Abstract
Manifestations of bone disease—osteopenia, osteolytic lesions, and fractures—are the hallmark of multiple myeloma (MM) and occur clinically in the vast majority of patients. These abnormalities can have devastating clinical effects by increasing both the morbidity and mortality of patients. Bone disease is usually found when patients are diagnosed with active MM; however, recent data suggest that it is present in early myelomagenesis, including patients with myeloma precursor disease, monoclonal gammopathy of undetermined significance (MGUS). The primary mechanisms of abnormal bone remodeling are increased osteoclastic activity, which occurs in close proximity to active myeloma cells, and decreased activity of the surrounding osteoblasts. Better understanding of the pathogenesis of bone disease in MM will allow us to enhance our current therapeutic options in the treatment of bone disease. In patients with active MM and at least one lytic lesion, intravenous bisphosphonates have been shown to decrease skeletal-related events and pain, improve performance status, and maintain quality of life. Emerging evidence suggests that intervention at earlier stages of disease may prevent skeletal-related events at time of progression, but there is no evidence that bisphosphonates in this setting change the natural history of the disease.
Multiple myeloma (MM) is a plasma cell dyscrasia, with 20,000 new cases diagnosed annually in the United States.1 It is characterized by plasma cell infiltration in the bone marrow along with the production of a monoclonal immunoglobulin in the serum and/or the urine. The diagnosis of active MM requires the demonstration of end-organ damage, which includes hypercalcemia, anemia, renal insufficiency, and/or bone involvement. MM is known to evolve from two precursor conditions: monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM).2
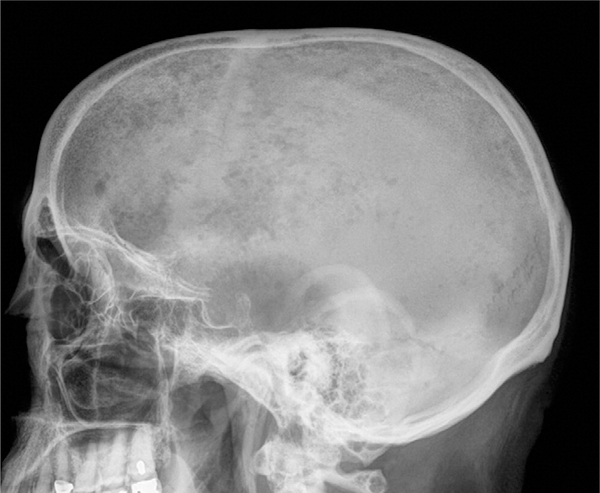
Bone disease and subsequent destruction is the hall-mark of MM and occurs in the vast majority of patients with reported incidences varying, according to the literature, from 70% to 95%.3 Overall, MM is the most common cancer to involve the bone. The manifestations of bone involvement in patients with MM include osteopenia, osteolytic lesions, and fractures, which can have devastating clinical effects by increasing both the morbidity and mortality of patients (Figure 1). In a retrospective study by Saad et al in 2007, MM had the highest incidence of bone fractures (53%) compared with breast (35%), prostate (19%), and lung cancer (17%).4 Other studies, such as a retrospective review of patients from 1945 to 2001 by Melton et al, have demonstrated fracture rates in MM as high as 81%.5 In fact, MM patients who develop pathologic fractures have a 20% increased risk of death.4
Figure 1.
Plain radiographs of the skull demonstrating lytic lesions in MM.
In MM, bone remodeling is abnormal resulting in increased bone resorption combined with decreased new bone formation. The primary cause of abnormal bone remodeling is increased osteoclastic activity, which occurs in close proximity to active MM cells, and decreased activity of the surrounding osteoblasts.6,7 Histomorphometric studies have demonstrated an increase in the activity and number of osteoclasts.7,8 In sharp contrast, balanced bone remodeling with increased osteoclastogenesis combined with normal to increased bone formation exists in myeloma patients in areas of bone without plasma cell invasion.9 Studies demonstrating increased markers of bone resorption (C-terminal telopeptide of collagen type I, N-terminal telopeptide of collagen type I) and decreased markers of bone production (alkaline phosphatase, osteocalcin) provide additional evidence of dysregulated bone resorption in patients with MM.10–12 The interaction between MM cells, osteoclasts, osteoblasts, and other cells of the bone marrow microenvironment play key roles in the development of bone disease in MM and in the growth and survival of MM cells in the bone marrow.13 Research over the past decade has increased our understanding of the bone marrow microenvironment and the crucial mediators in the pathophysiology of the disease.
Bone disease is usually detected in patients with MM, traditionally by the skeletal survey. However, this test requires significant bone destruction to occur (around 30% of the bone architecture) to reveal osteolytic lesions. More recent data using more sensitive laboratory techniques suggest that bone resorption is present in the precursor states of MM. Increased markers of bone resorption have been demonstrated in some patients with MGUS compared with normal healthy controls.11–13 Evidence of imbalanced or uncoupled bone remodeling has also been demonstrated by histomorphometric studies in patients with MGUS.14 Small studies have shown that the prevalence of osteoporosis is high in MGUS patients, increasing the risk of fracture.15–18 A recent study by Kristinsson et al looking at 5,326 MGUS patients in Sweden demonstrated that when compared with matched controls, MGUS patients had a significant increase in risk of fractures (hazards ratio = 1.74; 95% confidence interval [CI], 1.58–1.92) at 5 and 10 years.19 This data from both laboratory and clinical studies again highlight the importance of bone dysregulation in the pathophysiology of patients with plasma cell dyscrasias and suggest that abnormal bone remodeling is an early rather than late finding.
Another intriguing aspect of MM is that bone involvement results in permanent scarring of the bone. Persistent lytic lesions can be found in patients in remission with no evidence of marrow infiltration of malignant MM cells.20 Tian and colleagues demonstrated that an antagonist of the Wnt signaling pathway, DKK1, is produced by MM cells and prevents the osteoblast differentiation from bone marrow stromal cells.21 Increased bone production at sites of injury to repair the damage occurs by the differentiation of mesenchymal stem cells into osteoblasts.22 Bone repair appears inhibited at sites of osteolytic lesions in MM patients most likely because of inhibition of osteoblastic activity by DKK1.3 Further understanding of the bone microenvironment may allow repair of existing osteolytic sites in bone by pathway interruption of inhibitory signals. Some studies suggest that bortezomib may have a potential role in enabling bone repair by promoting bone formation, but these findings need to be confirmed by studies looking at clinical end points specific to the bone.23
Thus, understanding the biology of bone disease has implications not only in diagnostics and the implementation of therapeutic strategies but also in the design of future trials looking at both treatment outcomes and prevention. This review discusses the role of osteoblasts and osteoclasts in myelomagenesis, and it summarizes current treatment guidelines for bone disease in patients diagnosed with MM. Also it discusses concepts of bone disease in early myelomagenesis, and it proposes directions for new research and future early interventions.
OSTEOBLASTIC DYSREGULATION AND ACTIVITY IN MYELOMA
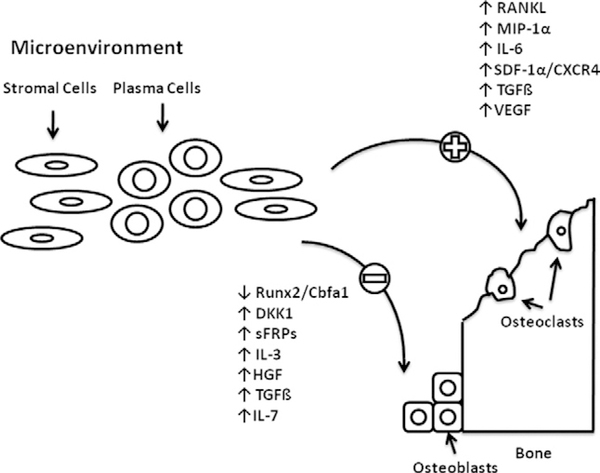
Initial theories on the development of bone disease in MM centered on increased osteoclast activity and subsequent bone degradation. However, we now know that osteoblast impairment plays a key role in the pathophysiology of MM bone disease. This finding is evidenced by the inability of bone scans to detect abnormalities in the bones of MM patients. While the interactions between MM cells and osteoblasts are complex and not entirely understood, recent insights in the pathways involved in these interactions have been elucidated (Figure 2). These pathways include both direct cell-to-cell interactions and soluble osteoblast-inhibiting factors.24
Figure 2.
Key mediators in the inhibition of osteoblasts and activation of osteoclasts.
In bone histomorphometric studies in a murine model of MM, Hjorth-Hansen found significant osteoblastopenia (99% reduction in osteoblasts counts) along with decreased bone formation in areas of tumor growth.25 Furthermore, Silvestris and colleagues showed that osteoblasts isolated from patients with MM with active bone involvement are more likely to undergo apoptosis due to high levels of cytokines and by direct interactions with MM cells, resulting in ineffective new bone formation.26 Apoptosis of osteoblasts was also demonstrated when the osteoblasts were co-cultured with MM cells.27 The impairment in osteoblasts also results from the primary blockade of osteogenic differentiation of mesenchymal progenitors into osteoblasts.28
Osteoblasts also may play a key role in both the growth and survival of MM cells. For example, secretion of interleukin (IL)-6 is known to support the growth of MM cells. IL-6 secretion by osteoblasts in co-culture with MM cells suggests that osteoblasts may contribute to the high IL-6 levels found in the bone marrow microenvironment.29 Osteoblasts may also contribute to the survival of MM cells through secretion of osteoprotegerin, resulting in inhibition of TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis.30 However, studies by Yaccoby et al demonstrate that osteoblasts may also inhibit the growth of MM cells in vivo.31 Thus, osteoblasts may have multiple actions in the pathogenesis of MM, and conditions in the fluid microenvironment may dictate these roles at various stages of the disease.
Role of Cell-to-Cell Interactions in Inhibition of Osteoblasts in MM Cells
Close cellular interactions between MM cells and osteoblast progenitors inhibit the formation of mature osteoblasts.28 The differentiation of mesenchymal cells into osteoblasts is mediated through the activity and function of a transcription factor Runx2/Cbfal.32 New bone formation is stimulated by the interactions of Runx2/Cbfal with other transcriptional factors such as Osterix.33 The importance of Runx2 in the formation of osteoblasts is demonstrated in animal models where Runx2/Cbfal knockout mice demonstrated complete absence of osteoblasts and bone formation.34 Increased activity of Runx2/Cbfa1 is associated with differentiation of osteoblasts in humans without significant changes in protein levels.32 However, bone formation can also be inhibited by the overexpression of Runx2, suggesting that Runx2 may play a dual role in osteoblast differentiation depending on the stage of development.32
Decreased Runx2/Cbfa 1 activity and subsequent inhibition of osteoblast differentiation in MM bone disease has been demonstrated in humans.35 MM cells co-cultured with osteoblast progenitors inhibited the formation and subsequent differentiation of osteoblasts.36 A reduction in both early precursors of osteoblasts, fibroblast colony-forming units (CFU-F) and more differentiated precursors such as the colony-forming osteoblasts units (CFU-OB) were demonstrated along with decreased expression of biologic markers of osteoblast differentiation such as collagen I genes, osteocalcin, and alkaline phosphatase.36 The inhibition of the osteoblast progenitors was induced in human osteoprogenitor cells by blocking the activity of Runz/Cbfa 1.32,36 The results are also supported by observations of significant reductions in the proportion of Runx2-positive osteoblasts confirmed by immunohistochemistry in osteolytic bone lesions of patients with bone disease when compared to patients with no evidence of bone disease.36
Cell-to-cell interactions between osteoprogenitor cells and MM cells appear to play a key role in the inhibition of osteoblast differentiation and the decreased activity of Runx2/Cbfa 1.33 Vascular cellular adhesion molecule-1 (VCAM-1) on stromal cells of the marrow and very late antigen-4 (VLA-4) on myeloma cells appear to control the interaction between the cells.37 Inhibition of Runx2/Cbfa 1 activity by myeloma cells was shown by adding an anti-VLA-4 antibody to co-cultures of marrow stromal cells and myeloma cells.37 Additional mediators of cell-to-cell interactions may be involved in the suppression of osteoblasts by MM cells.33 Suppression of bone matrix production can be decreased by neural cell adhesion molecule (NCAM)-NCAM interactions between osteoblast and MM cells.33 These interactions may play a role in the development of osteolytic lesions in the bone of patients with MM.38
Role of Soluble Factors in Osteoblast Inhibition
Wnt Signaling Pathway Inhibition and Dkk1
The Wnt pathway plays a key role in bone formation through the growth and development of both immature and mature osteoblasts.39 This pathway has been implicated in the pathogenesis of diseases with dysregulated bone remodeling such as osteoporosis and MM.40 The pathway involves binding of WNT to a soluble mediator, LRP5 or LRP6, creating a complex that then binds to the fizzled receptor.41 Dephosphorylation and stabilization of β-catenin is promoted by signal transduction from the fizzled receptor and subsequent localization of β-catenin to the nucleus leading to increased expression of target genes.41 In vitro studies have shown that osteoblast differentiation can be initiated by activation of the β-catenin pathway.41 Soluble factors such as Dkkl, Wnt inhibitory factor-1 (Wif1), and soluble-frizzled receptor-like proteins (sFRPS) have been shown to inhibit the Wnt pathway, resulting in osteoblast suppression and progression of bone disease in MM.40
Bone marrow stromal cells, osteoblasts and MM cells express Dkk1 and in vitro studies show that inhibition of the Wnt signaling pathway by Dkk1 leads to inhibition of new bone formation.42 Transgenic mice overexpressing Dkk1 developed severe osteopenia. In contrast, increases in bone mass were demonstrated by deletions of Dkk1 alleles.39,42 These in vivo studies highlight the importance of this Wnt antagonist in bone physiology.39,42 Reports from Tian et al demonstrated DKK1 is upregulated in MM cells and that there is a correlation between Dkk1 levels and extent of lytic bone lesions on imaging.20
Other soluble inhibitors of the Wnt signaling pathway include the sFRPS (sFRPs 1–4).32 The binding of Wnt to membrane-bound frizzled receptor is blocked by these decoy receptors.41 Both sFRP-2 and sFRP-3 have been investigated as possible mediators of osteoblast inhibition in myeloma. 41 Preliminary studies have demonstrated inhibition of murine osteoblast differentiation by sRFP-2, which was derived from myeloma cells.41 Furthermore, overexpression of sFRP3 in myeloma cells has been demonstrated. This factor, along with Dkk1, contributed to the development of bony lytic lesions seen in patients with myeloma.32
IL-3
The cytokine IL-3 has inhibitory properties on osteoblast formation and differentiation and is elevated in the serum and bone marrow of patients with MM.41,42 In both human and animal models, IL-3 was shown to have inhibitory effects on the differentiation of osteoblasts, and the bone marrow plasma from myeloma patients with high IL-3 levels was shown to inhibit the differentiation of osteoblasts in human cultures.41,43 IL-3 has also been shown to have stimulatory effects on osteoclast activity.39 These data suggest that the actions of IL-3 in MM are complex and that it likely has a dual role in the pathophysiology of MM bone disease.
Hepatocyte Growth Factor
Hepatocyte growth factor (HGF) is found in high levels in patients with MM due to secretion from myeloma cells.42 The role of HGF in bone disease in patients with MM is suggested by a negative association of serum HGF levels with outcomes in patients and alkaline phosphatase levels.44 This suggestion is further supported by the demonstration of the inhibition of bone morphogenetic protein (BMP)-induced osteoblastogenesis and suppression of Runx2 by HGF.44
Transforming Growth Factor-β
The growth factor transforming growth factor-β (TGF-β) is secreted by bone matrix during osteoclast-mediated bone resorption and has inhibitory actions on osteoblast differentiation.42 In vitro studies inhibiting TGF-β signaling blocked the inhibition of osteoblast differentiation by MM cells.45 Thus, TGF-β has a key role in bone pathology and represents another potential target in the dysregulated bone of patients with myeloma.
IL-7
Inhibition of osteoblasts can also be mediated by the actions of IL-7. IL-7 levels are elevated in the marrow of patients with MM.46 Inhibition of osteoblasts was demonstrated to occur in cultures of human osteoblasts.46 IL-7 also has inhibitory effects on Runx2.41,46 Also, neutralizing antibodies to IL-7 partially suppress this inhibition of osteoblast differentiation by myeloma cells.46
PATHOPHYSIOLOGY OF INCREASED OSTEOCLAST ACTIVITY IN MYELOMA
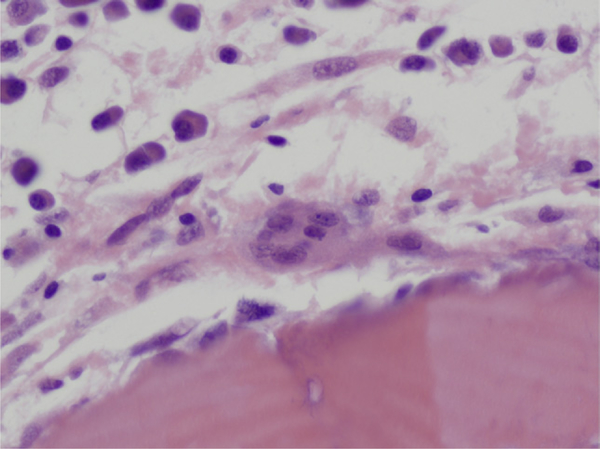
MM is characterized by increased bone resorption resulting from the increased osteoclast activity. This increased activity and resorption by osteoclasts occurs in close proximity to myeloma cells as demonstrated in histologic studies of bone biopsies from patients with MM.47 However, studies have shown that the number of osteoclasts is not increased in areas of bone without MM involvement.48 The findings from histologic studies imply that local mediators released by MM cells play key roles in the stimulation of local osteoclasts. Key factors identified as contributing to increased osteoclastic activity in MM include receptor activator of nuclear factor-κB ligand (RANKL), macrophage inflammatory protein-1a (MIP-1α), IL-6, and stromal-derived factor-1α (SDF-1α) (Figure 3).
Figure 3.
Osteoclast in area of bone remodeling in marrow of a patient with MM with surrounding plasma cells.
The RANK/RANKL/OPG System
Receptor activator of nuclear factor κB (RANK), RANKL, and osteoprotegerin (OPG), members of the tumor necrosis factor (TNF) and TNF receptor super-family, play key roles in the development and activity of osteoclasts.42 Both osteoblasts and bone marrow stromal cells express RANKL.49 The interaction of RANKL with RANK, found on both mature and early osteoclasts, stimulates osteoclast growth and resorption of bone.49 OPG acts as antagonist of this pathway and is secreted by bone marrow stromal cells and osteoblasts. OPG is a soluble decoy receptor and prevents the interaction by RANKL and RANK by binding to RANKL, thus resulting in inhibition of bone resorption by osteoclasts.50 In vivo studies illustrate the vital role these molecules play by demonstrating extensive osteoporosis in mice deficient in RANK or RANKL and osteoporosis in mice deficient in OPG.42
The ratio of RANKL and OPG is altered in patients with MM where an increased RANKL expression and decreased OPG expression is found, which is in sharp contrast to patients without MM who exhibit a low ratio of RANKL to OPG.42 MM cells stimulate increased RANKL expression by bone marrow stromal cells by direct cell-to-cell interactions.51 Osteoclast growth was correlated with this increased expression of RANKL by bone marrow stromal cells and inhibition was demonstrated by RANKL antagonists.52 The increase in the serum RANKL to OPG ratio has also been associated with increased bone disease and survival in patients.53
MIP-1α
MIP-1α is a chemokine produced by MM cells, which induces the differentiation of osteoclast progenitors, leading to the proliferation of osteoclasts.54 MM cells produce and secrete MIP-1α, which has been associated with bone destruction and inversely correlated with severity of bone disease and prognosis.41,55 Choi et al demonstrated increased levels of MIP-1α in the bone marrow plasma of patients with MM, as well as osteoclast inhibition using an antibody to block MIP-1α.56 In addition, MIP-1α has indirect actions on bone resorption. Studies have shown that MIP-1α leads to increased expression of RANKL on stromal cells, leading to increased production of osteoclasts.54 MIP-1α also exerts direct effects on MM cells, which express CCR1, a receptor for MIP-1α.54 MIP-1α stimulates adhesion between myeloma and bone marrow stromal cells by increasing MM cell expression of β1-integrins, leading to increased production by marrow stromal cells mediators of MM cell growth and angiogenesis such as IL-6, VEGF, and TNF-α.47 Because of these effects and interactions, MIP-1α represents a potential important target for drug development in myeloma.
IL-6
While IL-6 has known activity in stimulating the growth and survival of plasma cells, it also is a potent induction agent of osteoclast production.47 Studies have demonstrated a correlation between elevated IL-6 levels and lytic lesions in MM patients with bone disease compared with those without known bone disease.47 Production of IL-6 appears to occur primarily from cells in the microenvironment through interactions with MM cells rather than directly by the MM cells themselves.47 While the exact role of IL-6 in bone disease is not clearly defined, it likely has a dual role both directly, through enhancement of osteoclast-mediated bone destruction, and indirectly, via stimulation of plasma cells.
SDF-1α/CXC Chemokine Receptor-4
SDF-1α is a chemokine of the CXC family and its receptor is expressed on many cells of the microenvironment, including osteoclast precursors, lymphocytes, and stem cells, as well as on malignant cells.42 Evidence suggests that the SDF-1α/CXC chemokines receptor 4 (CXCR-4) complex plays a vital role in the migration and growth of MM cells.42 There is also evidence suggesting that, in addition to its role as a mediator of the tumor microenvironment, SDF-1 also increases both osteoclast induced bone resorption and migration.57 The reduction of MM induced osteoclast activation by agents that inhibit CXCR-4 further supports the role of SDF-1α in the pathophysiology of MM bone disease.42
VEGF
VEGF has an established role in the growth and development of new vasculature in non-hematologic neoplasms. MM cells have the ability to secrete VEGF and the density of microvessels in the bone marrow of MM patients is associated with adverse clinical outcomes.42 VEGF may also play a role in MM as a mediator of osteoclasts.42 In vitro data suggest that VEGF can induce the growth and development of osteoclasts and that inhibition of VEGF suppressed angiogenesis and bone resorption.58
Cell-to-Cell Interactions
As previously mentioned, sites of increased osteoclast activity and bone destruction in MM occur in close association with MM cells. (Figure 3) In addition to soluble mediators, cell-to-cell interactions are critical components in the increased osteoclast activity and bone destruction seen in MM bone disease. Direct interactions between MM cells and stromal cells lead to the secretion of mediators such as IL-6 and RANKL, which stimulate osteoclast growth and development.42 In addition, evidence suggests an increase in the growth of MM cells and osteoclast proliferation as a result of direct interactions between the MM cells and osteoclasts.59
CURRENT TREATMENT GUIDELINES FOR BONE DISEASE IN MM
Better understanding of the pathogenesis of bone disease in MM will allow us to enhance our current therapeutic options in the treatment of bone disease and open up the opportunity to explore intervention at early stages of the disease. For clinicians in practice, the diagnosis and treatment of bone disease are critical components in caring for patients with MM. Current guidelines in the treatment of bone disease in MM emphasize the use of intravenous bisphosphonates in the United States while the use of clodronate (Bonefos; Boehringer Ingelheim, Ingelheim, Germany) is supported by some in Europe. The primary mechanism of action of bisphosphonates is osteoclast inhibition, which leads to reduced bone resorption. In patients with active MM and at least one lytic lesion, intravenous bisphosphonates have been shown in large, double-blind, randomized trials to decrease skeletal-related events and pain, improve performance status, and maintain quality of life.60–62
To our knowledge, there are four peer-reviewed guidelines in the literature from the United States and Europe on the use of bisphosphonates in MM63–66 (see Table 1). The choice of the two intravenous bisphosphonates, pamidronate (Aredia; Novartis, Basel, Switzerland) and zoledronic acid (Zometa; Novartis, Basel, Switzerland), approved for treatment of MM in the United States, in general, is left to the discretion of the treating physician and the patient. Zoledronic acid is more potent than pamidronate and has the advantage of a more rapid infusion time. However, some of the guidelines, for example, the Mayo Clinic guidelines, favor the use of pamidronate due to concern about the increased risks for osteonecrosis with zoledronic acid compared with pamidronate based on findings by Zervas et al, which showed a 9.5-fold greater risk with zoledronic acid.67
Table 1.
Bisphosphonate Guidelines in Multiple Myeloma
| ASCO63 | NCCN64 | Mayo65 | EMN66 | |
|---|---|---|---|---|
| Indications | MM with lytic lesion or osteopenia or osteoporosis Not indicated in SMM or MGUS | MM with lytic lesion or osteopenia or osteoporosis Not indicated in SMM or MGUS | MM with lytic lesion or osteopenia or osteoporosis Not indicated in SMM or MGUS | MM with lytic lesion or osteopenia or osteoporosis Not indicated in SMM or MGUS |
| Duration | 2 years, stop if stable disease/responsive disease. Continuing beyond 2 years at discretion of treating physician. Restart at relapse, no guidelines on duration or frequency | N/A | Monthly x2 years then discontinue if in remission or stable/plateau phase. If MM still active, recommend continuing but decrease frequency to every 3 months. | Recommend 2 years of therapy by most of panel. Some on panel prefer to continue beyond 2 years at reduced dose or frequency. Resume on relapse, no recommendation on duration or frequency in this setting. |
| Surveillance Renal | Creatinine with each dose. | N/A | N/A | Monitor creatinine in all patients. Moniter CrCL, electrolytes and U/A in patient with CKD. |
| ONJ | Comprehensive dental exam and preventive dentistry prior to starting. While on therapy, maintain good oral hygiene and avoid invasive procedures if possible. | N/A | Comprehensive exam before initiating. Complete required invasive procedures prior to starting. See dentist annually. Manage new dental procedures conservatively. See oral/ maxillofacial surgeon if surgery required. | Clinical dental exam prior to treatment. Treat infections. Complete required dental procedure prior to starting therapy. Dental exam yearly. Avoid elective procedures. Maintain good oral hygiene on therapy. |
| Other | Ca, electrolytes, Hgb/Hct regularly but no defined time intervals. U/A every 3–6 months. | N/A | N/A | Calcium (1,600 mg/d) and vitamin D (400 lU/d) in patients in areas of decreased sun. |
| Choice of bisphosphanate | Pamidronate or zoledronic acid.Choice up to patient/physician. | Pamidronate or zoledronic acid. | Favor pamidronate over zoledronic acid due to risks of ONJ. | Pamidronate and zoledronic acid equally effective. Cloforobate (available in Europe) can be considered as well. |
Abbreviations: MM, multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance; SMM, smouldering multiple myeloma; ONJ, osteonecrosis of the jaw; N/A, not applicable; CrCl, creatinine clearance; U/A, urinalysis; CKD, chronic kidney disease; Ca, calcium; Hgb, hemoglobin; Hct, hematocrit; ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; EMN, European Myeloma Network.
Areas of controversy in the current treatment guidelines that are important to practicing clinicians are the duration and frequency of bisphosphonate therapy. Most of the treatment guidelines restrict the duration of bisphosphonate therapy to 2 years in patients with responsive or stable disease due to the risk of osteonecrosis from cumulative exposure to bisphosphonates. The Mayo Clinic provides guidelines for continuation of bisphosphonate therapy beyond 2 years in patients with active disease, who have not achieved a response or who have threatening bone disease. Their recommendation in this setting is to decrease the frequency to every 3 months. The other guidelines do not provide provisions beyond the initial 2 years and leave this decision to the discretion of the treating physician. Another area of controversy is in the treatment of patients with relapsed disease who had previously received bisphosphonate therapy for 2 years. While it is common practice to restart active therapy of bone disease at relapse there are no societal recommendations in regard to frequency or duration of therapy in this setting. For example, the American Society of Clinical Oncology (ASCO) guidelines recommend restarting bisphosphonates at this time, but do not recommend specific frequency or duration of the therapy.
Most of the guidelines provide various recommendations for monitoring the potential toxic effects of bisphosphonate therapy. In regard to potential development of nephrotoxicity, the ASCO guidelines recommend monitoring creatinine prior to each dose of bisphosphonate. These guidelines suggest the drug should be withheld in patients who develop renal insufficiency (defined as increase in creatinine of 0.5 mg/dL or two times above baseline value) and can be resumed when the serum creatinine returns to within 10% of baseline. The European Myeloma Network and the National Comprehensive Cancer Network guidelines (NCCN) also recommend monitoring the creatinine, particularly in patients with underlying renal insufficiency, but do not provide specific interventions in the event of the development of an adverse event. The Mayo guidelines do not provide any official recommendation regarding the monitoring of creatinine.
In the setting of moderate renal insufficiency (creatinine clearance of 30–60 mL/min), dosing guidelines provided in the package insert for zoledronic acid should be followed. These guidelines recommend decreasing the dose of zoledronic acid, generally to 3.0 or 3.5 mg, based on the patient’s creatinine clearance. No dosing guidelines are presently available for pamidronate in the setting of renal insufficiency. However, most clinicians do consider decreasing the dose of pamidronate in a patient with known renal insufficiency. Decreasing the dose to 30 to 60 mg and infusing over 4 hours are reasonable considerations.64 The use of zoledronic acid or pamidronate is not recommended in the setting of a severe renal insufficiency (creatinine clearance <30 mL/min).
Osteonecrosis of the jaw is one of the most feared complications of bisphosphonate therapy and all four guidelines recommend monitoring for the possible development of this adverse side effect of therapy. Patients should receive a comprehensive dental evaluation prior to starting therapy and have any necessary dental procedures performed prior to the initiation of bisphosphonate therapy. Patients should maintain excellent oral hygiene while on therapy and see their dentist at least annually. Elective procedures should be avoided while on therapy. Dental problems that develop should be managed conservatively during the course of treatment.
CURRENT EXPERIENCE FROM TREATMENT OF BONE DISEASE IN MYELOMA PRECURSOR DISEASE
Overall, there are few studies in the published literature examining the effects of bisphosphonates in SMM or MGUS and all of the current guidelines restrict the use of bisphosphonate therapy to patients with active MM and evidence of bone disease. However, there is emerging evidence that intervention at earlier stages of the disease may prevent skeletal-related events at time of progression, but there is no evidence that bisphosphonates in this setting change the natural history of the disease. Musto et al examined the use of zoledronic acid in previously untreated patients with SMM in an open-label, phase III, randomized trial.68 One hundred sixty-three patients were enrolled and randomized to zoledronic acid (n = 81 patients) for 1 year at a monthly dose of 4 mg versus placebo for 1 year (n = 82 patients).68 The rates of progression to MM were similar at a median follow-up of 64.7 months with 44.4% of patients in the zoledronic groups versus 45.1% in the placebo control arm (P = .9307).68 In the zoledronic acid group the median time to progression was 67 months and in the control arm the median time to progression was 59 months (P = .8312).68 At the same time, the authors found that, at time of progression to MM, skeletal-related events were significantly lower in the zoledronic acid groups compared with the control arm: 55.5% and 78.3%, respectively (P = .041).68 A few other studies have examined the effects of bisphosphonates in SMM, MGUS, and early-stage MM (see Table 2).
Table 2.
Studies Examining Bisphosphonates in Patients With MGUS, SMM, or Early MM
| Study | Study Design | Bisphosphonate | No. of Patients | Results |
|---|---|---|---|---|
| Musto et al68 (2008) | Prospective, phase III, randomized,placebo control | Zoledronic acid 4 mg monthly x1 year | 163 SMM | Decreased skeletal-related events at progression.No change in natural history. |
| Berenson et al69 (2008) ASH abstract | Prospective, single arm | Zoledronic acid 4 mg at months 0, 6, 12 | 58 MGUS | Increased bone density at 1 year. |
| Musto et al70 (2003) | Prospective, randomized,placebo control | Pamidronate 60 mg monthly x1 year | 66 stage IA Durie-Salmon MM 24 Stage IIA Durie- Salmon MM |
Decreased skeletal-related events in treatment arm. No change in progression. |
| Musto et al71 (2003) | Prospective, historical control | Pamidronate 60 mg monthly x1 year then every 3 mo until progression | 43 Stage IA Durie-Salmon MM or SMM | No change in rates of disease progression or time to progression.Decreased skeletal-related events in treatment arm. |
| Martin et al72 (2002) | Prospective, single arm | Pamidronate 60–90 mg monthly x1 year | 12 SMM | Increased bone density at 1 year. |
Abbreviations: MM, multiple myeloma; SMM, smoldering multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance.
In a study presented at the 2008 ASCO meeting, Berenson et al examined the use of zoledronic acid in patients with osteopenia/osteoporosis (T score <−1.0) and who met the criteria for MGUS.69 Zoledronic acid was administered intravenously at a dose of 4 mg over 15 minutes at months 0, 6, and 12.69 Dexa scans and skeletal surveys were performed at time of screening and 1 month after the final zoledronic acid injection. A total of 54 patients were enrolled with an average age of 68 without prior exposure to bisphosphonate therapy.69 The mean baseline T score was −2.21 in the L spine and −1.89 in the hip.69 T scores after therapy improved +0.58 (mean increase of 26%, P = .0021) in the L spine and +0.26 (mean increase of 14%, P = .0020) in the hip.67 MGUS patients appear to have a high prevalence of osteopenia and osteoporosis and recent data suggest that they are at increased risk of fractures as well.15–19 Taken together, the role of bisphosphonates in myeloma precursor disease (MGUS and SMM)—with the aim to prevent future skeletal-related events—needs to be better defined in randomized, prospective studies.
SUMMARY AND FUTURE DIRECTIONS
On a clinical note, bone disease remains a critical component in the diagnosis and treatment of patients with MM. There are data to support the use of bisphosphonates in the treatment of bone disease in MM in order to decrease skeletal-related complications and maintain quality of life. However, the use of bisphosphonates has to be balanced against the complications of prolonged therapy. The exact duration and frequency of bisphosphonates in patients with stable disease, on active treatment, and at relapse needs to be further defined.
Efforts to find ways to reduce the toxicity associated with bisphosphonate therapy are being explored. Gimsing et al investigated the effect of 30 mg of pamidronate versus 90 mg in patients with newly diagnosed MM.73 In this double-blind, randomized phase III trial patients were randomized to receive either 30 mg or 90 mg of pamidronate monthly for at least 3 years of therapy.73 The primary outcome of the study was physical function after 12 months of therapy assessed by a quality-of-life questionnaire.73 There were no significant differences in the mean physical function between the two groups.73 In the patients who developed a skeletal-related event, the median time was 9.2 months in the 90-mg group and 10.2 months in the 30-mg group (P = .63).73 In a retrospective analysis, eight of the 157 patients in the 90-mg group developed osteonecrosis of the jaw, while only two of the 156 patients in the 30 mg group developed this condition.73
On a research basis, there are emerging data to support a role for other agents to target various; pathways involved in the pathophysiology of bone disease in MM. Their role remains to be defined in the treatment paradigm. Emerging data also suggest that bone disease is found in both precursor states of myeloma: MGUS and SMM. One interesting area of future investigation is to define a role of intervention in these myeloma precursor states with standard therapy for MM (ie, bisphosphonates), as well as newer agents.
Newer agents targeting other pathways in the pathogenesis of bone disease are being developed and tested in patients with myeloma bone disease and include drugs such as denosumab (Prolia; Amgen, Thousand Oaks, CA). Denosumab, a fully human monoclonal antibody, binds RANKL preventing the interaction of RANKL-RANK imitating the effects of OPG.47 Phase II studies of denosumab in MM demonstrated a significant decrease in skeletal-related events and phase III trials (NCT00330759, www.clinicaltrials.gov) are currently underway.47 Murine studies demonstrate decreased bone destruction and decreased tumor burden with MIP-1 a-blocking antibodies.74 Other potential targets for drug development include inhibitors of osteoblast differentiation such as DKK1, IL-3, and IL-7.74 The agents provide further opportunities to explore early intervention in myeloma bone disease in an attempt to improve outcomes and influence the natural history of the disease.
Footnotes
Disclosures: None of the authors have anything to disclose; there is no conflict of interest related to this review. These views are those of the authors and not the official views of the Department of Defense or the National Institutes of Health.
REFERENCES
- 1.Dispenzieri A, Kyle RA. Multiple myeloma: clinical features and indications for therapy. Best Pract Res Clin Haematol. 2005;19:553–68. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23: 1691–7. [DOI] [PubMed] [Google Scholar]
- 3.Roodman GD. Pathogenesis of myeloma bone disease. Blood Cells Mol Dis. 2005;32:192–8. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Lipton A, Cook R, Chen Y, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007;110: 1860–7. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ III, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20:487–93. [DOI] [PubMed] [Google Scholar]
- 6.Giuliani N, Colla S, Rizzoli V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NG-kappaB ligand (RANKL). Exp Hematol. 2005;32:685–91. [DOI] [PubMed] [Google Scholar]
- 7.Osteoblastogenesis Yaccoby S. and tumor growth in myeloma. Leuk Lymphoma. 2010;51:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bataille R, Chappard D, Marcelli C, et al. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentin OA, Charhon SA, Meunier PF, Edouard CM, Arlot ME. Quantitative histology of myeloma-induced bone changes. Br J Hematol. 1982;52:601–10. [DOI] [PubMed] [Google Scholar]
- 10.Jakob C, Zavrski L, Heider U, Bollow M, Schulz CO, Fleissner C. Serum levels of carboxy-terminal telopeptide of type-I collagen are elevated in patients with multiple myeloma showing skeletal manifestations in magnetic resonance imaging but lacking bone lesions in conventional radiography. Clin Cancer Res. 2003;9:3047–51. [PubMed] [Google Scholar]
- 11.Vejlgaard T, Abildgaard N, Jans H, Nielsen JL, Heickendorff L. Abnormal bone turnover in monoclonal gammopathy of undetermined significance: analysis of type I collage telopeptide osteocalcin, bone-specific alkaline phosphatase and propeptides of type I and type III pro-collagens. Eur J Haematol. 1997;58:104–8. [DOI] [PubMed] [Google Scholar]
- 12.Jakob C, Zavrski I, Heider U, et al. Bone resporption parameters [carboxy-terminal telopeptide of type-I collagen (ICTP), amino-terminal collagen type-I telopeptide (NTx), and deoxypyridinoline (Dpd)] in MGUS and multiple myeloma. Eur J Haematol. 2002;69:37–42. [DOI] [PubMed] [Google Scholar]
- 13.Mundy GR. Myeloma bone disease. Eur J Cancer. 1998; 34:246–51. [DOI] [PubMed] [Google Scholar]
- 14.Battaille R, Chappard D, Basle MF. Quantifiable excess of bone resorption in monoclonal gammopathy is an early symptom of malignancy: a prospective study of 87 bone biopsies. Blood. 1996;87:4762–9. [PubMed] [Google Scholar]
- 15.Golombick T, Diamond T. Prevelance of monoclonal gammopathy of undetermined significance/myeloma in patients with acute osteoporotic vertebral fractures. Acta Heamatol. 2008;120:87–90. [DOI] [PubMed] [Google Scholar]
- 16.Pepe J, Petrucci MT, Nofroni I, et al. Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:485–90. [DOI] [PubMed] [Google Scholar]
- 17.Gregersen H, Jensen P, Gislum M, Jorgensen B, Sorensen HT, Norgaard M. Fracture risk in patents with monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;135:62–7. [DOI] [PubMed] [Google Scholar]
- 18.Bida JP, Kyle RA, Therneau TM, et al. Dissease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17/398 patients. Mayo Clin Proc. 2009;84:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristinsson SY, Tang M, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010. July 7 Available from bloodjournal.hematologylibrary.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson KC, Shaughnessy JD Jr, Barlogie B, Harousseau JL, Roodman GD. Multiple Myeloma. Hematology Am Soc Hematol Educ Program. 2002;2202:214–40. [DOI] [PubMed] [Google Scholar]
- 21.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349:2483–94. [DOI] [PubMed] [Google Scholar]
- 22.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–91. [DOI] [PubMed] [Google Scholar]
- 23.Terpos E, Sezer O, Croucher P, Dimopoulos MA. Myeloma bone disease and proteasome inhibition therapies. Blood. 2007;110:1098–104. [DOI] [PubMed] [Google Scholar]
- 24.Evans CE, Galasko CS, Ward C. Does myeloma secrete and osteoblast inhibiting factor? J Bone Joint Surg Br. 1989;71:288–90. [DOI] [PubMed] [Google Scholar]
- 25.Hjorth-Hansen H, Seifert MF, Borset M, et al. Marked osteoblastopenia and reduced bone formation in a model of multiple myeloma bone disease in severe combined immunodeficiency mice. J Bone Miner Res. 1999;14: 256–63. [DOI] [PubMed] [Google Scholar]
- 26.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of up-regulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004;126:475–86. [DOI] [PubMed] [Google Scholar]
- 27.Tinhofer I, Biedermann R, Krismer M, Crazzolara K, Greil R. A role of TRAIL in killing osteoblasts by myeloma cells. FASEBJ. 2006;20:759–61. [DOI] [PubMed] [Google Scholar]
- 28.Roodman GD. Osteoblast function in myeloma. Bone. Epub 2010. June 19 Available from: www.elsevier.com/locate/bone. [DOI] [PubMed] [Google Scholar]
- 29.Karadag A, Oyajobi BO, Apperley JF, Russell RG, Croucher PI. Human myeloma cells promote the production of interleukin-6 by primary human osteoblasts. Br J Haematol. 200;108:383–90. [DOI] [PubMed] [Google Scholar]
- 30.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–6. [PubMed] [Google Scholar]
- 31.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliani N, Rizzoli V, Roodman GD. Mutiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–6. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani N, Rizzoli V. Myeloma cells and bone marrow osteoblast interactions: role in the development of osteolytic lesions in multiple myeloma. Leuk Lymphoma. 2007;48:2323–9. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Kronenberg H. Minireview: transcriptional regulation in development of bone. Endocrinology. 2005;146:1012–7. [DOI] [PubMed] [Google Scholar]
- 35.Roodman GD. Pathogenesis of myeloma bone disease. J Cell Biochem. 2010;109:283–91. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani N, Colla S, Morandi F, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–83. [DOI] [PubMed] [Google Scholar]
- 37.Michigami T, Shimizu N, Williams PJ, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast stimulating activity. Blood. 2000;96:1953–60. [PubMed] [Google Scholar]
- 38.Ely SA, Knowles DM. Expression of CD56/neural cell adhesion molecule correlates with the presence of lytic lesions in multiple myeloma and distinguishes myeloma from monoclonal gammopathy of undetermined significance and lymphomas with plasmacytoid differentiation. AmJ Pathol. 2002;160:1293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadopoulou EC, Batzios SP, Dimitriadou M, Perifanis V, Garipidou V. Multiple myeloma and bone disease: pathogenesis and current therapeutic approaches. Hippokratia. 2010;14:76–81. [PMC free article] [PubMed] [Google Scholar]
- 40.Westendorff JJ, Kahler RA, Schroeder TM. Wnt signaling in osteblasts and bone diseases. Gene. 2004;341:19–29. [DOI] [PubMed] [Google Scholar]
- 41.Lentzch D Ehrlich LA, Roodman GD. Pathophysiology of multiple myeloma bone disease. Hematol Oncol Clin N Am. 2007;21:1035–49. [DOI] [PubMed] [Google Scholar]
- 42.Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma. Bone. 2008;42: 1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehrlich LA, Chung HY, Chobrial I, et al. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–14. [DOI] [PubMed] [Google Scholar]
- 44.Standal T, Abildgaard N, Fagerli UM, et al. HGF inhibits BMP-induced osteoblastogenesis: possible implications for bone disease of multiple myeloma. Blood. 2007; 109:3024–30. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi K, Abe M, Oda A, et al. Enhancement of osteoblast differentiation by inhibition of TGF-beta signaling suppresses myeloma cell growth and protects from destructive bone lesions. J Bone Miner Res. 2006;21:1101. [Google Scholar]
- 46.Giuliani N, Colla S, Morandi F, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–83. [DOI] [PubMed] [Google Scholar]
- 47.Roodman GC. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–44. [DOI] [PubMed] [Google Scholar]
- 48.Bataille R, Chappard D, Basle M. Excessive bone resorption in human plasmacytomas: direct induction by tumour cells in vivo. Br J Haematol. 1995;90:721–4. [DOI] [PubMed] [Google Scholar]
- 49.Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997; 390:175–9. [DOI] [PubMed] [Google Scholar]
- 50.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. [DOI] [PubMed] [Google Scholar]
- 51.Sezer O, Heider U, Zavriski I, Kuhne CA, Hofbauer LC. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003;101:2094–8. [DOI] [PubMed] [Google Scholar]
- 52.Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axs to stimulate bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98:11581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terpos E, Szydlo R, Apperley JF, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for novel prognostic index. Blood. 2003;102: 1064–9. [DOI] [PubMed] [Google Scholar]
- 54.Abe M, Hiura K, Wilde J, et al. Role for macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–02. [PubMed] [Google Scholar]
- 55.Terpos E, Politou M, Szydlo R, Goldman JM, apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol. 2003;123:106–9. [DOI] [PubMed] [Google Scholar]
- 56.Choi SJ, Cruz JC, Craig F, et al. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–75. [PubMed] [Google Scholar]
- 57.Zannettino AC, Farrugia AN, Kortesdis A, et al. Elevated serum levels of stromal-derived factor-1 alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65:1700–9. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka Y, Abe M, Hiasa M, et al. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007;13:816–23. [DOI] [PubMed] [Google Scholar]
- 59.Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–91. [DOI] [PubMed] [Google Scholar]
- 60.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:529–30. [DOI] [PubMed] [Google Scholar]
- 61.Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. J Clin Oncol. 1998;16:2572–73. [DOI] [PubMed] [Google Scholar]
- 62.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind comparative trial. Cancer J. 2001;7:377–87. [PubMed] [Google Scholar]
- 63.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–72. [DOI] [PubMed] [Google Scholar]
- 64.Anderson KC, Alsira M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Cancer Netw. 2009;7:908–42. [DOI] [PubMed] [Google Scholar]
- 65.Lacy MQ, Deispnzieri A, Gertz MA, et al. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047–53. [DOI] [PubMed] [Google Scholar]
- 66.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European myeloma network. Ann Oncol. 2009;20:1303–17. [DOI] [PubMed] [Google Scholar]
- 67.Zervas K, Verrou E, Teleioudis Z, et al. Incidenc, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol. 2006;134:620–3. [DOI] [PubMed] [Google Scholar]
- 68.Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–95. [DOI] [PubMed] [Google Scholar]
- 69.Berenson JR, Yellin O, Boccia RV, et al. Marked improvement in bone mineral density (BMD) for patients (pts) with monoclonal gammopathy of undetermined significance (MGUS) using zoledronic acid (ZOL) [abstract]. ASCO Annual Meeting Abstracts. 2008;26:8550. [Google Scholar]
- 70.Musto P, Falcone A, Grazia S, et al. Pamidronate reduces skeletal events but does not improve progression-free survival in early-stage untreated myeloma: results of a randomized trial. Leuk Lymphoma. 2003;44:1545–8. [DOI] [PubMed] [Google Scholar]
- 71.Musto P, Falcone A, Sanpaolo G, Bodenizza C, Carella AM. Pamidronate for early-stage, untreated myeloma. J Clin Oncol. 2003;21:3177–8. [DOI] [PubMed] [Google Scholar]
- 72.Martin A, Garcia-Sanz R, Hernandez J, et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant antitumor effect. Br J Hematol. 2002;118:239–42. [DOI] [PubMed] [Google Scholar]
- 73.Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30mg versus 90mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomized controlled trial. Lancet Oncol. 2010;11:973–82. [DOI] [PubMed] [Google Scholar]
- 74.Roodman GD. New potential targets for treating myeloma bone disease. Clin Cancer Res. 2006;12:6270s–73s. [DOI] [PubMed] [Google Scholar]