Abstract
Post-stroke depression (PSD) is a common and serious complication following stroke. Both stroke and depression have independently been associated with pathologically elevated glutamate levels in the brain’s extra-cerebral fluid (ECF). Here we evaluate an alternative therapeutic approach to PSD with pyruvate. Rats were randomly assigned into one of 3 groups: Middle Cerebral Artery Occlusion (MCAO) plus pyruvate treatment, MCAO plus placebo treatment, and sham operated rats. Post-MCAO depressive and anxiety-like behavior was assessed, along with neurological status, brain infarct zone, brain edema, blood brain barrier (BBB) breakdown, cerebrospinal fluid and blood glutamate levels. Anxiety-like behavior and levels of blood alanine and α-ketoglutarate were measured in naïve rats treated with pyruvate, as a control. Post-stroke neurological deficit with concurrent elevation in glutamate levels were demonstrated, with peak glutamate levels 24 h after MCAO. Treatment with pyruvate led to reduced glutamate levels 24 h after MCAO and improved neurologic recovery. Pyruvate treatment reduced lesion volume, brain edema and the extent of BBB permeability 24 h post-MCAO. Naïve rats treated with pyruvate showed increased levels of α-ketoglutarate. Rats demonstrated post-stroke depressive behavior that was improved by the administration of pyruvate. There was less anxiety-like behavior in post-stroke rats treated with placebo in comparison to the post-stroke rats treated with pyruvate or sham operated rats. Glutamate scavenging with pyruvate appears to be an effective as a method in providing neuroprotection following stroke and as a therapeutic option for the treatment of PSD by reducing the consequent elevations in CNS glutamate levels.
Keywords: Glutamate scavenging, Pyruvate, Post-stroke depression, Neuroprotection, Antidepressants, Anxiety
1. Introduction
Post-stroke depression (PSD) is a common and serious complication of stroke. While the exact prevalence rate of PSD is difficult to define, it is estimated that it can be seen in up to 30–35% of patients, ranging from 20 to 60% (Dafer et al., 2008; Lenzi et al., 2008), with a peak prevalence between six months and two years (Dafer et al., 2008). Despite its significant impact on functional recovery and quality of life following stroke, this psychiatric consequence is often overlooked and untreated.
Stroke is accompanied by a sharp three to four hundred-fold increase in the brain’s extra-cerebral fluid (ECF) and cerebrospinal fluid (CSF) glutamate concentrations (Benveniste et al., 1984; Castillo et al, 1996, 1997, 2002; Guyot et al., 2001). The glutamate spreads and subsequently causes neuronal damage in areas well beyond the infarcted tissue (Han et al., 2008). A toxic excess of glutamate in the brain’s ECF stimulates glutamate receptors, which in turn leads to swelling of the cell, apoptosis, and neuronal death. These increased glutamate levels have been strongly correlated with poor neurological outcomes (Koura et al., 1998; Zauner et al., 1996; Zhang et al., 2001).
Similarly, there is a growing body of evidence that the glutamatergic system is central to the development of many mood disorders, including anxiety and depression (Altamura et al., 1995; Hashimoto et al., 2007; Kucukibrahimoglu et al., 2009; Levine et al., 2000; Mauri et al., 1998; Mitani et al., 2006). There are a number of studies reporting alterations in glutamate levels in blood (Mauri et al., 1998) and CSF (Levine et al., 2000) in patients with major depression. There is also a positive correlation between plasma glutamate levels and severity of depressive symptoms in patients with major depression (Mitani et al., 2006). In a study of postmortem tissue from the human frontal cortex, glutamate levels were elevated in subjects with a history of depression compared with controls (Hashimoto et al., 2007).
Clinical studies have demonstrated that the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine, which interferes with glutamate receptor activation, has rapid antidepressant effects in patients suffering from treatment-resistant major depressive disorder (Diazgranados et al., 2010). As such, the U.S. Food and Drug Administration recently approved esketamine for treatment-resistant depression (Kim et al., 2019). Animal models have demonstrated the anti-depressive effects of interfering with glutamate activation at a variety of receptors (Hashimoto, 2011). However, while agents such as NMDA receptor antagonists showed initial promise for neuroprotection in animal models (Lee et al., 1999; McCulloch, 1992), studies with glutamate antagonists have failed to demonstrate clinical neuroprotective efficacy in human clinical studies (Ikonomidou and Turski, 2002; Muir, 2006).
An alternative method of reducing glutamate is to reduce excess toxic glutamate, rather than antagonize the receptors. One method of eliminating excess glutamate from the brain’s interstitial fluids is by utilizing the naturally occurring brain-to-blood glutamate efflux via the endothelial transport systems (Teichberg, 2007; Teichberg et al., 2009). Glutamate co-substrates, pyruvate and oxaloacetate, via blood resident enzymes glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase convert glutamate into its inactive form 2-ketoglutarate (Gonzalez et al., 2005; Gray et al., 2014; Leibowitz et al., 2012). This method of reducing excess glutamate, known as blood glutamate scavenging (BGC), has been shown to be effective in various animal studies (Leibowitz et al., 2012). BGC is achieved by activating several mechanisms including the catalyzation of the enzymatic process involved in glutamate metabolism, the redistribution of glutamate into tissue, and as an acute stress response (Leibowitz et al., 2012).
We have previously demonstrated that a decrease in blood glutamate concentrations in the injured brain results in improved neurological deficit (Zlotnik et al., 2012), reduced cerebral edema (Zlotnik et al., 2007), reduced infarct zone (Boyko et al., 2011c), and reduced BBB breakdown (Boyko et al., 2012a). Additional studies, based on the intravenous injection of pyruvate, demonstrated faster and greater neurological recovery after closed head injury in a rat model (Zlotnik et al., 2007). Pyruvate may provide neuroprotection by other mechanisms as well, including via hydroxyl radical scavenging, stimulating NADPH-dependent peroxide scavenging systems, inhibiting poly (ADP-ribose) polymerase activity, and by serving as an alternative energy source for brain cells (Leibowitz et al., 2012).
The activity of BGS in stimulating the brain-to-blood glutamate efflux is a self-limiting process; as excess glutamate levels decrease to concentrations below the threshold of activation of the brain vasculature glutamate transporters (i.e below their Km values), the process of glutamate efflux slows and eventually stops. Thus, BGS preserves the physiological effects of glutamate in regulating the metabolic and electrolyte balance, maintains neuronal integrity, and exerts beneficial effects in neurologic repair after brain injury. This method maintains a balance between eliminating the undesirable effects of excess glutamate and preserving its positive effects that are necessary to sustain life (Teichberg et al., 2009).
The objective of this study was to investigate the efficacy of long-term pyruvate treatment on post-stroke depression, as determined by neurologic status and performance on several behavioral tests. Additionally, this study examines this treatment modality on neurological outcomes as determined by the volume of infarcted zone, blood brain barrier (BBB) breakdown, real-time monitoring of brain ECF concentrations via biosensors and by concentration of blood glutamate. The therapeutic effects were also examined by magnetic resonance imaging (MRI) techniques, which included in vivo MRI spectroscopy, MRI monitoring of BBB disruption and MRI evaluation of infarcted zone and brain edema.
2. Methods
2.1. Animals
The experiments were conducted in accordance with the recommendation of the Declarations of Helsinki and Tokyo and to the Guidelines for the use of Experimental Animals of the European Community. The experiments were approved by the Animal Care Committee of Ben-Gurion University of Negev, Israel. A total of one hundred-forty male Sprague-Dawley rats (Harlan Laboratories, Israel) were used in this experiment. All rats weighed between 300 and 400 g. Purina Chow and water were made available ad libitum. The temperature in the room was maintained at 22 °C, with a 12 h light–dark cycle.
2.2. Experimental design
Eighty rats were randomly assigned into one of 3 groups: Middle Cerebral Artery Occlusion (MCAO) plus pyruvate treatment (n = 30 rats), MCAO plus placebo treatment (n = 30), and sham operated rats (n = 20). Rats who died or still had neurological deficits after 4 weeks were excluded from the study to avoid the effect of a motor deficit on rat behavioral performance. The final number of animals in each group was 23, 20, and 20, respectively.
After induction of anesthesia, BD Neoflon 24G catheters had been introduced into the tail artery for blood pressure monitoring and blood sampling. Neurological status was tested before surgery (baseline) and at 1 h, 24 h, 1 week, 2 week and 4 weeks following stroke. Magnetic resonance imaging (MRI) with magnetic resonance spectroscopy (MRS) was performed 0 h, 3 h, 24 h and 1 week following stroke. Based on data from the MRI/MRS, we evaluated the development of the infarct zone, brain edema, BBB breakdown, and ischemic penumbra.
Rats were assessed for depressive behavior via a sucrose preference test at 0, 1, 2, 3 and 6 months post-MCAO, and assessed for anxiety-like behavior with the Vogel and elevated plus maze tests, at 0, 1, 3 and 6 months post-MCAO.
Sixty separate rats were also assigned to three additional groups for monitoring CSF and blood glutamate levels 24 h and 1-week post-stroke, respectively: MCAO plus pyruvate treatment (n = 19 rats), MCAO plus placebo treatment (n = 22), and naive rats (n = 19).
Thirty-two additional rats were used as a control group. In 16 of these naïve rats, the Vogel and plus maze tests were performed at baseline and then again after 1 month of pyruvate treatment. In the other 16 naïve rats, blood samples for alanine and α-ketoglutarate were collected at baseline and then again after 1 month of treatment with pyruvate.
2.3. Middle Cerebral Artery Occlusion (MCAO) procedure
Rats were anesthetized with a mixture of isoflurane (4% for induction, 2% for surgery, 1.3% for maintenance) in 24% oxygen (2 L/min) without tracheotomy, and allowed to breathe spontaneously. Rats were anesthetized for 35–40 min during each procedure. There were no differences in the time allotted for anesthesia between groups in order to control the effects of isoflurane, pO2, or pCO2. The anesthesia was considered sufficient for surgery when the tail reflex was abolished. Physiological parameters, including mean arterial pressure, heart rate and O2 saturation of arterial blood were monitored. A heating plate was used to maintain a core body temperature of 37 °C, measured via a probe placed in the rats’ rectum. Body temperature was kept constant between rats, thus minimizing any effect of hypothermia or hyperthermia on neurological outcome and neurological injury. Blood samples were collected to measure arterial blood gas tensions (pCO2, pO2, and HCO3), pH, and concentrations of glucose, hemoglobin, and electrolytes (Na, K, and Cl). Measurements of physiological parameters were recorded before anesthesia and after recovery from anesthesia and surgery. The body temperature before surgery was measured at 8:00 to 9:00 a.m. in all animals and at 24 h after the onset of MCAO. The MCA was occluded in our modified technique by inserting the catheter directly through the ICA (Boyko et al., 2010).
Following a careful dissection and exposure of the common and internal carotid artery, and after separation of these arteries from the vagus nerve, the ICA was permanently blocked (a 4–0 silk suture was tied loosely around ICA just above the CCA bifurcation) proximal to the filament insertion point and temporarily blocked distal to the filament insertion point. The purpose of the proximal ligation was to occlude the ICA while the additional distal ligation reduced the bleeding around the filament and secured it in place. A heat-blunted monofilament was introduced via the ICA into the circle of Willis, effectively occluding the MCA. The silk suture in the ICA was fastened around the intraluminal thread to prevent bleeding. The suture was inserted approximately 18–18.5 mm from the bifurcation of the CCA until a sensation of mild resistance was reached to occlude the MCA. The thread was then fixed in position by tying a silk filament over the ICA, just above the pterygopalatine artery. Rats were allowed to fully recover after the surgery to prevent any potential motor impairment that could impact the performance of the behavior tests (Boyko et al., 2010).
2.4. Drugs and doses
Pyruvate was purchased from Sigma Israel Chemicals (Rehovot, Israel) and was stored at 2–4 °C until use and was dissolved in drinking water immediately prior to administration. A fresh solution was prepared every 8 h. In rats that received pyruvate, doses of 180 mg/kg/day (in three divided doses) was administered in their drinking water for 30 days. Doses of pyruvate were chosen based on our previous work from MRS data, that demonstrated that doses of 180 mg/kg/day were optimal to produce a blood and brain glutamate reduction of about 25–35% (under publication). Equal volumes of drinking water without pyruvate were given to the placebo groups.
2.5. Determination of blood glutamate
Whole blood (200 μl aliquot) was de-proteinized by adding an equal volume of ice-cold 1 M perchloric acid and then centrifuging at 10 000 × g for 10 min at 4 °C. The pellet was discarded and the supernatant was collected, adjusted to pH 7.2, with 2 M K2CO3, and stored at −80 °C for later analysis, if needed. Glutamate concentration was measured using the fluorometric method of Graham and Aprison. A 60 μl aliquot from the perchloric acid supernatant was added to 90 μl of a 0.3 M glycine; 0.25 M hydrazine hydrate buffer adjusted to pH 8.6 with 1 M H2SO4 and containing 11.25 U of glutamate dehydrogenase in 10 mM Nicotinamide adenine dinucleotide (NAD). After incubation for 30–45 min at room temperature, the fluorescence was measured at 460 nm with excitation at 350 nm. A glutamate standard curve was established with concentrations ranging from 0 to 6 μM. All determinations were done at least in duplicates (Boyko et al., 2012b).
2.6. Determination of CSF glutamate
Fresh CSF (110 μl) was mixed with perchloric acid (25 μl) of 0.3 M, and then centrifuged at 10 000 × g for 10 min at 4 °C. The pellet was discarded and the supernatant was collected, adjusted to pH 7.2 with 12.5 μl of 2 M K2CO3 and stored at −80 °C for later analysis (Boyko et al., 2012a). Analysis was performed by fluorometric method as described above for blood samples.
2.7. Determination of alanine
Whole blood (200 μl aliquot) was collected into biochemical microtubes and centrifuging at 13 000 × g for 10 min at 4 °C. Serum samples was deproteinized with a 10 kDa MWCO spin filter. Samples was collected into Eppendorf microtubes and stored at −80 °C for later analysis. For fluorometric analyzes, we used 50 μl of the sample for each reaction (well). Samples were diluted to optimal concentration for readings within the linear range of the standard curve. Alanine concentration was performed using the coupled enzymatic assay, according to the manufacturer’s instructions (Sigma; catalog #: MAK001 - Alanine Assay Kit https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/1/mak001bul.pdf), as previously described (Cao et al., 2013). We used a blank sample for each sample by omitting the Alanine Converting Enzyme in the Reaction Mix. After incubation for 60 min at 37 °C, the fluorescence intensity was measured at λex = 535/λem = 587 nm. An alanine standard curve was established with concentrations ranging from 0 to 10 nmole/well.
2.8. Determination of α-ketoglutarate
Whole blood (200 μl aliquot) was collected into biochemical microtubes and centrifuging at 13 000 × g for 10 min at 4 °C. Serum samples was deproteinized with a 10 kDa MWCO spin filter. Samples was collected into Eppendorf microtubes and stored at −80 °C for later analysis. For fluorometric analyzes, we used 50 μl of the sample for each reaction (well). Samples were diluted to optimal concentration for readings within the linear range of the standard curve. The α-ketoglutarate concentration was performed using the coupled enzymatic assay according to the manufacturer’s instructions (Sigma; catalog #: MAK054 α-Ketoglutarate Assay Kit https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/1/mak054bul.pdf), as previously described (Bergmeyer et al., 1974). We used a blank sample for each sample by omitting the α-Ketoglutarate Converting Enzyme in the Reaction Mix. After incubation for 30 min at 37 °C, the fluorescence intensity was measured at λex = 535/λem = 587 nm. A α-Ketoglutarate standard curve was established with concentrations ranging from 0 to 10 nmole/well.
2.9. Imaging protocol (MRS/MRI)
MRS was used to measure brain glutamate concentrations (Cai et al., 2012), and MRI was used for the determination of Ktrans, DWI, T2, and perfusion-weighted imaging (PWI). The experimental procedure was performed as described previously at 1 h, 24 h, and 1 week after MCAO. Measurements were performed in injured hemispheres and symmetric area of the contralateral hemisphere in the penumbra area in close proximity to the necrotic core. Animals were maintained under general anesthesia (1.5% isoflurane in oxygen). A tail vein catheter was introduced and connected to a syringe containing a solution of Gd-DTPA (Dotarem, 0.5 mmoL/ml Guerbet, France). A 3T MRI was used (Ingenia, Philips Medical Systems, Best, The Netherlands) using an eight-channel receive-only coil. Localising T2w TSE sequences were acquired in sagittal and coronal planes with TR/TE = 3000/80 msec, turbo factor = 15, water-fat shift = 1.6 pixels, resolution (freq × phase × slice) = 0.47 × 0.41 × 2.0 mm and one average for a scan time of 1:00 min. In the axial direction the scan parameters were TR/TE = 3000/80 msec, turbo factor = 14, water-fat shift = 1.6 pixels, resolution (freq × phase × slice) = 0.37 × 0.33 × 2.0 mm. Four averages were acquired for a scan time of 4:54 min. Diffusion tensor imaging in 6 directions was performed in the axial direction using a multi-shot STEAM spin-echo, echo-planar sequence with TR/TM/TE = 1355/15.0/143 msec, SENSE reduction factor = 1.5, turbo factor = 19, b = 1000 s/mm2, resolution (freq × phase × slice) = 0.55 × 0.55 × 2.0 mm with spectrally-selective fat suppression. Five signal averages were acquired for a scan time of 8:40 min. T1 permeability studies were performed using a segmented 3D T1w-FFE sequence with 50 dynamics for a total scan time of 25:52 min. The scan parameters were TR/TE = 16/4.9 msec, turbo factor = 48, SENSE factor 1.5, resolution (freq × phase × slice) = 0.30 × 0.37 × 2.0 mm, tip angle = 80 and two signal averages for a scan time of 31 s/dynamic. Three calibration scans with identical resolution preceeded the dynamic sequence with tip angles 50, 100 and 150. The contrast agent was injected after the 5th dynamic scan. T2 perfusion studies were carried out using a dynamic, single-shot gradient-echo epi sequence with spectrally-selective fat suppression. The scan parameters were TR/TE = 1300/40 msec, resolution (freq × phase × slice) = 0.64 × 0.69 × 2.0 mm, and one signal average giving a scan time of 1.3sec/dynamic. A total of 150 dynamics were acquired for a scan time of 3:19 min. For PWI we used a typical multimodal stroke MRI protocol consists of PWI adjusted to clinical MRI 3T scanner technique (Ulmer S et al., 2008). The contrast agent (Dotarem – Gadoteric acid 0.5 mmol/ml) was injected after the 5th dynamic (0.03 ml Gd in 0.4 ml saline).
The Intellispace Portal workstation (V5.0.0.20030, Philips Medical Systems, Best, The Netherlands) was used for the post-processing of the permeability and perfusion studies. The MRS acquisitions were performed using a multi-voxel, 2D PRESS acquisition. A VOI 5.0 × 10.0 mm was graphically placed in a FOV of 40 × 40 mm divided into 16 × 16. The scan parameters were TR/TE = 2000/32 msec, SENSE = 2 × 2 (AP × RL), slab thickness = 10 mm for an acquisition resolution (AP × RL × FH) of 2.5 × 2.5 × 10.0 mm. Second-order shimming were used yielding a water line width of approximately 25 Hz. Water suppression was achieved by applying two bandwidth selective rf pulses. The acquisition bandwidth was 2000 Hz giving a spectral resolution of 0.98 Hz. A total of 23 averages were acquired for a scan time of 38:48 min. The spectra was analyzed using the Philips SpectroView software. To identify and assess the peak of glutamate from glutamine we used modern methods (Hancu, 2009; Prescot et al., 2012).
2.10. MR image Analysis
Image analysis was performed by an expert, who was blind to the group assignment. Quantitative CBF and apparent Diffusion coefficient (ADC) maps, in units of square millimeters per second, were generated in Philips software package (Ingenia, Philips Medical Systems, Best, The Netherlands) and subsequently analyzed using Image J software 1.50i version (http://rsb.info.nih.gov/ij/), as previously described (Boyko et al., 2019; Reid et al., 2012).
These thresholds were used to identify all pixels with abnormal CBF and ADC characteristics on each slice. The viability thresholds were 0.53 × 10–3mm2/s for ADC images (Boyko et al., 2019) and for CBF maps (Bardutzky et al., 2005): 0–6 mL/100 g/min was associated with damage brain tissue and called infarct core, 0–15 mL/100 g/min was associated with damage brain tissue and include 2 abnormal zones-infarct core and penumbra, 0–70 mL/100 g/min was associated with damage brain tissue and include 3 abnormal zones: infarct core, penumbra and benign oligemia.
Calculation of infarcted zone was performed by the Ratios of Ipsilateral and Contralateral Cerebral Hemispheres (RICH) method. The calculation of the lesion volume with the correction for tissue swelling by the RICH technique was done using the following formula:
The infarcted brain volume was measured as a percentage of the total brain (Boyko et al, 2013, 2019).
Calculation of brain edema was performed by the RICH method. The calculation of brain edema by the RICH technique was done by comparing the contralateral and ipsilateral hemispheres, and performed using the following formula:
The infarcted brain volume was measured as a percentage of the total brain (Boyko et al, 2011a, 2013).
2.11. Examination of neurologic status
Two observers, who were blinded as to which surgical procedure each rat had received, tested the animals for neurological deficits following MCAO. All animals were tested according to the advanced neurological severity score (NSS), as previously described (Boyko et al., 2011b). The testing procedure did not take more than 7–10 min per rat. The rat’s neurological status was examined as baseline before MCAO and then at 1 h, 24 h, 1 week, 2 weeks and 4 weeks following MCAO. Due to a fast recovery after Isoflurane anesthesia, rats were fully awake 1 h after surgery.
2.12. Elevated plus maze test
The plus maze was situated in a darken room and consisted of two open and two closed arms (each of dimensions 16 × 46 cm). It was constructed from black plastic and positioned 100 cm above the floor. The closed arms, opposite to one another, had a surrounding wall of height 40 cm. Experiments were recorded by a video camera (CC TV Panasonic, Japan) suspended approximately 200 cm above the center of the plus maze. 5% ethanol was used to clean the maze prior to the introduction of each animal. Rats were tested on the maze in a randomized order. Each rat was placed in the center of the plus maze facing one of the open arms, and the rat’s behavior was videotaped for 5 min for future analysis.
The number of entries into the various arms and time spent in arms and in the center of the elevated plus maze was recorded with a video camera (CC TV Panasonic, Japan) and subsequently analyzed using Ethovision XT software (Noldus, Wageningen, Netherlands) (Boyko et al., 2013). The elevated plus maze test was performed at 0, 1, 3 and 6 months.
2.13. Conflict drinking test (Vogel test)
Rats were water-deprived before testing. Food was available in the home cage at all times. After 24 h of water deprivation, rats were habituated to the testing cages to assess licking behavior and allowed to drink for 15 min (training session), with an additional 15 min drinking in their home cages. Water deprivation then continued for another 24 h. On the test day, rats were placed in the testing cages. When the rats licked the water spigot on the bottle, they receiving an electric shock of 0.5 mA with pulse duration of 0.2 s. The number of punished responses was automatically recorded for each rat during the 5 min of testing (Belcheva et al., 1997). The Vogel test was performed at 0, 1, 3 and 6 months.
2.14. Sucrose preference test
The sucrose preference test was performed as described previously (Boyko et al., 2015). The rats were allowed to consume1% (w/v) by placing two bottles of sucrose solution in each cage for 24 h. The rationale for the two bottles is that the appearance of an additional bottle in the rat’s cage during the initial part of the sucrose preference test may frighten the rat. Afterwards, one of the bottles was replaced with water for 24 h. As such, this design may help avoid the effects of neophobia. Following the adaptation procedure, the rats were deprived of water and food for12 h. The sucrose preference test was conducted at 9:00 a.m. The rats were housed in individual cages and given free access to the two bottles containing 100 mL of sucrose solution (1%, w/v) and 100 ml of water, respectively. After 4 h, the volume (ml) of both the consumed sucrose solution and water was recorded, and sucrose preference was calculated as sucrose preference (%) = sucrose consumption (ml)/(sucrose consumption (ml) + water consumption (ml)) × 100%. The sucrose preference test was performed at 0, 1, 2, 3 and 6 months.
2.15. Statistical analysis
Statistical analysis was performed with the SPSS 22 package (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used, considering the number of rats in each group for deciding the appropriate test for the comparisons between the different parameters. For non-parametric data, we used the transformation or applied the appropriate tests suitable for non-parametric data. The significance of comparisons between groups had been determined using the Kruskal–Wallis followed by Mann–Whitney (for nonparametric data) and one-way ANOVA with Bonferroni post hoc test or the Student’s t-tests (for parametric data). Mortality rate was analyzed with chi-square, Fisher’s exact test. Results were considered statistically significant when P < 0.05, and highly significant when P < 0.01.
3. Results
3.1. Survival rate
The eighty original rats were divided randomly into three groups: MCAO plus pyruvate treatment (n = 30 rats), MCAO plus placebo treatment (n = 30), and sham operated rats (n = 20). After rats that were excluded, as noted above, the final number of animals in each group was 23, 20, and 20, respectively. The mortality rate was lower in the rats treated with pyruvate compared to the control group, although the difference was not statistically significant (10% vs 13.3%, respectively).
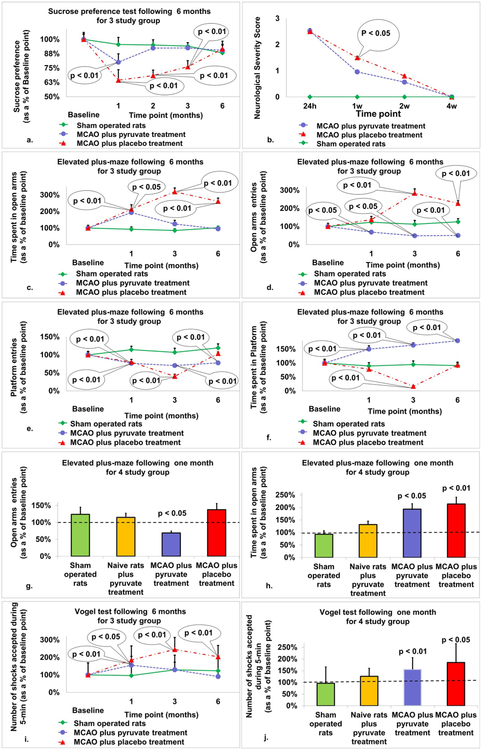
3.2. Sucrose preference test
Performance over time on the sucrose preference test post-MCAO is illustrated in Fig. 1a. At 1-month post-MCAO there was a significant difference in sucrose preference for all three groups (64.4% ± 9.2 and 80.1% ± 7.3 vs. 95.6% ± 6.1, p < 0.01). There was also a significant difference between post-stroke rats given placebo and post-stroke rats given pyruvate at 2 (68.4% ± 4.8 and 92.4% ± 3.6, p < 0.01) and 3 (76.1% ± 5.4 and 92.6% ± 4.6, p < 0.01) months post-MCAO. By month 6, there were no differences between all 3 groups.
Fig. 1.
The neuro-behavioral profile for post-stroke rats treated with pyruvate and post-stroke rats treated with placebo compared to naïve rats. a. Sucrose preference test: At 1-month post-MCAO there was a significant difference in sucrose preference for all three groups (p < 0.01). There was also a significant difference between post-stroke rats given placebo and post-stroke rats given pyruvate at 2 and 3 months post-MCAO (p < 0.01). By month 6, there were no differences between all 3 groups. The data are measured in ml and expressed as a mean percentage ± SD. b. Neurologic severity score: Neurological severity deficit at one week after MCAO was significantly greater for the 20 post-stroke rats treated with placebo than for the 23 post-stroke rats treated with pyruvate (median = 1.5 vs. 0.96, p < 0.05). c–h. Elevated plus maze: There was a significant difference in the time spent on the open arms for post-stroke rats treated with pyruvate compared to sham operated rats at 1 month (p < 0.01) and post-stroke rats treated with placebo compared to either post-stroke rats treated with pyruvate or sham operated rats at 1, 3, and 6 months post-MCAO (p < 0.01). There was a significant difference in open arm entries in post-stroke rats treated with placebo compared to either post-stroke rats treated with pyruvate or sham operated rats at 1 (p < 0.01 and p < 0.05, respectively), 3 (p < 0.01), and 6 (p < 0.01) months post-MCAO. There was also a difference found for post-stroke rats treated with pyruvate compared to sham operated rats at 3 (p < 0.05) and 6 (p < 0.01) months post-MCAO. There was a significant difference in platform entries by sham operated rats compared to post-stroke rats treated with placebo at 1 (p < 0.01) and 3 (p < 0.01) months post-MCAO and compared to post-stroke rats treated with pyruvate at 1 (p < 0.01), 3 (p < 0.01), and 6 (p < 0.01) months post-MCAO. A significant difference was also found between post-stroke rats treated with pyruvate and post-stroke rats treated with placebo at 3 months (p < 0.05). There was a significant difference in the time spent on the platform by post-stroke rats treated with pyruvate compared to both post-stroke rats treated with placebo and sham operated rats at 1, 3, and 6 months post-MCAO (p < 0.01). A significant difference was also found between post-stroke rats treated with placebo and sham operated rats at 3 months (p < 0.01). The data are measured in seconds and expressed as a mean percentage of the baseline ± SEM. i–j. Vogel test: At 1-month post-MCAO, there was a significant difference between post-stroke rats treated with placebo (p < 0.05) and post-stroke rats treated with pyruvate (p < 0.01) compared to sham operated rats. At 3- and 6-months post-stroke rats treated with placebo scored significantly different than both rats treated with pyruvate (p < 0.05) and sham operated rats (p < 0.01). Number of shocks were estimated as a percentage from baseline. The data are measured in seconds or count and expressed as a mean percentage of the baseline ± SEM.
3.3. Neurological severity score
Neurological deficit at one week after MCAO was significantly greater for the 20 post-stroke rats treated with placebo than for the 23 post-stroke rats treated with pyruvate (median = 1.5 vs. 0.96, p < 0.05, Fig. 1b). Two weeks after MCAO administration, there was also less neurological deficit in rats treated with pyruvate than in rats treated with placebo, but the difference wasn’t statistically significant.
3.4. The elevated plus maze
Performance over time on the elevated plus maze post-MCAO is illustrated in Fig. 1c–h, broken down into time spent on open arms, open arm entries, platform entries and time spent on the platform.
At 1-month post-MCAO, there was a significant difference in the time spent on the open arms between post-stroke rats treated with placebo (214% ± 27, p < 0.01) and post-stroke rats treated with pyruvate (193% ± 22, p < 0.01) compared to sham operated rats (93% ± 15). Additionally, there was a significant difference for post-stroke rats treated with placebo compared to either post-stroke rats treated with pyruvate or sham operated rats at 3 months (319% ± 23 vs. 124% ± 22 and 85% ± 11, p < 0.01), and 6 (261% ± 20 and 95% ± 15 vs. 102% ± 10, p < 0.01) months post-MCAO.
There was a significant difference in open arm entries in post-stroke rats treated with placebo compared to either post-stroke rats treated with pyruvate or sham operated rats at 1 (138% ± 18 vs. 69% ± 5 and 124% ± 21, p < 0.01 and p < 0.05, respectively), 3 (284% ± 24 vs. 48% ± 7 and 112% ± 21, p < 0.01), and 6 (228% ± 14 vs. 50% ± 7 and 126% ± 17, p < 0.01) months post-MCAO. There was also a difference found for post-stroke rats treated with pyruvate compared to sham operated rats at 3 (p < 0.05) and 6 (p < 0.01) months post-MCAO.
There was a significant difference in platform entries by sham operated rats compared to post-stroke rats treated with placebo at 1 (114% ± 9 vs. 80% ± 8, p < 0.01) and 3 (107% ± 11 vs. 40% ± 6, p < 0.01) months post-MCAO and compared to post-stroke rats treated with pyruvate at 1 (114% ± 9 vs. 76% ± 4, p < 0.01), 3 (107% ± 11 vs. 70% ± 4, p < 0.01), and 6 (119% ± 11 vs. 78% ± 4, p < 0.01) months post-MCAO. A significant difference was also found between post-stroke rats treated with pyruvate and post-stroke rats treated with placebo at 3 months (p < 0.05).
There was a significant difference in the time spent on the platform by post-stroke rats treated with pyruvate compared to both post-stroke rats treated with placebo and sham operated rats at 1 (150% ± 12 vs. 78% ± 7 and 89% ± 12, p < 0.01), 3 (164% ± 9 vs. 17% ± 2 and 94% ± 13, p < 0.01), and 6 (180% ± 13 vs. 93% ± 7 and 91% ± 12, p < 0.01) months post-MCAO. A significant difference was also found between post-stroke rats treated with placebo and sham operated rats at 3 months (p < 0.01).
In naïve rats treated with 1 month of pyruvate, there were no significant differences in performance on the elevated plus maze compared to baseline.
3.5. The Vogel conflict test
Performance over time on the Vogel conflict test post-MCAO is illustrated in Fig. 1i and j. At 1-month post-MCAO, there was a significant difference between post-stroke rats treated with placebo (185% ± 80, p < 0.05) and post-stroke rats treated with pyruvate (155% ± 50, p < 0.01) compared to sham operated rats (96% ± 70). At 3- and 6-months post-stroke rats treated with placebo scored significantly different than both rats treated with pyruvate (245% ± 69 and 204% ± 65 vs 129% ± 43 and 90% ± 26, p < 0.05) and sham operated rats (245% ± 69 and 204% ± 65 vs. 129% ± 84 and 123% ± 85, p < 0.01).
In naïve rats treated with 1 month of pyruvate, there were no significant differences in performance on the Vogel test compared to baseline.
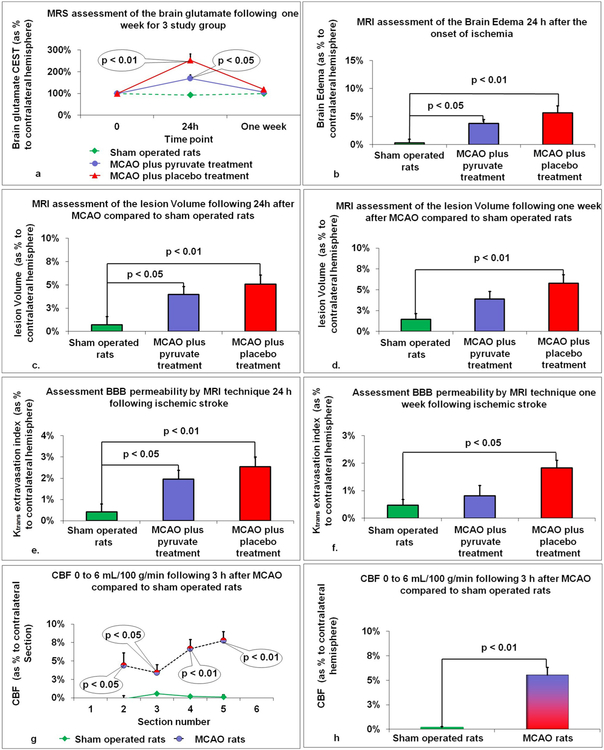
3.6. Determination of brain glutamate by MRS
Glutamate levels by Chemical Exchange Saturation Transfer (CEST) imaging were estimated as a ratio to creatinine and expressed as a percentage to the contralateral hemisphere. 24 h post-MCAO, both groups, post-stroke rats treated with pyruvate (169.9% ± 15.6, p < 0.05) and post-stroke rats treated with placebo (253.5% ± 15.8, p < 0.01) demonstrated increased brain glutamate concentrations compared to sham operated rats (92.7% ± 6.8, Fig. 2a). There was a trend towards higher brain glutamate in rats treated with placebo compared to pyruvate or sham operated rats 118.7% ± 6 vs. 106.9% ± 5.6 and 99.6% ± 5.6), although the difference was not statistically significant.
Fig. 2.
The effect of long-term treatment with pyruvate using MRS techniques. a. Brain glutamate: 24 h post-MCAO, both groups, post-stroke rats treated with pyruvate (p < 0.05) and post-stroke rats treated with placebo (p < 0.01) demonstrated increased brain glutamate concentrations compared to sham operated rats. The data is presented as mean ± SEM. b. Brain edema: Post-stroke rats treated with either placebo or pyruvate demonstrated increased brain edema 24 h post-MCAO compared to sham operated rats. The data are measured as a percentage to the contralateral hemisphere and expressed as mean ± SEM. c–d. Lesion volume: Post-stroke rats treated with either placebo (p < 0.01) or pyruvate (p < 0.05) demonstrated increased lesion volume 24 h post-MCAO compared to sham operated rats. Furthermore, there was a significant increase in lesion volume 1 week following MCAO in the rats treated with placebo compared to sham operated rats (p < 0.01). The data are measured as a percentage to the contralateral hemisphere and expressed as mean ± SEM. e–f. BBB breakdown: Post-stroke rats treated with either placebo (p < 0.01) or pyruvate (p < 0.05) demonstrated increased BBB permeability 24 h post-MCAO compared to sham operated rats. Moreover, there was a significant increase in BBB permeability 1 week following MCAO in the rats treated with placebo compared to sham operated rats (p < 0.05). The data are measured as a percentage to the contralateral hemisphere and expressed as mean ± SEM. g–h. Cerebral blood flow: There was a significant increase in CBF in all rats who underwent MCAO 3 h after injury in the second, third, fourth, and fifth brain sections. There was a significant increase in CBF 3 h post-MCAO in the injured hemisphere as a percentage to the contralateral hemisphere compared to sham operated rats. The data are measured as a percentage to the contralateral hemisphere and expressed as mean ± SEM.
3.7. Determination of brain edema by MRI
Post-stroke rats treated with either placebo (5.69% ± 1.19, p < 0.01) or pyruvate (3.78% ± 0.68, p < 0.05) demonstrated increased brain edema 24 h post-MCAO compared to sham operated rats (0.32% ± 0.62, Fig. 2b). There were no significant differences in brain edema between the 3 experimental groups one-week post-MCAO.
3.8. Determination of lesion volume by MRI
Post-stroke rats treated with either placebo (5.07% ± 0.98, p < 0.01) or pyruvate (3.96% ± 0.86, p < 0.05) demonstrated increased lesion volume 24 h post-MCAO compared to sham operated rats (0.72% ± 0.89, Fig. 2c). Furthermore, there was a significant increase in lesion volume 1 week following MCAO in the rats treated with placebo compared to sham operated rats (5.78% ± 1.01 vs. 1.47% ± 0.68, p < 0.01, Fig. 2d). Post-stroke rats treated with pyruvate had no statistical difference in lesion volume compared with sham operated rats (3.9% ± 0.9 vs. 1.47% ± 0.68).
3.9. Determination of BBB breakdown by MRI
Post-stroke rats treated with either placebo (2.55% ± 0.45, p < 0.01) or pyruvate (1.96% ± 0.41, p < 0.05) demonstrated increased BBB permeability 24 h post-MCAO compared to sham operated rats (0.42% ± 0.38, Fig. 2e). Moreover, there was a significant increase in BBB permeability 1 week following MCAO in the rats treated with placebo compared to sham operated rats (1.83% ± 0.28 vs. 0.47% ± 0.21, p < 0.05, Fig. 2f). Post-stroke rats treated with pyruvate had no statistical difference in BBB permeability compared with sham operated rats (0.81% ± 0.38 vs. 0.47% ± 0.21).
3.10. Determination cerebral blood flow by MRI
An independent-samples t-test indicated that the regions of reduced CBF below 6 mL/100 g/min were associated with damaged brain tissue (p < 0.05). CBF at each brain section, as a percentage to the contralateral section, is illustrated in Fig. 2g. There was a significant increase in CBF in all rats who underwent MCAO 3 h after injury in the second (4.38% ± 1.7 vs. −0.15% ± 0.44, p < 0.05), third (3.4% ± 1.1 vs. 0.54% ± 0.14, p < 0.05), fourth (6.64% ± 1.24 vs. 0.17% ± 0.17, p < 0.01), and fifth (7.76% ± 1.19 vs. 0.09% ± 0.32, p < 0.01) brain sections. There was also a significant increase in CBF 3 h post-MCAO in the injured hemisphere as a percentage to the contralateral hemisphere compared to sham operated rats (5.55% ± 0.78 vs. 0.17% ± 0.1, p < 0.01, Fig. 2h).
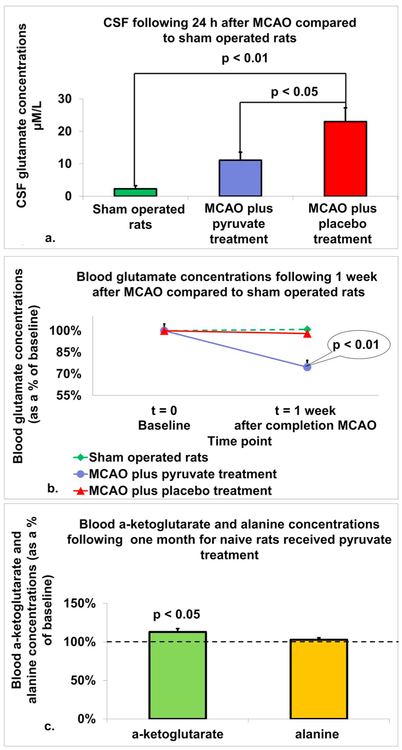
3.11. CSF glutamate concentrations
There was a significant increase in CSF glutamate concentration 24 h post-MCAO in both post-stroke rats treated with placebo (23 μM/L ± 4.4) and post-stroke rats treated with pyruvate (11.1 μM/L ± 2.6), compared to sham operated rats (2.3 μM/L ± 1, p < 0.01, Fig. 3a). Furthermore, CSF glutamate concentrations were significantly lower in post-stroke rats treated with pyruvate compared to post-stroke rats treated with placebo (p < 0.05).
Fig. 3.
In-vivo effects of pyruvate on blood and brain glutamate levels in a rat model of MCAO. a. Concentrations of glutamate in CSF: There was a significant increase in CSF glutamate concentration 24 h post-MCAO in both post-stroke rats treated with placebo and post-stroke rats treated with pyruvate, compared to sham operated rats (p < 0.01). Furthermore, CSF glutamate concentrations were significantly lower in post-stroke rats treated with pyruvate compared to post-stroke rats treated with placebo (p < 0.05). The data are measured in μM/L and presented as mean ± SEM. b. Concentrations of glutamate in blood: There was a significant decrease in concentrations of glutamate in the blood 1-week post-MCAO in rats treated with pyruvate compared to post-stroke rats treated with placebo and sham operated rats (p < 0.01). The data are measured as μM/l and expressed as a percentage of the baseline ± SD. c. Serum blood concentrations of alanine and α-Ketoglutarate: In naïve rats treated with pyruvate for 1 month, concentration of α-ketoglutarate in blood serum was significantly increased compared to baseline levels (p < 0.05). While concentration of alanine in blood serum was increased compared to baseline, the difference was not statistically significant. The data is presented as average percentage of baseline ± SEM.
3.12. Blood glutamate concentrations
There was a significant decrease in concentrations of glutamate in the blood 1-week post-MCAO in rats treated with pyruvate (74.7%, ± SD 22.5) compared to post-stroke rats treated with placebo (98.1%, ± SD 20.6) and sham operated rats (101%, ± SD 16.1, p < 0.01, Fig. 3b).
3.13. Blood serum α-Ketoglutarate concentrations
In naïve rats treated with pyruvate for 1 month, concentration of α-ketoglutarate in blood serum was significantly increased compared to baseline levels (113%, 4.4 vs. 100% ± 2.6, p < 0.05, Fig. 3c).
3.14. Blood serum alanine concentrations
In naïve rats treated with pyruvate for 1 month, concentration of alanine in blood serum was increased compared to baseline (103%, ± 2.7 vs. 100% ± 2.5, Fig. 3c) although the difference was not statistically significant.
4. Discussion
These studies demonstrated some important findings about the connection between post-stroke elevation of glutamate levels, development of post-stroke anxiety and depression, and the impact of pyruvate on glutamate scavenging for these disorders in a rat model.
First, a significant elevation of glutamate levels in ischemic brain tissue was demonstrated using non-invasive MRS imaging and invasive CSF measurements, methods established in previous trials. Peak levels of glutamate were recorded 24 h after ischemia was induced by MCAO. Gradually, glutamate levels began to decline and returned to near-normal levels 1 week after ischemia induction. The net amount of exposure in the brain to glutamate during the first week post-stroke was recorded as markedly higher than in a perfused brain.
Second, MRS and CSF measuring confirmed that pyruvate served as a useful method to reduce brain glutamate levels. Despite differences in the estimates of glutamate reduction 24 h post-ischemia induction (approximately 33% by MRS imaging and approximately 50% by CSF measurement), there is a clear efficacy of pyruvate as a blood glutamate scavenger.
Third, the development of post-stroke mood disorders was demonstrated in the rats treated with placebo, as suggested by previous trials (Boyko et al., 2013; Soares et al., 2016). Although mood disorders can be difficult to measure, the data suggests that the significantly elevated post-stroke glutamate levels are responsible for the development of the mood disorders. The disorders normalized when the glutamate scavenger pyruvate was administered.
In the sucrose preference test, post-stroke rats given placebo showed significantly less voluntary sucrose consumption during the first three months compared to naïve rats, which is a sign of depressive behavior. The ischemic rats treated with pyruvate showed less of a reduction in voluntary sucrose consumption than the rats given placebo. After a month of observation, the rats given pyruvate demonstrated behavior that did not differ from the sham operated rats, emphasizing the effectiveness of pyruvate in eliminating signs of depressive behavior following stroke.
On the other hand, we received unexpected results from the Vogel and elevated plus maze tasks, aimed to evaluate anxiety in post-stroke rats. These results revealed less anxiety behavior in post-stroke rats treated with placebo in comparison to sham operated rats. When the stroke rats were treated with the blood glutamate scavenger, pyruvate, there was an increase in anxiety level. Given the concurrent reduction of depressive behavior with this increase in anxious behavior after pyruvate application, it does not appear that errors were made that produced the conflicting results. The results are consistent with our procedural models, and we propose two possible explanations for this seemingly-paradoxical post-stroke behavior:
It is possible that the post-stroke rats in our experiment developed severe depression that could lead to a loss of fear and anxiety in itself. Severe depression can include a reduction of anxiety and behavior that lacks fear, given the depressed individual’s apathy for the value of life (Dutton and Karakanta, 2013; Painuly et al., 2005; Piko and Pinczés, 2014). Therefore, post-stroke rats treated with placebo may have experienced reduced fear, demonstrated through less anxiety behaviors, as a result of severe depression. The group treated with pyruvate showed less anxious behavior than the group treated with placebo, but more anxious behavior than the sham operated group. These results confirm pyruvate administration for depression which, when severe, includes reduction in fear and consequently reduction in anxiety.
It is also possible that post-stroke, rats may have developed not only depression but bipolar disorder with manic states as well. Bipolar is a less common though well-known post-stroke or post-brain injury complication, consisting of periods of mania and depression with euthymic moods between episodes (Ferro et al., 2009; Rege et al., 1993; Santos et al., 2011). Depressive episodes consist of persistent sadness, fatigue, eating disturbances, sleep disturbances, suicidal thoughts, guilt and social withdrawal, while manic episodes consist of hyperactivity, elevated mood or agitation, racing thoughts, reckless behavior, little need for sleep, and sometimes psychosis (Association, 2013). Rats and mice brought to a manic state through pharmacological methods, environment and genetic modification displayed reduced anxiety and elevated hedonia (Logan and McClung, 2016). A manic state explains the reduced anxiety and elevated risk behavior seen in the Vogel and elevated plus maze tasks, and the depressed state would account for the increase in depressive behaviors demonstrated in the sucrose preference test for the post-stroke rats on placebo. Treatment with pyruvate, could reduce the effect of mania, allowing anxiety-like behavior to become more prominent. A related possibility is that many of the rats treated with placebo were in a manic state after the stroke, and the depression rate according to the sucrose test for the control group would be higher without including the manic rats.
It is important to note, however, that although depression following brain insults, such as stroke and traumatic brain injury, has clearly been established and depends on the severity and location of the brain insult (Boyko et al., 2013; Ifergane et al., 2018; Kuts et al., 2019), there is no clear consensus in the literature about the development of anxiety-like behavior following brain insult. Evidence of the development of anxiety-like behavior following stroke or brain injury has been inconstant in our laboratory. Further studies examining the effects of BGS on anxiety-like behavior are warranted.
Pyruvate treatment reduced lesion volume, brain edema, and the extent of BBB permeability compared to the placebo-treated group, as measured 24 h after ischemia. In addition, lesion volume and BBB permeability were less pronounced in the pyruvate-treated group than in the placebo group. Although those measurements remained higher than in the naïve group one week following the ischemia induction, they were not statistically different, confirming the success of pyruvate for neuroprotection. This improvement aligns with previously published data suggesting glutamate reduction in the brain’s ECF after pyruvate administration (Boyko et al, 2011c, 2012a). A significant decrease of NSS was recorded in the group administered pyruvate 1 week after stroke induction compared to the placebo group. The pyruvate-treated group displayed reduction of depression as a result of the neuroprotective effects of pyruvate to reduce excess glutamate.
One of the limitations of our study was that we used only one protocol of pyruvate treatment with a fixed pyruvate dose and measuring the subsequent level of reduced brain glutamate. In future studies, levels of brain glutamate reduction should be varied to better determine the impact of pyruvate glutamate scavenging.
Despite the mood stabilization and neuroprotective effects achieved by the reduction of CNS glutamate with oral pyruvate administration, and despite the use of native mechanisms of removing glutamate from the CNS, the existence of possible harmful effects of prolonged elevated blood ketoglutarate and alanine (due to exaggeration of pyruvate with glutamate enzymatic reaction by pyruvate administration) were not excluded by this study. Additional studies should include a control group of naïve rats treated with pyruvate supplementation, along with prolonged monitoring of ketoglutarate and alanine blood levels, while measuring neurological and behavior outcomes.
In conclusion, glutamate scavenging by oral pyruvate administration seems to be an efficient method for reducing post-stroke pathologically elevated CNS glutamate levels and for the treatment of post-stroke depression (unipolar disorder) and manic disorders (bipolar disorder). Additionally, glutamate scavenging by oral pyruvate administration displays neuroprotective effects of subsequent post-stroke reduction of lesion volume, brain edema and BBB breakdown. The impact of oral pyruvate admission on post-stroke anxiety disorder and the exclusion of possible harmful effects of prolonged blood ketoglutarate and alanine elevation should be further investigated.
HIGHLIGHTS.
Post-stroke neurological deficit with concurrent elevation in glutamate levels were demonstrated, with peak glutamate levels 24 h after MCAO.
Treatment with pyruvate led to reduced glutamate levels 24 h after MCAO and improved neurologic recovery.
Rats demonstrated post-stroke depressive behavior that was improved by the administration of pyruvate.
There was less anxiety-like behavior in post-stroke rats treated with placebo in comparison to the post-stroke rats treated with pyruvate or sham operated rats.
Our main conclusion was that glutamate scavenging with pyruvate appears to be an effective as a method in providing neuroprotection following stroke and as a therapeutic option for the treatment of PSD by reducing the consequent elevations in CNS glutamate levels.
Acknowledgements/conflict of interest disclosure/compliance with ethical standards
We thank Dr. Nataly Zueva, Division of Internal Medicine, Soroka Medical Center, Ben-Gurion University, Beer-Sheva, Israel, Dr. Svetlana Li, Department of Radiology, Soroka University Medical Center, Ben-Gurion University, Beer-Sheva, Israel and Dr. Ruslan Biliar, Department of Urology, Soroka Medical Center, Ben-Gurion University, Beer-Sheva, Israel for their outstanding help in behavioral examination and analysis of results by computer software. We thank Valeria Frishman, laboratory assistant and Prof. Amos Douvdevani Head, Research Lab., from the Department of Clinical Biochemistry, Soroka Medical Center, Ben-Gurion University, for her help with the biochemical analysis. We thank Dr. Kaziev Eleonora, Dr. Ibrahim Al Saif, Dr. Elena Braun, Dr. Tomer Kotek and the all staff at the Department of Anesthesiology and Critical Care, Soroka University Medical Center, for their support and helpful discussions. We thank PhD candidate Ohad Stoler and Prof. Ilya Fleidervish Head, Research Lab., from Department of Physiology and Cell Biology, Faculty of Health Sciences, Ben–Gurion University, Beer Sheva 84105, Israel for her help with the electrophysiological assessment. The data obtained are part of D.F.’s PhD thesis. This research was supported by the Israel Science Foundation (grant No. 1490/15) awarded to Matthew Boyko and Alexander Zlotnik. Authors Matthew Boyko and Alexander Zlotnik declare that they do not have a conflict of interest.
Abbreviations
- PSD
Post-stroke depression
- ECF
extra-cerebral fluid
- MCAO
Middle Cerebral Artery Occlusion
- BBB
blood brain barrier
- CNS
central nervous system
- NMDA
N-methyl-d-aspartate
- BGC
blood glutamate scavenging
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- ICA
Internal carotid artery
- CCA
common carotid artery
- NAD
Nicotinamide adenine dinucleotide
- PWI
perfusion weighted imaging
- ADC
apparent diffusion coefficient
- CBF
cerebral blood flow
- RICH
Ratios of Ipsilateral and Contralateral Cerebral Hemispheres
- NSS
neurological severity score
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2019.05.035.
References
- Altamura C, Maes M, Dai J, Meltzer HY, 1995. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol 5 (Suppl. l), 71–75. [DOI] [PubMed] [Google Scholar]
- Association AP, 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. (Washington, DC: ). [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M, 2005. Differences in ischemic lesion evolution in different rat strains using Diffusion and perfusion imaging. Stroke 36 (9), 2000–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva I, Belcheva S, Petkov VV, Hadjiivanova C, Petkov VD, 1997. Behavorial responses to the 5-HT1A receptor antagonist NAN190 injected into rat CA1 hippo-campal area. Gen. Pharmacol 28 (3), 435–441. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH, 1984. Elevation of the extra-cellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem 43 (5), 1369–1374. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bergmeyer J, Grassl M, 1974. Methods of Enzymatic Analysis.
- Boyko M, Kutz R, Grinshpun J, Zvenigorodsky V, Gruenbaum SE, Gruenbaum BF, et al. , 2015. Establishment of an animal model of depression contagion. Behav. Brain Res 281, 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko M, Kutz R, Gruenbaum BF, Cohen H, Kozlovsky N, Gruenbaum SE, et al. , 2013. The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cognit. Affect Behav. Neurosci 13 (4), 847–859. [DOI] [PubMed] [Google Scholar]
- Boyko M, Melamed I, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A, et al. , 2012a. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics 9 (3), 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Douvdevani A, Gruenbaum BF, Melamed I, et al. , 2011a. Cell-free DNA–a marker to predict ischemic brain damage in a rat stroke experimental model. J. Neurosurg. Anesthesiol 23 (3), 222–228. [DOI] [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Novack L, Novack V, Perry ZH, et al. , 2011b. Morphological and neuro-behavioral parallels in the rat model of stroke. Behav. Brain Res 223 (1), 17–23. [DOI] [PubMed] [Google Scholar]
- Boyko M, Stepensky D, Gruenbaum BF, Gruenbaum SE, Melamed I, Ohayon S, et al. , 2012b. Pharmacokinetics of glutamate-oxaloacetate transaminase and gluta-mate-pyruvate transaminase and their blood glutamate-lowering activity in naive rats. Neurochem. Res 37 (10), 2198–2205. [DOI] [PubMed] [Google Scholar]
- Boyko M, Zlotnik A, Gruenbaum BF, Gruenbaum SE, Ohayon S, Goldsmith T, et al. , 2010. An experimental model of focal ischemia using an internal carotid artery approach. J. Neurosci. Methods 193 (2), 246–253. [DOI] [PubMed] [Google Scholar]
- Boyko M, Zlotnik A, Gruenbaum BF, Gruenbaum SE, Ohayon S, Kuts R, et al. , 2011c. Pyruvate’s blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur. J. Neurosci 34 (9), 1432–1441. [DOI] [PubMed] [Google Scholar]
- Boyko M, Zvenigorodsky V, Grinshpun J, Shiyntum HN, Melamed I, Kutz R, et al. , 2019. Establishment of novel technical methods for evaluating brain edema and lesion volume in stroked rats: a standardization of measurement procedures. Brain Res. 1718, 12–21. [DOI] [PubMed] [Google Scholar]
- Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, et al. , 2012. Magnetic resonance imaging of glutamate. Nat. Med 18 (2), 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Zhao C, Shen C, Wang Y, 2013. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS One 8 (12), e82092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva Y, et al. , 2002. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 58 (4), 624–629. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Naveiro J, Noya M, 1996. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 27 (6), 1060–1065. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Noya M, 1997. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet 349 (9045), 79–83. [DOI] [PubMed] [Google Scholar]
- Dafer RM, Rao M, Shareef A, Sharma A, 2008. Poststroke depression. Top. Stroke Rehabil. 15 (1), 13–21. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. , 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatr 67 (8), 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton DG, Karakanta C, 2013. Depression as a risk marker for aggression: a critical review. Aggress. Violent Behav 18 (2), 310–319. [Google Scholar]
- Ferro JM, Caeiro L, Santos C, 2009. Poststroke emotional and behavior impairment: a narrative review. Cerebrovasc. Dis 27 (Suppl. 1), 197–203. [DOI] [PubMed] [Google Scholar]
- Gonzalez SV, Nguyen NH, Rise F, Hassel B, 2005. Brain metabolism of exogenous pyruvate. J. Neurochem 95 (1), 284–293. [DOI] [PubMed] [Google Scholar]
- Gray LR, Tompkins SC, Taylor EB, 2014. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci 71 (14), 2577–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot LL, Diaz FG, O’Regan MH, McLeod S, Park H, Phillis JW, 2001. Real-time measurement of glutamate release from the ischemic penumbra of the rat cerebral cortex using a focal middle cerebral artery occlusion model. Neurosci. Lett 299 (1–2), 37–40. [DOI] [PubMed] [Google Scholar]
- Han F, Shioda N, Moriguchi S, Qin ZH, Fukunaga K, 2008. Downregulation of glutamate transporters is associated with elevation in extracellular glutamate concentration following rat microsphere embolism. Neurosci. Lett 430 (3), 275–280. [DOI] [PubMed] [Google Scholar]
- Hancu I, 2009. Optimized glutamate detection at 3T. J. Magn. Reson. Imaging 30 (5), 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, 2011. The role of glutamate on the action of antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35 (7), 1558–1568. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M, 2007. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry 62 (11), 1310–1316. [DOI] [PubMed] [Google Scholar]
- Ifergane G, Boyko M, Frank D, Shiyntum HN, Grinshpun J, Kuts R, et al. , 2018. Biological and behavioral patterns of post-stroke depression in rats. Can. J. Neurol. Sci 45 (4), 451–461. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L, 2002. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1 (6), 383–386. [DOI] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R, 2019. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N. Engl. J. Med [DOI] [PubMed] [Google Scholar]
- Koura SS, Doppenberg EM, Marmarou A, Choi S, Young HF, Bullock R, 1998. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir. Suppl 71, 244–246. [DOI] [PubMed] [Google Scholar]
- Kucukibrahimoglu E, Saygin MZ, Caliskan M, Kaplan OK, Unsal C, Goren MZ, 2009. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol 65 (6), 571–577. [DOI] [PubMed] [Google Scholar]
- Kuts R, Melamed I, Shiyntum HN, Frank D, Grinshpun J, Zlotnik A, et al. , 2019. A middle cerebral artery occlusion technique for inducing post-stroke depression in rats. J. Vis. Exp 147, e58875. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW, 1999. The changing landscape of ischaemic brain injury mechanisms. Nature 399 (6738 Suppl. l), A7–A14. [DOI] [PubMed] [Google Scholar]
- Leibowitz A, Boyko M, Shapira Y, Zlotnik A, 2012. Blood glutamate scavenging: insight into neuroprotection. Int. J. Mol. Sci 13 (8), 10041–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi GL, Altieri M, Maestrini I, 2008. Post-stroke depression. Rev. Neurol. (Paris) 164 (10), 837–840. [DOI] [PubMed] [Google Scholar]
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW, 2000. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 47 (7), 586–593. [DOI] [PubMed] [Google Scholar]
- Logan RW, McClung CA, 2016. Animal models of bipolar mania: the past, present and future. Neuroscience 321, 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. , 1998. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 37 (3), 124–129. [DOI] [PubMed] [Google Scholar]
- McCulloch J, 1992. Excitatory amino acid antagonists and their potential for the treatment of ischaemic brain damage in man. Br. J. Clin. Pharmacol 34 (2), 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr., Kawahara R, 2006. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 30 (6), 1155–1158. [DOI] [PubMed] [Google Scholar]
- Muir KW, 2006. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Pharmacol 6 (1), 53–60. [DOI] [PubMed] [Google Scholar]
- Painuly N, Sharan P, Mattoo SK, 2005. Relationship of anger and anger attacks with depression: a brief review. Eur. Arch. Psychiatry Clin. Neurosci 255 (4), 215–222. [DOI] [PubMed] [Google Scholar]
- Piko BF, Pinczés T, 2014. Impulsivity, depression and aggression among adolescents. Pers. Indiv. Differ 69, 33–37. [Google Scholar]
- Prescot AP, Richards T, Dager SR, Choi C, Renshaw PF, 2012. Phase-adjusted echo time (PATE)-averaging 1 H MRS: application for improved glutamine quantification at 2.89 T. NMR Biomed. 25 (11), 1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege K, Powles R, Norton J, Mahendra P, Mitchell P, Agrawal S, et al. , 1993. An unusual presentation of acute myeloid leukaemia with pericardial and pleural effusions due to granulocytic sarcoma. Leuk. Lymphoma 11 (3–4), 305–307. [DOI] [PubMed] [Google Scholar]
- Reid E, Graham D, Lopez-Gonzalez MR, Holmes WM, Macrae IM, McCabe C, 2012. Penumbra detection using PWI/DWI mismatch MRI in a rat stroke model with and without comorbidity: comparison of methods. J. Cereb. Blood Flow Metab 32 (9), 1765–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CO, Caeiro L, Ferro JM, Figueira ML, 2011. Mania and stroke: a systematic review. Cerebrovasc. Dis 32 (1), 11–21. [DOI] [PubMed] [Google Scholar]
- Soares LM, De Vry J, Steinbusch HWM, Milani H, Prickaerts J, Weffort de Oliveira RM, 2016. Rolipram improves cognition, reduces anxiety- and despair-like behaviors and impacts hippocampal neuroplasticity after transient global cerebral ischemia. Neuroscience 326, 69–83. [DOI] [PubMed] [Google Scholar]
- Teichberg VI, 2007. From the liver to the brain across the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A 104 (18), 7315–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A, 2009. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience 158 (1), 301–308. [DOI] [PubMed] [Google Scholar]
- Zauner A, Bullock R, Kuta AJ, Woodward J, Young HF, 1996. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir. Suppl 67, 40–44. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang X, Zhang T, Chen L, 2001. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin. Chem 47 (8), 1458–1462. [PubMed] [Google Scholar]
- Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI, 2007. Brain neuroprotection by scavenging blood glutamate. Exp. Neurol 203 (1), 213–220. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Sinelnikov I, Gruenbaum BF, Gruenbaum SE, Dubilet M, Dubilet E, et al. , 2012. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology 116 (1), 73–83. [DOI] [PubMed] [Google Scholar]