To the Editor,
Therapy-related acute myeloid leukemias (t-AMLs) following therapy are well described in the literature, but only rare cases of therapy-related acute lymphoblastic leukemia (t-ALL) have been reported previously. Cases of multiple myeloma (MM) terminating in ALL are even rarer. Herein, we report the clinicopathological, immunological, cytogenetic, and molecular features of two patients diagnosed with B-cell acute lymphoblastic leukemia (B-ALL) and MM who presented with MM at the initial diagnosis.
Patient 1, a 68-year-old male, was diagnosed with MM in 2015. He received 2 cycles of PD (bortezomib and dexamethasone) with a good response, and then maintenance with thalidomide.
Patient 2, a 65-year-old female, was diagnosed with MM in 2012. She received 4 cycles of VAD (vincristine, epirubicin, and dexamethasone) with a partial response. She then relapsed and received treatment with one cycle of TAD (thalidomide, epirubicin, and dexamethasone). After that, she achieved complete remission. In 2016, the patient relapsed again. She continued treatment with BTD (bortezomib, dexamethasone, and thalidomide) and achieved partial response.
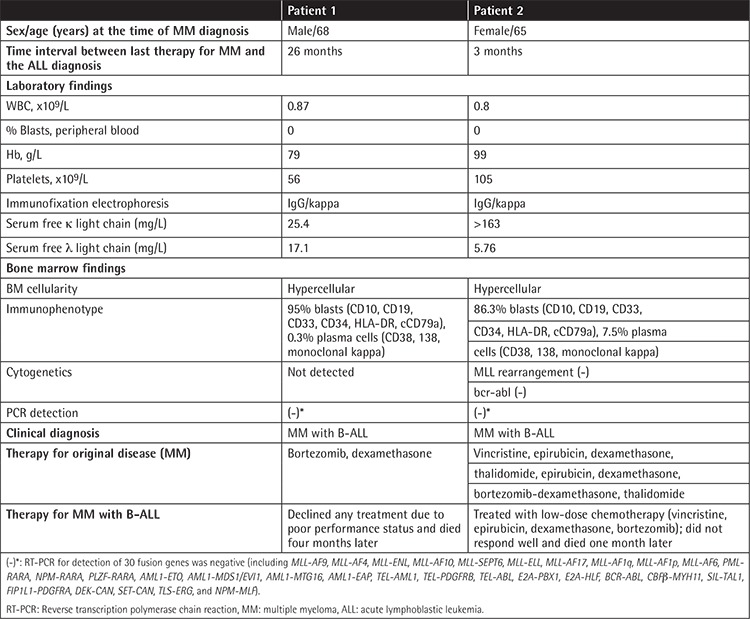
In 2017, the two patients both presented with leukopenia. Immunofixation electrophoresis showed monoclonal IgG and K light chain. The bone marrow was heavily infiltrated by lymphoblasts and a few malignant plasma cells. Flow cytometry analysis demonstrated that malignant plasma cells with CD38, CD138, and monoclonal K chain and B-cell lymphoblasts expressed CD10, CD19, CD34, HLA-DR, cCD79a, and CD33. No other aberrant expression of myeloid or T lymphocyte-associated antigens was identified (Figures 1A-1C). The female patient’s G-banding cytogenetic results revealed a hypodiploid and complex karyotype. Reverse transcription-polymerase chain reaction for detection of fusion genes in the two patients was negative. Both patients were diagnosed with B-ALL with MM. The male patient declined any treatment due to poor performance status and died four months later. The female patient was treated with low-dose chemotherapy (vincristine, epirubicin, dexamethasone, and bortezomib) and did not respond well; she died one month later. The clinical features of the two patients are summarized in Table 1.
Figure 1.
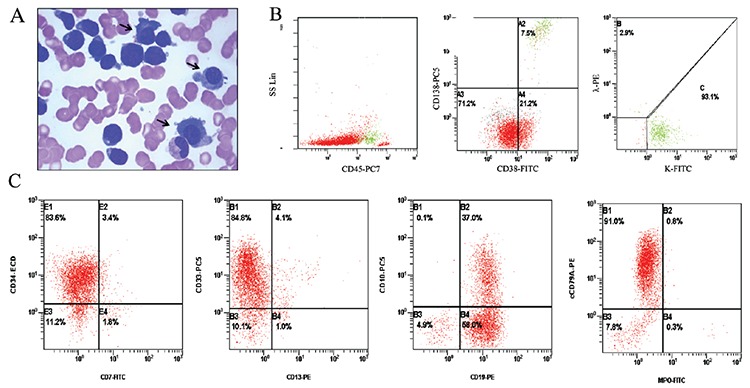
Patient 1: A) Black arrows point at malignant plasma cells, which are very different from other lymphoblasts (Wright-Giemsa staining, 100x). B) Malignant plasma cells were positive for CD38, CD138, and monoclonal kappa (green region of the scatter plot). C) The lymphoblasts were immunophenotyped as B-cell and expressed CD10, CD19, CD34, and cCD79a with aberrant coexpression of CD33. The morphologic and immunological characteristics of Patient 2 were similar.
Table 1. Clinical features of acute lymphoblastic leukemia in patients with previously treated multiple myeloma (MM) from our institution.
It has been reported in the literature that therapy-related acute leukemia comprises 2 major types: alkylating agent/radiotherapy-related and topoisomerase II inhibitor-related [1]. Alkylating agent-related acute leukemia is associated with abnormalities of chromosomes 5 and/or 7, while topoisomerase II inhibitor-related acute leukemia has been linked to 11q23 [2,3]. The female patient received a topoisomerase II inhibitor while the male did not, and neither of them showed specific genetic abnormalities. Intriguingly, the clinical, morphologic, and immunological characteristics of the two patients were similar. MM is a plasma cell neoplasm derived from mature B-lymphocytes, whereas B-ALL is a B-cell neoplasm derived from early B-precursors. It is possible that MM and B-ALL may derive from the same stem cell clones, or MM cells dedifferentiate into immature B cells that develop B-ALL [4]. They may have identical karyotypes and immunophenotyping, and they may share some cytogenetic abnormalities [5,6]. The possibility of MM and B-ALL deriving from two independent clones cannot be excluded, either. Future studies such as molecular and cytogenetic studies to explore their relationship would be intriguing.
Acknowledgments
This work was supported by the Applied Research Training Program of Jiangxi Province (No. 20181BBG78057) and the National Natural Science Foundation of China (No. 81760539).
Footnotes
Conflict of Interest: The authors of this paper have no conflicts of interest including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
References
- 1.Vardiman JW, Thiele J, Arber DA. Acute myeloid leukaemia (AML) and related precursor neoplasms. In: Swerdlow SH, Campo E, Harris NL, (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, IARC Press. 2008. [Google Scholar]
- 2.Cortes J, O’Brien S, Kantarjian H, Cork A, Stass S, Freireich EJ, Keating M, Pierce S, Estey E. Abnormalities in the long arm of chromosome 11 (11q) in patients with de novo and secondary acute myelogenous leukemias and myelodysplastic syndromes. Leukemia. 1994;8:2174–2178. [PubMed] [Google Scholar]
- 3.Hawkins MM, Wilson LM, Stovall MA, Marsden HB, Potok MH, Kingston JE, Chessells JM. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ. 1992;304:951–958. doi: 10.1136/bmj.304.6832.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau LG, Tan LK, Koay ES, Liu TC. Acute lymphoblastic leukemia after tandem autologous stem cell transplantations for multiple myeloma. Leukemia. 2005;19:299–301. doi: 10.1038/sj.leu.2403587. [DOI] [PubMed] [Google Scholar]
- 5.Foon KA, Thiruvengadam R, Saven A, Bernstein ZP, Gale RP. Genetic relatedness of lymphoid malignancies. Transformation of chronic lymphocytic leukemia as a model. Ann Intern Med. 1993;119:63–73. doi: 10.7326/0003-4819-119-1-199307010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Makower D, Venkatraj U, Dutcher JP, Wiernik PH. Occurrence of myeloma in a chronic lymphocytic leukemia patients after response to differentiation therapy with interleukin-4. Leuk Lymphoma. 1996;23:617–619. doi: 10.3109/10428199609054873. [DOI] [PubMed] [Google Scholar]


