Abstract
Significance: The proangiogenic mediator vascular endothelial growth factor (VEGF) plays an important role in cutaneous wound repair. Most of the work on VEGF and wound healing has focused on its role in mediating angiogenesis and how this affects wound closure rates. Less is known about how VEGF affects other phases of wound healing, including scar formation.
Recent Advances: Over the last 10 years, mounting evidence suggests that VEGF plays an important role in regulating scar tissue production. Multiple studies have linked high VEGF levels with scar formation in normal, hypertrophic, and keloid scars. In addition, there is experimental evidence that VEGF inhibition can reduce scar tissue deposition.
Critical Issues: While there is evidence that VEGF can promote scar formation in the skin, there are several unanswered questions that remain. First, the mechanisms by which VEGF promotes scar formation have not been completely characterized. While both indirect and direct mechanisms could be involved, clear evidence for a specific mechanism is lacking. In addition, despite the availability of anti-VEGF drugs, the potential value in targeting VEGF to attenuate scar formation clinically is not yet known.
Future Directions: While there are a significant number of studies examining the effects of VEGF on angiogenesis and wound closure, much less attention has been paid to the contribution of VEGF to scar tissue production. Additional studies are required to learn more about how VEGF regulates scar formation and whether VEGF inhibition could be used clinically to manage scars.
Keywords: VEGF, scar, angiogenesis, hypertrophic scar, keloid
Traci A. Wilgus, PhD.

Scope and Significance
Wound healing in fully developed skin is an imperfect process that leads to the formation of scar tissue rather than normal tissue. Scars are characterized by disorganized collagen and a lack of skin appendages. The biomechanical and architectural properties of scars are unique; as a result, scar tissue is weaker and functions at a suboptimal level compared to normal skin. Many different proteins produced during wound healing have been shown to regulate scar formation in the skin with the most well-studied fibrogenic mediator being transforming growth factor-beta. Another protein that is produced at high levels during the repair process is vascular endothelial growth factor (VEGF or VEGF-A), and there has been mounting evidence over the last 10 years that VEGF stimulates scar tissue production. This review will discuss published data implicating VEGF in the regulation of scar formation, as well as possible underlying mechanisms and potential clinical implications.
Translational Relevance
VEGF has been studied extensively in the context of cutaneous wound healing. Most studies have focused on the role of VEGF as a proangiogenic mediator, which is understandable given the importance of angiogenesis in wound repair. However, recent studies have indicated that VEGF may also act as a profibrotic mediator. The mechanisms by which VEGF promotes scar formation are not entirely clear, but several have been proposed. These include indirect effects associated with the stimulation of endothelial cells or amplification of inflammation and possible direct effects on fibroblasts. Understanding more about how VEGF influences scar formation could aid in the development of new therapeutic strategies to minimize scar formation.
Clinical Relevance
Scarring is a significant clinical problem that affects millions of patients. Unfortunately, there is currently a lack of clinically beneficial noninvasive antiscar therapies, so finding new therapeutic targets to control scar formation is important. Several studies in animal models suggest that blocking the biological activity of VEGF may be beneficial for limiting scar formation; however, the clinical utility of this approach is not yet known. There are a number of FDA-approved anti-VEGF drugs on the market for the treatment of cancer and other diseases, so there are options available to investigate clinically whether blocking the function of VEGF can prevent or treat scars. Further studies are needed to explore this possibility.
Overview
The cutaneous wound repair process is characterized by a series of distinct stages that begins with an acute inflammatory response and is followed by periods of cellular proliferation and extracellular matrix (ECM) deposition/remodeling, ultimately leading to the formation of a scar.1–3 Scars are produced to quickly patch the damaged area in the dermal layer of the skin, but the deposition of scar tissue as a replacement for the original tissue is not ideal. Scars are weaker than normal skin and, depending on the location and severity, they can cause restricted tissue growth and mobility of the joints.4 Scars are also a cause of significant morbidity and the cosmetic issues associated with scarring can have substantial consequences. Studies have shown that scars can have psychosocial implications and can significantly affect quality of life by negatively affecting confidence, mood, and personal relationships.5 Unfortunately, clinically beneficial antiscar therapies are limited, and there is currently no standardized, accepted method for treatment.6 Given that millions of people are affected by scars each year, either from traumatic events or surgical procedures, the identification of new therapeutic targets has the potential to make an impact on the lives of a significant number of patients. In the sections below, published studies suggesting that VEGF may be a beneficial target to reduce scarring will be discussed.
Vascular Endothelial Growth Factor
Angiogenesis, or the generation of new blood vessels, is a prominent feature of healing wounds.7 New blood vessels are needed during the repair process to deliver oxygen and nutrients present in the blood to the site of injury. One of the most potent and well-studied proangiogenic mediators is VEGF (or VEGF-A).
VEGF-A (referred to as VEGF throughout this article) is a 45 kDa heterodimeric heparin-binding protein that is one of several proteins in a family of VEGF. VEGF was initially described as a vascular permeability factor,8 and later it was found to act as a potent proangiogenic mediator, in part, by stimulating endothelial cell proliferation, migration, differentiation, and survival.9–11 Several isoforms of the VEGF protein can be produced from alternatively spliced variants of the VEGF mRNA.12 Other members of the VEGF family include VEGF-B, VEGF-C, VEGF-D, and placental growth factor, and each family member binds to different tyrosine kinase receptors.13 VEGF-A binds to VEGF receptor-1 (VEGFR-1) and VEGF receptor-2 (VEGFR-2) on endothelial cells.14 These receptors are characterized by seven extracellular immunoglobulin-like domains, a transmembrane region, and an internal tyrosine kinase domain.15 When VEGF binds to its receptors on endothelial cells, autophosphorylation of tyrosine residues activates various signaling pathways that stimulate the proliferation, migration, and survival needed for a strong angiogenic response.16
VEGF and Wound Healing
Numerous studies have shown that VEGF is important for wound healing in the skin. VEGF is highly upregulated in healing animal17 and human18 wounds. VEGF production has been demonstrated in epidermal keratinocytes, mast cells, monocytes/macrophages, and activated fibroblasts after injury.17–23 The overall importance of VEGF in wound healing has been demonstrated by studies in which treatment with VEGF/VEGFR inhibitors or genetic deletion of VEGF in specific cell types (i.e., keratinocytes and myeloid cells) results in delayed wound closure.22–25 In addition, diabetic wounds, which heal more slowly than normal wounds, generally have less VEGF,26–28 and enhancing VEGF levels in diabetic wounds can accelerate healing or improve the quality of the healed wound.28–31 While most of these effects have been attributed to the stimulation of angiogenesis by VEGF, it should be noted that multiple nonendothelial cell types have been reported to contain VEGFRs (reviewed in13); therefore, it is possible that some of the effects of VEGF are not related to angiogenesis. While most functional studies on VEGF in wound healing have focused on its ability to accelerate epidermal closure, VEGF has also been implicated in dermal repair processes. Based on the results of multiple studies, there appears to be a strong correlation between VEGF levels or VEGF activity and the amount of granulation tissue formed in both healthy and diabetic animals.20,28–33 In addition, VEGF has been shown to affect wound breaking strength in some cases.31,34 This could result from alterations in collagen production or the organization/arrangement of the deposited collagen. The ability of VEGF to influence dermal aspects of repair suggests that VEGF may alter fibroblast behavior during wound healing, ultimately affecting the scar formation process.
VEGF and Scar Formation
During the terminal stages of wound healing, fibroblasts restore the dermal layer of the skin by depositing and remodeling collagen, eventually forming a mature scar. In most cases, a normal scar will form; however, abnormal scars such as hypertrophic scars and keloids can develop when fibroblasts produce an overabundance of collagen. Hypertrophic scars are raised red scars that are generally confined to the borders of the original wound, whereas keloids are fibroproliferative lesions characterized by the formation of scar tissue that extends beyond the boundaries of the initial wound. A number of studies have now suggested that VEGF contributes to the formation of normal scars, hypertrophic scars, and keloids.
Normal Scars
Studies using both human samples and animal models have linked VEGF to normal scars. Kumar, et al. showed a time-dependent increase in microvessel density and VEGF levels in human surgical scars.35 Vessel density and VEGF followed similar patterns, with the highest levels observed between 2 and 12 weeks postsurgery. Although VEGF levels declined over time, they remained significantly elevated in scars out to 2 years after surgery compared to normal skin.35 The relationship between VEGF and scarring has also been examined in animal studies. Using a murine model of fetal wound healing, in which incisional wounding of the skin at embryonic day 15 (E15) results in scarless healing and wounding at embryonic day 18 (E18) results in scar formation, our laboratory showed that VEGF protein levels were higher in scar-forming E18 wounds compared with scarless E15 wounds.36 In contrast, a fetal wound healing study in a rat excisional wound model suggested an increase in VEGF mRNA levels in scarless wounds.37 It should be noted that the murine and rat fetal wound studies differed in the type of wound (incisional vs. excisional) and how VEGF was measured (protein vs. mRNA), which could explain the differences in the reported results between the two studies. Interestingly, a study on oral mucosal wound healing demonstrated a reduction in VEGF in scarless wounds38 similar to what was observed in scarless murine fetal wounds.
In addition to correlative studies, functional studies also implicate VEGF in the process of scar formation in vivo. Injection of recombinant VEGF into E15 fetal wounds, which normally heal scarlessly, caused them to heal with prominent scars.36 In addition, in a model of incisional wound healing in adult mice, neutralization of VEGF and inhibition of angiogenesis by injection of anti-VEGF antibodies caused a decrease in the size of scars that formed and also led to a more normal structure of collagen fibrils.36 Overall, data from both human and animal studies support a role for VEGF in normal scar formation.
Hypertrophic scars
With the exception of one study that did not report differences between human hypertrophic and normal scars between 4 and 7 months after burn injury,39 all other studies examining VEGF in hypertrophic scars have reported that high VEGF levels are associated with hypertrophic scarring. Cao, et al. showed that VEGF levels are higher in proliferative hypertrophic scars from trauma or burn injury and that VEGF levels decline in more mature scars.40 In another study looking at sternotomy incisions, microvascular density was increased in hypertrophic scars at 12 and 52 weeks postsurgery compared to normal skin and normal scars.41 Although no significant changes in VEGF mRNA levels were observed, immunostaining for VEGF showed more intense staining in keratinocytes and blood vessels in hypertrophic scar samples.41 Wang, et al. showed an increase in angiogenesis and VEGF mRNA and protein expression in hypertrophic scars compared to normal skin, with VEGF localization observed primarily in keratinocytes and endothelial cells.42 VEGF levels have also been examined in a large animal model of hypertrophic scarring. A red Duroc pig model, in which shallow wounds form normal scars and deep wounds form scars that resemble human hypertrophic scars, VEGF protein levels were shown to be elevated in deep wounds compared with shallow wounds and normal skin.43
Several studies have also suggested that VEGF may be a viable target to reduce hypertrophic scarring. In patients receiving interferon-alpha2b treatment for burn injury-induced hypertrophic scars, clinical improvement was associated with a reduction in angiogenesis.42 In addition, cultured endothelial cells treated with interferon-alpha2b displayed reduced responses to VEGF as measured by tube formation and proliferation, possibly due to inhibiting the expression of VEGFRs on endothelial cells.42 This suggests that the ability of interferon to improve hypertrophic scarring may depend, in part, on inhibition of VEGF. A more recent study by Kwak, et al. demonstrated the potential of VEGF as a therapeutic target for hypertrophic scars more directly.44 Using a rabbit ear model of hypertrophic scarring, the authors showed that local injection of bevacizumab, a humanized anti-VEGF antibody that is used to treat various malignancies, reduced the appearance of scars.44 The scars treated with bevacizumab had fewer blood vessels, were less red and less raised, and had a less dense collagen arrangement based on histology.44 Taken together, the studies showing a correlation between VEGF levels and hypertrophic scarring along with studies indicating that VEGF inhibition can minimize scarring suggest that VEGF could be a viable target to treat hypertrophic scars.
Keloids
Multiple studies have linked VEGF with keloid scars. Gira, et al. demonstrated higher VEGF mRNA expression in keloids by in situ hybridization, with the keratinocytes of hyperplastic epidermis of expressing higher VEGF compared with keratinocytes in normal adjacent epidermis.45 Mogili, et al. found an increase in serum VEGF levels as well as increases in VEGF mRNA and protein in keloid versus normal tissue.46 Several other studies have also reported an increase in VEGF protein levels in keloid versus normal tissue,47,48 and immunohistochemistry results from one of these studies suggested that there may be a change in VEGF localization in keloid keratinocytes.47 Nuclear staining was observed in the keratinocytes of normal skin with an increase in cytoplasmic staining in keloid keratinocytes47; however, the functional significance of this finding is not entirely clear. In addition to higher tissue levels, VEGF gene expression and protein production have also been reported to be higher in cultured keloid fibroblasts.47–49
While there are no good in vivo models to study the potential effects of VEGF inhibition on keloid development/growth, several human studies have hinted that VEGF levels may be connected to therapeutic efficacy. Salem, et al. examined VEGF levels in biopsies taken before and after treatment with either intralesional injection with a corticosteroid (triamcinolone acetonide) or with cryotherapy.50 Immunostaining showed that there was a reduction in dermal VEGF staining after treatment compared with before treatment, and a decrease in VEGF levels were associated with resolution of nodular collagen and a favorable clinical response.50 Wu, et al. showed a similar reduction in VEGF staining after intralesional steroid injection and demonstrated in in vitro studies that VEGF reversed the ability of dexamethasone to reduce keloid fibroblast proliferation.51 Dexamethasone also reduced VEGF mRNA and protein levels and inhibited tube formation in cultured endothelial cells,51 suggesting that the therapeutic effects of steroid treatment on keloids may be related to the drug's ability to reduce VEGF and inhibit neovascularization.
Potential Mechanisms for the Promotion of Scarring By VEGF
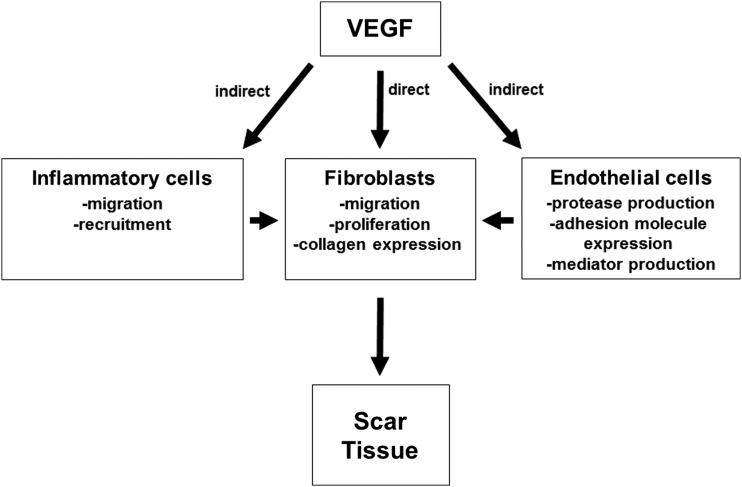
As discussed in the previous section, there are several lines of evidence suggesting that VEGF plays a role in scar formation (summarized in Table 1). Despite the positive association between VEGF levels and scar formation, little is known about the mechanisms responsible. However, it is possible that VEGF regulates scar formation indirectly, through its ability to stimulate endothelial cells or by affecting inflammation, or directly by influencing fibroblasts. (Fig. 1).
Table 1.
Summary of evidence that vascular endothelial growth factor stimulates scar formation
| SCAR Type | Finding |
|---|---|
| Normal scars | Higher VEGF levels in scar tissue35,36 Lower VEGF levels in scarless wounds36,38 VEGF inhibition reduces scarring36 |
| Hypertrophic scars | Higher VEGF levels in hypertrophic scar tissue40–43 VEGF inhibition reduces scarring44 |
| Keloids | Higher VEGF levels in keloid tissue45,47,48,50 Higher VEGF levels in keloid fibroblasts47,49 Lower VEGF levels in treated keloid tissue50,51 |
VEGF, vascular endothelial growth factor.
Figure. 1.
Potential mechanisms of VEGF in scar tissue production. VEGF could promote scar formation via indirect or direct mechanisms. VEGF could enhance the migration and recruitment of inflammatory cells to the site of injury, and these cells could produce factors that stimulate scar tissue formation by fibroblasts. VEGF could also stimulate endothelial cells to produce proteases, express adhesion molecules that aid in inflammatory cell recruitment, or produce mediators that stimulate scar tissue production by fibroblasts. Finally, VEGF could directly promote scar tissue production by affecting migration, proliferation, or collagen production by fibroblasts. VEGF, vascular endothelial growth factor.
Indirect mechanisms: Endothelial and inflammatory cells
Endothelial cells are likely the primary target of VEGF and the cell type able to most efficiently respond to VEGF in a healing wound. Therefore, it is possible that VEGF promotes scar tissue by stimulating endothelial cells. During the process of sprouting angiogenesis, matrix metalloproteinases and other proteases must be produced to degrade the ECM surrounding the original vessel so that the tip cells can migrate into the tissue and guide the formation of a new sprout. This degradation of ECM components may stimulate compensatory collagen production by neighboring fibroblasts, leading to more scar tissue deposition. VEGF could also enhance the production of profibrotic mediators (cytokines, growth factors, etc.) by endothelial cells that promote collagen production by fibroblasts. It is also known that VEGF stimulates the expression of adhesion molecules by endothelial cells that facilitate the recruitment inflammatory cells from the bloodstream into the skin.52 In fact, transgenic mice, in which VEGF is overexpressed in epidermal keratinocytes, display an increase in leukocyte–endothelial interactions such as rolling and adhesion53 which likely contribute to the increase in inflammation seen in these mice. Furthermore, high VEGF levels in the skin result in an increase in the presence of various inflammatory cell types in the dermis, including mast cells and macrophages,53,54 cell types that have been linked to granulation tissue and/or scar formation21,55–59 and are found in high numbers in hypertrophic scars and keloids.60–65 It should be noted that VEGF may also lead to direct recruitment and/or activation of some inflammatory cells, such as monocytes/macrophages, which have been shown to express VEGFRs and migrate in response to VEGF.66–68
Direct mechanism: Fibroblasts
Despite the lack of a comprehensive study determining whether VEGF can directly affect fibroblasts during wound healing to enhance scar formation, several studies have suggested that VEGF can stimulate cultured dermal fibroblasts. Wu, et al. observed an increase in fibroblast proliferation upon treatment with VEGF.51 They also showed that VEGF treatment could reverse dexamethasone-induced suppression of keloid fibroblast proliferation.51 In another set of studies, Brem, et al. showed that VEGF treatment increased migration of dermal fibroblasts isolated from human wounds using a scratch assay.31 In cultured human fibroblasts from normal or scleroderma patients, VEGF was shown to increase collagen production.69 In addition to stimulating proliferation, migration, and collagen production, VEGF treatment was shown to increase plasminogen activator inhibitor-1 levels in cultured keloid fibroblasts through ERK1/2 signaling,48 suggesting that VEGF may stimulate the production of mediators by fibroblasts that regulate the production and degradation of collagen.
Altering VEGF for Therapeutic Purposes
The data supporting a role for VEGF in the development of scar formation suggest that VEGF could be targeted to minimize scarring and fibrosis. In fact, several studies in animal models support the idea that treatment with anti-VEGF drugs can reduce scarring.36,44 These studies used anti-VEGF antibodies and showed a reduction in scar formation. While there are currently no published clinical studies evaluating anti-VEGF drugs to prevent or treat scars, there are several FDA-approved drugs for cancer therapy that could be tested. For example, bevacizumab is a humanized monoclonal anti-VEGF antibody that neutralizes the bioactivity of VEGF by preventing it from binding to its receptor, and there are a number of small molecule tyrosine kinase receptors, such as sunitinib and sorafenib, which block VEGFR signaling.
There would be several potential caveats to using a VEGF inhibitor in a wound setting to reduce scarring, as blocking the activity of VEGF could delay wound closure. The risk to benefit ratio would have to be carefully considered, and the patient would have to be willing to sacrifice any potential delays in healing rates for a favorable cosmetic outcome. Characteristics of the patient, such as age and other conditions such as diabetes that might increase risk of wound healing complications, and characteristics of the wound, such as size, type (traumatic vs. surgical wound), and location, would have to be carefully considered.
Conclusions
Multiple published studies suggest that VEGF can influence the amount of scar tissue produced in a healing wound. These include studies linking higher VEGF levels with normal, hypertrophic, and keloid scarring, studies demonstrating that VEGF inhibition can limit scar formation in normal and hypertrophic scar models, and studies showing reduced VEGF levels are associated with clinical improvements in keloids after therapy. Together, the evidence suggests VEGF could be a viable target to minimize scarring clinically. More work will have to be done and clinical testing will have to be performed to determine whether VEGF inhibition would be an effective antiscarring strategy. Since there are few proven treatment options available for scars, therapies with new mechanisms of action could have a significant impact in this area.
Take-Home Messages.
VEGF is known for its role as a proangiogenic mediator in healing skin wounds, but recent studies have suggested that it also helps mediate scar formation.
VEGF levels correlate with scar formation in several types of scars. Reduced VEGF levels are observed in scarless wounds, and elevated VEGF levels are associated with scar formation, especially in abnormal scars such as hypertrophic scars and keloids.
Inhibition of VEGF can reduce scar formation in animal models, suggesting that VEGF could be targeted clinically to limit scar formation.
Further studies are needed to evaluate the clinical efficacy of VEGF inhibitors as antiscarring agents.
Acknowledgments and Funding Sources
Dr. Wilgus acknowledges previous funding for work on VEGF and scar formation from National Institutes of Health and Wound Healing Foundation-3M fellowships. Current funding from the National Institutes of Health (AR071115) is also acknowledged.
Abbreviations and Acronyms
- E15
embryonic day 15
- E18
embryonic day 18
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
About the Author
Traci A.Wilgus, PhD, is an Associate Professor in the Department of Pathology at The Ohio State University Wexner Medical Center. Research in her laboratory focuses on the contribution of inflammation and angiogenesis to skin carcinogenesis, wound healing, and scar formation.
Author Disclosure and Ghostwriting
The author declares that there are no competing financial interests to disclose. The content of this article was expressly written by the author listed and no ghostwriters were used.
References
- 1. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 2. Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 3. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 4. Zhu Z, Ding J, Tredget EE. The molecular basis of hypertrophic scars. Burns Trauma 2016;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 2008;61:1049–1058 [DOI] [PubMed] [Google Scholar]
- 6. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg 2014;40:825–831 [DOI] [PubMed] [Google Scholar]
- 7. DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol 2016;100:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–985 [DOI] [PubMed] [Google Scholar]
- 9. Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 1998;273:30336–30343 [DOI] [PubMed] [Google Scholar]
- 10. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–1309 [DOI] [PubMed] [Google Scholar]
- 11. Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309–1312 [DOI] [PubMed] [Google Scholar]
- 12. Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 1993;4:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carmeliet P, Ruiz de Almodovar C. VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci 2013;70:1763–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676 [DOI] [PubMed] [Google Scholar]
- 16. Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2012;2:a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown LF, Yeo KT, Berse B, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992;176:1375–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nissen NN, Polverini PJ, Gamelli RL, DiPietro LA. Basic fibroblast growth factor mediates angiogenic activity in early surgical wounds. Surgery 1996;119:457–465 [DOI] [PubMed] [Google Scholar]
- 19. Kishimoto J, Ehama R, Ge Y, et al. In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am J Pathol 2000;157:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012;120:613–625 [DOI] [PubMed] [Google Scholar]
- 21. Shiota N, Nishikori Y, Kakizoe E, et al. Int Arch Allergy Immunol 2010;151:80–88 [DOI] [PubMed] [Google Scholar]
- 22. Rossiter H, Barresi C, Pammer J, et al. Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res 2004;64:3508–3516 [DOI] [PubMed] [Google Scholar]
- 23. Stockmann C, Kirmse S, Helfrich I, et al. A wound size-dependent effect of myeloid cell-derived vascular endothelial growth factor on wound healing. J Invest Dermatol 2011;131:797–801 [DOI] [PubMed] [Google Scholar]
- 24. Wilgus TA, Matthies AM, Radek KA, et al. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol 2005;167:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobi J, Tam BY, Sundram U, et al. Discordant effects of a soluble VEGF receptor on wound healing and angiogenesis. Gene Ther 2004;11:302–309 [DOI] [PubMed] [Google Scholar]
- 26. Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem 1995;270:12607–12613 [DOI] [PubMed] [Google Scholar]
- 27. Stallmeyer B, Pfeilschifter J, Frank S. Systemically and topically supplemented leptin fails to reconstitute a normal angiogenic response during skin repair in diabetic ob/ob mice. Diabetologia 2001;44:471–479 [DOI] [PubMed] [Google Scholar]
- 28. Romano Di Peppe S, Mangoni A, Zambruno G, et al. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther 2002;9:1271–1277 [DOI] [PubMed] [Google Scholar]
- 29. Galeano M, Deodato B, Altavilla D, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia 2003;46:546–555 [DOI] [PubMed] [Google Scholar]
- 30. Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004;164:1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brem H, Kodra A, Golinko MS, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol 2009;129:2275–2287 [DOI] [PubMed] [Google Scholar]
- 32. Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg 1999;134:200–205 [DOI] [PubMed] [Google Scholar]
- 33. Deodato B, Arsic N, Zentilin L, et al. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther 2002;9:777–785 [DOI] [PubMed] [Google Scholar]
- 34. Christoforidis JB, Wang J, Jiang A, et al. The effect of intravitreal bevacizumab and ranibizumab on cutaneous tensile strength during wound healing. Clin Ophthalmol 2013;7:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg 2009;96:1484–1491 [DOI] [PubMed] [Google Scholar]
- 36. Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 2008;88:579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colwell AS, Beanes SR, Soo C, et al. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg 2005;115:204–212 [PubMed] [Google Scholar]
- 38. Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 2005;84:309–314 [DOI] [PubMed] [Google Scholar]
- 39. Hakvoort T, Altun V, van Zuijlen PP, de Boer WI, van Schadewij WA, van der Kwast TH. Transforming growth factor-beta(1), -beta(2), -beta(3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burn scars. Eur Cytokine Netw 2000;11:233–239 [PubMed] [Google Scholar]
- 40. Cao PF, Xu YB, Tang JM, Yang RH, Liu XS. HOXA9 regulates angiogenesis in human hypertrophic scars: induction of VEGF secretion by epidermal stem cells. Int J Clin Exp Pathol 2014;7:2998–3007 [PMC free article] [PubMed] [Google Scholar]
- 41. van der Veer WM, Niessen FB, Ferreira JA, et al. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen 2011;19:292–301 [DOI] [PubMed] [Google Scholar]
- 42. Wang J, Chen H, Shankowsky HA, Scott PG, Tredget EE. Improved scar in postburn patients following interferon-alpha2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res 2008;28:423–434 [DOI] [PubMed] [Google Scholar]
- 43. Zhu KQ, Engrav LH, Armendariz R, et al. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig-further similarities between female, Duroc scar and human hypertrophic scar. Burns 2005;31:5–10 [DOI] [PubMed] [Google Scholar]
- 44. Kwak DH, Bae TH, Kim WS, Kim HK. Anti-vascular endothelial growth factor (Bevacizumab) therapy reduces hypertrophic scar formation in a rabbit ear wounding model. Arch Plast Surg 2016;43:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gira AK, Brown LF, Washington CV, Cohen C, Arbiser JL. Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J Am Acad Dermatol 2004;50:850–853 [DOI] [PubMed] [Google Scholar]
- 46. Mogili NS, Krishnaswamy VR, Jayaraman M, Rajaram R, Venkatraman A, Korrapati PS. Altered angiogenic balance in keloids: a key to therapeutic intervention. Transl Res 2012;159:182–189 [DOI] [PubMed] [Google Scholar]
- 47. Ong CT, Khoo YT, Tan EK, et al. Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. J Pathol 2007;211:95–108 [DOI] [PubMed] [Google Scholar]
- 48. Wu Y, Zhang Q, Ann DK, Akhondzadeh A, Duong HS, Messadi DV, Le AD. Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts. Am J Physiol Cell Physiol 2004;286:C905–C912 [DOI] [PubMed] [Google Scholar]
- 49. Fujiwara M, Muragaki Y, Ooshima A. Upregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activity. Arch Dermatol Res 2005;297:161–169 [DOI] [PubMed] [Google Scholar]
- 50. Salem A, Assaf M, Helmy A, et al. Role of vascular endothelial growth factor in keloids: a clinicopathologic study. Int J Dermatol 2009;48:1071–1077 [DOI] [PubMed] [Google Scholar]
- 51. Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol 2006;126:1264–1271 [DOI] [PubMed] [Google Scholar]
- 52. Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol 2006;80:247–257 [DOI] [PubMed] [Google Scholar]
- 53. Detmar M, Brown LF, Schon MP, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998;111:1–6 [DOI] [PubMed] [Google Scholar]
- 54. Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J 2004;18:1111–1113 [DOI] [PubMed] [Google Scholar]
- 55. Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184:3964–3977 [DOI] [PubMed] [Google Scholar]
- 56. Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen 2008;16:226–233 [DOI] [PubMed] [Google Scholar]
- 58. Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 2012;132:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One 2014;9:e85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–3261 [DOI] [PubMed] [Google Scholar]
- 61. Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns 2006;32:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kischer CW, Bunce H, 3rd, Shetlah MR. Mast cell analyses in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol 1978;70:355–357 [DOI] [PubMed] [Google Scholar]
- 63. Smith CJ, Smith JC, Finn MC. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J Burn Care Rehabil 1987; 8:126–131 [DOI] [PubMed] [Google Scholar]
- 64. Wang J, Ding J, Jiao H, et al. Human hypertrophic scar-like nude mouse model: characterization of the molecular and cellular biology of the scar process. Wound Repair Regen 2011;19:274–285 [DOI] [PubMed] [Google Scholar]
- 65. Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol 2012;167:1053–1066 [DOI] [PubMed] [Google Scholar]
- 66. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996;87:3336–3343 [PubMed] [Google Scholar]
- 67. Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 2001;97:785–791 [DOI] [PubMed] [Google Scholar]
- 68. Shen H, Clauss M, Ryan J, et al. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993;81:2767–2773 [PubMed] [Google Scholar]
- 69. Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014;73:1880–1887 [DOI] [PubMed] [Google Scholar]