Abstract
Significance: Fractional resurfacing involves producing arrays of microinjuries on the skin, by thermal or mechanical means, to trigger tissue regeneration. Originally developed for cosmetic enhancement, fractional resurfacing induces a broad array of improvements in the structural and functional qualities of the treated skin and is especially effective at returning defective skin to a more normal state. In addition to fascinating questions about the nature of this remarkable regenerative capacity, there may be potential utility in ulcer prevention by halting or even reversing the progressive decline in overall skin quality that usually precedes chronic wound development.
Recent Advances: Photoaging and scarring are the two skin defects most commonly treated by fractional resurfacing, and the treatment produces profound and long-lasting improvements in skin quality, both clinically and at the cellular/histologic level. Chronic wounds usually occur in skin that is compromised by various pathologic factors, and many of the defects found in this ulcer-prone skin are similar to those that have seen improvements after fractional resurfacing.
Critical Issues: The mechanisms responsible for the regenerative capacity of fractional resurfacing are mostly unknown, as is how ulcer-prone skin, which is usually afflicted by stressors external to the skin tissue itself, would respond to fractional resurfacing.
Future Directions: Better understanding of the cellular and molecular mechanisms underlying the unique healing response to fractional resurfacing could reveal fundamental information about adult tissue regeneration, lead to improvements in current applications, as well as new therapies in other pathologic conditions.
Keywords: fractional resurfacing, skin quality, regeneration, scar, chronic wounds
Joshua Tam, PhD.

Scope and Significance
Fractional resurfacing induces improvements in a broad array of structural and functional qualities in the treated skin. Here, we will review the clinical effects of fractional resurfacing, putative mechanisms involved, key unanswered questions, and potential application in chronic wound prevention.
Translational Relevance
Microinjuries induced by fractional resurfacing appear to have a unique regenerative capacity, perhaps best exemplified by their ability to produce dramatic improvements in scar tissue. The cellular processes and molecular mechanisms involved in this rare example of adult tissue regeneration, and its applicability in other defective skin conditions are promising avenues for translational investigations.
Clinical Relevance
Since many options for fractional resurfacing are already clinically available, expanding their utility to other skin defects should be relatively straightforward. Better understanding of the underlying mechanisms could enable more rational selection of treatment modality and parameters, which are currently widely variable and based mostly on the individual practitioner's clinical experience.
Overview
Fractional resurfacing to induce skin remodeling
Outside of early gestation, human skin wounds mostly heal by scarring, which is characterized by disorganized microanatomy, absence of dermal appendages, and myriad other structural and functional defects.1 There is, however, one notable exception to this generalization: small (nominally 500 μm or less in diameter) wounds can regenerate completely, without scarring, even when total tissue loss is substantial—up to 50% of skin tissue within a treatment area can be removed in the form of microinjuries, and the subsequent remodeling process can actually lead to better-quality skin (Fig. 1).2–4 Various clinical modalities have been developed to take advantage of this phenomenon (Fig. 2), whereby arrays of microinjuries are produced by thermal (lasers and radiofrequency) or mechanical (microneedling) means, to induce tissue remodeling via a process known as fractional resurfacing. Our group has also utilized this principle to develop a new technology to harvest autologous skin tissue for wound repair5,6—this technology was the subject of our 2016 WHF 3M Fellowship research, and is now in early stages of clinical use. In this article, we will focus mainly on laser-based treatments, namely ablative fractional laser (AFL) and nonablative fractional laser (NAFL), as these have been studied more extensively than other modalities.
Figure 1.
Size-dependent dichotomy in healing response. Untreated skin tissue is depicted in (A), with keratinocytes shown in brown, dermal collagen fibers shown in pink, and elastin fibers in purple. When skin is injured by a series of nonconnecting small wounds (B), the tissue heals by completely regenerating the composition and architecture of normal tissue and can even bring about improvements in tissue quality (C). In contrast, when the wound is large in any one dimension (even if there is equal or less total tissue loss-(D) depicts a wound that is the exact combination of the two small wounds in (B), with the dotted line denoting where the edges of the two small wounds would be if they remained discrete injuries), the wound heals by scarring (E).
Figure 2.
Current clinical options for producing microinjuries. (A) Untreated skin tissue. (B) Ablative fractional laser: laser microbeam vaporizes a thin column of tissue, and also leaves behind a zone of thermal damage in the adjacent tissue (depicted in purple). (C) Nonablative fractional laser: tissue is heated and denatured in a fractional pattern. (D) Microneedling: piercing injury made by small needles, no tissue is removed. (E) Radiofrequency microneedling: in addition to piercing injury, radiofrequency heating is used to cause thermal damage in the surrounding tissue. (F) Fractional tissue harvesting: small harvesting needles are used to extract tissue, causing tissue loss without thermal damage.
Discussion
Fractional resurfacing is a popular procedure to “rejuvenate” skin by reducing the visible signs of photoaging, such as rhytids and dyschromia,4 but beyond cosmetic enhancements, the treatment also leads to broad improvements in skin quality, including increased dermal thickness, synthesis and remodeling of extracellular matrix (ECM) components, reduced atypical keratinocytes, increased vascularity, improved rete ridge formation, reduced senescent fibroblast population, and restoration of proper response to ultraviolet light.7–9 Fractional resurfacing also has a unique ability to induce dramatic and lasting functional improvements in skin scars, including normalizing scar thickness, reducing pain and pruritus (suggesting resolution of underlying inflammation), increasing tissue pliability and range of motion, and improving overall quality of life.10–14 There have even been anecdotal reports of functional hair follicles and sweat glands regenerating in scars that had been devoid of these structures.15,16
Curiously, fractional resurfacing seems to stimulate different remodeling responses in different defects. In hypertrophic scars consisting of dense, thick, disorganized collagen bundles, AFL induces looser but more organized collagen.14,17,18 Conversely, atrophic scars—characterized by a paucity of collagen leading to depressions in the skin—respond to AFL by thickening of the epidermis and increasing dermal collagen deposition.19 These findings suggest that, rather than inducing a constant set of effects, fractional resurfacing elicits a homeostatic response that returns the tissue to a more normal state. Consistent with this view, healthy skin showed no histological evidence of fractional resurfacing after 3 months2,20 (whereas beneficial effects on scar remodeling persist for years11), and pretreatment of normal skin in a large animal model provided no benefit toward the subsequent surgical scarring response.21
Mechanisms
Despite strong clinical evidence of efficacy in a variety of dermatological conditions, the mechanism by which fractional resurfacing asserts its effects is still unclear. A few studies have detailed the histological and biochemical changes following fractional resurfacing, with particular focus on ECM arrangement, and known regulators of tissue remodeling, such as the transforming growth factor-β (TGFβ) pathway, matrix metalloproteinases (MMPs), and heat shock proteins (HSPs). These findings provide some insights into the potential mechanism of fractional resurfacing, but perturbation studies that could demonstrate which of these components are causal versus correlative are still lacking. It should also be noted that there is little clinical consensus on treatment settings (e.g., microinjury density and energy settings), and these vary widely in the literature. In this discussion, we will disregard specific treatment settings, and simply categorize treatments according to the modality used to produce microinjuries.
Transforming growth factor-β
TGFβ is important for normal wound healing, and a key regulator of fibrosis and scarring as it promotes expression of type I collagen (COL1A1) and tissue inhibitors of metalloproteinases (TIMPs).22 TGFβ is secreted by immune cells during wound healing to stimulate fibroblast differentiation and collagenesis.23 Latent TGFβ is stored in the ECM and can later be activated by matrix turnover, thereby coupling collagenesis to the mechanical properties of the ECM.23 There are three TGFβ isoforms with distinct functions. TGFβ1 and TGFβ2 promote both wound healing and scar formation, while TGFβ3 inhibits scar formation and is considered particularly important for scarless healing of the fetus.24 TGFβ signaling is often suppressed in chronic wounds.25 TGFβ is also an important chemokine for macrophages and neutrophils following acute injury.22
In photodamaged skin, TGFβ is upregulated 3 days post-AFL and expression decreases over 30 days.26 Studies in burn scars using more frequent timepoints found biphasic TGFβ expression after fractional resurfacing. TGFβ1 and TGFβ3 are transiently upregulated 1 h postop.27 TGFβ1 returns to baseline at 24–48 h, while TGFβ3 and TGFβ2 are downregulated compared to baseline.27,28 TGFβ1 and TGFβ3 expression increases again 96 and 168 h postop.27 One study on long-term TGFβ expression, using immunohistochemistry at baseline and 6 months postop, found that staining intensity for TGFβ1 is reduced at 6 months postop compared to pretreatment, and that TGFβ1 is primarily found in fibroblasts and in the ECM.18
Interestingly, neutralization of TGFβ1 and TGFβ2 reduces scarring, but inhibition of just one of these has no effect,29 highlighting the need to understand changes in each TGFβ isoform. However, the three TGFβ isoforms are frequently not distinguished in the literature, and TGFβ2 is often neglected even when individual isoforms are investigated. A better understanding of how each isoform is affected by fractional resurfacing, especially in the long term, is needed.
Heat shock protein
HSPs can be activated by many forms of stress, including heat, trauma, toxins, and hypoxia.30 Classically, HSPs act as chaperones and promote cell survival by correcting stress-induced protein misconformations.30,31 More recently, HSPs have been recognized as regulators of the wound healing response.32 Dysregulation of HSPs is involved in a number of fibrotic conditions and different HSPs can either promote or inhibit TGFβ signaling.33–37 HSPs have also been investigated as potential therapeutic targets for fibrosis, either through inhibition (e.g., HSP90 inhibition reduces hepatic38 and renal fibrosis39) or induction (e.g., HSP70 induction reduces hepatic40 and pulmonary fibrosis41). These findings indicate competing roles for HSPs, and the relative importance of HSPs in fibrosis and wound healing is an area of ongoing research.
HSP47 is a chaperone involved in collagen processing, and its expression correlates with collagen synthesis and fibrosis progression in many organs.33,42 The HSP70 family, primarily consisting of the constitutively expressed HSP73 and inducible HSP72, has also been studied in some detail. In photodamaged skin, AFL causes HSP70 upregulation from 1 h postop through day 14.43 NAFL showed similar results.44 The pattern of HSP expression has been further studied in healthy skin. NAFL caused upregulation of HSP47 and HSP70 in tissue immediately surrounding the microinjury at 1 day postop.2,45 At day 7, HSP47 and HSP70 are expressed inside the treatment zone as well as more diffusely throughout the dermis.45 NAFL and fractional radiofrequency microneedling have both been reported to induce HSP70 expression,46,47 which is severely attenuated in diabetic skin,48 with an expression time course that correlates with delayed healing.49 Long-term upregulation of HSPs has been reported after large-area ablative laser resurfacing.20 If a similar response occurs after fractional resurfacing, it could account for the ongoing remodeling of scars for months after treatment, although experimental confirmation is needed.
Collagen synthesis and degradation
In healthy tissue, there is a balance between collagen synthesis and degradation.50 Tipping that balance either way is pathologic—excessive collagen synthesis leads to hypertrophic scarring, while excessive degradation is characteristic of both skin aging and chronic wounds.51–53
Collagen degradation is commonly estimated through MMP expression, but many factors influence MMP activity—most notably TIMPs and synthesis in an inactive proenzyme form—and the more accurate method for determining collagen degrading activity is zymography54; however, such studies have yet to be performed for laser resurfacing techniques. MMPs are a large group of enzymes with varying activities for the many components of the ECM (collagens, fibronectin, laminin, etc.). In photoaged skin, MMP-1, MMP-3, MMP-9, MMP-10, MMP-11, and MMP-13 are upregulated between day 7 and 14 post-AFL. MMP-1, MMP-3, and MMP-13 expression drops off after day 14, while MMP-9, MMP-10, and MMP-11 expression continues to increase through day 21.55 Similar effects have been reported for other modalities. NAFL caused upregulation of MMP-1, MMP-3, and MMP-9 at day 1 postop, with significant decay at day 7.44 It was also found that MMP-1 and MMP-3 were localized to the treatment zone, while MMP-9 was dispersed throughout the dermis.44 It is likely that there are similar patterns of MMP expression across the modalities, although with varying magnitudes. Far fewer MMPs have been investigated for AFL in scar tissue. One study reported MMP-1 upregulation at 48 h postop and no significant change in MMP-13.28
Collagen synthesis is typically approximated by collagen mRNA levels. In scars, collagen mRNA exhibits a biphasic response to AFL in line with TGFβ expression. Both (COL1A1) and type III collagen (COL3A1) are upregulated at 1 h postop, fall below or near baseline at 24–48 h, then are again upregulated at 168 h.27,28 NAFL in photodamaged skin reduced COL1A1 and COL3A1 at 1 day postop, followed by upregulation from day 14 through 28, with procollagen I protein levels following the same trend.56 Immunohistochemical investigation of NAFL in healthy skin showed gradual increase in COL3A1 staining inside the treatment zone until 1 week postop.2 In a healthy mouse model, AFL caused increased protein levels of COL3A1 from day 3 through 56 postop and COL1A1 from day 28 through 56.57
These findings are not altogether surprising—as with any acute injury, laser resurfacing promotes matrix turnover in the short-term. While the remodeling phase of wound healing can continue for over a year after injury,51 this on its own does not fully explain why there is continued scar regression in the months following treatment. Long-term measurements are required to determine how fractional resurfacing changes the balance between collagen synthesis and degradation.
Apoptosis
Fractional resurfacing causes tissue damage through thermal and/or mechanical injury. Apoptosis is an essential component of the biological responses to tissue damage. Once viewed as a largely cell-autonomous process, there is now increasing understanding that apoptosis has significant impact on the local milieu, inducing proliferation and remodeling of adjacent cells and tissue, at least, in part, via ectopic expression of wingless/integrated (Wnt) and induction of p53.58 Apoptosis plays a key role in wound healing by inducing mitogenic signals that drive regeneration,59,60 but there is currently little data on how apoptosis may be involved in the regenerative effects of fractional resurfacing.61 Apoptosis was observed within 24 h of AFL and steadily declined over 7 days. The authors concluded that apoptosis induces a paracrine cascade that leads to cell proliferation and stimulates stem or progenitor cells to participate in tissue regeneration.61 Another study compared five different laser devices using TUNEL assay. Thermal tissue damage and penetration depth differed widely, as did the extent of apoptosis and necrosis,62 and it is unclear whether clinical outcomes correlate with the extent, location, or distribution of apoptosis. It has been proposed that apoptosis can alter local tissue tension, induce remodeling in nearby tissue, and influence the behavior of neighboring cells through paracrine signaling.58 Given that scars are characterized by increased stiffness that can be substantially improved by fractional resurfacing, apoptosis could play a role in treatment-induced tension relief in fibrotic tissues. Wnt proteins, which are often secreted by apoptotic cells, can regulate the cytoskeleton63 and are also important signaling molecules in epidermal skin stem cells.64 Whether those two mechanisms merely coexist or actually act in concert is unclear.58
Other findings
Where overlap exists in the literature, cytokine expression between the different laser resurfacing modalities is generally similar. The proinflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNFα), and monocyte chemoattractant protein 1 (MCP-1) are all upregulated shortly after treatment. IL-6 and MCP-1 return to baseline in 96 and 24 h, respectively.27 TNFα expression significantly decays within 1 week.44 IL-1β upregulation is longer-lived, with expression sustained up to 14 days postop.44 One study reported basic fibroblast growth factor (FGF) expression decreased at 48 h,28 while another found increased expression at 3 and 30 days postop.26,57
Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis and vascular permeability in the early stages of the response to injury. AFL caused significant upregulation of VEGF at day 1 and 3 postop in a murine model, with no significant difference from controls after day 7,57 while mild upregulation of VEGF up to 30 days postop was reported in photodamaged human skin.26 These findings are consistent with reports of increased vascularity after fractional resurfacing.9,65 It has also been reported that VEGF can influence MMP activity and collagen deposition66 and there is some evidence that it may promote scar formation in later stages of wound healing.67 Further investigation of VEGF is required to better understand its role in scar remodeling.
Qu et al. showed that miR-18a and miR-19a expression is increased after AFL in scars.28 These miRNAs belong to the miR-17/92 cluster, which has been shown to suppress TGFβ signaling.68 Most research to date has focused on the role of miR-17/92 in cancer, but a few studies have also indicated a potential protective role in fibrosis.69,70
Kim et al. performed the only transcriptome level study to date for fractional laser resurfacing, although their analysis of the dataset was limited to a handful of genes.71 This study looked at AFL in healthy skin and emphasized Wnt5a, an antiapoptotic gene and fibroblast mitogen, and cysteine-rich angiogenic inducer 61 (CYR61), which has been shown to inhibit COL1A1 synthesis and promote degradation. It was also shown that CYR61 expression was upregulated throughout the dermis, rather than isolated to the injury sites.
Other modalities for fractional resurfacing
Microneedling is another common method for producing microinjuries. It is generally not as effective as laser resurfacing but has lower risk of negative outcomes, so it has achieved some popularity as a treatment for individuals seeking minor improvements. Microneedling involves a drum covered in fine needles (usually 0.5–1.5 mm in length) repeatedly rolled over the skin to create hundreds of punctures per square centimeter.72,73 It is most commonly used for treatment of atrophic scars from acne, as it has shown limited success for hypertrophic scars.74
The mechanism of microneedling has received even less attention than that of laser resurfacing. At one point, a theory for microneedling was proposed based on the needles creating electrical field perturbations to stimulate proliferation and release of growth factors.74 However, recent publications suggest that microneedling functions similarly to fractional laser resurfacing. Microinjuries induce a minor acute immune response, with associated release of inflammatory cytokines. TGFβ and FGF promote fibroblast proliferation and differentiation, increased matrix turnover, and angiogenesis. The result is normalization of the matrix architecture with increased deposition of collagen and elastin.73,75
Fractional radiofrequency irradiation is another common microinjury method. Similar to NAFL, this technique creates small, isolated columns of thermal injury. The main benefits of radiofrequency over laser resurfacing are less disruption of the epidermis, lower risk of dyspigmentation, and avoidance of side effects caused by light absorption by melanin in darker skin types.78 Broadly speaking, fractional radiofrequency causes similar effects to laser resurfacing, with upregulation of HSPs, MMPs, TGFβ, and proinflammatory cytokines leading to increased collagen type I, collagen type III, and elastin in the dermis.79,80
Mechanically removing small cores of skin tissue by fractional skin harvesting is a relatively new technique. Our group has primarily focused on using it to obtain autologous tissue for wound repair, but others have shown that the procedure leads to improvements in skin qualities in the porcine model, including thickening of both epidermis and papillary dermis, increased rete ride undulation at the dermal–epidermal junction, and increased synthesis of collagen and elastin fibers.65 This raises the potential for the treatment to have dual benefits—fractional skin harvesting from areas surrounding a wound could provide anatomically matched autologous skin tissue to be transplanted into the wound to accelerate healing, while the harvesting procedure could induce beneficial skin changes leading to increased resistance to further wounding.
Potential utility in chronic wound prevention
Chronic wounds typically occur in skin compromised by various underlying etiologies, such as complications associated with venous insufficiency (e.g. stasis dermatitis,81 lipodermatosclerosis,82 atrophie blanche83), diabetes (e.g. microangiopathy, neuropathy,84 necrobiosis lipoidica85), prolonged pressure, and aging. Although the underlying etiology may differ, chronic wounds are almost always preceded by a progressive decline in the overall skin quality, which renders the skin more susceptible to injuries and less able to heal from them. Defects found in ulcer-prone skin include effacement of rete ridges (the undulating projections at the dermal–epidermal junction that anchor the epidermis to the underlying dermis),86 abnormal epidermal structure and weakening of the epidermal barrier,87,88 breakdown of ECM components89,90 and disorganization of ECM structure,86,91,92 fibrotic changes,93,94 persistent inflammation,92 accumulation of advanced glycation endproducts,95 and senescent cells.96,97 These deteriorations in skin quality often lead directly to negative functional consequences. Diabetes and venous insufficiency are both associated with a host of skin symptoms.82,98 Flattening of the dermal–epidermal junction and disruption of the dermal ECM organization lead to significant declines in biomechanical properties of the skin.99 The accumulation of senescent cells appears to be at least partially responsible for age-related disorders, as removal of senescent cells attenuates many of these disorders in animal models.100 Prolonged and dysregulated inflammation is a major contributing factor to impaired ulcer healing.101 The period of skin degradation before ulcer formation makes chronic wounds somewhat predictable, and also presents a potential window of opportunity for the application of prophylactic measures to prevent ulceration. This is not a new concept—various modalities such as barrier creams, compression therapy, and preventative offloading are routinely applied clinically for ulcer prevention by reducing the harmful impacts of external stressors on the skin. However, relatively few treatments are designed to directly improve the quality of the skin itself.
There have been a few reports on using fractional resurfacing to treat chronic wounds, with enhanced healing after fractional laser treatments reported in an animal study of delayed diabetic healing102 and several clinical cases of chronic wounds associated with scars,103–105 epidermolysis bullosa,105 and traumatic lesions in the elderly.106 More extensive studies are needed to determine if this treatment is broadly applicable to different chronic wound types. In particular, wounds associated with defects that are known to be ameliorated by fractional resurfacing (some examples listed in Table 1) may make for promising targets. Chronic wounds may respond differently to different fractional resurfacing modalities and treatment parameters, depending on specific underlying etiologies, disease stage, and wound care regimen (dressings, compression, topical and systemic therapies, debridement, and so on), and information on these differences should be helpful for deciding whether/when fractional resurfacing may be beneficial.
Table 1.
Skin defects found in ulcer-prone skin, and reports of similar defects ameliorated by fractional resurfacing
| Skin Defects Present in Ulcer-Prone Skin | Examples of Similar Defects Ameliorated by Fractional Resurfacing | ||
|---|---|---|---|
| Defects | References | Effects of Fractional Resurfacing | References |
| Effacement of dermal–epidermal junction | 86 | Increase formation of rete ridges | 4,7 |
| Fragmentation of dermal collagen | 89,90 | Improved collagen formation | 7,19,115,116 |
| Breakdown of elastin fibers | 86 | Increase elastin formation | 115,116 |
| Disorganization of ECM | 86,93,94,117 | Improve ECM organization | 17,118 |
| Accumulation of senescent cells | 96,119 | Reduce senescent fibroblasts | 8 |
| Attenuation of TGFβ signaling | 25 | Upregulation of TGFβ | 26,27 |
| Lack of HSP 70 protein expression | 48 | HSP 70 induction | 46,47 |
ECM, extracellular matrix; HSP, heat shock protein; TGFβ, transforming growth factor-β.
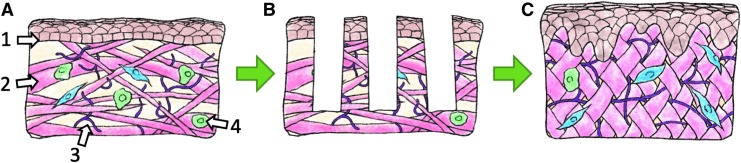
Given the ability of fractional resurfacing treatments to improve skin quality in different skin disorders, and particularly in ameliorating various defects (e.g., deficiencies in vascularization, ECM production and organization, accumulation of senescent cells) that are similar to those found in chronic wounds (Fig. 3; Table 1), it may be a potential approach to prevent skin ulceration by prophylactically reversing at least some of the defective qualities of ulcer-prone skin. In addition, using it to induce remodeling in scars from previously closed ulcers is another application that may positively impact the chronic wound trajectory, since scars are functionally defective and prone to reinjury107—properties that are likely to contribute to the high recurrence rate of chronic wounds. It should be noted that ulcer-prone skin is almost always afflicted by pathologies that extend beyond the local skin tissue (e.g., impaired circulation, nutritional deficiencies, biomechanical abnormalities), and these are unlikely to be affected by fractional resurfacing (or any other local treatment). However, to the extent that improved skin quality could benefit disease trajectory, fractional resurfacing may be a useful addition to the armamentarium of comprehensive chronic wound management. How ulcer-prone skin would respond to fractional resurfacing, and whether this treatment could reduce susceptibility to ulceration, should make for intriguing subjects for scientific and clinical investigations.
Figure 3.
Corrective effects of fractional resurfacing on defects similar to those found in ulcer-prone skin. (A) Defective skin with atypical epidermal structure, flattening of the dermal–epidermal junction (1), diminished and disorganized collagen (2) and elastin fibers (3), and accumulation of senescent cells (4, senescent fibroblasts shown in green, “normal” fibroblasts in blue). Fractional resurfacing (B) is known to produce broad improvements in skin structure and function, including normalization of epidermal structure, restoration of rete ridges at the dermal–epidermal junction, increased production and improved organization of collagen and elastin fibers, and reduction of senescent cell population (C).
Key remaining questions
While the clinical evidence is unequivocal that fractional resurfacing can induce dramatic regenerative effects, its mechanism(s) of action is still poorly understood. One fundamental unknown is why there is this size-dependent dichotomy in wound repair (Fig. 1), toward regeneration versus scarring. Considering that simple surgical incisions still heal by scarring, despite there being no tissue loss, it appears that factors such as wound volume, oxygen diffusion limit, and proximity of the wound area to uninjured tissue, are not primarily responsible. One potential contributor is the difference in local tissue mechanics–mechanical stresses have a profound effect on scarring,108 and different wound shapes are likely to result in different mechanical cues, for example, small circular wounds may cause less disruption to Langer's lines than a longer wound of similar total volume. Other possibilities include different cell populations involved in repair (local cells from neighboring areas vs. those recruited from the systemic circulation), different activation status and behaviors in the same cell populations, and differences in the postinjury inflammatory response. Detailed, well-controlled comparisons of the healing responses following different wound sizes/shapes may yield important insights about deciding factors governing scarring versus regeneration in adult tissues.
There are a number of foundational questions whose answers should have broad implications for whether/how fractional techniques could be utilized to prevent/treat chronic wounds. One critical question is whether the various underlying etiologies associated with chronic wounds could change the threshold at which the skin could spontaneously heal and remodel after fractional resurfacing, since that ability is a prerequisite for applying fractional treatments. There is at least one clinical report of deficient healing after fractional resurfacing in skin damaged by exposure to radioactive phosphorus.109 While arguably an extreme example, it does demonstrate the possibility for the skin quality to be so compromised that it has difficulty healing even very small wounds. This may also affect the choice of treatment modality, for example, nonablative treatments are generally considered to be less aggressive than ablative treatments, and the former may be more suitable for strengthening friable skin that may not tolerate ablative injuries. Conversely, ablative fractional treatments at more aggressive settings may provide a more potent “jolt” to push stalled wounds toward an acute healing response. The effectiveness of fractional resurfacing in treating photoaged skin is at least partly attributed to its ability to remove tissue with decades' worth of accumulated UV damage and replacing it with new tissue that had not (yet) been exposed to the sun. A similar mechanism may be beneficial for skin with accumulated damage due to aging, diabetes, and so on—this could be verified experimentally by measuring levels of known molecular byproducts associated with these etiologies. Other pertinent unknowns include potential differences in response based on body site (e.g., shin vs. plantar surface), the cellular sources of the various signaling molecules elicited by fractional resurfacing (described above in section “Mechanisms”), and whether there are specific differences in the healing response to microinjuries induced by thermal versus mechanical means. A better understanding of these practical questions could aid in more rational selection of treatment parameters and decisions regarding preventative versus therapeutic applications.
There remains a crucial missing link between the short-term response and long-term results of fractional resurfacing. The sequence of events immediately following treatment and the eventual histological changes are both well described, but the mechanism by which one leads to the other is poorly understood. Through speculation and bringing in elements from general fibrosis research, the pieces can be connected in a few different ways to formulate potential mechanisms. For scar remodeling, mechanical changes in the ECM may again play a substantial role. Microinjuries enable infiltration of the densely packed, highly cross-linked collagen fibrils that are resistant to degradation.110,111 The acute inflammatory response recruits a population of cells (macrophages and neutrophils) that strongly promote matrix turnover as a part of the normal wound healing process. This short but potent response is sufficient to significantly reduce scar stiffness, as shown by immediate clinical improvements (e.g., patient showing improved range of motion in 3 days13). But improvements continue long after resolution of inflammation. The long-term response could be initiated by the precipitous drop in tissue stiffness provided by the short-term activity. Collagen deposition and degradation is a dynamic process, even in long-healed scars.52,53,112 Furthermore, tissue stiffness forms a crucial positive feedback loop with fibrosis; greater stiffness promotes collagen deposition and inhibits degradation, therefore promoting further increases in tissue stiffness.113 A reduction in stiffness could tip the balance to be less in favor of collagen deposition, enabling long-term remodeling toward normal ECM. Changes in tissue stiffness could also explain the reduction in pruritis, as it has recently been shown that substrate stiffness can regulate mast cell recruitment and activation.114 A summary of the putative mechanisms involved is depicted in Fig. 4. The importance of other factors discussed previously, such as HSPs, Wnt, and CYR61, and how they may fit into a potential mechanism needs to be addressed by future research.
Figure 4.
Putative mechanisms involved in fractional resurfacing-induced remodeling. Solid arrows denote direct involvement in specific processes, dotted arrows denote regulatory roles. The TGFβ pathway regulates fibroblast function both directly and indirectly through effects on immune cells. Both fibroblasts and immune cells also produce TGFβ. Paracrine signaling from apoptotic cells stimulates proliferation and differentiation of neighboring cells, and also modulate the inflammatory response. HSPs interact with TGFβ signaling and also serve as chaperones for the production of ECM components. Tissue biomechanics may be altered by both the microinjuries from fractional resurfacing and the ECM remodeling process, resulting in feedback regulation of fibroblast functions through mechanotransduction pathways. ECM, extracellular matrix; HSP, heat shock protein; TGFβ, transforming growth factor-β.
Summary
Fractional resurfacing is effective at correcting a wide range of defective skin qualities, most prominently in photoaging and scarring. Better understanding of the cellular and molecular mechanisms underlying the unique healing response to fractional resurfacing could reveal fundamental information about adult tissue regeneration, lead to improvements in current applications, as well as new therapies in other pathologic conditions.
Take Home Messages.
Small wounds can regenerate completely, with no scarring
Clinical fractional resurfacing entails producing arrays of small wounds, by thermal or mechanical means, to induce tissue regeneration
Fractional resurfacing can produce homeostatic corrections in many structural and functional skin defects, particularly in photoaged skin and scars
Ulcer-prone skin is plagued by many similar defects, the potential utility of fractional resurfacing in the chronic wound setting remains to be explored.
Acknowledgments and Funding Sources
J.T. was supported by the 2016 Wound Healing Foundation 3M Fellowship Award. J.T. is partially supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-13-2-0054. The U.S. Army Medical Research Acquisition Activity, Fort Detrick, Maryland, is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. J.T. and C.F. are partially supported by the Military Medicine Technology Transformation Collaborative, Award Number HU0001-17-2-0009. The Uniformed Services University of the Health Sciences (USU), Bethesda, Maryland is the awarding and administering office. The information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred on the part of, USU, the Department of Defense, or the U.S. Government.
Abbreviations and Acronyms
- AFL
ablative fractional laser
- COL1A1
type I collagen
- COL3A1
type III collagen
- CYR61
cysteine-rich angiogenic inducer 61
- ECM
extracellular matrix
- HSP
heat shock protein
- IL
interleukin
- MCP-1
monocyte chemoattractant protein 1
- MMP
matrix metalloproteinase
- NAFL
nonablative fractional laser
- TGFβ
transforming growth factor-β
- TIMP
tissue inhibitors of metalloproteinase
- TNFα
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- Wnt
wingless/integrated
Author Disclosure and Ghostwriting
The authors have no competing financial interests related to this article. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Ben D. Leaker, BASc, is a PhD student in Medical Engineering and Medical Physics through the Harvard-MIT Program in Health Sciences and Technology. He completed his degree in Biomedical Engineering at the University of Toronto in 2017. His research interests include the mechanisms of scar resurfacing and conserved elements of fibrosis across organs. Christiane Fuchs, PhD, is a Research Fellow at the Wellman Center for Photomedicine. She received her doctorate in genetics and microbiology from the University of Vienna, Austria, and performed postdoctoral work on muscle tissue engineering and shockwave therapy for wound healing at the University of Applied Sciences Technikum Wien, Austria. Her current research interests include therapies to improve wound healing and mechanisms involved in skin regeneration. Joshua Tam, PhD, is an Instructor in Dermatology at the Wellman Center for Photomedicine. He received his doctorate in Biomedical Engineering from the Harvard-MIT Division of Health Sciences and Technology. His main research interest is in new technologies to enhance wound healing and tissue regeneration. He coinvented the technology for harvesting autologous, full-thickness skin tissue in a fractionated pattern, using the tissue for wound repair. His 2016 Wound Healing Foundation 3M Fellowship project was entitled “Reassembly of full-thickness skin from micro skin columns.”
References
- 1. Moore AL, Marshall CD, Barnes LA, Murphy MP, Ransom RC, Longaker MT. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip Rev Dev Biol 2018;7 [Epub ahead of print]; DOI: 10.1002/wdev.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg Med 2006;38:142–149 [DOI] [PubMed] [Google Scholar]
- 3. Carniol PJ, Hamilton MM, Carniol ET. Current status of fractional laser resurfacing. JAMA Facial Plast Surg 2015;17:360–366 [DOI] [PubMed] [Google Scholar]
- 4. Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004;34:426–438 [DOI] [PubMed] [Google Scholar]
- 5. Tam J, Wang Y, Farinelli WA, et al. Fractional skin harvesting: autologous skin grafting without donor-site morbidity. Plast Reconstr Surg Glob Open 2013;1:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tam J, Wang Y, Vuong LN, Fisher JM, Farinelli WA, Anderson RR. Reconstitution of full-thickness skin by microcolumn grafting. J Tissue Eng Regen Med 2017;11:2796–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadick NS, Smoller B. A study examining the safety and efficacy of a fractional laser in the treatment of photodamage on the hands. J Cosmet Laser Ther 2009;11:29–33 [DOI] [PubMed] [Google Scholar]
- 8. Spandau DF, Lewis DA, Somani AK, Travers JB. Fractionated laser resurfacing corrects the inappropriate UVB response in geriatric skin. J Invest Dermatol 2012;132:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connolly KL, Chaffins M, Ozog D. Vascular patterns in mature hypertrophic burn scars treated with fractional CO2 laser. Lasers Surg Med 2014;46:597–600 [DOI] [PubMed] [Google Scholar]
- 10. Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns 2017;43:573–582 [DOI] [PubMed] [Google Scholar]
- 11. Hultman CS, Friedstat JS, Edkins RE, Cairns BA, Meyer AA. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg 2014;260:519–529; discussion 529–532. [DOI] [PubMed] [Google Scholar]
- 12. Alster T, Tanzi E, Lazarus M. The use of fractional photothermolysis for the treatment of atrophic scars. Dermatol Surg 2007;33:295–299 [DOI] [PubMed] [Google Scholar]
- 13. Schumaker P, Kwan J, Landers J, Uebolhoer N. Functional improvements in traumatic scars and scar contractures using an ablative fractional laser protocol. J Trauma Acute Care Surg 2012;73:S116–S121 [DOI] [PubMed] [Google Scholar]
- 14. El-Zawahry BM, Sobhi RM, Bassiouny DA, Tabak SA. Ablative CO2 fractional resurfacing in treatment of thermal burn scars: an open label controlled clinical and histopathological study. J Cosmet Dermatol 2015;14:324–331 [DOI] [PubMed] [Google Scholar]
- 15. Beachkofsky TM, Henning JS, Hivnor CM. Induction of de novo hair regeneration in scars after fractionated carbon dioxide laser therapy in three patients. Dermatol Surg 2011;37:1365–1368 [DOI] [PubMed] [Google Scholar]
- 16. Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol 2011;65:661–663 [DOI] [PubMed] [Google Scholar]
- 17. Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol 2013;149:50–57 [DOI] [PubMed] [Google Scholar]
- 18. Makboul M, Makboul R, Abdelhafez A, Hassan S, Youssif S. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J Cosmet Dermatol 2014;13:169–179 [DOI] [PubMed] [Google Scholar]
- 19. Magnani L, Schweiger E. Fractional CO2 lasers for the treatment of atrophic acne scars: a review of the literature. J Cosmet Laser Ther 2014;16:48–56 [DOI] [PubMed] [Google Scholar]
- 20. Xu X, Luo Y, Wu Y, et al. Immunohistological evaluation of skin responses after treatment using a fractional ultrapulse carbon dioxide laser on back skin. Dermatol Surg 2011;37:1141–1149 [DOI] [PubMed] [Google Scholar]
- 21. Baca M, Neaman K, Rapp D, Burton M, Mann R, Renucci J. Reduction of post-surgical scarring with the use of ablative fractional CO2 lasers: a pilot study using a porcine model. Lasers Surg Med 2017;49:122–128 [DOI] [PubMed] [Google Scholar]
- 22. Douglas H. TGF-β in wound healing: a review. J Wound Care 2010;19:403–406 [DOI] [PubMed] [Google Scholar]
- 23. Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 2015;47:54–65 [DOI] [PubMed] [Google Scholar]
- 24. Lichtman M, Otero-Vina M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen 2016;24:215–222 [DOI] [PubMed] [Google Scholar]
- 25. Ramirez H, Patel SB, Pastar I. The role of TGFbeta signaling in wound epithelialization. Adv Wound Care (New Rochelle) 2014;3:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prignano F, Campolmi P, Bonan P, et al. Fractional CO2 laser: a novel therapeutic device upon photobiomodulation of tissue remodeling and cytokine pathway of tissue repair. Dermatol Ther 2009;22:S8–S15 [DOI] [PubMed] [Google Scholar]
- 27. DeBruler D, Blackstone B, Baumann B, et al. Inflammatory responses, matrix remodelling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med 2017;49:675–685 [DOI] [PubMed] [Google Scholar]
- 28. Qu L, Liu A, Zhou L, et al. Clinical and molecular effects on mature burn scars after treatment with a fractional CO2 laser. Lasers Surg Med 2012;44:517–524 [DOI] [PubMed] [Google Scholar]
- 29. Shah M, Foreman D, Ferguson M. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108:985–1002 [DOI] [PubMed] [Google Scholar]
- 30. Feder M, Hofmann G. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 1999;61:243–282 [DOI] [PubMed] [Google Scholar]
- 31. Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 2015;14:630–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci 2009;10:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellaye PS, Burgy O, Causse S, Garrido C, Bonniaud P. Heat shock proteins in fibrosis and wound healing: good or evil? Pharmacol Ther 2014;143:119–132 [DOI] [PubMed] [Google Scholar]
- 34. Wrighton KH, Lin X, Feng XH. Critical regulation of TGFbeta signaling by Hsp90. Proc Natl Acad Sci U S A 2008;105:9244–9249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yun CH, Yoon SY, Nguyen TT, et al. Geldanamycin inhibits TGF-beta signalinng through induction of Hsp70. Arch Biochem Biophys 2010;495:8–13 [DOI] [PubMed] [Google Scholar]
- 36. Sakai G, Tokuda H, Fujita K, et al. Heat shock protein 70 negatively regulates TGF-beta stimulated VEGF synthesis via p38 MAP kinase in osteoblasts. Cell Physiol Biochem 2017;44:1133–1145 [DOI] [PubMed] [Google Scholar]
- 37. Shang Y, Xu X, Duan X, et al. Hsp70 and Hsp90 oppositely regulate TGF-beta signaling through CHIP/Stub1. Biochem Biophys Res Commun 2014;446:387–392 [DOI] [PubMed] [Google Scholar]
- 38. Abu-Elsaad NM, Serrya MS, El-Karef AM, Ibrahim TM. The heat shock protein 90 inhibitor, 17-AAG, attenuates thioacetamide induced liver fibrosis in mice. Pharmacol Rep 2016;68:275–282 [DOI] [PubMed] [Google Scholar]
- 39. Noh H, Kim HJ, Yu MR, et al. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradtaion of transforming growth factor beta type II receptor. Lab Invest 2012;92:1583–1596 [DOI] [PubMed] [Google Scholar]
- 40. He W, Zhuang Y, Wang L, et al. Geranylgeranylacetone attenuates hepatic fibrosis by increasing the expression of heat shock protein 70. Mol Med Rep 2015;12:4895–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanaka K, Tanaka Y, Namba T, Azuma A, Mizushima T. Heat shock protien 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol 2010;80:920–931 [DOI] [PubMed] [Google Scholar]
- 42. Nagata K. Expression and function of heat shock protein 47: a collagen-specific molecular chaperone in the endoplasmic reticulum. Matrix Biol 1998;16:379–386 [DOI] [PubMed] [Google Scholar]
- 43. Helbig D, Bodenforf M, Grunewald S, Kendler M, Simon J, Paasch U. Immunohistochemical investigation of wound healing in response to fractional photothermolysis. J Biomed Opt 2009;14:06044. [DOI] [PubMed] [Google Scholar]
- 44. Orringer J, Rittié L, Baker D, Voorhees J, Fisher G. Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol 2010;163:757–768 [DOI] [PubMed] [Google Scholar]
- 45. Hantash B, Bedi V, Struck S, Chan K. Immunohistochemical evaluation of the heat shock response to nonablative fractional resurfacing. J Biomed Opt 2010;15:068002. [DOI] [PubMed] [Google Scholar]
- 46. Helbig D, Mobius A, Simon JC, Paasch U. Heat shock protein 70 expression patterns in dermal explants in response to ablative fractional phothothermolysis, microneedle, or scalpel wounding. Wounds 2011;23:59–67 [PubMed] [Google Scholar]
- 47. Manuskiatti W, Pattanaprichakul P, Inthasotti S, et al. Thermal response of in vivo human skin to fractional radiofrequency microneedle device. Biomed Res Int 2016;2016:6939018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oberringer M, Baum HP, Jung V, et al. Differential expression of heat shock protein 70 in well healing and chronic human wound tissue. Biochem Biophys Res Commun 1995;214:1009–1014 [DOI] [PubMed] [Google Scholar]
- 49. McMurty A, Cho K, Young L, Nelson C, Greenhalgh D. Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res 1999;86:36–41 [DOI] [PubMed] [Google Scholar]
- 50. Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011;4:165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Broughton GI, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006;117:12S–34S [DOI] [PubMed] [Google Scholar]
- 52. Nimni M. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum 1983;13:1–86 [DOI] [PubMed] [Google Scholar]
- 53. Cohen I, Keiser H. Disruption of healed scars in scurvy—the result of a disequilibrium in collagen metabolism. Plast Reconstr Surg 1976;57:213–215 [DOI] [PubMed] [Google Scholar]
- 54. Kupai K, Szucs G, Cseh S, et al. Matrix metalloproteinase activity assays: importance of zymography. J Pharmacol Toxicol Methods 2010;61:205–209 [DOI] [PubMed] [Google Scholar]
- 55. Reilly M, Cohen M, Hokugo A, Keller G. Molecular effects of fractional carbon dioxide laser resurfacing on photodamaged human skin. Arch Facial Plast Surg 2010;12:321–325 [DOI] [PubMed] [Google Scholar]
- 56. Orringer J, Kang S, Johnson T, et al. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. JAMA 2004;140:1326–1332 [DOI] [PubMed] [Google Scholar]
- 57. Jiang X, Ge H, Zhou C, Chai X, Ren QS. The role of vascular endothelial growth factor in fractonal laser resurfacing with the carbon dioxide laser. Lasers Med Sci 2012;27:599–606 [DOI] [PubMed] [Google Scholar]
- 58. Perez-Garijo A, Steller H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development 2015;142:3253–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal 2010;3:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vriz S, Reiter S, Galliot B. Cell death: a program to regenerate. Curr Top Dev Biol 2014;108:121–151 [DOI] [PubMed] [Google Scholar]
- 61. Prignano F, Ricceri F, Bonan P, Cannarozzo G, Campolmi P. Induction of apoptosis by fractional CO2 laser treatment. J Cosmet Laser Ther 2012;14:267–271 [DOI] [PubMed] [Google Scholar]
- 62. Farkas JP, Richardson JA, Burrus CF, Hoopman JE, Brown SA, Kenkel JM. In vivo histopathologic comparison of the acute injury following treatment with five fractional ablative laser devices. Aesthet Surg J 2010;30:457–464 [DOI] [PubMed] [Google Scholar]
- 63. Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 2004;164:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012. [DOI] [PubMed] [Google Scholar]
- 65. Fernandes JR, Samayoa JC, Broelsch GF, et al. Micro-mechanical fractional skin rejuvenation. Plast Reconstr Surg 2013;131:216–223 [DOI] [PubMed] [Google Scholar]
- 66. Bao P, Kodra A, Tomic-Canic M, Golinko M, Ehrlich H, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson K, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dews M, Fox JL, Hultine S, et al. The myc-miR17 ∼ 92 axis blunts TGFbeta signaling and production of multiple TGFbeta dependent antiangiogenic factors. Cancer Res 2010;70:8233–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dakhlallah D, Batte K, Wang Y, et al. Epigenetic regulation of miR-17 ∼ 92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2013;187:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Geng H, Guan J. MiR-18a-5p inhibits endothelial-mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem Biophys Res Commun 2017;491:329–336 [DOI] [PubMed] [Google Scholar]
- 71. Kim J, Won C, Bak H, et al. Gene profiling analysis of the early effects of ablative fractional carbon dioxide laser treatment on human skin. Dermatol Surg 2013;39:1033–1043 [DOI] [PubMed] [Google Scholar]
- 72. Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg 2009;2:110–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hou A, Cohen B, Haimovic A, Elbuluk N. Microneedling: a comprehensive review. Dermatol Surg 2017;43:321–339 [DOI] [PubMed] [Google Scholar]
- 74. Liebl H, Kloth LC. Skin cell proliferation stimulated by microneedles. J Am Coll Clin Wound Spec 2012;4:2–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alster TS, Graham PM. Microneedling: a review and practical guide. Dermatol Surg 2018;44:397–404 [DOI] [PubMed] [Google Scholar]
- 76. Sadick N, Rothaus KO. Minimally invasive radiofrequency devices. Clin Plast Surg 2016;43:567–575 [DOI] [PubMed] [Google Scholar]
- 77. Hantash BM, Ubeid AA, Chang H, Kafi R, Renton B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med 2009;41:1–9 [DOI] [PubMed] [Google Scholar]
- 78. Sun W, Zhang C, Zhao J, Wu J, Xiang L. Comparison of moderate and high energy of a nano-fractional radiofrequency treatment on a photoaging hairless mice model. Dermatol Surg 2018;44:569–575 [DOI] [PubMed] [Google Scholar]
- 79. Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol 2017;18:383–390 [DOI] [PubMed] [Google Scholar]
- 80. Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010;23:375–388 [DOI] [PubMed] [Google Scholar]
- 81. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care 2014;27:518–524; quiz 525–526. [DOI] [PubMed] [Google Scholar]
- 82. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 83. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin 2015;33:343–360 [DOI] [PubMed] [Google Scholar]
- 84. Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist 2016;56 Suppl 2:S230–S242 [DOI] [PubMed] [Google Scholar]
- 85. Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Human skin stem cells and the ageing process. Exp Gerontol 2008;43:986–997 [DOI] [PubMed] [Google Scholar]
- 86. Chao CY, Zheng YP, Cheing GL. Epidermal thickness and biomechanical properties of plantar tissues in diabetic foot. Ultrasound Med Biol 2011;37:1029–1038 [DOI] [PubMed] [Google Scholar]
- 87. Argyropoulos AJ, Robichaud P, Balimunkwe RM, et al. Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLoS One 2016;11:e0153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009;174:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tahrani AA, Zeng W, Shakher J, et al. Cutaneous structural and biochemical correlates of foot complications in high-risk diabetes. Diabetes Care 2012;35:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tellechea A, Kafanas A, Leal EC, et al. Increased skin inflammation and blood vessel density in human and experimental diabetes. Int J Low Extrem Wounds 2013;12:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nikkels-Tassoudji N, Henry F, Letawe C, Pierard-Franchimont C, Lefebvre P, Pierard GE. Mechanical properties of the diabetic waxy skin. Dermatology 1996;192:19–22 [DOI] [PubMed] [Google Scholar]
- 92. Herrick SE, Treharne LJ, deGiorgio-Miller AM. Dermal changes in the lower leg skin of patients with venous hypertension. Int J Low Extrem Wounds 2002;1:80–86 [DOI] [PubMed] [Google Scholar]
- 93. Van Putte L, De Schrijver S, Moortgat P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: a systematic review. Scars Burn Heal 2016;2:2059513116676828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mendez MV, Stanley A, Phillips T, Murphy M, Menzoian JO, Park HY. Fibroblasts cultured from distal lower extremities in patients with venous reflux display cellular characteristics of senescence. J Vasc Surg 1998;28:1040–1050 [DOI] [PubMed] [Google Scholar]
- 95. Wang AS, Dreesen O. Biomarkers of cellular senescence and skin aging. Front Genet 2018;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol 2017;18:541–553 [DOI] [PubMed] [Google Scholar]
- 97. Langton AK, Graham HK, McConnell JC, Sherratt MJ, Griffiths CEM, Watson REB. Organization of the dermal matrix impacts the biomechanical properties of skin. Br J Dermatol 2017;177:818–827 [DOI] [PubMed] [Google Scholar]
- 98. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care (New Rochelle) 2018;7:209–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee HN, Bae JM, Goo BCL, Park YM. Promotion of wound healing through low-fluence ablative fractional laser treatment in diabetic mice. Lasers Med Sci 2019;34:421–425 [DOI] [PubMed] [Google Scholar]
- 101. Krakowski AC, Diaz L, Admani S, Uebelhoer NS, Shumaker PR. Healing of chronic wounds with adjunctive ablative fractional laser resurfacing in two pediatric patients. Lasers Surg Med 2016;48:166–169 [DOI] [PubMed] [Google Scholar]
- 102. Shumaker PR, Kwan JM, Badiavas EV, Waibel J, Davis S, Uebelhoer NS. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol 2012;148:1289–1293 [DOI] [PubMed] [Google Scholar]
- 103. Krakowski AC, Ghasri P. Case report: rapidly healing epidermolysis bullosa wound after ablative fractional resurfacing. Pediatrics 2015;135:e207–e210 [DOI] [PubMed] [Google Scholar]
- 104. Phillips TJ, Morton LM, Uebelhoer NS, Dover JS. Ablative fractional carbon dioxide laser in the treatment of chronic, posttraumatic, lower-extremity ulcers in elderly patients. JAMA Dermatol 2015;151:868–871 [DOI] [PubMed] [Google Scholar]
- 105. Buchanan PJ, Kung TA, Cederna PS. Evidence-based medicine: wound closure. Plast Reconstr Surg 2014;134:1391–1404 [DOI] [PubMed] [Google Scholar]
- 106. Barnes LA, Marshall CD, Leavitt T, et al. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care (New Rochelle) 2018;7:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tran TN, Hoang MV, Phan QA, et al. Fractional epidermal grafting in combination with laser therapy as a novel approach in treating radiation dermatitis. Semin Cutan Med Surg 2015;34:42–47 [DOI] [PubMed] [Google Scholar]
- 108. Vater CA, Harris EDJ, Siegel RC. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J 1979;181:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Issa R, Zhou X, Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 2004;126:1795–1808 [DOI] [PubMed] [Google Scholar]
- 110. Eckersley J, Dudley H. Wounds and wound healing. Br Med Bull 1988;44:423–436 [DOI] [PubMed] [Google Scholar]
- 111. Liu F, Mih JD, Shea BS, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010;190:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang H, Liu X, Shen Z, et al. An investigation of the distribution and location of mast cells affected by the stiffness of substrates as a mechanical niche. Int J Biol Sci 2018;14:1142–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO(2) fractional laser: a randomized controlled trial. Ann Dermatol 2011;23:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. de Angelis F, Kolesnikova L, Renato F, Liguori G. Fractional nonablative 1540-nm laser treatment of striae distensae in Fitzpatrick skin types II to IV: clinical and histological results. Aesthet Surg J 2011;31:411–419 [DOI] [PubMed] [Google Scholar]
- 115. Osman OS, Selway JL, Harikumar PE, et al. A novel method to assess collagen architecture in skin. BMC Bioinformatics 2013;14:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. El-Hoshy K, Abdel-Halim MRE, Dorgham D, El-Din Sayed SS, El-Kalioby M. Efficacy of fractional carbon dioxide laser in the treatment of mature burn scars: a clinical, histopathological, and histochemical study. J Clin Aesthet Dermatol 2017;10:36–43 [PMC free article] [PubMed] [Google Scholar]
- 117. Golomb L, Sagiv A, Pateras IS, et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ 2015;22:1764–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]