Abstract
HIV-HCV co-infection causes rapid progression of liver fibrosis. These outcomes to liver cirrhosis can be improved, but not stopped by specific antiviral therapies. Due to high significance of HIV-HCV interactions for morbidity and mortality in co-infected patients, our attention was attracted to the multi-component pathogenesis of fibrosis progression as the transition to end-stage liver disease development. In this study, we hypothesize that increased matrix stiffness enhances apoptosis in HCV-HIV-co-infected hepatocytes and that capturing of apoptotic bodies (AB) derived from these infected hepatocytes by hepatic stellate cells (HSC) drives the fibrosis progression. As the source of viruses, JFH1 (HCV genotype 2a) and HIV-1ADA (either purified or containing in infected macrophage supernatants) were chosen. Using Huh7.5-CYP (RLW) cells and primary human hepatocytes mono-infected with HCV and HIV or co-infected, we have shown that both HCV and HIV RNA levels were increased in co-infected cells, which was accompanied by hepatocyte apoptosis. This apoptosis was attenuated by azidothymidine treatment. The levels of both infections and apoptosis were more prominent in primary hepatocytes cultured on substrates mimicking fibrotic stiffness (24 kPa-stiff) compared to substrates mimicking healthy liver (2.4 kPa-soft). The engulfment of AB from pathogen-exposed hepatocytes activated pro-fibrotic mRNAs in HSC. Overall, the increased matrix stiffness is not only a consequence of liver inflammation/fibrosis, but the condition that further accelerates liver fibrosis development. This is attributed to the switching of HSC to pro-fibrotic phenotype by capturing of excessive amounts of apoptotic HCV- and HIV-infected hepatocytes.
Keywords: Hepatocytes, HCV, HIV, apoptosis, matrix stiffness, hepatic stellate cells
INTRODUCTION
Liver injury became one of the leading causes of death among HIV-infected non-AIDS patients. The rapid progress of liver disease in HIV-infected patients usually is associated with HCV-and HBV-co-infections or alcohol abuse. Among the estimated 40 million HIV carriers worldwide, about 4–5 million of them are chronically co-infected with HCV [1]. These co-infected patients have frequent end-stage disease outcomes, including liver cirrhosis and hepatocellular carcinoma (HCC) [2]. Although liver injury at HIV mono-infection is possible, there is higher incidence of liver damage in HIV-HCV co-infections, and the mechanisms of rapid fibrosis progression are not well-defined. Modern antiretroviral therapy (ART) suppresses HIV replication and improves disease outcomes, but does not stop liver damage progression [3]. In HCV infection, modern direct antiviral agents (DAA) efficiently block HCV replication, but sustained virologic response does not guarantee the outcome to HCC [4]. Natural history studies of HIV-HCV co-infection have demonstrated that the progression of liver fibrosis to cirrhosis is rapid, and these patients with decompensated cirrhosis have higher mortality rate [5]. As demonstrated by earlier studies, liver fibrosis development was primarily attributed to immune-mediated events related to HIV infection of non-parenchymal cells, induction of oxidative stress, mitochondrial injury, cytotoxicity and gut microbial translocation [6, 7]. The toxic effects of HIV on hepatocytes, which represents about 80% of liver cells, were mainly linked to signal transduction events initiated by interactions between viral envelop protein gp120 and chemokine HIV co-receptors, CCR5 and CXCR4 expressed on liver resident cells [8, 9]. However, it is still questionable whether hepatocytes present these receptors at the density that is sufficient to induce cell death or some other mechanisms are involved when they are exposed to infectious virus. It is not quite clear whether the death of parenchymal cells depends on matrix stiffness and contributes to hepatic stellate cells (HSC) activation since HSC activation is crucial for fibrosis.
In this study, we hypothesize that HCV pre-sensitizes hepatocytes to HIV-mediated apoptosis, and capturing of apoptotic bodies (AB) derived from infected hepatocytes by HSC drives the fibrosis progression. We also anticipate that these events will be more pronounced when hepatocytes are cultured on environment mimicking the fibrotic stiffness.
MATERIALS AND METHODS
Reagents and Media
High glucose Dulbecco’s Modified Eagle Medium (DMEM) and Williams Medium with supplements (insulin, holo-transferin, L-ascorbic acid, dexamethazone, selenium and antibiotics) were from Invitrogen (Carlsbad, CA), fetal bovine serum was from Atlanta Biological (Flowery Branch, GA). Antibody to the cleaved caspase-3 was from Cell Signaling (Beverly, MA); Antibody to β-actin was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). M30 Apoptosense® CK18 ELISA Kit was from Diapharma (West Chester, OH, USA).
Cells and culturing and treatments
We used Huh 7.5 cells stably transfected with CYP2E1 (designated as RLW cells) [10] as well as primary human hepatocytes obtained from University of Pittsburgh Medical Center Liver Tissue Cell Distribution System funded by NIH. Cells were cultured on polydimethyl siloxane (PDMS) gels coated with polyelectrolyte multilayer films (PEMs) with 2 kPa (soft) and 24 kPa (stiff) rigidity mimicking healthy and fibrotic liver environment [11–16]. Sylgard 527 and Sylgard 184 (Fisher Scientific, USA) were blended in various weight ratios for creating PDMS substrates with the desired stiffness for culturing cells (Table 1). According to our prior study, the two Sylgard precursors were mixed in varying weight ratios for engineering different stiffness and poured into tissue culture plates. The plates were incubated at 65°C overnight to ensure complete crosslinking of the mixture, to yield uniform PDMS substrates. PDMS surfaces were then coated with 10 bilayers of PEM films using two polyelectrolytes, Poly(diallyldimethylammonium chloride) (PDAC) (Mw~100,000–200,000) as a 20 wt % solution and sulfonated poly(styrene), sodium salt (SPS) (Mw~70,000) to facilitate cell adhesion. This platform provides an ideal in vitro environment to noninvasively study and assess the effects of liver stiffness as a sole condition on pro-apoptotic/fibrotic effects of HIV and HCV in hepatocytes. We tested the effects of co-infection on “healthy” and “diseased” liver, thereby mimicking HIV-HCV-induced pro-fibrotic and pro-inflammatory changes at early stage of co-infection (2 kPasoft gels) and at advanced liver cirrhosis (24 kPa-stiff gels). For co-infection, the cells were exposed to HIV-1ADA at MOI 0.1, then the virus was removed and washed out after overnight incubation, and in 24 h, cells were infected with HCV (JFH1 virus [17]) at MOI 0.1 for 3 days. Alternatively, the cells were mono-infected with HIV and HCV, for 4 and 3 days, respectively or used uninfected.
Table 1.
Young’s Modulus of PEM coated PDMS substrates used for primary hepatocyte culture as determined using Indentation load technique.
| Substrate | Young’s Modulus (in KPa) |
|---|---|
| Soft (100% Sylgard 527) | 2.4 ± 0.03 |
| Stiff (91% wt Sylgard 527 + 9% wt Sylgard 184) | 24.2 ± 0.03 |
RNA isolation, Real Time (RT)- PCR and immunoblotting
RNA was isolated and HCV RNA was measured by RT-PCR as described [18]; gag HIV RNA was measured by both quantitative RT-PCR (RT-qPCR) and as a relative quantity [19]. For immunoblotting (IB), cells were lysed and then IB was performed as described [20].
Apoptotic bodies
(AB) were generated from RLW cells by exposure to UV light and characterized as shown previously [18].
Hepatic stellate cells (HSC) treatment with AB
AB from RLW cells (AB Hep) were incubated with HSC cell line, LX2 cells (EMD Millipore). Cells were incubated with AB for 2 −8 h at 1:3 ratio and then pro-fibrotic markers (Col1A1, TGFβ, TIMP-1) and a pro-inflammatory marker IL-1 β) were quantified by RT-PCR.
Statistical analyses
Data from at least three independent experiments were expressed as mean values ± standard error. Comparisons among multiple groups were determined by one-way ANOVA, using a Tukey post-hoc test. For comparisons between two groups, we used Student’s t-test. A probability value of 0.05 or less was considered significant.
RESULTS
HCV-HIV co-infection in RLW cells
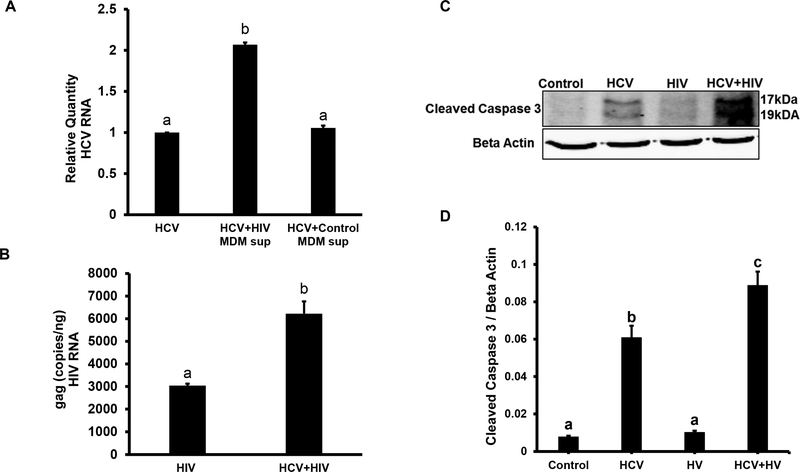
Because canonic liver cells for HCV replication are hepatocytes and for HIV are immune cells (namely liver macrophages, Kupffer cells), we cultured HCV-infected RLW cells with medium from human monocyte derived macrophages (MDM) infected or not with HIV. MDM produced high amount of HIV in supernatants and the reverse transcriptase activity in supernatant used for all experiments was <25,000 cpm/ml. Co-infection of RLW cells was assessed by the levels of HCV and HIV RNAs (RT-PCR and Q-PCR). As shown on Fig. 1A, HCV RNA expression in RLW cells exposed to HIV-containing supernatants from MDMs was higher than in HCV-mono-infected cells and those exposed to supernatants from non-infected MDMs. Also, the level of gag HIV RNA was higher in RLW cells co-infected with HCV (Fig.1B). Furthermore, this highest expression of HCV and HIV RNAs in co-infected RLW cells plated on plastic surface (with no gels) was accompanied by apoptosis induction (caspase 3 cleavage detected by IB, Fig.1C, D).
Fig. 1. HCV and HIV RNAs and caspase 3 cleavage in liver cells.
RLW cells were plated on plastic surface and then exposed to JFH1 (HCV) and supernatants from HIV-infected or uninfected macrophages as specified. A. HCV RNA (RT-PCR); B. gag HIV RNA (RT-qPCR); C. Cleaved caspase 3 (IB); D. Cleaved caspase 3 (IB quantification). All data (representative results and quantification) were conducted in duplicate and obtained from 3 independent experiments, and presented as Mean ± SEM. Bars with different letters are significantly different at p ≤ 0.05.
Matrix stiffness regulates HIV RNA and HCV RNA levels in HCV-HIV co-infected liver cells
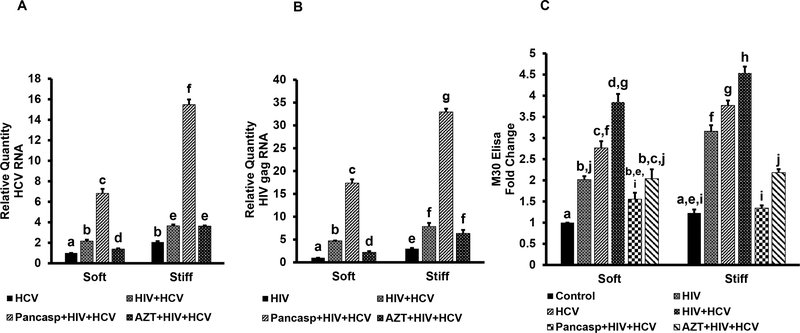
Next, we confirmed the results obtained with co-infected RLW cells on primary human hepatocytes and studied the effects of matrix stiffness on intracellular infection levels and apoptotic cell death. Hepatocytes were plated on soft (2 kPa) and stiff gels (24 kPa) to mimic healthy and fibrotic liver environment. Cells were infected with HCV or HIV or both in the presence or absence of pan-caspase inhibitor (50uM, Z-VAD-EMK. UBPBio, Aurora, CO). To demonstrate that HIV RNA content in hepatocytes is attributed to replicating virus, the experiment has been done with or without azidothymidine (AZT, 100 μM) treatment [21]. Then HCV RNA and HIV RNA were measured by RT-PCR. Consistent with the results on RLW cells, we observed that co-infection with HIV with HCV increased both HCV and HIV RNAs in hepatocytes (Fig.2 A, B). This effect was more pronounced when hepatocytes were plated on fibrotic like-stiff gels. The levels of HIV and HCV RNAs were up to 2-fold increased by treatment with pan-caspase inhibitor, suggesting that a substantial portion of co-infected cells undergo apoptosis. Importantly, expression of HCV and HIV RNA was lower in the presence of AZT underlining the contribution of HIV replication in hepatocytes to the regulation of HCV RNA expression. However, AZT was not effective under stiff gel conditions. To confirm apoptosis in mono- or co-infected hepatocytes, we measured cytokeratin 18 (apoptotic marker M30) in cell supernatants by ELISA. As shown on Fig. 2 C, apoptosis levels were higher in co-infection vs mono-infections, and they were abrogated by treatment with pan-caspase inhibitor. Furthermore, AZT attenuated the effects of HIV on apoptosis induction in hepatocytes. Apoptosis induction was increased when hepatocytes were plated on stiff gels compared with soft gels.
Fig. 2. HCV-HIV co-infection in primary human hepatocytes.
Primary hepatocytes were plated on PDMS gels coated with (PDAC/SPS)10 PEM films with 2 kPa (soft) and 24 kPa (stiff) stiffness mimicking healthy and fibrotic liver environment and infected with HCV and HIV, in the presence or absence of pan-caspase inhibitor and AZT. A. HCV RNA (RT-PCR); B. gag HIV RNA (RT-PCR); C. M30 levels in hepatocyte supernatants (fold change, ELISA). All data were generated from 3 independent duplicate experiments and presented as Mean ± SEM. Bars with different letters are significantly different at p ≤ 0.05.
Hepatocyte apoptotic bodies (AB) and HSC
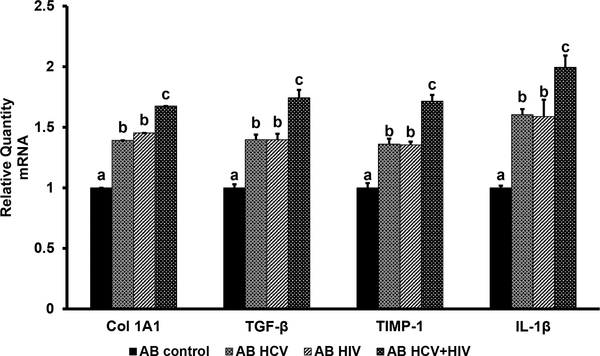
To mimic the pro-apoptotic effects of HCV-HIV co-infection on hepatocytes, ABs from RLW cells (uninfected or infected with either HIV or HCV or HIV+HCV) were exposed to HSC, and then pro-fibrotic and pro-inflammatory changes were measured by RT-PCR. We found that in HSC, AB from mono-infected RLW cells increased Col1A1, TGFβ, TIMP-1 and IL-1 β mRNAs compared with the effects of AB from uninfected cells; the levels of these mRNAs were significantly higher when AB were generated from co-infected RLW cells (Fig. 3).
Fig. 3. Activation of HSC by AB from uninfected and HCV-HIV-infected liver cells.
AB were made from infected or uninfected RLW cells and incubated with HSC as described. Then mRNAs of Col 1A1, TGFβ, TIMP-1 and IL-1β were measured by RT-PCR. All data were generated from 3 independent duplicate experiments and presented as Mean ± SEM. Bars with different letters are significantly different at p ≤ 0.05.
DISCUSSION
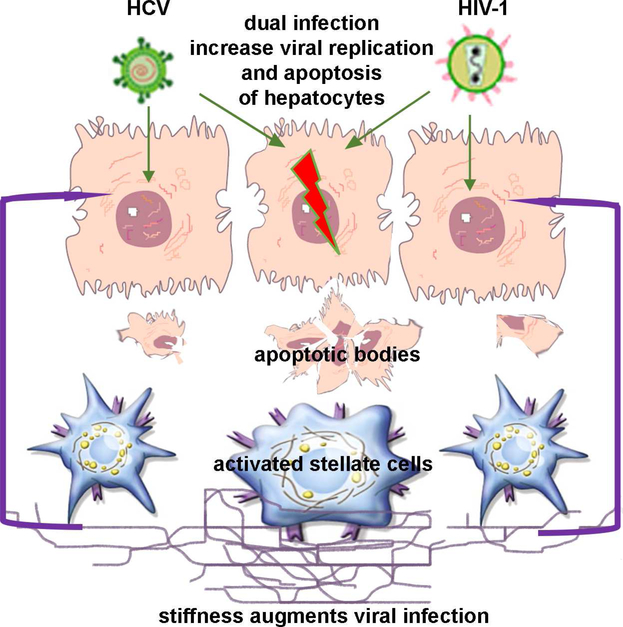
HCV-HIV co-infections have high incidence of decompensated liver cirrhosis and hepatocellular carcinoma exceeding the frequency of these outcomes reported for mono-infections [22]. Many mechanisms explaining this scenario were suggested, including the effects of HIV and HCV on cell death or direct infection of non-parenchymal liver cells, namely, HSC responsible for matrix deposition [23–25]. However, in addition to these reasons, there are more options explaining why HCV-HIV co-infection promotes the rapid fibrosis progression, and one of suggested mechanisms might be a pro-fibrotic activation of HSC by apoptotic pathogen-expressing hepatocytes. In this study, we examined the effects of infectious HIV and HCV on hepatocytes. By using experimental model of hepatocyte-like CYP2E1-expressing RLW cells, we established HCV-HIV co-infection in these cells accompanying by apoptotic cell death. While hepatocytes are known as the permissive cells for HCV, HIV-infection in these cells is questionable or low [21]. To investigate whether the persistence of double-infected hepatocytes is restricted by apoptosis, we blocked apoptosis with pan-caspase inhibitor and only then have been able to observe the elevated levels of co-infected hepatocytes. In our model, HIV serves as a second hit for pushing of HCV-infected hepatocytes to apoptosis. This resembles the situation of HCV-infection combined with ethanol treatment (as a second hit) when we also observed massive apoptosis in HCV-infected hepatocytes as well as dissemination of HCV to uninfected cells with these ABs [18]. Although studies by others demonstrated the role of gp120 interactions with HIV co-receptor CXCR4 for apoptosis induction in the absence of whole virus [26], our current results indicate the involvement of the infectious HIV to trigger cell death. In fact, the blockade of HIV replication by AZT is crucial for apoptosis suppression in co-infected hepatocytes. Interestingly, the levels of infections and apoptosis were more prominent when hepatocytes were cultured on fibrotic-like stiff matrix, indicating that co-infected hepatocytes at the late stage of liver disease (corresponding to liver cirrhosis) become more prone to apoptotic cell death triggered by higher intracellular viral load. Interestingly, in our hands, AZT provided no protection to Hep plated on stiff gels, indicating that at late stage of liver fibrosis, co-infected Hep might be less sensitive to ART. These novel data on the role of matrix stiffness in hepatocyte survival can complement our understanding of the pathogenesis of accelerated liver fibrosis progression induced by continues activation of liver non-parenchymal cells after exposure to infectious hepatocyte AB. As appeared, an enhanced matrix stiffness is not only a consequence, but also the condition that promotes fast fibrosis development. In fact, elevated levels of immune activation biomarkers have been reported in HCV-HIV co-infected patients in conjunction with the increased liver stiffness [27]. Induction of pro-fibrotic and pro-inflammatory mRNAs in HSC by exposure to pathogen-containing hepatocyte ABs provides one more matrix stiffness-regulated mechanism of fibrosis acceleration in HCV-HIV co-infected patients (summarized in Fig.4).
Fig. 4. Acceleration of fibrosis progression in HCV-HIV co-infection via AB release.
HCV-HIV co-infection induces prominent apoptotic cell death in hepatocytes. These apoptotic bodies engulfed by HSC induce pro-fibrotic activation. Increased matrix stiffness corresponding to more advanced stages of liver disease further promoted apoptosis of infected hepatocytes, thereby accelerating fibrosis development.
We conclude that the levels of intracellular HIV and HCV infections and apoptotic cell death are higher in HIV-HCV-co-infected than in mono-infected hepatocytes and can be further enhanced under increased matrix stiffness conditions during progression of fibrosis. Apoptotic bodies from pathogen-expressing hepatocytes trigger pro-fibrotic and pro-inflammatory changes in HSC, thereby promoting fibrosis development.
Highlights.
HIV-HCV co-infection increases HIV/HCV RNAs levels and apoptosis in hepatocytes;
Fibrotic matrix stiffness enhances HIV/HCV RNAs and apoptosis in hepatocytes;
Apoptotic infected hepatocytes induce pro-fibrotic changes in hepatic stellate cells
Acknowledgment
We thank Dr. C. Rice for Huh7.5 cells, Dr. T. Wakita for JFH1 virus. This work was supported by Merit Review BX001673 from the Department of Veterans Affairs, Office of Research and Development (Biomedical Laboratory Research and Development).
Abbreviations
- AB
Apoptotic bodies
- AZT
Azidothymidine
- ART
antiretroviral therapy
- CYP2E1
cytochrome P4502E1
- HCV
hepatitis C virus
- HIV
human Immunodeficiency Virus
- HSC
hepatic stellate cells
- JFH1
Japanese fulminant hepatitis virus-1
- IB
immunoblotting
- MDM
Macrophages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alter MJ, Epidemiology of viral hepatitis and HIV co-infection, J Hepatol, 44 (2006) S6–9. [DOI] [PubMed] [Google Scholar]
- [2].Barreiro P, Martin-Carbonero L, Nunez M, Rivas P, Morente A, Simarro N, Labarga P, Gonzalez-Lahoz J, Soriano V, Predictors of liver fibrosis in HIV-infected patients with chronic hepatitis C virus (HCV) infection: assessment using transient elastometry and the role of HCV genotype 3, Clin Infect Dis, 42 (2006) 1032–1039. [DOI] [PubMed] [Google Scholar]
- [3].Pascual-Pareja JF, Caminoa A, Larrauri C, Gonzalez-Garcia J, Montes ML, Diez J, Grande M, Arribas JR, HAART is associated with lower hepatic necroinflammatory activity in HIV-hepatitis C virus-coinfected patients with CD4 cell count of more than 350 cells/microl at the time of liver biopsy, AIDS, 23 (2009) 971–975. [DOI] [PubMed] [Google Scholar]
- [4].Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S, Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with directacting antivirals, J Hepatol, 65 (2016) 727–733. [DOI] [PubMed] [Google Scholar]
- [5].Pineda JA, Romero-Gomez M, Diaz-Garcia F, Giron-Gonzalez JA, Montero JL, Torre-Cisneros J, Andrade RJ, Gonzalez-Serrano M, Aguilar J, Aguilar-Guisado M, Navarro JM, Salmeron J, Caballero-Granado FJ, Garcia-Garcia JA, Grupo I Andaluz para el Estudio de las Enfermedades, H. Grupo Andaluz para el Estudio del, HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis, Hepatology, 41 (2005) 779–789. [DOI] [PubMed] [Google Scholar]
- [6].Crane M, Iser D, Lewin SR, Human immunodeficiency virus infection and the liver, World J Hepatol, 4 (2012) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin W, Weinberg EM, Chung RT, Pathogenesis of accelerated fibrosis in HIV/HCV co-infection, J Infect Dis, 207 Suppl 1 (2013) S13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bruno R, Sacchi P, Puoti M, Maiocchi L, Patruno SF, Cima S, Filice G, Pathogenesis of liver damage in HCV-HIV patients, AIDS Rev, 10 (2008) 15–24. [PubMed] [Google Scholar]
- [9].Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, Mendez-Navarro J, Chen WC, Jilg N, Zhao H, Goto K, Zhang L, Brockman MA, Schuppan D, Chung RT, HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB, J Biol Chem, 286 (2011) 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ganesan M, Zhang J, Bronich T, Poluektova LI, Donohue TM Jr., Tuma DJ, Kharbanda KK, Osna NA, Acetaldehyde accelerates HCV-induced impairment of innate immunity by suppressing methylation reactions in liver cells, Am J Physiol Gastrointest Liver Physiol, 309 (2015) G566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kidambi S, Chan C, Lee I, Selective depositions on polyelectrolyte multilayers: self-assembled monolayers of m-dPEG acid as molecular template, J Am Chem Soc, 126 (2004) 4697–4703. [DOI] [PubMed] [Google Scholar]
- [12].Kidambi S, Chan C, Lee I, Tunable resistive m-dPEG acid patterns on polyelectrolyte multilayers at physiological conditions: template for directed deposition of biomacromolecules, Langmuir, 24 (2008) 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kidambi S, Lee I, Chan C, Controlling primary hepatocyte adhesion and spreading on protein-free polyelectrolyte multilayer films, J Am Chem Soc, 126 (2004) 16286–16287. [DOI] [PubMed] [Google Scholar]
- [14].Kidambi S, Sheng L, Yarmush ML, Toner M, Lee I, Chan C, Patterned co-culture of primary hepatocytes and fibroblasts using polyelectrolyte multilayer templates, Macromol Biosci, 7 (2007) 344–353. [DOI] [PubMed] [Google Scholar]
- [15].Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y, Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance, Proc Natl Acad Sci U S A, 106 (2009) 15714–15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Natarajan V, Berglund EJ, Chen DX, Kidambi S, Substrate stiffness regulates primary hepatocyte functions, RSC Advances, 5 (2015) 80956–80966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kato T, Date T, Murayama A, Morikawa K, Akazawa D, Wakita T, Cell culture and infection system for hepatitis C virus, Nat Protoc, 1 (2006) 2334–2339. [DOI] [PubMed] [Google Scholar]
- [18].Ganesan M, Natarajan SK, Zhang J, Mott JL, Poluektova LI, McVicker BL, Kharbanda KK, Tuma DJ, Osna NA, Role of apoptotic hepatocytes in HCV dissemination: regulation by acetaldehyde, Am J Physiol Gastrointest Liver Physiol, 310 (2016) G930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arainga M, Su H, Poluektova LY, Gorantla S, Gendelman HE, HIV-1 cellular and tissue replication patterns in infected humanized mice, Sci Rep, 6 (2016) 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ganesan M, Poluektova LY, Tuma DJ, Kharbanda KK, Osna NA, Acetaldehyde Disrupts Interferon Alpha Signaling in Hepatitis C Virus-Infected Liver Cells by Up-Regulating USP18, Alcohol Clin Exp Res, 40 (2016) 2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kong L, Cardona Maya W, Moreno-Fernandez ME, Ma G, Shata MT, Sherman KE, Chougnet C, Blackard JT, Low-level HIV infection of hepatocytes, Virol J, 9 (2012) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brau N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, Trikha A, Sherman M, Sulkowski MS, Dieterich DT, Rigsby MO, Wright TL, Hernandez MD, Jain MK, Khatri GK, Sterling RK, Bonacini M, Martyn CA, Aytaman A, Llovet JM, Brown ST, Bini EJ, Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study, J Hepatol, 47 (2007) 527–537. [DOI] [PubMed] [Google Scholar]
- [23].Chen JY, Feeney ER, Chung RT, HCV and HIV co-infection: mechanisms and management, Nat Rev Gastroenterol Hepatol, 11 (2014) 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sagnelli C, Uberti-Foppa C, Pasquale G, De Pascalis S, Coppola N, Albarello L, Doglioni C, Lazzarin A, Sagnelli E, Factors influencing liver fibrosis and necroinflammation in HIV/HCV coinfection and HCV monoinfection, Infection, 41 (2013) 959–967. [DOI] [PubMed] [Google Scholar]
- [25].Saracino A, Cozzi-Lepri A, Shanyinde M, Ceccherini Silberstein F, Nozza S, Di Biagio A, Cassola G, Bruno G, Capobianchi M, Puoti M, Monno L, d’Arminio Monforte A, Study IF, HIV-1 co-receptor tropism and liver fibrosis in HIV-infected patients, PLoS One, 13 (2018) e0190302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV, Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4, J Infect Dis, 188 (2003) 1455–1460. [DOI] [PubMed] [Google Scholar]
- [27].Medrano LM, Garcia-Broncano P, Berenguer J, Gonzalez-Garcia J, Jimenez-Sousa MA, Guardiola JM, Crespo M, Quereda C, Sanz J, Canorea I, Carrero A, Hontanon V, Munoz-Fernandez MA, Resino S, Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/HCV-coinfected patients, Aids, (2018). [DOI] [PubMed] [Google Scholar]