Abstract
HIV and methamphetamine (MA) use disorder are commonly comorbid and individually associated with adverse health consequences, including frailty; however, less is known about the combined effects of both conditions. The current cross-sectional study examined how HIV and lifetime MA use disorder relate to frailty and explored associations between frailty and relevant clinical outcomes (i.e., neurocognitive and everyday functioning). Participants were categorized into three groups based on HIV status and lifetime MA diagnosis: HIV+/MA+ (n = 43), HIV+/MA− (n = 75), and HIV−/MA− (n = 92). A frailty index score (representing proportion of accumulated multisystem deficits) was calculated from 27 medical and psychiatric deficits. Multiple regression was used to examine frailty index score by HIV/MA group. Additional multiple regression models examined the interaction between frailty and HIV/MA group on cognitive and everyday functioning. Comorbid HIV+/MA+ participants had higher frailty index scores than both HIV−/MA− (b = −0.13, p < .001) and HIV+/MA− participants (b = −0.06, p = .007). Additional models linked higher frailty index score to worse global neurocognition (b = −17.6, p = .018) and greater likelihood of everyday functioning dependence (odds ratio = 1.56, p = .021). Although these relationships did not significantly differ by HIV/MA status, group-stratified analyses showed that associations of frailty with neurocognitive and everyday functioning were strongest among the HIV+/MA+ group. Multimodal public health interventions aimed at reducing frailty may help to decrease the likelihood of neurocognitive and everyday functioning problems. Current findings additionally lay groundwork for future longitudinal research examining whether frailty predicts onset of neurocognitive and functional decline in individuals with comorbid HIV and MA use disorder.
Keywords: HIV/AIDS, substance use, aging, cognition, activities of daily living
Introduction
Methamphetamine (MA) is a highly addictive psychostimulant that can detrimentally impact neurocognition, real-world functioning, and medical outcomes.1–3 MA misuse is particularly common among persons living with HIV (PLWH) and represents a major obstacle for public health efforts aimed at reducing the prevalence and severity of HIV.4,5 Chronic MA misuse not only enhances risk of HIV transmission, through needle-sharing and unprotected sex,6,7 but can also exacerbates HIV disease burden by disrupting antiretroviral therapy (ART) efficacy and adherence.8 Consequently, a myriad of clinical complications can arise from comorbid HIV disease and MA use disorders (HIV+/MA+).
It has been widely documented that MA and HIV independently inflict neuropathophysiological damage, with downstream decrements in neurocognitive and everyday functioning abilities.9–11 Although less well studied, there is also evidence to suggest that additive neurotoxic effects of MA and HIV exist and operate via the convergence of MA- and HIV-related mechanisms of neural injury to preferentially damage frontostriatal regions.12,13 The coupling of MA misuse with HIV is characterized by neurocognitive deficits in executive functions, learning and memory, and motor skills,14–16 which can translate to impairments in real-world daily functioning. These challenges in everyday functioning typically reflect high-stakes outcomes such as unemployment, dependence in instrumental activities of daily living (IADLs), and poor ART adherence.17,18 Recent studies highlight an additive deleterious effect on everyday functioning such that HIV+/MA+ individuals exhibit the highest rates of IADL dependence compared to singly affected groups (i.e., HIV−/MA+ and HIV+/MA−).19 Given the severity of impairments associated with MA misuse, a greater understanding of mechanisms underlying MA-associated neurobehavioral dysfunction is needed.
Frailty is one mechanism that may explain the detrimental effect of HIV+/MA+ on neurobehavioral impairment and has not been previously examined in this population. The construct of frailty, which first emerged through geriatric research, is conceptualized as vulnerability to multisystem damage and is most commonly used to predict risk for adverse health outcomes.20,21 There are several different approaches to operationalizing frailty, including as an index representing the accumulation of multisystem health deficits (e.g., diabetes, chronic inflammation, anemia) associated with the erosion of homeostatic processes.22,23 One gerontological model for the role of frailty in neurocognition details a complex environment, in which the gradual draining of physiological reserve dynamically interacts with psychosocial and functional factors to promote neurocognitive decline.24 Given the extensive evidence that PLWH are at risk for the acquisition of multiple comorbidities potentially reflective of an accelerated aging process,25 frailty indices have been developed for PLWH across the lifespan and are predictive of mortality independent of markers of HIV disease burden.26,27
Although the relationship between frailty and MA misuse is poorly characterized, MA misuse is linked to widespread toxicity of multiple organ systems (e.g., cardiovascular, renal, pulmonary) and enhances risk for mortality and multimorbidity.28,29 Furthermore, models of cellular senescence provide support that the physiological dysregulation caused by MA exposure may also reflect accelerated biological aging.30 Indications that MA and HIV independently diminish the capacity to respond to physiological stressors, coupled with gerontological evidence linking frailty to incident neurocognitive decline,31–33 necessitate the examination of frailty in the context of neurocognitive and everyday functioning among HIV+/MA+ persons.
An understanding of the temporal trends concerning MA misuse, frailty, and neurobehavioral impairment is critical for modeling their relationship. Although younger PLWH are more likely to actively use MA than older PLWH,34,35 older PLWH are more likely to exhibit frailty due to the strong relationship between frailty and age.20,22 Thus, we aimed to study the lasting effects of past MA use disorder on frailty among middle-aged to older PLWH. Thus, the current study examined differences in frailty across three groups: a group with comorbid HIV and lifetime MA use disorder (HIV+/MA+), an HIV+/MA− group, and an HIV−/MA− control group and explored relationships between frailty and neurocognitive and everyday functioning among the three groups. We hypothesized that the comorbid HIV+/MA+ group would demonstrate the highest frailty compared to the other two groups. We also hypothesized that frailty would be negatively associated with neurocognitive and everyday functioning, and that these relationships would be strongest in the comorbid HIV+/MA+ group.
Materials and Methods
Participants
Participants consisted of 118 HIV+ and 92 HIV− adults, aged 35 to 65 [M = 50.8, standard deviation (SD) = 7.97], enrolled in the 5-year Multi-Dimensional Successful Aging among HIV-Infected Adults study conducted at the University of California San Diego (UCSD).36,37 The current study represents a secondary analysis on available MA use data; thus, participants were not specifically recruited by lifetime MA status. Baseline data were included in this analysis. Study exclusion criteria were diagnosis of a psychotic disorder, presence of a neurological condition known to impact neurocognitive functioning (e.g., stroke), and positive urine toxicology for alcohol or drugs (excluding marijuana) on the day of testing. The study protocol was approved by the UCSD Institutional Review Board. Participants provided written, informed consent.
Measures
Psychiatric assessment
The Composite International Diagnostic Interview (CIDI, v2.1) World Health38 was administered to assess for presence of current and lifetime (occurring >12 months ago) substance use and mood disorders [e.g., major depressive disorder (MDD)] based on the Diagnostic and Statistical Manual of Mental Disorders.39 For this analysis, participants were categorized into three groups based on HIV status and lifetime MA abuse or dependence diagnosis: HIV+/MA+ (n = 43); HIV+/MA− (n = 75); and HIV−/MA− (n = 92). No HIV− participants met criteria for lifetime MA use disorder in our available sample; therefore, our analysis is confined to the three groups identified above. Within the HIV+/MA+ group, 37 met criteria for lifetime MA dependence and 6 met criteria for lifetime MA abuse. No individuals in this sample met criteria for a current MA use disorder.
Neuromedical assessment
Study participants completed a standardized medical history interview, which assessed for the presence of medical comorbidities (e.g., hyperlipidemia; diabetes mellitus) using a combination of self-report and laboratory measurements; structured neurological and medical evaluation; and collection of blood and urine samples. All participants were screened for HIV infection using a fingerstick test (Medmira, Nova Scotia, Canada) and confirmed with an Abbott RealTime HIV-1 test (Abbott Laboratories, IL) or by submitting specimens to a Clinical Laboratory Improvement Amendments-certified laboratory (ARUP Laboratories, UT) for HIV-1 viral load quantitation. Additional HIV characterization included AIDS status, plasma viral load, CD4+ T cell counts (nadir and current), estimated duration of HIV disease, and current ART regimen.
Frailty assessment
Based on previously established methods,26,27 the frailty index was calculated as the proportion of health deficits from a group of 27 health variables, including markers of general health and comorbidities (Table 1). Thus, frailty index scores have a possible range from 0 (no deficits) to 1 (all 27 deficits).
Table 1.
Variables and Deficit Criteria for Frailty Index
| Variable | Deficit criteria | |
|---|---|---|
| 1. | High or low BMI | >25 or <18 kg/m2 |
| 2. | High total cholesterol | >200 mg/dL |
| 3. | High LDL cholesterol | >100 mg/dL |
| 4. | Low HDL cholesterol | <40 mg/dL |
| 5. | High triglycerides | >150 mg/dL |
| 6. | Abnormal white blood cell count | <4,000 cells/μL |
| 7. | Hemoglobin | Male: <13.5 μmol/L; female: <12 μmol/L |
| 8. | Elevated AST | >31 U/L |
| 9. | Elevated ALT | >31 U/L |
| 10. | Abnormal ALP | <38 or >126 U/L |
| 11. | Low platelets | <150 billion/L |
| 12. | Abnormal potassium | <3.5 or >5.3 mEq/L |
| 13. | Elevated total bilirubin | >1.1 mg/dL |
| 14. | CRP | >0.7 mg/L |
| 15. | Elevated IL-6 | >50th percentile of control samplea |
| 16. | Elevated MCP-1 | >50th percentile of control samplea |
| 17. | Elevated sCD14 | >50th percentile of control samplea |
| 18. | Elevated TNF-a | >50th percentile of control samplea |
| 19. | Elevated d-dimer | >50th percentile of control samplea |
| 20. | HCV | Positiveb |
| 21. | DM | Positiveb |
| 22. | HTN | Positiveb |
| 23. | Hyperlipidemia | Positiveb |
| 24. | Smoking (ever) | Positive |
| 25. | Unemployment | Positive |
| 26. | Current MDD | Positive |
| 27. | Lifetime MDD | Positive |
The control sample only includes the 92 participants who were HIV-uninfected without a lifetime history of methamphetamine use disorder (HIV−/MA−).
Determined by one or a combination of sources, including self-report (if previously diagnosed by an outside provider), having a prescription medication for the condition, and/or in-laboratory blood test values indicative of the condition (HCV = standard clinical antibody detection; DM = elevated A1C or fasting glucose values; HTN = elevated systolic or diastolic blood pressure; hyperlipidemia = elevated total cholesterol, LDL, or triglycerides).
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; DM, diabetes mellitus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HTN, hypertension; IL-6, interleukin-6; LDL, low-density lipoprotein; MA, methamphetamine; MCP-1, monocyte chemoattractant protein 1; MDD, major depressive disorder; sCD14, soluble CD14; TNF-a, tumor necrosis factor alpha.
Each variable within the frailty index was dichotomized as “1,” when a deficit was present, and “0,” when absent, based on criteria displayed in Table 1. The 27 selected variables were chosen based on all available neuromedical and neuropsychiatric data from the parent study that were consistent with variables included in previous frailty indexes.26,27 In addition, following published guidelines for creating a frailty index,23 we excluded factors that (1) had greater than 5% missing data (i.e., greater than 10 missing cases in this sample) and (2) had less than 1% of participants meeting criteria for the deficit (i.e., fewer than two cases in this sample). Factors not included in the index due to having greater than 5% missing data in our sample included hemoglobin A1C and fasting insulin level. Factors not included in the index due to insufficient number of participants (<1%) meeting criteria for the deficit included abnormal sodium, albumin, and phosphorous levels.
Neurocognitive assessment
Participants completed a standardized battery of neuropsychological tests to evaluate neurocognitive functioning. The battery assesses seven neurocognitive domains commonly affected by HIV, that is, verbal fluency, working memory, processing speed, verbal and visual learning and delayed recall, executive functioning, and motor skills.10 Raw test scores were converted to T-scores (M = 50; SD = 10) adjusted to correct for age, gender, education, and race/ethnicity.40–42 T-scores were averaged in each domain to obtain domain T-scores, and all test T-scores were averaged to obtain a Global T-score.
Everyday functioning assessment
To determine dependence in IADL, participants completed a modified version of the Lawton and Brody ADL questionnaire.43 This self-report questionnaire queried 11 IADL domains (e.g., managing finances and managing medications) and asked participants to rate their current and highest levels of functioning. Participants were categorized as IADL dependent if they reported a decline or need for assistance in ≥2 IADL domains, regardless of whether the difficulties were attributed to cognitive or physical factors. This methodology has previously been validated in a normative sample,43 and it has been shown to be associated with objective measures of functional loss in persons with HIV and/or MA use disorders.44,45
Statistical analyses
To compare HIV/MA groups on demographic, clinical, and primary outcome variables, one-way analysis of variance (ANOVA) was used. To follow up on significant omnibus results, pairwise comparisons were examined using Tukey's honestly significant difference (HSD) (α = 0.05) for continuous outcomes or Bonferroni adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes. Next, a multiple regression model was used to examine group differences in frailty, covarying for demographic and clinical factors that differed between groups. Pearson correlations were used to explore associations between frailty index score and specific MA use disorder characteristics. Finally, multiple linear and logistic regressions were used to examine the interactions between frailty and HIV/MA group on global neurocognitive T-score and IADL dependence. Due to potential lack of power to find significant interaction effects, exploratory group-stratified analyses were conducted examining the relationships of frailty index score to global neurocognitive T-score and IADL dependence within each HIV/MA group. Pearson r correlations were also used to examine relationships between frailty and domain-specific neurocognitive functioning within the HIV+/MA+ group.
Results
Participant characteristics
Demographic and clinical characteristics for each HIV/MA group are presented in Table 2. The HIV+/MA− group had the fewest non-Hispanic whites, compared to the HIV+/MA+ and HIV−/MA− groups (p < .001), and the HIV+/MA+ group had fewer years of education compared to the other two groups (p < .001). PLWH (regardless of MA status) were more likely to be unemployed than persons without HIV (p < .001). Regarding neuropsychiatric characteristics, both HIV+ groups were more likely to have current and lifetime MDD than the HIV−/MA− group (ps < .001). The HIV+/MA+ group had the highest proportion of other (non-MA) lifetime substance use disorders (SUDs) (p < .001). Regarding HIV disease severity, both HIV+ groups were comparable with fairly well-controlled HIV (ps > .05). Regarding MA use disorder characteristics in the HIV+/MA+ group, participants reported meeting criteria for an MA use disorder for the first time at 34 years of age on average (SD = 10.1), and most recently met criteria for an MA use disorder at 43 years of age on average (SD = 8.7).
Table 2.
Participant Characteristics by HIV/Methamphetamine Groups (N = 210)
|
A HIV−/MA− (n = 92) |
B HIV+/MA− (n = 75) |
C HIV+/MA+(n = 43) |
p | Pairwise comparisonsa | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 51.2 (7.45) | 50.8 (8.95) | 50.0 (7.35) | .74 | |
| Sex (male) | 64 (70%) | 62 (83%) | 37 (86%) | .04 | |
| Race/ethnicity (White) | 64 (70%) | 33 (44%) | 32 (74%) | <.001 | B < A, C |
| Education (years) | 15.0 (2.32) | 14.4 (2.61) | 13.3 (2.01) | <.001 | A, B < C |
| WRAT-readingb | 106.8 (13.78) | 103.2 (14.06) | 102.0 (12.89) | .09 | |
| Unemployed | 26 (28%) | 48 (64%) | 34 (79%) | <.001 | A < B, C |
| Psychiatric diagnoses | |||||
| Current MDD | 0 (0%) | 5 (7%) | 8 (20%) | <.001 | A < B, C |
| LT MDD | 19 (21%) | 34 (46%) | 28 (67%) | <.001 | A < B, C |
| LT other SUDc | 33 (36%) | 39 (52%) | 36 (84%) | <.001 | A < B < C |
| HIV characteristics | |||||
| History of AIDS | — | 46 (61%) | 26 (60%) | .93 | |
| Detectable viral loadd | — | 6 (8%) | 3 (7%) | .86 | |
| Current CD4 counte | — | 638 [417–901] | 633 [445–779] | .72 | |
| Nadir CD4 counte | — | 189 [59–353] | 150 [19–332] | .29 | |
| Estimated duration of infection (years) | — | 17.4 (9.09) | 16.6 (8.31) | .65 | |
| On antiretroviral therapy | — | 68 (93%) | 43 (100%) | .08 | |
| MA characteristics | |||||
| Age of first MA dx | — | — | 34.2 (10.05) | ||
| Age of most recent MA dx | — | — | 42.7 (8.69) | ||
| Years since most recent MA dx | — | — | 4 [2–11] | ||
Values are presented as mean (SD), median [IQR], or N (%). Bold values indicate p-values significant at <0.05.
Pairwise comparisons were examined using Tukey's HSD (α = 0.05) for continuous outcomes or Bonferroni adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes.
Used as an indicator of education quality.
Other SUDs include alcohol, cannabis, cocaine, hallucinogens, opioids, PCP, and sedatives.
Plasma; defined as >50 copies/mL.
Values were compared by Wilcoxon Test because of skewed distributions.
dx, diagnosis; HSD, honestly significant difference; IQR, interquartile range; LT, lifetime; PCP, phencyclidine; SD, standard deviation; SUD, substance use disorder; WRAT-reading, wide range achievement test, reading subtest.
Frailty, neurocognition, and everyday functioning across HIV/MA groups
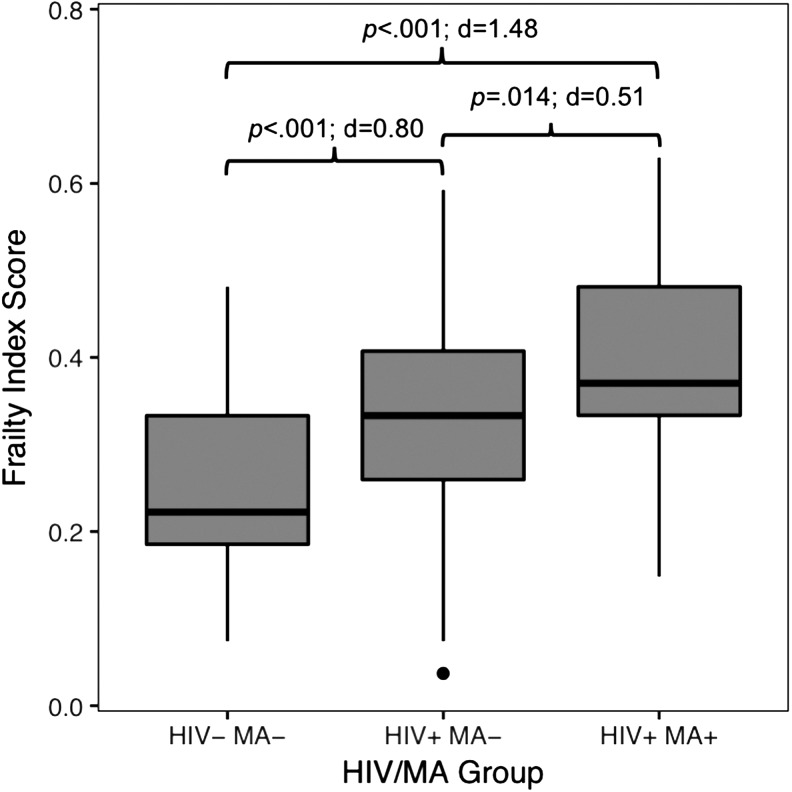
Frailty index scores, neurocognitive impairment rates and T-scores, and IADL dependence rates for each group are displayed in Table 3. In univariable analyses, frailty index score differed between all groups such that the comorbid HIV+/MA+ group had a higher mean frailty index score than the HIV+/MA− group, and the HIV+/MA− group had a higher mean frailty index score than the control group (p < .001; Fig. 1). See Supplementary Table S1 for analyses exploring the proportions of participants meeting deficit criteria for individual frailty variables across each group. Regarding neurocognition, both HIV+ groups displayed worse global and executive functioning than the HIV−/MA− group (ps < .01). Compared to HIV−/MA−, the HIV+/MA− group performed worse on tests of learning, and the HIV+/MA+ group performed worse on tests of motor skills (ps < .05). Notably, the HIV+/MA+ and HIV+/MA− groups did not differ on any domain of neurocognitive functioning. Finally, a stairstep pattern of everyday functioning by group was observed, such that the HIV+/MA+ group displayed the highest rate of IADL dependence, followed by the HIV+/MA− group, and then the control group (p < .001).
Table 3.
Frailty, Cognition, and Everyday Functioning by HIV/Methamphetamine Groups (N = 210)
|
A HIV−/MA− (n = 92) |
B HIV+/MA− (n = 75) |
C HIV+/MA+ (n = 43) |
p | Pairwise comparisonsa | |
|---|---|---|---|---|---|
| Frailty | |||||
| Frailty index score | 0.23 (0.10) | 0.33 (0.12) | 0.39 (0.10) | <.001 | A < B < C |
| Cognition | |||||
| GDS-impaired | 24 (26%) | 31 (41%) | 19 (44%) | .05 | |
| Global T-score | 50.1 (6.01) | 47.0 (7.33) | 46.8 (6.54) | .003 | A > B, C |
| Verbal fluency T-score | 50.5 (6.63) | 48.6 (9.27) | 49.7 (6.64) | .29 | |
| Executive functioning T-score | 52.8 (9.49) | 47.7 (8.95) | 47.4 (11.00) | <.001 | A > B, C |
| Processing speed T-score | 52.1 (8.5) | 49.0 (8.53) | 48.7 (8.75) | .03 | |
| Learning T-score | 44.9 (9.22) | 40.5 (9.81) | 41.0 (7.79) | .005 | A > B |
| Delayed recall T-score | 44.2 (9.34) | 41.4 (9.97) | 40.8 (8.38) | .07 | |
| Working memory T-score | 49.4 (10.36) | 48.3 (9.18) | 47.5 (9.40) | .56 | |
| Motor skills T-score | 53.5 (10.64) | 50.3 (10.73) | 48.4 (10.58) | .02 | A > C |
| Everyday functioning | |||||
| IADL dependent | 8 (9%) | 20 (27%) | 19 (44%) | <.001 | A < B < C |
Values are presented as mean (SD) or N (%). Bold values indicate p-values significant at <0.05.
Pairwise comparisons were examined using Tukey's HSD (α = 0.05) for continuous outcomes or Bonferroni adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes.
GDS, global deficit score; IADL, instrumental activities of daily living.
FIG. 1.
Frailty index score by HIV/MA group. p-Values were determined using Tukey's HSD for multiple comparisons. d, Cohen's d; HSD, honestly significant difference; MA, methamphetamine.
Next, a multiple linear regression analysis was conducted to further explore the differences in frailty index score across groups, covarying for specified demographic and clinical characteristics. Covariates included age (to account for the known positive relationship between frailty and age) and any characteristic that differed between groups that were not included in the creation of the frailty index: race/ethnicity, years of education, and lifetime other (non-MA) SUD. Of note, we collapsed all non-MA SUDs into one representative variable to seek parsimony in the regression models. Results of the multiple linear regression revealed that HIV/MA group membership was significantly associated with frailty index score, such that both the HIV−/MA− (b = −0.13; p < .001) and HIV+/MA− (b = −0.06; p = .007) groups had significantly lower frailty index scores than the HIV+/MA+ group (Table 4).
Table 4.
Results of Multivariable Linear Regression Predicting Frailty Index Score
| Predictor variables | Estimate | 95% CI | p |
|---|---|---|---|
| HIV−/MA− (vs. HIV+/MA+) | −0.13 | −0.17 to −0.09 | <.001 |
| HIV+/MA− (vs. HIV+/MA+) | −0.06 | −0.10 to −0.02 | .007 |
| Age (years) | 0.003 | 0.001 to 0.005 | .005 |
| White (vs. nonwhite) | 0.02 | −0.01 to 0.06 | .142 |
| Education (years) | −0.008 | −0.015 to −0.002 | .010 |
| Lifetime other SUD (yes vs. no)a | 0.001 | −0.03 to 0.03 | .996 |
Bold values indicate p-values significant at <0.05.
Other SUDs include alcohol, cannabis, cocaine, hallucinogens, opioids, PCP, and sedatives.
CI, confidence interval.
We also examined relationships between frailty and MA use disorder characteristics. Frailty index score was not significantly related to age at which participants first met criteria for MA use disorder (r = 0.08, p = .67), age at which participants most recently met criteria for MA use disorder (r = 0.08, p = .67), or years since last MA use disorder (r = 0.20, p = .26).
Relating frailty index score to neurocognitive and everyday functioning by HIV/MA group
The multiple linear regression examining the interaction between frailty and HIV/MA group on global neurocognitive T-score showed a main effect of frailty, such that higher frailty index score was associated with lower global T-score (b = −17.60; p = .02; Table 5). However, there was no main effect of HIV/MA group (ps > .05) and no interaction effect between frailty and group on global T-score (ps > .05). Exploratory follow-up analyses, however, showed that the HIV+/MA+ group had the strongest negative relationship between frailty index score and global T-score (r = −0.28, p = .06), followed by HIV−/MA− (r = −0.17, p = .11), then HIV+/MA− (r = −0.07, p = .54). Domain-specific analyses within the HIV+/MA+ group revealed that frailty index score had the strongest negative association with executive functioning (r = −0.34, p = .03) and working memory (r = −0.33, p = .03), followed by processing speed (r = −0.25, p = .10), learning (r = −0.21, p = .18), verbal fluency (r = −0.15, p = .34), delayed recall (r = −0.02, p = .88), and motor skills (r = −0.01, p = .94).
Table 5.
Results of Multiple Linear and Logistic Regressions Examining the Interaction Between Frailty and HIV/Methamphetamine Group on Global Neurocognition and Everyday Functioning
| Outcome: global T-score | |||
|---|---|---|---|
| Predictor variables | Estimate | 95% CI | p |
| Frailty index score | −17.60 | −33.62 to −1.58 | .018 |
| HIV−/MA− (vs. HIV+/MA+) | 1.26 | −1.75 to 4.27 | .410 |
| HIV+/MA− (vs. HIV+/MA+) | −1.14 | −4.12 to 1.83 | .448 |
| Frailty index score × HIV−/MA− | 7.05 | −16.84 to 30.93 | .561 |
| Frailty index score × HIV+/MA− | 13.25 | −9.59 to 36.09 | .254 |
| Outcome: IADL dependence | |||
|---|---|---|---|
| Predictor variables | Logit | 95% CI | p |
| Frailty index score (per 0.10 U change) | 0.45 | 0.07 to 0.83 | .021 |
| HIV−/MA− (vs. HIV+/MA+) | −0.84 | −1.58 to −0.21 | .014 |
| HIV+/MA− (vs. HIV+/MA+) | 0.25 | −0.33 to 0.83 | .398 |
| Frailty index score × HIV−/MA− | −0.42 | −10.22 to 1.51 | .155 |
| Frailty index score × HIV+/MA− | 0.10 | −0.36 to 0.58 | .680 |
Bold values indicate p-values significant at <0.05.
Finally, the multiple logistic regression predicting IADL dependence showed main effects of frailty index score [b = 0.45; odds ratio (OR) = 1.56 (per 0.10 U increase); p = .02] and HIV/MA group, such that HIV−/MA− controls were less likely to be IADL dependent compared to the HIV+/MA+ group (b = −0.84; p = .01); however, the HIV+/MA− group was comparable to the HIV+/MA+ group (p > .05; Table 5). There was also no interaction between frailty index score and HIV/MA group (ps > .05). Exploratory follow-up analyses showed that the HIV+/MA+ group had the strongest positive relationship between frailty index score and likelihood of IADL dependence [χ2 = 5.63, OR = 2.15 (per 0.10 U increase in frailty index score), p = .03], followed by HIV+/MA− [χ2 = 5.56, OR = 1.72 (per 0.10 U increase in frailty index score), p = .03], then HIV−/MA− [χ2 = 0.004, OR = 1.02 (per 0.10 U increase in frailty index score), p = .95].
In an effort to ensure that certain items within our frailty index were not redundant, and that results were not driven by psychosocial variables within the frailty index (i.e., unemployment and depression), we reran primary analyses with different variations of the frailty index. Specifically, we examined whether the stairstep effect of frailty by HIV/MA group held, and whether frailty index score still related to global neurocognition and IADL dependence. First, to ensure that items 2–5 (lipid panel values) were not redundant with item 23 (hyperlipidemia), we created new frailty indexes removing either hyperlipidemia or all lipid panel values, and all results held. Next, to ensure that lifetime MDD was not redundant with current MDD, we created new frailty indexes removing either of these, and all results held. Finally, to ensure that psychosocial variables were not driving the relationship between HIV/MA group and frailty, we removed unemployment, current MDD, and lifetime MDD from the frailty index. Although the mean frailty index score was slightly lowered among the HIV+/MA+ group (M = 0.38, SD = 0.10) compared to our original 27-item frailty index, the ANOVA (p < 0.001) and post hoc Tukey HSD (ps < 0.05) analyses still showed a significant stairstep effect by group.
Discussion
Understanding the biological and functional consequences of HIV and lifetime MA use disorder is essential to provide optimal care for persons living with these conditions comorbidly. In support of our hypothesis, we found a stairstep pattern such that persons with comorbid HIV and MA use disorder had the highest frailty index scores, followed by HIV+/MA−, then HIV−/MA−. Although we did not find worse neurocognitive outcomes among those with comorbid HIV and MA compared to those with HIV only in our adult to older adult sample, there were meaningful relationships between frailty index scores and neurocognitive and everyday functioning, particularly within the comorbid group. Given prior research showing that frailty is related to future neurocognitive and functional decline among the general population,31–33 in addition to the higher levels of frailty in the comorbid group may indicate heightened risk for future neurocognitive, and everyday functioning declines as individuals age; however, longitudinal research is needed to explore this hypothesis. Overall, the current results lay the groundwork for a greater understanding of the complex relationships between HIV and lifetime MA use disorder on frailty, neurocognition, and everyday functioning.
The current study is one of the first to examine frailty in the context of comorbid HIV infection and lifetime MA use disorder diagnosis. Our findings, showing highest levels of frailty among HIV+/MA+ individuals are consistent with literature examining physiological consequences of each condition independently. Several reports have documented increased frailty among PLWH compared to their seronegative counterparts,46–48 as HIV portends a heightened risk for early acquisition of age-related conditions and physiological decrements.49 Although the effect of MA on “frailty” per se is less studied, the consequences of MA use on multiple physiological systems, including those that are captured in our frailty index, are well documented.28,29 Our results are also consistent with research showing greater risk for physiological damage in comorbid HIV disease and other substance use (e.g., alcohol).50 Importantly, the association between HIV/MA group and frailty remained significant even after covarying for other factors related to frailty (i.e., age) and factors that differed between groups. Our novel results suggest that comorbid HIV and MA relates to heightened physiological damage compared to those with HIV only, and that damage to these physiological systems remains detectable even when MA diagnosis is remote (i.e., more than 1 year before evaluation). Further understanding of the lasting effects of past MA misuse, with and without comorbid HIV infection, on frailty is warranted.
Notably, we found that frailty index score was not significantly related to MA use disorder characteristics (i.e., age at which participants first met criteria for MA use disorder, age at which participants most recently met criteria for MA use disorder, and years since last MA use disorder) within the HIV+/MA+ group. Although we do not have data to examine the relationship between specific MA use parameters (e.g., total lifetime grams of MA used) and frailty, our finding is consistent with prior research demonstrating that characteristics of MA use do not reliably predict neurocognitive functioning.51 Previous work indicates that such variability in the relationship between MA use and health outcomes may be explained by moderators on this relationship, including genetic susceptibility.52
Finally, we examined relationships between frailty index score and neurocognitive and everyday functioning and explored whether these relationships differ across HIV/MA groups. Consistent with hypotheses, higher frailty index score was related to worse neurocognitive functioning and greater likelihood of IADL dependence. Notably, the interaction terms within multiple regression models were not significant, indicating that the associations of frailty to neurocognitive and everyday functioning did not significantly differ by HIV/MA group; however, our exploratory within-group analyses revealed that these relationships were strongest among participants with comorbid HIV and MA. These findings support the clinical relevance of our frailty index, particularly among HIV+/MA+ individuals, and suggest that it is composed of components that represent physiological mechanisms underlying neurobehavioral functioning in this population. For example, the frailty index is hypothesized to capture erosion of homeostatic processes (e.g., metabolic factors, chronic inflammation) that likely result in adverse neurological outcomes (e.g., apoptosis, poor cerebral perfusion).33 Neurocognitive domain-specific analyses within the HIV+/MA+ group further indicate that domains of executive functioning and working memory may be especially affected by frailty, which is consistent with domains known to be impaired among individuals who are HIV+ and/or MA users.14–16
The current study is novel in its examination of the impact of HIV and lifetime MA on frailty; however, addressing its limitations is necessary to improve the methodology of future studies. First, there were no HIV-uninfected individuals who met criteria for a lifetime MA diagnosis in our sample, and thus, we cannot conclude whether our HIV+/MA+ group findings were simply reflective of an MA effect rather than a potential combined effect. Inclusion of a HIV−/MA+ group in future research is needed to uncover whether there may be an additive or interactive effect of HIV+/MA+ on frailty. We also did not collect data on specific MA or other substance use parameters (e.g., total grams used or total days of use) for our participants. Although MA use parameters do not reliably predict neurocognitive functioning,51 future work may benefit from exploring dose–response relationships between MA and frailty. Next, we had a relatively limited sample size for HIV+/MA+ individuals. Although this limited our power to detect statistically significant interaction effects between frailty and HIV/MA group on neurocognitive and everyday functioning, we also utilized group-stratified analyses. In addition, our frailty index, being composed of 27 variables, contains slightly below published recommendations to include at least 30 variables to best capture relationships between health and mortality23; however, less than 30 variables have been used in previous frailty indices.27
Given the heightened risk for acquired age-related declines with the increasing lifespan of PLWH, there is a need to understand factors that contribute to frailty and risk for impaired neurocognitive and everyday functioning to increase healthspan. Our results demonstrate that individuals with comorbid HIV and lifetime MA use disorder have high levels of frailty. Clinically, our findings highlight the need for more comprehensive medical assessment and care in this population, even when individuals do not demonstrate impairments in neurocognitive and everyday functioning. The variables included in a frailty index are often commonly collected in primary care settings, and could be compiled to evaluate risk for neurocognitive and functional decrements. Furthermore, because research among other clinical populations (e.g., older adults at risk for Alzheimer's disease) has found that frailty is associated with subsequent onset of neurocognitive and functional decline,33 our current findings indicate the need for future research to examine whether frailty may be a preclinical marker of subsequent neurocognitive and everyday functioning impairment among persons living with comorbid HIV and lifetime MA use disorder.
Funding
This research was supported by the National Institute of Mental Health [grant numbers R01 MH099987, P30 MH062512, and K23 MH107260 (salary support to R.C.M.)], the National Institute on Drug Abuse [grant number T32 DA031098 (stipend support to E.W.P., J.L.M., and L.M.C.)], and the National Institute on Alcohol Abuse and Alcoholism [grant number T32 AA013525 (stipend support to R.S.)].
Supplementary Material
Acknowledgments
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH.
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes the following: Director: Robert K. Heaton, PhD; Codirector: Igor Grant, MD; Associate Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and Scott Letendre, MD; Center Managers: Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, MPH; and Melanie Sherman; Neuromedical Component: Ronald J. Ellis, MD, PhD (PI), Scott Letendre, MD, J. Allen McCutchan, MD, Brookie Best, PharmD, Rachel Schrier, PhD, and Debra Rosario, MPH; Neurobehavioral Component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, Thomas D. Marcotte, PhD, Mariana Cherner, PhD, David J. Moore, PhD, and Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, PhD (PI), Monte S. Buchsbaum, MD, John Hesselink, MD, Sarah L. Archibald, MA, Gregory Brown, PhD, Richard Buxton, PhD, Anders Dale, PhD, and Thomas Liu, PhD; Neurobiology Component: Eliezer Masliah, MD (PI) and Cristian Achim, MD, PhD; Neurovirology Component: David M. Smith, MD (PI) and Douglas Richman, MD; International Component: J. Allen McCutchan, MD (PI) and Mariana Cherner, PhD; Developmental Component: Cristian Achim, MD, PhD (PI) and Stuart Lipton, MD, PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI) and Jennifer Marquie-Beck, MPH; Data Management and Information Systems Unit: Anthony C. Gamst, PhD (PI) and Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD (Co-PI), Anya Umlauf, MS, and Bin Tang, PhD.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Darke S, Kaye S, McKetin R, Duflou J: Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008;27:253–262 [DOI] [PubMed] [Google Scholar]
- 2. Henry BL, Minassian A, Perry W: Effect of methamphetamine dependence on everyday functional ability. Addict Behav 2010;35:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nordahl TE, Salo R, Leamon M: Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. J Neuropsychiatry Clin Neurosci 2003;15:317–325 [DOI] [PubMed] [Google Scholar]
- 4. Malta M, Strathdee SA, Magnanini MM, Bastos FI: Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction 2008;103:1242–1257 [DOI] [PubMed] [Google Scholar]
- 5. Degenhardt L, Mathers B, Guarinieri M, et al. : Meth/amphetamine use and associated HIV: Implications for global policy and public health. Int J Drug Policy 2010;21:347–358 [DOI] [PubMed] [Google Scholar]
- 6. Colfax G, Santos GM, Chu P, et al. : Amphetamine-group substances and HIV. Lancet 2010;376:458–474 [DOI] [PubMed] [Google Scholar]
- 7. Santos GM, Coffin PO, Das M, et al. : Dose-response associations between number and frequency of substance use and high-risk sexual behaviors among HIV-negative substance-using men who have sex with men (SUMSM) in San Francisco. J Acquir Immune Defic Syndr 2013;63:540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore DJ, Blackstone K, Woods SP, et al. : Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care 2012;24:1504–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott JC, Woods SP, Matt GE, et al. : Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol Rev 2007;17:275–297 [DOI] [PubMed] [Google Scholar]
- 10. Heaton R, Clifford D, Franklin D, et al. : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dean AC, Groman SM, Morales AM, London ED: An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 2013;38:259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langford D, Adame A, Grigorian A, et al. : Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr 2003;34:467–474 [DOI] [PubMed] [Google Scholar]
- 13. Maragos WF, Young KL, Turchan JT, et al. : Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem 2002;83:955–963 [DOI] [PubMed] [Google Scholar]
- 14. Rippeth JD, Heaton RK, Carey CL, et al. : Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 2004;10:1–14 [DOI] [PubMed] [Google Scholar]
- 15. Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I: Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav 2006;10:185–190 [DOI] [PubMed] [Google Scholar]
- 16. Kesby JP, Heaton RK, Young JW, et al. : Methamphetamine exposure combined with HIV-1 disease or gp120 expression: Comparison of learning and executive functions in humans and mice. Neuropsychopharmacology 2015;40:1899–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber E, Blackstone K, Iudicello JE, et al. : Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend 2012;125:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgan EE, Doyle KL, Minassian A, et al. : Elevated intraindividual variability in methamphetamine dependence is associated with poorer everyday functioning. Psychiatry Res 2014;220:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blackstone K, Iudicello JE, Morgan EE, et al. : Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med 2013;7:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitnitski AB, Mogilner AJ, Rockwood K: Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buta BJ, Walston JD, Godino JG, et al. : Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rockwood K, Mitnitski A: Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727 [DOI] [PubMed] [Google Scholar]
- 23. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K: A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salem BE, Nyamathi A, Brecht ML, et al. : Constructing and identifying predictors of frailty among homeless adults-a latent variable structural equations model approach. Arch Gerontol Geriatr 2014;58:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wing EJ: HIV and aging. International journal of infectious diseases: Int J Infect Dis 2016;53:61–68 [DOI] [PubMed] [Google Scholar]
- 26. Guaraldi G, Brothers TD, Zona S, et al. : A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015;29:1633–1641 [DOI] [PubMed] [Google Scholar]
- 27. Oppenheim H, Paolillo EW, Moore RC, et al. : Neurocognitive functioning predicts frailty index in HIV. Neurology 2018;91:e162–e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blaker AL, Northrop NA B.K. Y: Chapter 30—Peripheral Influences of Methamphetamine Neurotoxicity. In: Neuropathology of Drug Addictions and Substance Misuse, Vol. 2 (Preedy VR, ed.) Academic Press, San Diego, 2016, pp. 309–319 [Google Scholar]
- 29. Volkow ND, Fowler JS, Wang GJ, et al. : Distribution and pharmacokinetics of methamphetamine in the human body: Clinical implications. PLoS One 2010;5:e15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Astarita G, Avanesian A, Grimaldi B, et al. : Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PLoS One 2015;10:e0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA: Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc 2010;58:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA: Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med 2007;69:483–489 [DOI] [PubMed] [Google Scholar]
- 33. Robertson DA, Savva GM, Kenny RA: Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev 2013;12:840–851 [DOI] [PubMed] [Google Scholar]
- 34. Substance Abuse and Mental Health Services Administration (SAMHSA): Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. SAMHSA, Rockville, MD, 2012 [Google Scholar]
- 35. Montoya JL, Cattie J, Morgan E, et al. : The impact of age, HIV serostatus and seroconversion on methamphetamine use. Am J Drug Alcohol Abuse 2016;42:168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore RC, Paolillo EW, Heaton A, Fazeli PL, Jeste DV, Moore DJ: Clinical utility of the UCSD Performance-Based Skills Assessment-Brief (UPSA-B) in adults living with HIV: Associations with neuropsychological impairment and patient-reported everyday functioning difficulties. PLoS One 2017;12:e0183614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore RC, Hussain MA, Watson CW, et al. : Grit and ambition are associated with better neurocognitive and everyday functioning among adults living with HIV. AIDS Behav 2018;22:3214–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Composite International Diagnostic Interview (CIDI, version 2.1) [computer program]. World Health Organization, Geneva, Switzerland, 1997 [Google Scholar]
- 39. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). American Psychiatric Association, Washington, DC, 2000 [Google Scholar]
- 40. Heaton R, Miller SW, Taylor MJ, Grant I: Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Lutz, FL, 2004 [Google Scholar]
- 41. Heaton RK, Taylor M, Manly J: Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky D.S., Heaton R.K., Chelune G., Ivnik R., Bornstein R.A., Prifitera A., & Ledbetter M. (Eds.), Clinical Interpretation of the WAIS-III and WMS-III. Academic Press, San Diego, CA, 2003. p. 181–210 [Google Scholar]
- 42. Norman MA, Moore DJ, Taylor M, et al. : Demographically corrected norms for African Americans and Caucasians on the Hopkins verbal learning test–revised, brief visuospatial memory test–revised, Stroop color and word test, and Wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol 2011;33:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heaton RK, Marcotte TD, Mindt MR, et al. : The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10:317–331 [DOI] [PubMed] [Google Scholar]
- 44. Obermeit LC, Beltran J, Casaletto KB, et al. : Evaluating the accuracy of self-report for the diagnosis of HIV-associated neurocognitive disorder (HAND): Defining “symptomatic” versus “asymptomatic” HAND. J Neurovirol 2017;23:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doyle KL, Morgan EE, Morris S, et al. : Real-world impact of neurocognitive deficits in acute and early HIV infection. J Neurovirol 2013;19:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Althoff KN, Jacobson LP, Cranston RD, et al. : Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014;69:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Desquilbet L, Jacobson LP, Fried LP, et al. : HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62:1279–1286 [DOI] [PubMed] [Google Scholar]
- 48. Piggott DA, Muzaale AD, Mehta SH, et al. : Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013;8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guaraldi G, Palella FJ, Jr.: Clinical implications of aging with HIV infection: Perspectives and the future medical care agenda. AIDS 2017;31 Suppl 2:S129–S135 [DOI] [PubMed] [Google Scholar]
- 50. Justice AC, McGinnis KA, Tate JP, et al. : Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016;161:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cherner M, Suarez P, Casey C, et al. : Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend 2010;106:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cherner M, Bousman C, Everall I, et al. : Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: Preliminary findings. J Int Neuropsychol Soc 2010;16:890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.