Abstract
Background and Objective: The ideal hemostatic agent for laparoscopic partial nephrectomy (LPN) would provide complete hemostasis and sealing of the collecting system at a low cost. Chitosan (CS) is an established topical hemostatic agent, but standard sterilization techniques affect its functional and biologic properties, thereby preventing parenteral uses. This study sought to characterize the safety and efficacy of an implanted CS hemostat sterilized with either a standard technique, electron beam (e-beam) irradiation, or a novel technique, nonthermal nitrogen plasma, in a porcine LPN model.
Methods: Laparoscopic partial nephrectomies were performed on six farm pigs and hemostasis achieved using only a CS hemostatic agent (Clo-Sur P.A.D.) that was e-beam (n = 3) or plasma sterilized (PS) (n = 3). Number of pads needed to achieve hemostasis, estimated blood loss, operative time, mass of kidney resection, and warm ischemia time were measured. Animals were monitored for 14 weeks and at harvest, retrograde ureteropyelography and histologic analysis were performed.
Results: Complete hemostasis and collection system sealing were achieved in both groups. There was a trend toward less pads required for hemostasis (p = 0.056) and reduced blood loss (p = 0.096) with PS pads, although this did not achieve statistical significance. No complications were observed for 14 weeks and gross examination showed the implanted CS was encapsulated in a fibrous capsule. Histologic analysis revealed a healed nephrectomy site with residual CS and associated chronic inflammation, reactive fibrosis, and foreign body giant cell formation. Importantly, the adjacent renal tissue was intact and viable with no residual parenchymal inflammation or cytologic damage.
Conclusion: CS pads alone provided safe and effective hemostasis in a porcine LPN model. PS may enhance hemostatic efficacy and resorption compared with e-beam.
Keywords: chitosan, plasma sterilization, hemostasis, hemostatic agents, nonthermal nitrogen plasma, laparoscopic partial nephrectomy, LPN, electron beam, e-beam
Introduction
Partial nephrectomy has become the treatment of choice for small clinical T1 tumors (<7 cm) in patients who are candidates for nephron-sparing surgery.1–4 Laparoscopic partial nephrectomy (LPN), including robot-assisted LPN, is the preferred approach to partial nephrectomy because of comparable oncologic outcomes and reduced morbidity.5,6 Achieving hemostasis during LPN is technically challenging and may result in higher rates of bleeding and urine leak compared with open partial nephrectomy.6–8 Current methods to achieve hemostasis and sealing of the collecting system in LPN include electrocautery, harmonic scalpel, suturing, argon beam coagulation, and hemostatic agents. The ideal hemostatic agent for LPN has yet to be identified.9
In this feasibility study, a chitosan (CS) hemostatic agent (Clo-Sur P.A.D.™; Scion Biomedical, Inc., Miami, FL) was evaluated in a hypertensive porcine survival model of LPN. Although CS has an established safety profile in humans—including in urologic procedures—and is an effective topical hemostatic agent, it is yet to be used as an implantable hemostat because of concerns regarding pyrogen contamination and adverse alterations to the material caused by conventional sterilization methods.10–18
To create a completely sterile form of CS that remains highly active as a hemostatic agent, a novel technique for nonthermal nitrogen plasma (NtNP) sterilization was conceived and tested. Plasma is considered the fourth state of matter, after solid, liquid, and gas, and has emergent properties that gases do not, such as quasineutrality, collective behavior, and controlled motion by electromagnetism.19 The mechanism(s) by which NtNP kills microbes, and inactivates spores, viruses, and pyrogens, is debated, but the leading hypothesis is that ultraviolet radiation (UV), reactive chemical species, and physical etching all play a role.20 Plasma is used widely in a number of industries, but has only recently been introduced to the medical field as a dry decontamination technique.21
Thus, this study sought to characterize the hemostatic efficacy of a lyophilized CS hemostatic pad in LPN when sterilized by a conventional method—electron beam irradiation—or a novel NtNP method.
Materials and Methods
Materials
Lyophilized foam-like CS pads that are currently manufactured for topical control of bleeding and infection control were obtained (Clo-Sur P.A.D.). Both electron beam-sterilized (ES) and unsterilized pads were obtained. The unsterilized CS pads were sterilized for 30 minutes with NtNP using the IoN 40 plasma system (PVA-TePla America, Inc., Corona, CA). Per standard practice in the sterilization field, the sterilization process was validated by placing spore strips with 5.5 × 106 Bacillus atrophaeus and Geobacillus stearothermophilus spores (Steris Corporation, Mentor, OH) in the IoN 40 with the CS pads and subsequently culturing the spores and pads with tryptic soy agar for 48 to 72 hours at 37°C and 58°C. Colony-forming units were quantitated with the minimum sterility assurance level considered six log.
Animals and surgeries
All animal procedures in this study were carried out in accordance with the 1996 National Research Council's “Guide for the Care and Use of Laboratory Animals.” After approval by the Loma Linda University Institutional Animal Care and Use Committee, six female farm pigs (S&S Farms, Santee, CA) were randomized into two treatment groups. In group 1, ES CS pads were used to control hemostasis during porcine LPN, whereas NtNP-sterilized (plasma-sterilized or PS) pads were employed in group 2.
Procedure
After a 4-day acclimation, animals were sedated with intramuscular telazole (0.5 mg/kg), intubated, and placed in the right lateral decubitus position. General anesthesia was maintained with isoflurane or sevoflurane (3%–5%). The animal was then sterilely prepped, draped, and a Veress insufflation was performed with pneumoperitoneum maintained at 15 mm Hg. Four 12 mm ports were placed in a diamond configuration. After mobilization of the bowel, the renal artery was dissected and clamped using an Aesculap small curved laparoscopic bulldog clamp (Aesculap, Inc., Center Valley, PA).
The lower pole of the left kidney was then removed with sharp dissection. The collecting system was entered in one animal from each treatment group. The CS pads, which are foam-like, were rolled and placed inside the Aesculap bulldog clamp removal tool and then passed down a 12-mm port, and applied to the freshly cut surface of the kidney (Fig. 1). At least 2 pads (4 cm × 4 cm) were applied to the cut surface of each kidney before bulldog clamp removal. If bleeding was observed after removing the bulldog clamp, then additional pads were applied until hemostasis was achieved. Epinephrine (1 μg through intravenous [IV] bolus per animal) was administered intravenously after bulldog clamp removal and initial hemostasis was achieved—to artificially raise the blood pressure to more closely mimic the higher mean arterial pressures in humans and to stress the CS pads—while pressure was applied to the CS pads using the laparoscopic instruments. Resected kidney was placed in a pouch and removed after morcellation through the 12-mm camera port. In four of six animals (two in each group), peritoneum was pulled over the kidney and closed using nonabsorbable hemo-clips. All ports were closed using a Carter-Thomason device and skin was closed with 4-0 Monocryl subcuticular suture and Dermabond (Ethicon, Somerville, NJ).
FIG. 1.
Deployment of the CS pad to the cut surface of the kidney under warm ischemia. The Clo-Sur P.A.D.™ was furled and passed through the 12 mm port (left, A) before being unfurled and applied to the cut surface of the kidney (right, B). CS = chitosan.
Evaluation of hemostasis
Primary outcomes were time to hemostasis, estimated blood loss (EBL), warm ischemia time (WIT), and number of pads used. Secondary outcomes included operative time, pre- vs postoperative blood counts/chemistries, complications (including pyrogenic responses identified by daily temperature readings for 72 hours postoperatively and before imaging procedures), and histologic abnormalities. Complete abdominal ultrasonographies and blood counts/chemistries were performed immediately postoperatively and on postoperative day 6. Retrograde pyelography was performed immediately before euthanasia on day 98 using a 6F endhole catheter to inject half strength contrast (Omnipaque 300; GE Healthcare, Inc., Little Chalfont, UK). After pyelograms were obtained, animals were sacrificed through IV injection of sodium pentobarbital. The LPN site and implanted CS were grossly examined before harvesting the kidneys and fixing them in 10% neutral buffered formalin.
Histologic analysis
For histology, paraffin tissue blocks were sectioned at 7 μm and mounted on lysine-coated microscope slides. Sections were stained with hematoxylin and eosin (Thermo Fisher Scientific, Waltham, MA) using standard methods and imaged. A board-certified pathologist blinded to the identity of the specimens analyzed each of the stained sections.
Statistical analysis
Data are presented as mean ± standard error of the mean and were analyzed using Student's t-test with p < 0.05 considered statistically significant.
Results
Complete hemostasis was achieved in all six cases using only CS pads and no sutures, cauterization, or other hemostatic agents. Some difficulty was encountered initially with PS pads because of brittleness, but the problem was solved by raising the relative humidity in the packaging of the PS pads.
In comparing surgical outcomes, fewer pads were needed to achieve hemostasis in the PS group than in the ES group, although the difference was not statistically significant (p = 0.056; Table 1). Similarly, there was a nonsignificant trend (p = 0.096) toward reduced bleeding (shown as EBL) in the PS pad group compared with the ES group (Table 1). Notably, two of the PS pad-treated animals had EBL <20 mL and nonhumidified PS pads were used in the one PS pad-treated animal that had increased EBL (110 mL). Other blood tests revealed significantly lower white blood cell counts postoperatively in the PS pad-treated group (9.13 ± 0.73) compared with the preoperative values (13.23 ± 1.47; p < 0.05) and the postoperative ES pad-treated group (17.85 ± 1.39; p < 0.05). No differences were observed between preoperative and postoperative values in the same group or in baseline chemistries between the two groups (Table 2). WITs, mean operative times, and specimen weights were not significantly different between groups (Table 1).
Table 1.
Surgical Data for Porcine Laparoscopic Partial Nephrectomy with Chitosan Hemostatic Agent
| ES pads (n = 3) (mean ± SEM) | PS pads (n = 3) (mean ± SEM) | p | |
|---|---|---|---|
| Number of pads | 3.67 ± 0.33 | 2.5 ± 0.29 | 0.056 |
| EBL (mL) | 201.67 ± 65.09 | 43.33 ± 33.33 | 0.096 |
| WIT (minutes) | 11.14 ± 1.54 | 8.27 ± 0.81 | 0.174 |
| Nephrectomy weight (g) | 6.65 ± 2.37 | 7.56 ± 1.83 | 0.776 |
| Operative time (hours) | 2.49 ± 0.32 | 2.39 ± 0.15 | 0.799 |
EBL = estimated blood loss; ES = electron beam-sterilized; PS = plasma-sterilized; SEM = standard error of the mean; WIT = warm ischemia time.
Table 2.
Blood Chemistry and Cell Counts Between Groups and Pre- vs Postoperatively
| ES pads (n = 3) | PS pads (n = 3) | Between groups, p | |
|---|---|---|---|
| RBC pre | 5.52 ± 0.23 | 6.44 ± 0.27 | >0.05 |
| RBC post | 5.60 ± 0.43 | 6.15 ± 0.63 | >0.05 |
| Hg pre | 8.0 ± 0.44 | 9.0 ± 0.44 | >0.05 |
| Hg post | 8.23 ± 0.48 | 8.23 ± 0.49 | >0.05 |
| HCT pre | 23.18% ± 1.05% | 25.22% ± 1.16% | >0.05 |
| HCT post | 23.67% ± 1.47% | 23.66% ± 1.08% | >0.05 |
| WBC pre | 14.33 ± 1.72 | 13.23 ± 1.47 | >0.05 |
| WBC post | 17.85 ± 1.39* | 9.13 ± 0.73* | <0.05 |
| CRE pre | 1.03 ± 0.07 | 1.0 ± 0.1 | >0.05 |
| CRE post | 0.80 ± 0.0 | 0.93 ± 0.12 | >0.05 |
| ALB pre | 3.80 ± 0.44 | 3.73 ± 0.13 | >0.05 |
| ALB post | 3.70 ± 0.153 | 3.77 ± 0.30 | >0.05 |
| BUN pre | 5.0 ± 1.0 | 4.33 ± 1.20 | >0.05 |
| BUN post | 5.0 ± 1.0 | 4.33 ± 1.20 | >0.05 |
Bold values are statistically significant as indicated by the *.
p < 0.05 for pre- vs postoperative comparison.
ALB = albumin; BUN = blood urea nitrogen; CRE = creatinine; pre = preoperative; post = postoperative; HCT = hematocrit; Hg = hemoglobin; RBC = red blood cell count; WBC = white blood cell count.
One ES pad-treated animal, with intentional collecting system breach, demonstrated signs of minor uroabdomen through ultrasound and paracentesis at 1 week and 30 days postoperatively. However, blood values remained normal and signs of overt toxicity or other complications—as assessed through twice daily observations/examinations by qualified veterinary staff—were absent throughout the postoperative period. The PS pad-treated animal with intentional collecting system breach exhibited minor hematuria for 5 days postoperatively, but hematocrit levels remained stable. Neither excess abdominal fluid nor urine leak was observed in any animals through ultrasonography and retrograde ureteropyelography, respectively, at 14 weeks (Fig. 2). There was no evidence of infection or pyrogenic responses in any animals up to 14 weeks after CS implantation.
FIG. 2.
Retrograde ureteropyelography. Representative pyelograms performed 14 weeks post-LPN of the right (A, C) and left (B, D) ureters and calices from both ES pad (A, B) and PS pad (C, D)-treated animals are shown. Urinary leakage was not observed in any animals. ES = electron beam-sterilized; LPN = laparoscopic partial nephrectomy; PS = plasma-sterilized.
Figure 3 shows representative examples from both treatment groups of the CS pads in relation to the left kidney intraoperatively (A and D), in situ at sacrifice (B and E), and after removal from the body and longitudinal sectioning (C and F). Gross examination at sacrifice suggested modest breakdown of the pads in both treatment groups with pads appearing as yellow masses with a paste-like consistency (Fig. 3B, E). The implanted CS appeared to be encapsulated (Fig. 3C, F).
FIG. 3.
Intraoperative, postmortem in situ, and ex vivo sectioned views of the CS pads. (A–C) show representative views of the ES pads and (D–F) show representative views of the PS pads from one animal each. (A, D) show the effect of epinephrine administration on hemostasis where epinephrine was given to the animal shown in (A), but not to the animal in (D). Note the appearance of the CS implant after 98 days in (B, C, E, F).
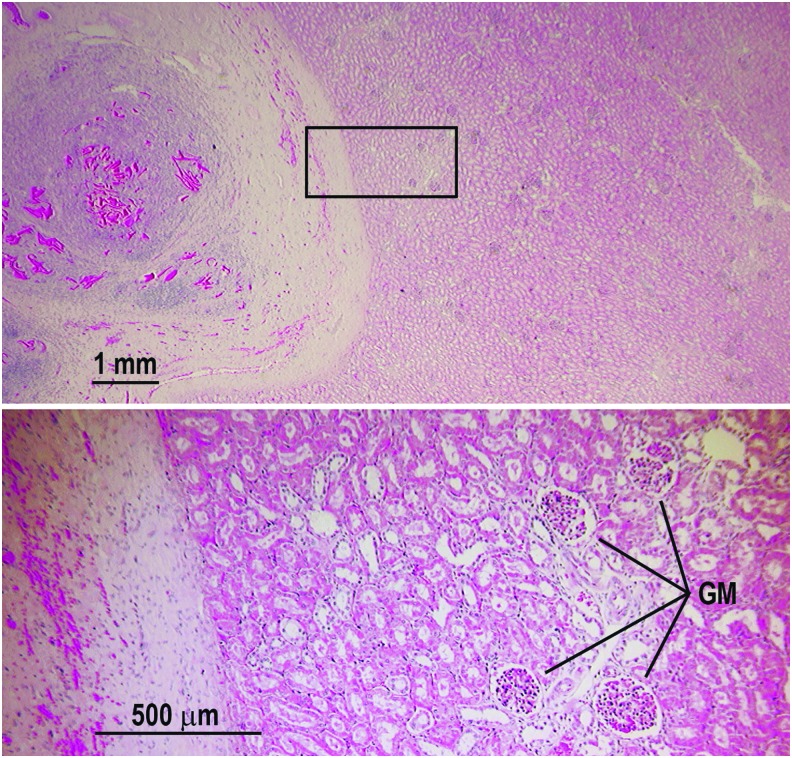
Histologic analysis demonstrated a well-healed nephrectomy site in all six animals (Fig. 4). The underlying renal parenchyma was intact and viable with normal morphology and no evidence of persistent parenchymal inflammation or cytologic damage. Residual CS was present at the nephrectomy site and associated with chronic inflammation, reactive fibrosis, and foreign body giant cell formation. Multinucleated giant cells displayed various stages of CS resorption (Fig. 5). Implanted PS CS was primarily filamentous, whereas implanted ES CS formed large amorphous collections that resisted cellular infiltration (Fig. 6). The ES CS did induce foreign body giant cell formation at its periphery, but was accompanied by a more robust lymphocytic infiltrate occasionally forming reactive germinal centers.
FIG. 4.
Viable normal-appearing renal parenchyma adjacent to the healed nephrectomy site. Upper panel shows the representative appearance of a kidney at the nephrectomy site 98 days postoperation and CS hemostasis. The site of nephrectomy is to the left showing residual CS surrounded by reactive fibrosis and chronic inflammation. The bottom panel is a magnified view of the boxed region in the upper panel showing normal appearing GM and tubules. No residual inflammation or damage was found. Similar findings were observed in all of the nephrectomized animals studied. GM = glomeruli.
FIG. 5.
Foreign body giant cell reaction to CS. (A) Various stages of CS (bright magenta material) resorption are illustrated; one multinucleated giant cell appears to have nearly completed its task with only fine granular material remaining (asterisk), whereas others appear to be in the early (triangle and octagon) to middle stages (diamond) of resorption. (B) Multiple sites of crater-like erosions (arrows) are evident; active giant cells are seen in this higher magnification view.
FIG. 6.
Histologic difference in CS following different sterilization methods. (A) NtNP sterilization (PS) yields a filamentous CS (triangle) that allows cellular infiltration (upwards white arrow). (B) While ES generates an amorphous material (triangle) that is resistant to cellular invasion (upwards white arrow). There is still giant cell formation at the periphery, but the majority of the CS remains acellular. NtNP = nonthermal nitrogen plasma.
None of the animals showed evidence of infection, hematoma, urinoma, excessive tissue reactivity, or other pathologic abnormalities at the time of harvest. There was also very little evidence of scar formation at the surgical site.
Discussion
This feasibility study showed that a 100% CS pad (Clo-Sur P.A.D.)—marketed as an antimicrobial barrier and hemostatic agent for arterial catheterization—is an effective hemostatic agent in a porcine LPN model. This is the first study to demonstrate that NtNP efficiently sterilizes and depyrogenates CS without adversely affecting its hemostatic properties. Histopathology revealed that PS pads were better infiltrated and produced a more robust giant cell reaction than did the ES pads. Owing to the small sample size in this study, many of the primary endpoints failed to reach statistical significance, but the safety profile of the implanted CS was unequivocal. Regardless of the sterilization technique, the CS pads alone provided complete hemostasis in this porcine model of LPN.
A major complication of NtNP treatment identified in this study was dehydration of the CS, which led to brittleness making it difficult to roll the pad for placement down the laparoscopic ports. Increasing the relative humidity in the packaging not only reduced the brittleness, but also enhanced the malleability of the pads. Since higher relative humidity accelerates and enhances hydrolytic depolymerization of CS within 6 months, alternative methods for increasing the malleability of CS are recommended.22
Intraoperatively, the PS pads demonstrated greater adhesion to the peritoneum and cut renal surface than the ES pads. This enhanced bioadhesivity made unfurling the PS pads more difficult because of increased adherence to the peritoneum. This feature may be beneficial by providing superior hemostasis through increasing adherence to the wound site and subsequently reducing the number of pads required to achieve hemostasis. In accordance with previous porcine LPN studies using a similar CS product from HemCon, Inc., the ES and PS CS pads ultimately sealed the urinary collecting system.23,24
Inducing hypertension through IV epinephrine bolus after applying the pads led to active hemorrhaging through the CS pads in all four animals receiving epinephrine (two each group), but blood loss remained low in these cases, including three with EBL ≤110 mL. It is unclear whether hemostasis was delayed by epinephrine or impaired until a certain blood pressure threshold was reached. Regardless, improving the physical form of the CS, possibly to a fibrous form, may overcome the issue of bleeding through the CS agent at higher blood pressures. Adding substances to the CS that enhance malleability may also enhance its hemostatic efficacy by improving its ease of manipulation and application.
Since pigs have higher levels of blood clotting factors V, VIII, IX, XI, and XII and, therefore, clot faster than humans, CS pads might be insufficient when used alone in human LPN procedures.25 However, the HemCon studies found that CS achieved hemostasis in porcine LPN procedures even with large preoperative doses of heparin, likely because of the fact CS induces hemostasis independent of the clotting cascade.23,24
The HemCon studies also found that residual CS was present at 6-month and 1-year post-LPN. Importantly, the immune response to CS was similar to the reaction induced by Surgical/Tisseel despite the prolonged degradation process.24 This study similarly showed that implanted CS is benign to the kidney parenchyma with an immune response similar to foreign body reactions elicited by commonly used absorbable materials such as suture. However, since residual foreign bodies can induce a prolonged chronic inflammatory response, alterations to the physical or chemical composition of the CS hemostat may be warranted to decrease the time to full resorption.26–28
Although zero ischemia approaches to LPN and robotic partial nephrectomy (RPN) are promising techniques, hemostatic agents will remain a critical tool in the performance of these operations.29 For example, Gill and colleagues report using a surgical bolster with zero ischemia RPN.30 Perhaps a CS bolster would provide superior hemostatic control than the oxidized regenerated cellulose bolsters currently used, making a complicated procedure such as zero ischemia LPN and RPN procedures easier to perform and, therefore, achievable for more surgeons.
This pilot study has limitations. First, the small sample size resulted in a small number of statistically significant findings and small effect sizes. A second limitation is the use of a porcine model with smaller blood vessels and enhanced clotting than humans. However, the use of the hypertensive model improves the adequacy of the model. Third, the fact that the collecting system was only entered in two animals limits the ability of this study to determine whether CS alone would adequately seal the collecting system. However, the agreement of our results with a previous study showing complete sealing of the collecting system with a similar CS hemostat bolsters this conclusion. Fourth, only one sacrifice time-point limits our understanding of the tissue and inflammatory responses to the implanted CS. Finally, the lack of a control arm in which hemostasis is achieved by only holding pressure or using the current standard of care limits the ability to determine the true effectiveness of the CS. Again, this limitation is partially overcome with the agreement of the results of this study with those of the HemCon study. In sum, this study provides sufficient evidence to support additional studies of PS CS as a hemostatic agent for use in LPN procedures and provide proof-of-concept data for the efficacy of PS and depyrogenation of CS. Several alterations to the CS may enhance its efficacy for LPN such as larger, yet thinner pad sizes, use as a bolster, or even a different physical or chemical form.
Conclusion
A 100% CS pad is an effective hemostatic agent for LPN procedures and NtNP effectively sterilizes and depyrogenates CS while preserving or potentially enhancing its hemostatic properties. Implantation of the CS pad, regardless of sterilization technique, did not produce complications and elicited tissue reactivity characteristic of foreign body implants. Active resorption of the CS pads was observed after 14 weeks with PS pads showing greater foreign body giant cell formation than ES pads. Future studies to optimize NtNP-sterilized CS use for LPN are warranted.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under SBIR award number R43CA186374. This grant was awarded to Scion Cardio-Vascular, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors express their gratitude to Audomaro Flores, Zachary Downs, and Dr. David Wolf for their assistance with the animal studies. The authors also thank Jackie Knecht and Louis Rose for administrative assistance. The authors are grateful to Demetri Chrysostomou, Michael Barden, Luke Turalitsch, Eduardo Mateo, and James Bond for assistance with the IoN40 plasma instrument and to Jim Shen and Salim Cheriyan for assistance with retrograde ureteropyelography.
Abbreviations Used
- ALB
albumin
- BUN
blood urea nitrogen
- CRE
creatinine
- CS
chitosan
- e-beam
electron beam
- EBL
estimated blood loss
- ES CS
electron beam-sterilized chitosan
- GM
glomeruli
- HCT
hematocrit
- Hg
hemoglobin
- LPN
laparoscopic partial nephrectomy
- NtNP
nonthermal nitrogen plasma
- PS CS
plasma-sterilized chitosan
- RBC
red blood cell count
- RPN
robotic partial nephrectomy
- SEM
standard error of the mean
- WBC
white blood cell count
- WIT
warm ischemia time
Author Disclosure Statement
A.C., S.H., and W.K. are board members and shareholders of Karamedica, Inc., a private for-profit company developing plasma machines for sterilizing and depyrogenating biopolymers and chitosan for parenteral uses. They assumed these roles in Karamedica, Inc. after this study was completed. They are also inventors on multiple issued and pending patents related to plasma depyrogenation and clinical uses of chitosan. All other authors have no relevant conflicts of interest to report.
Funding Information
No funding was received for this article.
References
- 1. Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med 2010;362:624–634 [DOI] [PubMed] [Google Scholar]
- 2. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000;163:730–736 [PubMed] [Google Scholar]
- 3. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-Year followup. J Urol 2000;163:442–445 [PubMed] [Google Scholar]
- 4. Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000;75:1236–1242 [DOI] [PubMed] [Google Scholar]
- 5. Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol 2007;177:70–74; discussion 4 [DOI] [PubMed] [Google Scholar]
- 6. Dominguez-Escrig JL, Vasdev N, O'Riordon A, Soomro N. Laparoscopic partial nephrectomy: Technical considerations and an update. J Minim Access Surg 2011;7:205–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aron M, Gill IS. Minimally invasive nephron-sparing surgery (MINSS) for renal tumours part I: Laparoscopic partial nephrectomy. Eur Urol 2007;51:337–346; discussion 46–47 [DOI] [PubMed] [Google Scholar]
- 8. Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178:41–46 [DOI] [PubMed] [Google Scholar]
- 9. Breda A, Stepanian SV, Lam JS, et al. Use of haemostatic agents and glues during laparoscopic partial nephrectomy: A multi-institutional survey from the United States and Europe of 1347 cases. Eur Urol 2007;52:798–803 [DOI] [PubMed] [Google Scholar]
- 10. Whang HS, Kirsch W, Zhu YH, Yang CZ, Hudson SM. Hemostatic agents derived from Chitin and Chitosan. J Macromol Sci C 2005;45:309–323 [Google Scholar]
- 11. Ulański P, Rosiak J. Preliminary studies on radiation-induced changes in chitosan. Int J Radiat Appl Instrum C Radiat Phys Chem 1992;39:53–57 [Google Scholar]
- 12. Dutkiewicz J, Judkiewicz L, Papiewski A, Kucharska M, Ciszewski R, eds. Some uses of krill chitosan as a biomaterial. Proceedings of the Fourth International Conference on Chitin and Chitosan, Trondheim, Norway, 1989 [Google Scholar]
- 13. Franca R, Mbeh DA, Samani TD, Le Tien C, Mateescu MA, Yahia L, Sacher E. The effect of ethylene oxide sterilization on the surface chemistry and in vitro cytotoxicity of several kinds of chitosan. J Biomed Mater Res B Appl Biomater 2013;101:1444–1455 [DOI] [PubMed] [Google Scholar]
- 14. Lim LY, Khor E, Koo O. Gamma irradiation of chitosan. J Biomed Mater Res 1998;43:282–290 [DOI] [PubMed] [Google Scholar]
- 15. Lim LY, Khor E, Ling CE. Effects of dry heat and saturated steam on the physical properties of chitosan. J Biomed Mater Res 1999;48:111–116 [DOI] [PubMed] [Google Scholar]
- 16. Marreco PR, da Luz Moreira P, Genari SC, Moraes AM. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J Biomed Mater Res B Appl Biomater 2004;71:268–277 [DOI] [PubMed] [Google Scholar]
- 17. Rao SB, Sharma CP. Sterilization of chitosan: Implications. J Biomater Appl 1995;10:136–143 [DOI] [PubMed] [Google Scholar]
- 18. Porpiglia F, Manfredi M, Checcucci E, et al. Use of chitosan membranes after nerve-sparing radical prostatectomy improves early recovery of sexual potency: Results of a comparative study. BJU Int 2018;123:465–473 [DOI] [PubMed] [Google Scholar]
- 19. Chen FF. Introduction to plasma physics and controlled fusion, 3rd Ed. Switzerland: Springer International Publishing, 2016, pp 1–17 [Google Scholar]
- 20. Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 2001;226:1–21 [DOI] [PubMed] [Google Scholar]
- 21. Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann JL. Plasma medicine: An introductory review. New J Phys 2009;11:115012 [Google Scholar]
- 22. Viljoen JM, Steenekamp JH, Marais AF, Kotze AF. Effect of moisture content, temperature and exposure time on the physical stability of chitosan powder and tablets. Drug Dev Ind Pharm 2014;40:730–742 [DOI] [PubMed] [Google Scholar]
- 23. Xie H, Khajanchee YS, Shaffer BS. Chitosan hemostatic dressing for renal parenchymal wound sealing in a porcine model: Implications for laparoscopic partial nephrectomy technique. JSLS 2008;12:18–24 [PMC free article] [PubMed] [Google Scholar]
- 24. Xie H, Lucchesi L, Teach JS, Virmani R. Long-term outcomes of a chitosan hemostatic dressing in laparoscopic partial nephrectomy. J Biomed Mater Res B Appl Biomater 2012;100:432–436 [DOI] [PubMed] [Google Scholar]
- 25. Roussi J, Andre P, Samama M, Pignaud G, Bonneau M, Laporte A, Drouet L. Platelet functions and haemostasis parameters in pigs: Absence of side effects of a procedure of general anaesthesia. Thromb Res 1996;81:297–305 [DOI] [PubMed] [Google Scholar]
- 26. Anderson JM. Biological responses to materials. Annu Rev Mater Res 2001;31:81–110 [Google Scholar]
- 27. Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997;18:567–575 [DOI] [PubMed] [Google Scholar]
- 28. Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005;26:5872–5878 [DOI] [PubMed] [Google Scholar]
- 29. Lamoshi AY, Salkini MW. Off-clamp robotic partial nephrectomy: Technique and outcome. Urol Ann 2015;7:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gill IS, Eisenberg MS, Aron M, et al. “Zero ischemia” partial nephrectomy: Novel laparoscopic and robotic technique. Eur Urol 2011;59:128–134 [DOI] [PubMed] [Google Scholar]