Abstract
Background:
Despite several neuroimaging studies in the past few years, the exact pathophysiology responsible for the development of bipolar disorder (BD) is still not completely known. Importantly, to the best of our knowledge, no study from India has examined resting state (RS) connectivity abnormalities in BD using regional homogeneity (ReHo). Hence, we examined spontaneous brain activity in patients with BD using RS functional magnetic resonance imaging (RS-fMRI).
Aim:
The aim of the study is to examine the spontaneous brain activity in patients with BD-I using ReHo approach and RS-fMRI compared to age- and gender-matched healthy control (HC).
Materials and Methods:
A total of 20 patients with BD and 20 age-, gender-, and education-matched HCs participated in the study. The fMRI data were obtained using 1.5T scanner. RS-fMRI abnormalities were analyzed using ReHo method.
Results:
Compared to healthy adults, significantly increased ReHo in the BD group was found in the right precuneus, right insula, right supramarginal gyrus, left superior frontal gyrus, right inferior frontal gyrus, right precentral gyrus, and right paracentral lobule. No region had significantly lower ReHo values in BD patients compared to controls.
Conclusion:
These results suggested that abnormal local synchronization of spontaneous brain activity is present in the frontoparietoinsular region which may be related to the pathophysiology of BD.
Keywords: Bipolar disorder, functional magnetic resonance imaging, regional homogeneity
INTRODUCTION
Bipolar disorder (BD) affects 1.5%–3% of the population and remains a significant source of morbidity, poor work functioning, and mortality.[1] BD is characterized by episodes of mania and depression. Despite several neuroimaging studies in the past few years, the exact pathophysiology responsible for the development of BD is still unknown. Functional magnetic resonance imaging (fMRI) studies use two modalities, i.e., task-related and resting-state (RS) method. Task-based studies lack consistency and applicability due to differences in neuropsychological paradigm and activation of specific task-based brain region rather than giving information about whole-brain functioning.[2] RS fMRI is an important tool to explore the spontaneous brain activity in BD.
Currently, the RS data processing methods include seed-based approaches, amplitude of low-frequency fluctuation (ALFF) and fractional ALFF, independent component analysis, regional homogeneity (ReHo) analysis, and restricted global brain connectivity.[3] ReHo, a newly developed method, reflects the temporal homogeneity of the regional blood oxygen level-dependent signal rather than its density and serves as a complement for resting-state functional connectivity. Abnormal ReHo may be relevant to the changes of temporal aspects of neural activity in the regional area and can be used to find abnormal activity in the whole brain.[4] The method does not demand a preliminary hypothesis such as a defined volume of interest for correlation analysis. Besides the functional connectivity analysis, the ReHo method can provide new insights in analyzing disease-related RS-fMRI data. An abnormal functional connectivity between two distinct regions indicates an abnormal relationship between these two regions; however, the method does not confirm which specific region is abnormal. In contrast, an abnormal ReHo indicates abnormal brain activity in a specific region. ReHo assumes that, within a functional cluster, the hemodynamic feature of each voxel would be similar or synchronous with that of others, and that this similarity could be altered or modulated in different situations. Higher ReHo indicates more synchronization in the functional cluster, which may illustrate functional synchronization in certain brain regions.[4] This method has already been used for investigation of functional modulations in the RS for patients with Alzheimer's disease,[5] schizophrenia,[6] and Parkinson's disease.[7]
Till date, there are very few studies in BD using ReHo approach.[8,9,10,11,12] Out of these five studies, two are in pediatric age group and three studies are in adult bipolar/unipolar depression. There is no study in adult BD in manic/euthymic phase. Importantly, to the best of our knowledge, no study from India has examined RS connectivity abnormalities in BD using ReHo. Hence, in this first-time study from India, we used ReHo approach to identify RS brain activity in patients with BD compared to healthy controls (HCs).
MATERIALS AND METHODS
Subjects
The study sample comprised 20 patients with DSM-IV BD Type I and 20 healthy volunteers. Patients meeting the DSM-IV diagnostic criteria for BD or first episode of mania were recruited from the Department of Psychiatry of a Government Medical College. The diagnosis was established by applying Mini-International Neuropsychiatric Interview-Plus (MINI-Plus).[13] An experienced psychiatrist confirmed the diagnosis through an independent clinical interview. Patients had no history of comorbid medical illness or any other psychiatric illness, including substance dependence. We collected relevant clinical information pertinent to illness onset, demographic and clinical details, and ascertained it via information obtained from the patient and family members. We assessed psychopathology using the Hamilton Depression Rating Scale[14] and Young Mania Rating Scale.[15] Healthy volunteers were assessed for Axis I psychiatric disorder using MINI Plus and none of them had any Axis I psychiatric disorder. They had no history of medical illness or substance dependence. There was no family history of psychiatric illness, including alcohol dependence syndrome in first-degree relatives. Patients were in different phases of illness with 8 being in remission and 12 being symptomatic at the time of assessment. Out of 20 patients, 5 patients had prior history of depressive episode and among the remaining 15 who had only episodes of mania, 8 were in the first episode. A total of 9 of the 20 had psychotic symptoms, while 11 had no psychotic symptoms. Considering the different stages of illness, patients were on treatment with combination of mood stabilizer and antipsychotic medications: 15 patients were treated with lithium, 13 patients with valproate, 1 patient with oxcarbazepine, 4 patients with lamotrigine, and 20 patients with antipsychotics. We assessed handedness using Edinburgh Handedness Inventory[16] and recruited right-handed participants. Written informed consent was obtained from all the participants beforethe assessment. The study was approved by the Institute Ethics Committee.
Data acquisition
An RS fMRI MR image using an EPI sequence was obtained using 1.5T MR scanner (Philips Achieva) with the following scanning parameters: 8 channel, 37 slices, TR = 2000 ms, echo time (TE) = 30 ms, acquisition matrix of 64 × 64 and slice thickness of 3 mm, field of vision (FOV) of 192 × 192 with resolution of 3 mm × 3 mm × 3 mm, and angulations anterior commissure (AC) - posterior commissure (PC).
Data analysis
Preprocessing of the fMRI images were done using SPM 12 (http://www.fil.ion.ucl.ac.uk/spm). The images were corrected for acquisition delay and head motion and normalized to a standard EPI template. A temporal band-pass filter was used to remove low-frequency drift and physiological noise. Linear trends were also removed. A ReHo analysis was done using Rest Toolbox (http://www.resting-fmri.sourceforge.net). ReHo is a method to examine the synchronization of a voxel's time series with its nearest neighbors. This involved calculating the Kendall's correlation coefficient (KCC) for each voxel's time series and its 26 neighboring voxels.
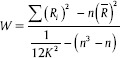
Where W is the KCC value of a given voxel; Ri the sum rank of the ith time point; 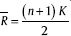 is the mean of the Ri's; K is the number of time series within a cluster; and n is the number of ranks. ReHo maps were created for each participant. Each map was divided by the global mean KCC value to standardize the maps. To examine the differences in ReHo patterns between patients and HCs, two-sample t-tests were performed. The results were threshold at P < 0.0001 (uncorrected) with cluster sizes more than 6 voxels. A mask was used to remove the nonbrain tissue. The schematic representation of analysis pipeline is given in Figure 1.
is the mean of the Ri's; K is the number of time series within a cluster; and n is the number of ranks. ReHo maps were created for each participant. Each map was divided by the global mean KCC value to standardize the maps. To examine the differences in ReHo patterns between patients and HCs, two-sample t-tests were performed. The results were threshold at P < 0.0001 (uncorrected) with cluster sizes more than 6 voxels. A mask was used to remove the nonbrain tissue. The schematic representation of analysis pipeline is given in Figure 1.
Figure 1.
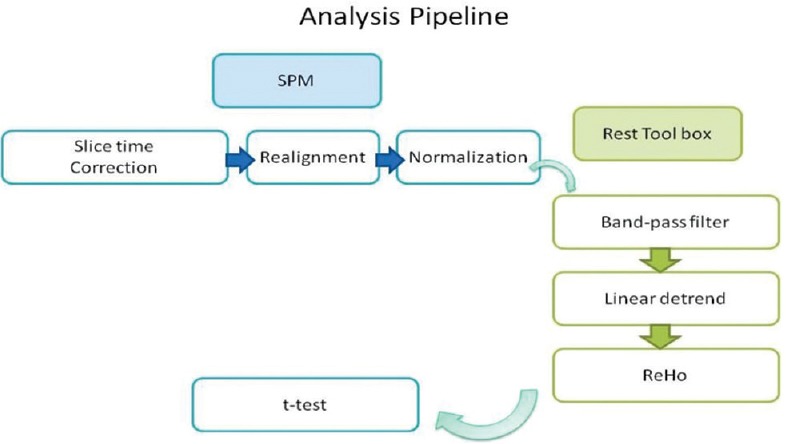
Schematic representation of steps involved in analysis and the software used
RESULTS
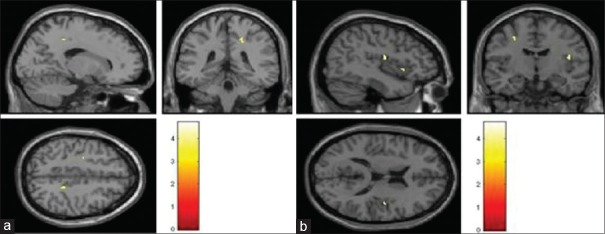
There was no difference between the two groups on age and sex [P > 0.5; Table 1]. Clinical details of patients are given in Table 1. The demographic and clinical features are described in Table 1. There was a significant difference between groups in ReHo values; on independent t-test, the patient group had significantly higher ReHo values in the right precuneus (BA 7), right insula (BA 13), right supramarginal gyrus (BA 40), left superior frontal gyrus (SFG) (BA 6), right inferior frontal gyrus (IFG) (BA 47), right precentral gyrus (BA 4), and right paracentral lobule (BA 5). No region had significantly lower ReHo values in patients compared to controls [Figure 2a and b].
Table 1.
Demographic and clinical characteristics comparison of subjects with bipolar disorder type I and healthy
| Characteristics | Patient | Controls | t/χ2 | P |
|---|---|---|---|---|
| Sex ratio (M:F) | 13:07 | 15:05 | 0.731 | |
| Age (Mean±SD) | 24.60±7.04 | 25.70±7.26 | 0.471 | 0.641 |
| HDRS (Mean±SD) | 1.4±3.06 | - | ||
| YMRS (Mean±SD) | 4.15±6.74 | - | ||
| Average no. of episodes (Mean±SD) | 2.30±1.68 | - | ||
| Patients with psychotic symptoms (n) | 9 | - | ||
| Patients in remission (n) | 8 | - | ||
| Patients with lifetime history of depressive episode (n) | 5 | |||
| Patients in first episode (n) | 8 | - | ||
| Patients exposed to lithium (n) | 15 | |||
| Patients exposed to valproate (n) | 13 | |||
| Patients exposed to oxcarbazepine (n) | 1 | |||
| Patients exposed to Lamotrigine(n) | 4 | |||
| Patients exposed to antipsychotic (n) | 20 |
Figure 2.
(a) Significantly higher regional homogeneity in the right precuneus in bipolar disorder compared to healthy volunteers. The difference in regional homogeneity between bipolar disorder and healthy volunteers is represented in yellow color and overlaid on structural T1 image for visualization. (b) Significantly higher regional homogeneity in the right insula and left superior frontal gyrus in bipolar disorder compared to healthy volunteers. The difference in regional homogeneity between bipolar disorder and healthy volunteers is represented in yellow color and overlaid on structural T1 image for visualization
DISCUSSION
To the best of our knowledge, this is the first Indian study in adult BD to examine neural synchronization of local brain areas during spontaneous brain activity using ReHo approach and RS fMRI. We found abnormal brain activity in the BD group relative to the HC group in several brain regions. Significantly increased ReHo in the BD group was mainly found in the right precuneus, right insula, right supramarginal gyrus, left SFG, right IFG, right precentral gyrus, and right paracentral lobule. No region had significantly lower ReHo values in patients compared to controls.
The increased ReHo in BD patients may indicate increased local synchronization of spontaneous functional activity. SFG is involved in mental manipulations, monitoring of information, spatial working memory, and executive functions.[17] Xiao et al.[10] have shown that decreased ReHo in SFG in pediatric BD. Taking together this data, along with our finding, suggest SFG is abnormal in BD. Differences in participant characteristics (adult vs. pediatric BD) may be the reason for differences in findings. Our study also found increased ReHo in the right IFG. IFG is involved in inhibitory and attention control, executive function.[18] IFG is also involved in subjective perception of the riskiness of the option.[19] The right-sided prefrontal lesion studies have demonstrated riskier behavior[20] in patients. Previous study by Liang et al.[9] has shown decreased ReHo in BD in depressive state. Right supramarginal gyrus (rSMG) is important for overcoming emotional egocentricity.[21] Egocentricity is a prominent feature in BD. rSMG was found to have increased coherence in BD patien, indicating dysfunctional rSMG in BD.
The precuneus is an intriguing cortical area involved in visuospatial imagery, episodic memory retrieval, self-processing, and consciousness.[22] Xiao et al.[10] have found decreased ReHo in pediatric BD patients and Liang et al.[9] reported increased ReHo in bipolar depression and unipolar depression, suggesting that self-awareness and introspection might be related to precuneus dysfunction. Precuneus is also a functional core of default mode network which is important in regulation of attention and cognition network. Our study found that ReHo value of precuneus was increased in BD which indicates that precuneus is dysfunctional in BD. Our study found increased ReHo in the right insular cortex. Human insular cortex is important for self-recognition, awareness of emotions, time perception, empathy, and decision-making under uncertainty, processing of music and language, intersubjective perspective-taking, cooperation, and guidance of social interactions.[23,24,25] Insular dysfunction may lead to pathophysiological processes predisposing BD patients to impaired integration of external emotional and interoceptive information,[26,27,28] that in turn may result in behavioral and emotional dysregulation. We also observed increased ReHo in the right precentral gyrus and right paracentral lobule. Xiao et al. 2013[10] reported aberrant coherence in precentral gyrus in pediatric BD compared to healthy adults. Increased ReHo may reflect neural hyperactivity in a regional brain area. Right precentral gyrus and right paracentral lobule play a part in many cognitive processes; ReHo increase observed in these regions may support previous findings of aberrant activity in BD during cognitive regulation.
Limitations
Our study findings need to be considered in the background of several limitations. First, the sample size of our study was small which limits the generalizability of the results. Second, all the patients in our study were on psychotropic medication. While the effect of psychotropic medication on the brain is still controversial, one cannot rule out the confounding effect of these medications.[29,30,31,32] Future studies in drug-naïve patients would possibly control the confounding effects of medication.
CONCLUSION
We used ReHo method to investigate the brain connectivity abnormalities between BD and HC. We found increased neuronal synchronization in the right precuneus, right insula, rSMG, left SFG, right IFG, right precentral gyrus, and right paracentral lobule. The areas implicated in the present study play an important role in the pathophysiology of BD.
Financial support and sponsorship
This study was supported by a grant from the Medical Research Council of Maharashtra, Government of Maharashtra, India under Star Research Project (PI: Rashmin Achalia). The funding agency did not have role in design of study or interpretation of results.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry. 2007;64:543–52. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keener MT, Phillips ML. Neuroimaging in bipolar disorder: A critical review of current findings. Curr Psychiatry Rep. 2007;9:512–20. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network – Functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–35. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 4.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, et al. Regional coherence changes in the early stages of Alzheimer's disease: A combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, et al. Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp. 2009;30:1502–10. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Jiao Q, Lu S, Zhong Y, Qi R, Lu D, et al. Alterations of regional homogeneity in pediatric bipolar depression: A resting-state fMRI study. BMC Psychiatry. 2014;14:222. doi: 10.1186/s12888-014-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang MJ, Zhou Q, Yang KR, Yang XL, Fang J, Chen WL, et al. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One. 2013;8:e79999. doi: 10.1371/journal.pone.0079999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Q, Zhong Y, Lu D, Gao W, Jiao Q, Lu G, et al. Altered regional homogeneity in pediatric bipolar disorder during manic state: A resting-state fMRI study. PLoS One. 2013;8:e57978. doi: 10.1371/journal.pone.0057978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CH, Ma X, Wu X, Zhang Y, Zhou FC, Li F, et al. Regional homogeneity of resting-state brain abnormalities in bipolar and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:52–9. doi: 10.1016/j.pnpbp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu CH, Ma X, Li F, Wang YJ, Tie CL, Li SF, et al. Regional homogeneity within the default mode network in bipolar depression: A resting-state functional magnetic resonance imaging study. PLoS One. 2012;7:e48181. doi: 10.1371/journal.pone.0048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 14.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 15.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. Functions of the left superior frontal gyrus in humans: A lesion study. Brain. 2006;129:3315–28. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 18.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage. 2010;50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574–83. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–83. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 21.Silani G, Lamm C, Ruff CC, Singer T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci. 2013;33:15466–76. doi: 10.1523/JNEUROSCI.1488-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 23.Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, et al. A volumetric comparison of the insular cortex and its subregions in primates. J Hum Evol. 2013;64:263–79. doi: 10.1016/j.jhevol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel – Now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 25.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 27.Cauda F, D'gata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: New insights from imaging. Surg Radiol Anat. 2003;25:113–9. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- 29.Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–75. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Atmaca M, Ozdemir H, Cetinkaya S, Parmaksiz S, Belli H, Poyraz AK, et al. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res. 2007;41:821–7. doi: 10.1016/j.jpsychires.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–20. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]