covariates
Baseline covariates included age, nadir CD4+ T lymphocyte count (CD4), history of AIDS-defining illness, CD4, HIV-1 RNA viral load, alcohol use (none, light, moderate, or heavy drinking), body mass index (BMI), waist circumference (elevated if >102 cm in men, >88 cm in women),32 physical activity (≥3 days moderate/vigorous activity in the last week vs. <3 days), peripheral neuropathy, cigarette use (never, former, and current), substance use within the past month, and use of antidepressant/anxiolytic medication. The presence of any comorbidity was considered as a single covariate. Comorbidities assessed included cardiovascular disease (myocardial infarction, stroke, and coronary artery disease), liver disease, kidney disease, malignancy (within the past 5 years), diabetes [diagnosis or hemoglobin A1C (HbA1C) ≥6.5%], hypertension (diagnosis or use of antihypertensive medications), and hepatitis C (positive HCV antibody or diagnosis). NCI was assessed using the A5001 NeuroScreen that includes Trailmaking tests A and B and the Wechsler Adult Intelligence Scale-Revised Digit Symbol tests.33 The NeuroScreen was previously validated by comparison with a comprehensive neuropsychological battery.34 NCI was defined by at least one z-score that was at least 2 standard deviations (SDs) below the mean or multiple z-scores that were at least 1 SD below the mean on separate tests within the A5001 NeuroScreen. Antiretroviral exposures examined were integrase strand transfer inhibitors, tenofovir disoproxil (TDF), efavirenz, and protease inhibitors at initial randomization and at HAILO entry as well as any previous exposure to didanosine (DDI), stavudine (D4T), or zidovudine (AZT).
Statistical analysis
Participants were categorized into four groups based on their gait speed over time: persistently normal, persistently slow, development of slow gait, or resolution of slow gait. Participants who developed slow gait were compared with those who maintained normal gait speed, and those who had resolution of slow gait with those who had persistently slow gait, by demographic, clinical, and behavioral characteristics. Chi-square tests were used for categorical variables and the Kruskal–Wallis test for continuous variables.
Logistic regression models were fit to assess associations between baseline covariates and development of slow gait (vs. being stable normal) and, separately, a return to normal speed (vs. being stable slow). Age was forced into all models, as it is strongly associated with gait speed. Each of the covariates defined above were included separately in the age-adjusted models, and those that were significantly associated with the outcome (p < .1) were included in the final, multivariable models. A two-sided 5% significance level was used for all analyses.
We also fit a series of age-adjusted models stratified by diabetes status to determine if effects of HbA1C were independent of diabetes status, and by sex and race/ethnicity, to determine if sex differences primarily explained by differences in race/ethnicity. Covariates were added to each model as described previously.
Results
Of 929 participants included in this analysis, the median age at baseline was 51 years [interquartile range (IQR) = 46–56]. Eighty-one percent were men, 31% black, and 20% Hispanic. Most individuals had well-controlled HIV infection at study entry: 92% had undetectable plasma HIV-1 RNA (viral load <50 copies/mL) with median CD4 631 cells/mm3 (IQR = 458–840). At study entry, 7% of participants had slow gait.
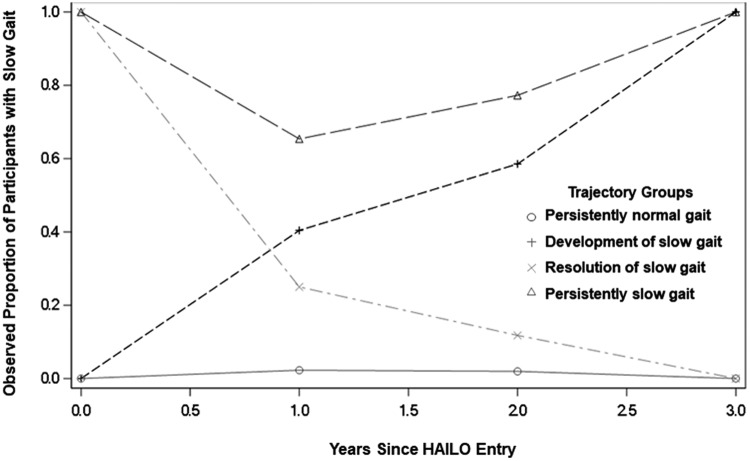
Over the 3 years of observation, 87% maintained normal gait speed, 3% maintained a slow gait, 6% developed a slow gait, and 4% improved from slow to normal gait speed. The prevalence of each gait speed trajectory group is given in Figure 1. Median time to complete the 4-m walk in each gait speed trajectory group at baseline and at each year of follow-up in the study is given in Supplementary Table S1. Characteristics of participants by gait speed trajectory group are given in Table 1. In brief, age, sex, race, HbA1C, BMI, waist circumference, level of physical activity, peripheral neuropathy, alcohol and illicit drug use, use of anxiolytic or antidepressant medications, TDF use at HAILO study entry, neurocognitive function, presence of kidney or liver disease, hypertension, and diabetes differed across groups. Between HAILO participants included in and excluded from this analysis, there were no significant differences in age; sex; HIV virologic control; current or nadir CD4; ART treatment history; BMI; reported physical activity; or use of alcohol, cigarettes, other substance use, or antidepressants or anxiolytics. Black non-Hispanic, white non-Hispanic, and Hispanic participants were more likely to be included in the analysis than Asian, Pacific Islander, and American Indian, Alaskan native participants. In addition, excluded participants had a higher median HbA1C (5.6 vs. 5.5, p = .01).
FIG. 1.
Prevalence of slow gait within each gait speed trajectory group. Over 3 years of observation, 87% of HAILO participants maintained normal gait speed, 3% maintained a slow gait speed, 6% developed a slow gait, and 4% improved from a slow to a normal gait speed. HAILO, HIV Infection, Aging, and Immune Function Long-term Observational Study.
Table 1.
Demographic and Clinical Characteristics by Gait Speed Trajectory Categories
| Persistently normal gait | Development of slow gait | Resolution of slow gait | Persistently slow gait | ||
|---|---|---|---|---|---|
| Characteristic | (n = 813) | (n = 52) | (n = 38) | (n = 26) | p |
| Age (years) | |||||
| Median (Q1–Q3) | 50 (46 to 56) | 51 (44 to 57) | 52 (47 to 55) | 56 (52 to 61) | .006 |
| Female sex | 140 (17%) | 19 (36%) | 8 (21%) | 10 (38%) | <.001 |
| Race/ethnicity | |||||
| White non-Hispanic | 423 (52%) | 13 (25%) | 14 (37%) | 7 (27%) | <.001 |
| Black non-Hispanic | 224 (28%) | 28 (54%) | 18 (47%) | 15 (58%) | |
| Hispanic (regardless of race) | 166 (20%) | 11 (21%) | 6 (16%) | 4 (15%) | |
| Less than high school education | 111 (14%) | 12 (23%) | 7 (18%) | 5 (19%) | .21 |
| Alcohol | |||||
| Abstainer | 299 (37%) | 28 (54%) | 24 (63%) | 19 (73%) | <.001 |
| Light drinker | 314 (39%) | 13 (25%) | 9 (24%) | 5 (19%) | |
| Moderate drinker | 57 (7%) | 3 (6%) | 0 (0%) | 0 (0%) | |
| Heavy drinker | 143 (18%) | 8 (15%) | 5 (13%) | 2 (8%) | |
| Cigarette use | |||||
| Never | 330 (41%) | 20 (38%) | 17 (45%) | 7 (27%) | .47 |
| Former | 268 (33%) | 14 (27%) | 11 (29%) | 10 (38%) | |
| Current | 193 (24%) | 18 (35%) | 10 (26%) | 9 (35%) | |
| Illicit substance use within the past month | 182 (22%) | 7 (13%) | 4 (11%) | 3 (12%) | .049 |
| Physical activity | |||||
| <3 days of both vigorous/moderate activity | 343 (42%) | 24 (46%) | 26 (68%) | 19 (73%) | <.001 |
| ≥3 days of vigorous/moderate activities | 429 (53%) | 25 (48%) | 12 (32%) | 6 (23%) | |
| Anti-anxiety or depression medications | 254 (31%) | 16 (31%) | 19 (50%) | 17 (65%) | <.001 |
| HbA1C (%) | |||||
| Median (Q1–Q3) | 5.50 (5.20 to 5.80) | 5.60 (5.20 to 6.00) | 5.60 (5.40 to 6.10) | 6.00 (5.40 to 6.40) | .011 |
| BMI (kg/m2) | |||||
| Median (Q1–Q3) | 27.1 (24.1 to 30.5) | 28.3 (25.4 to 32.2) | 27.8 (24.8 to 31.1) | 30.7 (25.7 to 36.3) | .025 |
| High waist circumference | 266 (33%) | 25 (48%) | 18 (47%) | 18 (69%) | <.001 |
| NCI | 108 (13%) | 16 (31%) | 12 (32%) | 8 (31%) | <.001 |
| NPZ3 score | |||||
| Median (Q1–Q3) | 0.40 (−0.30 to 1.07) | 0.03 (−1.12 to 0.87) | −0.13 (−1.00 to 0.97) | −0.38 (−1.10 to 0.60) | <.001 |
| Peripheral neuropathy | 314 (39%) | 27 (52%) | 23 (61%) | 17 (65%) | <.001 |
| Frailty | 18 (2%) | 3 (6%) | 17 (45%) | 19 (73%) | <.001 |
| Comorbidities | |||||
| Cardiovascular disease | 48 (6%) | 5 (10%) | 0 (0%) | 3 (12%) | .17 |
| Liver disease | 7 (1%) | 1 (2%) | 3 (8%) | 0 (0%) | .001 |
| Kidney disease | 69 (8%) | 10 (19%) | 8 (21%) | 9 (35%) | <.001 |
| History of cancer within past 5 years | 27 (3%) | 3 (6%) | 2 (5%) | 0 (0%) | .53 |
| Diabetes | 93 (11%) | 8 (15%) | 6 (16%) | 8 (31%) | .022 |
| Hypertension | 300 (37%) | 26 (50%) | 16 (42%) | 15 (58%) | .045 |
| Stroke | 15 (2%) | 2 (4%) | 0 (0%) | 2 (8%) | .12 |
| Hepatitis C | 92 (11%) | 6 (12%) | 8 (21%) | 6 (23%) | .097 |
| HIV-specific | |||||
| Any AIDS-defining conditions | 165 (20%) | 9 (17%) | 9 (24%) | 4 (15%) | .82 |
| Nadir CD4 count (cells/μL) | |||||
| Median (Q1–Q3) | 198 (63 to 300) | 154 (620 to 275) | 160 (61 to 318) | 264 (113 to 322) | .33 |
| <200 | 409 (50%) | 31 (60%) | 20 (53%) | 10 (38%) | .66 |
| 200–350 | 263 (32%) | 14 (27%) | 12 (32%) | 12 (46%) | |
| >350 | 141 (17%) | 7 (13%) | 6 (16%) | 4 (15%) | |
| CD4 count (cells/μL) | |||||
| Median (Q1–Q3) | 620 (463 to 838) | 678 (420 to 828) | 648 (419 to 906) | 677 (511 to 837) | .85 |
| Undetectable VL (<50 copies/mL) | 748 (92%) | 47 (90%) | 34 (89%) | 25 (96%) | .78 |
| Antiretrovirals | |||||
| ART use at HAILO entry | |||||
| EFV | 274 (34%) | 15 (29%) | 12 (32%) | 4 (15%) | .23 |
| INSTI | 184 (23%) | 12 (23%) | 9 (24%) | 7 (27%) | .96 |
| TDF | 634 (78%) | 40 (77%) | 28 (74%) | 14 (54%) | .036 |
| PI | 18 (2%) | 0 (0%) | 1 (3%) | 2 (8%) | .20 |
| ART use at randomization | |||||
| EFV | 295 (36%) | 20 (38%) | 17 (45%) | 10 (38%) | .75 |
| INSTI | 90 (11%) | 8 (15%) | 3 (8%) | 3 (12%) | .72 |
| TDF | 398 (49%) | 28 (54%) | 14 (37%) | 11 (42%) | .37 |
| PI | 461 (57%) | 24 (46%) | 20 (53%) | 13 (50%) | .43 |
| Still on initial randomized therapy at HAILO entry | 307 (38%) | 20 (38%) | 12 (32%) | 12 (46%) | .70 |
| Any exposure to DDI/D4T/AZT | 294 (36%) | 21 (40%) | 20 (53%) | 10 (38%) | .21 |
Chi-square tests were used for categorical variables and the Kruskal–Wallis test for continuous variable was used to assess differences in demographic and clinical characteristics between gait speed trajectory groups. Characteristics were measured at baseline unless otherwise indicated.
ART, antiretroviral therapy; AZT, zidovudine; BMI, body mass index; CD4, CD4+ T lymphocyte count; D4T, stavudine; DDI, didanosine; EFV, efavirenz; HAILO, HIV Infection, Aging, and Immune Function Long-term Observational Study; HbA1C, hemoglobin A1C; INSTI, integrase strand transfer inhibitor; NCI, neurocognitive impairment; PI, protease inhibitor; TDF, tenofovir disoproxil; VL, viral load.
Characteristics associated with development of slow gait
In the age-adjusted bivariate models, the development of slow gait was associated with female sex, black versus white race, Hispanic ethnicity versus white race, 12th grade and higher education, higher HbA1C percentage, higher BMI, elevated waist circumference, no alcohol versus light alcohol use, lower NPZ3 score, NCI, and peripheral neuropathy (Supplementary Table S2). Each of these covariates was included in the multivariable model.
In the multivariable model (Table 2), development of slow gait was associated with increase in baseline HbA1C percentage, per one unit increase [odds ratio (OR) = 1.36; 95% confidence interval (CI) = 1.03–1.81; p = .033], presence of NCI (OR = 3.47; 95% CI = 1.57–7.69; p = .002), and black versus white race (OR = 2.45; 95% CI = 1.08–5.59; p = .033). When the models were restricted to nondiabetic participants, development of slow gait was associated with NCI (OR = 4.14; 95% CI = 1.94–8.84; p < .001) and black race (OR = 2.56; 95% CI = 1.12–5.81; p = .025) but not HbA1C (age-adjusted OR = 1.24; 95% CI = 0.58–2.63; p = .57). In contrast, a multivariable model restricted to diabetic participants showed an association between HbA1C and development of slow gait (OR = 1.50; 95% CI = 0.99–2.27; p = .054).
Table 2.
Factors Associated with Gait Speed Change in Multivariable Models
| Characteristic at HAILO entry | Development of slow gait (OR, 95% CI) | Resolution of slow gait (OR, 95% CI) |
|---|---|---|
| NCI vs. no NCI | 3.47 (1.57–7.69); p = .0022 | |
| Age (years) | 1.01 (0.97–1.06); p = .57 | 0.92 (0.86–0.98); p = .013 |
| Heavy alcohol drinker vs. abstainer | 0.72 (0.27–1.89); p = .50 | |
| Light alcohol drinker vs. abstainer | 0.56 (0.26–1.2); p = .14 | |
| Moderate alcohol drinker vs. abstainer | 0.68 (0.15–3.13); p = .62 | |
| BMI (kg/m2) | 1.00 (0.93–1.07); p = .95 | 0.94 (0.84–1.04); p = .22 |
| Education (≥12 years vs. <12 years) | 0.70 (0.29–1.71); p = .43 | |
| HbA1C (per 1% increase) | 1.36 (1.03–1.81); p = .033 | |
| Waist circumference (high vs. normal) | 1.63 (0.65–4.07); p = .23 | 0.61 (0.16–2.41); p = .48 |
| Peripheral neuropathy | 1.59 (0.82–3.12); p = .17 | |
| Black, non-Hispanic vs. white, non-Hispanic race | 2.45 (1.08–5.59); p = .033 | |
| Hispanic vs. white, non-Hispanic race | 1.11 (0.38–3.20); p = .85 | |
| Female vs. male sex | 1.14 (0.49–2.65); p = .76 |
Covariates with p < 0.05 are italicized and in bold.
Characteristics were measured at baseline unless otherwise indicated.
CI, confidence interval; OR, odds ratio.
Sex and race/ethnicity-stratified models were also fit. Among men, the odds of developing slow gait were associated with NCI (OR = 3.69; 95% CI = 1.37–9.94; p = .01), HbA1C (OR = 1.58; 95% CI = 1.15–2.16; p = .004), and a marginal association with greater BMI (OR = 1.07; 95% CI = 0.99–1.16; p = .079). In women, only NCI (OR = 4.06, 95% CI = 1.36–12.2; p = .012) was associated with development of slow gait. In a multivariable model restricted to black non-Hispanic participants, the odds of developing slow gait were lower for those with at least a high school education (OR = 0.22; 95% CI = 0.07–0.73; p = .014) and greater with higher HbA1C (OR = 1.51; 95% CI = 1.07–2.14; p = .018). Among Hispanic participants, development of slow gait was associated with NCI (OR = 6.74; 95% CI = 1.62–28.10; p = .009) and high waist circumference (OR = 4.27; 95% CI = 1.10–16.5; p = .035). In this group, older age tended to be associated with greater odds of developing slow gait, although this was not statistically significant (OR = 1.07; 95% CI = 0.99–1.16; p = .09). No baseline characteristics were significantly associated with development of slow gait among white, non-Hispanic participants.
Characteristics associated with resolution of slow gait
The age-adjusted bivariate model (Supplementary Table S2) showed that resolution of slow gait was inversely associated with higher BMI and elevated waist circumference; these factors were included in the final, multivariable model. In the multivariable model (Table 2), age was the only factor associated with improvement from slow to normal gait speed, with older individuals less likely to improve (OR = 0.92 per year; 95% CI = 0.86–0.98; p = .013). In nondiabetic participants, no factors examined were significantly associated with resolution of slow gait in the multivariable model. Among participants with diabetes, resolution of slow gait was significantly associated with being physically active (OR = 26.0; 95% CI = 1.29–520; p = .033) and younger age (OR = 0.82 per year increase; 95% CI = 0.68–0.97; p = .024).
In the sex-stratified model of men alone, increasing age (OR = 0.90; 95% CI = 0.83–0.98; p = .011) and BMI (OR = 0.88; 95% CI = 0.79–0.99; p = .03) were inversely associated with resolution of slow gait. Among women, no characteristics were associated with improvement in slow gait. In a model restricted to black non-Hispanic participants, resolution of slow gait was inversely associated only with older age (OR = 0.85; 95% CI = 0.76–0.95; p = .003). Among Hispanics, older age also tended to be associated with lower odds of resolution of slow gait, although this was not statistically significant (OR = 0.88, 95% CI = 0.76–1.02; p = .09). No baseline characteristics were significantly associated with resolution of slow gait among white, non-Hispanic participants.
Discussion
Gait speed declines more rapidly with age among PWH than in HIV-uninfected individuals, but a longitudinal assessment of characteristics related to this functional outcome have previously been limited. In this large cohort of men and women living with HIV, we found that development of slow gait speed was significantly associated with higher HbA1C, NCI, and black race. These findings reveal a modifiable risk factor (HbA1C) and vulnerable populations (individuals who have NCI or black race) that may benefit from early screening and intervention to preserve physical function in PWH.
Associations between impaired neurologic and physical function have been noted among older populations of people with and without HIV.35,36 Previous cross-sectional analysis in PWH have shown a relationship between slowed gait and more advanced AIDS dementia complex stage.30 Structural changes in brain regions involved in both cognitive and motor function may provide a mechanistic explanation for this relationship. Both gait and cognition are vulnerable to impaired communication between brain regions in long white matter tracts. A small cross-sectional study of men and women (average age 50.6 years) in the Hawaii Aging with HIV cohort showed volumetric changes in brain regions (cerebellar white matter and subcortical gray matter) linked to cognition and motor control.37 In a previous cross-sectional analysis of the HAILO cohort, we reported an association between impaired cognitive function and gait speed.25 This study adds longitudinal evidence from this large, well-characterized cohort of PWH, where we similarly find that NCI is strongly associated with development of slow gait.
Similarly, abnormal glucose metabolism has been associated with gait speed decline in the general population.38–42 Decreased muscle quality and strength and mitochondrial dysfunction have been identified as potential causes of this relationship.43,44 Among diabetic participants, inflammation has been linked to increased risk of functional decline.45 Among men with HIV compared with seronegative controls, inflammation and insulin resistance have previously been associated with frailty, a complex phenotype of which gait speed is a key component.20 We found that the association between increased HbA1C and development of slow gait was most apparent among diabetic participants. These results suggest that individuals with both diabetes and HIV represent a particularly high-risk group for development of functional impairment.
In addition, studies of the general population have noted a complex interplay between insulin resistance and cognitive and physical dysfunction.46 Hyperglycemia, insulin resistance, and hyperinsulinemia have been associated both with frailty and cognitive performance in HIV cohorts.20,47,48 A recent study of MACS participants showed HIV and diabetes interact to produce white matter changes and that the extent of these structural alterations was significantly associated with psychomotor speed.49 Previous studies have shown that cerebral white matter disease links these three characteristics. White matter T2/FLAIR hyperintensities and abnormal diffusion tensor imaging are present in conditions such as cerebral microvascular disease and HIV.50,51 Because of the relatively low incidence of marked slowness as defined for this cohort, we were not sufficiently powered to detect interactions between A1C and NCI and the development of impaired gait speed. Additional analyses as this cohort continues to age will help to further characterize these relationships.
Our findings suggest that strategies to prevent and treat hyperglycemia may help avert functional impairment in PWH. Pharmacologic interventions, including metformin, to improve glucose control have shown some promise in elderly individuals with diabetes.52–54 However, the risks and benefits of such therapies must be carefully considered. Because of an increased risk of hypoglycemia, intensive glucose treatment may not be appropriate for aging PWH who are vulnerable to other geriatric syndromes such as polypharmacy and falls.55–57 Lifestyle interventions may help prevent insulin resistance in PWH and lead to improved glucose control and functional outcomes in individuals with known diabetes.58,59 Dietary and exercise interventions to thwart excess weight gain and reduce obesity are essential to prevent development of diabetes and physical limitations.60 In addition to weight benefits, exercise-based interventions promote physical function in older diabetic and nondiabetic participants alike.29,61–63 Indeed, in our analysis of participants with diabetes, physical activity was significantly associated with resolution of slow gait. This finding suggests that physical activity is a key intervention to maintain gait speed in older PWH, particularly those with diabetes.
In this study, we also found that black race had a significant association with development of slow gait. A previous analysis of gait trajectories among participants in the Multicenter AIDS Cohort Study also found significantly faster declines in nonwhite men with and at risk for HIV.5 Higher rates of impaired physical function among older individuals of black race have been previously noted in the general aging population.64,65 These differences are incompletely understood but have been linked to increased disease burden and socioeconomic inequalities.66 Indeed, increased education (completion of high school or beyond) was protective against development of slow gait among black participants, suggesting that socioeconomic disparity may in part be driving this difference. Furthermore, among black participants in this study, increasing HbA1C was significantly associated with development of slow gait, suggesting that black PWH should be prioritized for early screening and interventions for insulin resistance.
Several limitations of this study must be acknowledged. Although the HAILO cohort is diverse, its participants have been continuously enrolled in ACTG trials and observational studies since at least 2009. As a result, they may be more adherent and not be fully representative of the general population of older PWH. In addition, women remain underrepresented in this cohort and only account for 19% of participants included in this study. Similarly, the restricted number of participants in each subgroup analysis limits the power of our findings. As such, our preliminary study did not allow for more complex analysis of the relationship between NCI and gait speed. Without a control group of participants who do not have HIV, it remains unclear whether our findings are specific to or accentuated among PWH. Furthermore, there are limitations in obtaining some of the characteristics considered in this analysis. Minor differences in gait speed measurement may have persisted across sites, although all sites receive regular training and quality control procedures. Physical activity was assessed by self-report, which may be unreliable. Although the 4-m gait speed test is a useful screening tool, it is an acute stressor test and does not capture physiologic reserve and exercise tolerance in the setting of prolonged stressor conditions such as the 400-m walk test. Finally, the A5001 Neuroscreen is a limited assessment of neurocognitive function and may not capture all cognitive domains affected in participants.
The growing number of older PWH are vulnerable to poor health outcomes such as disability and reduced quality of life.7,22,67 Targeted interventions to preserve physical function and independence and promote resiliency is imperative in this population. As decline in gait speed was associated with increased HbA1C, NCI, and black race, we have identified those at the highest risk for physical function impairment who may benefit most from screening, prevention, and early intervention. Moreover, the association between baseline HbA1C and development of slow gait speed highlights an intervenable target to prevent progression of physical function limitations.
Supplementary Material
Acknowledgment
The authors thank the ACTG A5322 HAILO Study Team.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1 AI068634, UM1 AI068636, UM1 AI106701, and NIAID K24 120834 (to T.T.B), the National Institute of Aging K32 63008845 and R01 AG0504366 (to K.M.E); and National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007169 (to M.C.M.).
Supplementary Material
References
- 1. Deeks SG: HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hasse B, Ledergerber B, Furrer H, et al. : Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–1139 [DOI] [PubMed] [Google Scholar]
- 3. Smit M, Brinkman K, Geerlings S, et al. : Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis 2015;15:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desquilbet L, Jacobson LP, Fried LP, et al. : HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62:1279–1286 [DOI] [PubMed] [Google Scholar]
- 5. Schrack JA, Althoff KN, Jacobson LP, et al. : Accelerated longitudinal gait speed decline in HIV-infected older men. J Acquir Immune Defic Syndr 2015;70:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrack JA, Jacobson LP, Althoff KN, et al. : Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS 2016;30:2645–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johs NA, Wu K, Tassiopoulos K, et al. : Disability among middle-aged and older persons with human immunodeficiency virus infection. Clin Infect Dis 2017;65:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma A, Hoover DR, Shi Q, et al. : Longitudinal study of falls among HIV-infected and uninfected women: The role of cognition. Antivir Ther 2018;23:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tassiopoulos K, Abdo M, Wu K, et al. : Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS 2017;31:2287–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB: Relationship of physical function and quality of life among persons aging with HIV infection. AIDS 2014;28:1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Middleton A, Fritz SL, Lusardi M: Walking speed: The functional vital sign. J Aging Phys Act 2015;23:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cesari M, Kritchevsky SB, Penninx BW, et al. : Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2005;53:1675–1680 [DOI] [PubMed] [Google Scholar]
- 13. Guralnik JM, Ferrucci L, Pieper CF, et al. : Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Studenski S, Perera S, Patel K, et al. : Gait speed and survival in older adults. JAMA 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erlandson KM, Perez J, Abdo M, et al. : Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin Infect Dis 2019;68:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrucci L, Bandinelli S, Benvenuti E, et al. : Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 17. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L: The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc 2012;60:1811–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L: Rising energetic cost of walking predicts gait speed decline with aging. J Gerontol A Biol Sci Med Sci 2016;71:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB: Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erlandson KM, Ng DK, Jacobson LP, et al. : Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis 2017;215:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun J, Brown TT, Samuels DC, et al. : The role of mitochondrial DNA variation in age-related decline in gait speed among older men living with human immunodeficiency virus. Clin Infect Dis 2018;67:778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB: Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep 2014;11:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran T, Guardigni V, Pencina KM, et al. : Atypical skeletal muscle profiles in human immunodeficiency virus-infected asymptomatic middle-aged adults. Clin Infect Dis 2018;66:1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berner K, Morris L, Baumeister J, Louw Q: Objective impairments of gait and balance in adults living with HIV-1 infection: A systematic review and meta-analysis of observational studies. BMC Musculoskelet Disord 2017;18:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erlandson KM, Wu K, Koletar SL, et al. : Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017;215:933–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauer LO, Wu Z, Wolfson LI: An obese body mass increases the adverse effects of HIV/AIDS on balance and gait. Phys Ther 2011;91:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McInerney PA, Ncama BP, Wantland D, et al. : Quality of life and physical functioning in HIV-infected individuals receiving antiretroviral therapy in KwaZulu-Natal, South Africa. Nurs Health Sci 2008;10:266–272 [DOI] [PubMed] [Google Scholar]
- 28. Richert L, Brault M, Mercie P, et al. : Decline in locomotor functions over time in HIV-infected patients. AIDS 2014;28:1441–1449 [DOI] [PubMed] [Google Scholar]
- 29. Safeek RH, Hall KS, Lobelo F, et al. : Low levels of physical activity among older persons living with HIV/AIDS are associated with poor physical function. AIDS Res Hum Retroviruses 2018;34:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson KR, Parsons TD, Sidtis JJ, et al. : Timed Gait test: Normative data for the assessment of the AIDS dementia complex. J Clin Exp Neuropsychol 2006;28:1053–1064 [DOI] [PubMed] [Google Scholar]
- 31. Fried LP, Tangen CM, Walston J, et al. : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 32. Klein S, Allison DB, Heymsfield SB, et al. : Waist circumference and cardiometabolic risk: A consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007;30:1647–1652 [DOI] [PubMed] [Google Scholar]
- 33. Robertson KR, Smurzynski M, Parsons TD, et al. : The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921 [DOI] [PubMed] [Google Scholar]
- 34. Ellis RJ, Evans SR, Clifford DB, et al. : Clinical validation of the NeuroScreen. J Neurovirol 2005;11:503–511 [DOI] [PubMed] [Google Scholar]
- 35. Atkinson HH, Rosano C, Simonsick EM, et al. : Cognitive function, gait speed decline, and comorbidities: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–850 [DOI] [PubMed] [Google Scholar]
- 36. Rosso AL, Verghese J, Metti AL, et al. : Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology 2017;89:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kallianpur KJ, Sakoda M, Gangcuangco LM, et al. : Frailty characteristics in chronic HIV patients are markers of white matter atrophy independently of age and depressive symptoms: A Pilot Study. Open Med J 2016;3:138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Rekeneire N, Resnick HE, Schwartz AV, et al. : Diabetes is associated with subclinical functional limitation in nondisabled older individuals: The Health, Aging, and Body Composition study. Diabetes Care 2003;26:3257–3263 [DOI] [PubMed] [Google Scholar]
- 39. Gregg EW, Beckles GL, Williamson DF, et al. : Diabetes and physical disability among older U.S. adults. Diabetes Care 2000;23:1272–1277 [DOI] [PubMed] [Google Scholar]
- 40. Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL: Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: Results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc 2013;61:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuo CK, Lin LY, Yu YH, Wu KH, Kuo HK: Inverse association between insulin resistance and gait speed in nondiabetic older men: Results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002. BMC Geriatr 2009;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okoro CA, Zhong Y, Ford ES, Balluz LS, Strine TW, Mokdad AH: Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: A cross-sectional analysis. BMC Public Health 2006;6:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park SW, Goodpaster BH, Strotmeyer ES, et al. : Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes 2006;55:1813–1818 [DOI] [PubMed] [Google Scholar]
- 44. Phielix E, Mensink M: Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav 2008;94:252–258 [DOI] [PubMed] [Google Scholar]
- 45. Figaro MK, Kritchevsky SB, Resnick HE, et al. : Diabetes, inflammation, and functional decline in older adults: Findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care 2006;29:2039–2045 [DOI] [PubMed] [Google Scholar]
- 46. Umegaki H, Makino T, Uemura K, et al. : The Associations among insulin resistance, hyperglycemia, physical performance, diabetes mellitus, and cognitive function in relatively healthy older adults with subtle cognitive dysfunction. Front Aging Neurosci 2017;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang J, Jacobson LP, Becker JT, et al. : Impact of glycemic status on longitudinal cognitive performance in men with and without HIV infection. AIDS 2018;32:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khuder SS, Chen S, Letendre S, et al. : Impaired insulin sensitivity is associated with worsening cognition in HIV-infected patients. Neurology 2019;92:e1344–e1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu M, Fatukasi O, Yang S, et al. : HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 2018;32:1803–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Su T, Wit FW, Caan MW, et al. : White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS 2016;30:2329–2339 [DOI] [PubMed] [Google Scholar]
- 51. Levett TJ, Cresswell FV, Malik MA, Fisher M, Wright J: Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc 2016;64:1006–1014 [DOI] [PubMed] [Google Scholar]
- 52. Aro AK, Karjalainen M, Tiihonen M, et al. : Glycemic control and health-related quality of life among older home-dwelling primary care patients with diabetes. Prim Care Diabetes 2017;11:577–582 [DOI] [PubMed] [Google Scholar]
- 53. Rizzo MR, Barbieri M, Fava I, et al. : Sarcopenia in elderly diabetic patients: Role of dipeptidyl peptidase 4 inhibitors. J Am Med Dir Assoc 2016;17:896–901 [DOI] [PubMed] [Google Scholar]
- 54. Laksmi PW, Setiati S, Tamin TZ, et al. : Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: A double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med Indones 2017;49:118–127 [PubMed] [Google Scholar]
- 55. Guaraldi G, Malagoli A, Calcagno A, et al. : The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: A cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr 2018;18:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M, Moreno G, Mangione CM, Kimbro L, Vaisberg E: Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim TW, Walley AY, Ventura AS, et al. : Polypharmacy and risk of falls and fractures for patients with HIV infection and substance dependence. AIDS Care 2018;30:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yarasheski KE, Cade WT, Overton ET, et al. : Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab 2011;300:E243–E251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fitch KV, Anderson EJ, Hubbard JL, et al. : Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS 2006;20:1843–1850 [DOI] [PubMed] [Google Scholar]
- 60. Duncan AD, Goff LM, Peters BS: Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One 2018;13:e0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Byrne H, Caulfield B, De Vito G: Effects of self-directed exercise programmes on individuals with type 2 diabetes mellitus: A systematic review evaluating their effect on HbA1c and other metabolic outcomes, physical characteristics, cardiorespiratory fitness and functional outcomes. Sports Med 2017;47:717–733 [DOI] [PubMed] [Google Scholar]
- 62. Allet L, Armand S, de Bie RA, et al. : The gait and balance of patients with diabetes can be improved: A randomised controlled trial. Diabetologia 2010;53:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montoya JL, Jankowski CM, O'Brien KK, et al. : Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 2019;33:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koster A, Penninx BW, Bosma H, et al. : Is there a biomedical explanation for socioeconomic differences in incident mobility limitation? J Gerontol A Biol Sci Med Sci 2005;60:1022–1027 [DOI] [PubMed] [Google Scholar]
- 65. Thorpe RJ, Jr., Koster A, Kritchevsky SB, et al. : Race, socioeconomic resources, and late-life mobility and decline: Findings from the Health, Aging, and Body Composition study. J Gerontol A Biol Sci Med Sci 2011;66:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Louie GH, Ward MM: Socioeconomic and ethnic differences in disease burden and disparities in physical function in older adults. Am J Public Health 2011;101:1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leveille SG, Thapa S: Disability among persons aging with HIV/AIDS. Interdiscip Top Gerontol Geriatr 2017;42:101–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.