Abstract
Background:
Depression is common and results in a significant morbidity and economic burden. Depression is associated with pervasive impairments in social functioning, and antidepressant treatments are highly variable in improving these impairments. The objectives of this study were to test the effects of depression on social organization and behavior in a rodent model of depression, and to study the effectiveness of antidepressant medication in improving both symptoms of depression and the social function of depressed animals.
Methods:
One hundred-twenty male Sprague-Dawley rats were randomly and equally divided between the control group and depression group. After induction of depression by 5 weeks of chronic unpredictable stress, rats received either antidepressant treatment or placebo. In parallel with the initiation of drug therapy, 20 social groups of six rats were subjected to the complex diving-for-food situation to evaluate their social functioning. Four behavioral tests evaluated symptoms of depression and anxiety at 3 different time points.
Results:
We found that 1) depressed rats were significantly more active and aggressive in all parameters of social organization test compared with the control and antidepressant treatment groups, 2) depressed rats that received antidepressant treatment exhibited social behaviors like the control group, and 3) depression in the experimental groups was not accompanied by symptoms of anxiety.
Conclusions:
These results suggest that depression can significantly alter the social behavior and hierarchy in the social group in rats. Investigations of complex social group dynamics offer novel opportunities for translational studies of mood and psychiatric disorders.
Keywords: Depression, Social dynamics, Behavior
1. Introduction
Depression is common, with a lifetime incidence of 13.3–17.1% in the United States [1], and results in significant morbidity and economic burden [2]. Depression is characterized by a–persistent and overwhelming feeling of sadness, guilt or low self-worth, and a loss of pleasure or interest in normal activities [3]. The approach to treating depression is often multimodal, and includes both pharmacological and non-pharmacological interventions. Although antidepressant medications are among the most commonly prescribed drugs worldwide, up to 40% of patients are refractory to medical therapy [4].
Depression is associated with significant and pervasive impairments in social functioning, defined as an individual’s ability to perform and fulfill normal social roles [5]. The negative impact of depression on occupational and social functioning has been demonstrated in several large observational studies [6,7]. Patients with depression often exhibit poor psychosocial outcomes that include unemployment, living alone with few social contacts, and engagement in few leisure activities [5]. Social and interpersonal functioning is an important measure of successful treatment of depression. To this end, indices of social functioning have been used as an endpoint in recent clinical trials to evaluate the efficacy of antidepressants therapy [8]. Importantly, the effectiveness of antidepressant treatments in relieving depression-related impairments in social functioning is highly variable [5].
Like humans, many animals exhibit symptoms of depression including anhedonia and helplessness. As such, animal models can be used to study the impact of depression on social behavior, and to evaluate the efficacy of treatment modalities in relieving these symptoms. Rats are highly social animals, and are often used to study depression. Rats form hierarchical social groups that are organized by dominance relationships among group members. To better understand social interactions and decision making in individuals with depression, rats can be subjected to tasks that involve cooperation, deception, decisions about risk, and behavior adjustments in accordance with others’ behaviors.
Access to alimentary resources is an important factor for the social group’s social organization and structure, because it is necessary for the group’s survival [5]. The emergence of social interactions and differentiation has been previously described in naïve rats faced with environmental constraints that limited the accessibility of food [9]. In this situation, access to food was particularly difficult and required that the rats cross a water barrier and carry the food back to its cage. This situation allows individual rats within each group to be categorized by their method of obtaining food. In this paradigm, there are two main behavioral profiles when adult rats are subjected to this task: the carriers, which dive to the feeder, grab a pellet, hold it in its mouth, and swim back to the home cage, and the non-carriers, which never dive and obtain food only by stealing it from the carriers. The behavioral differentiation between carrier and non-carrier rats was observed in groups of six rats, with respective proportions that approached 50% [10]. When the animals were individually trained in the apparatus, the proportion of divers increased to 100% [11]. Subsequently, a more complex diving-for-food task was designed to allow divers to consume the food in a second cage away from non-divers [9]. In this task, diver rats could consume the food away from non-diver rats.
We propose that the complex diving-for-food test will be useful in investigating the effects of depression on social organization and interactions in rats. The objectives of this study were twofold: first, we studied the impact of depression on social organization and behavior in a rodent model of depression using a complex diving-for-food situation test. Second, we investigated whether antidepressant medication can improve both symptoms of depression and concomitant social dysfunction in depressed animals.
2. Materials and methods
2.1. Materials
Imipramine hydrochloride was obtained from Sigma-Aldrich (I7379) and were stored in closed bottle in cool and dry place until their use and were dissolved in isotonic saline immediately prior to the administration to the animals.
2.2. Animals
This study was conducted per the recommendations of the Declarations of Helsinki and Tokyo, and the Guidelines for the Use of Experimental Animals of the European Community. The Animal Care Committee at the Ben-Gurion University of the Negev approved the experiments. One hundred-twenty male Sprague-Dawley rats, aged 3 months old, (Harlan Laboratories, Israel) were used in this experiment. All rats weighed between 300 and 350 g. Rats were housed in cages, with three rats per cage for at least 2 weeks after their arrival to allow for adaptation. Rats were housed in accordance with weight, age, and the size of the cage. Purina Chow and water were made available ad libitum. The temperature in the room was maintained at 22 °C, with a 12 h light–dark cycle (light on at 8 a.m.). All animals were individually marked on the tail with permanent felt pen to allow for identification.
2.3. Experimental design
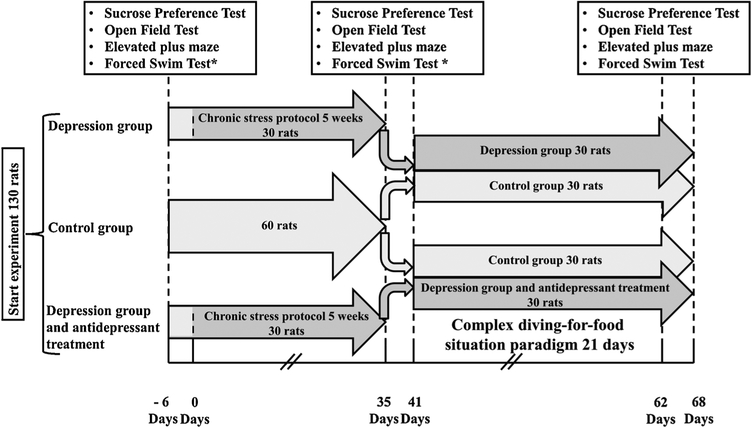
The timeline of the experiment is illustrated in Fig. 1. All experimental procedures were carried out between 8 a.m. and 5 p.m. Before starting the experiment, all animals were tested for the presence of depressive and anxiety behaviors using 3 tests: sucrose preference test, open field test, elevated plus maze test, as described below. At the beginning of this experiment, 130 rats were tested using a sucrose preference test. Four out of 130 rats showed markedly different results in the sucrose preference test from the average values for the group. These four rats were excluded from further experiments. From the 126 remaining rats, 120 were randomly selected. Subsequently, rats were randomly divided into 2 main groups: control group (60 rats) and depression group (60 rats). Rats in the depression group were subjected to additional testing for the presence of depressive-like behavior using the forced swimming test. The results of the swimming test in rats before the chronic unpredictable stress (CUS) procedure were subsequently used as a baseline for comparison between the groups that received and did not receive antidepressants (Fig. 6). Included rats were then subjected to several manipulations of chronic unpredictable stress (CUS), as described below, to induce depression for a duration of 5 weeks. After induction of depression, 4 preliminary tests were performed to confirm the development of depression-like behavior and to evaluate the level of anxiety. In the control group, 3 preliminary tests (sucrose preference test, open field test, elevated plus maze test) were performed (Fig. 1).
Fig. 1.
Timeline of the experimental protocol. A timeline of the experimental protocol. * Rats in the control group did not participate in the forced swim test. At the start of the experiment, 130 rats were tested using a sucrose preference test. Four out of 130 rats showed markedly different results in the sucrose preference test from the average values for the group. These four rats were excluded from further experiments. From the 126 remaining rats, 120 were randomly selected.
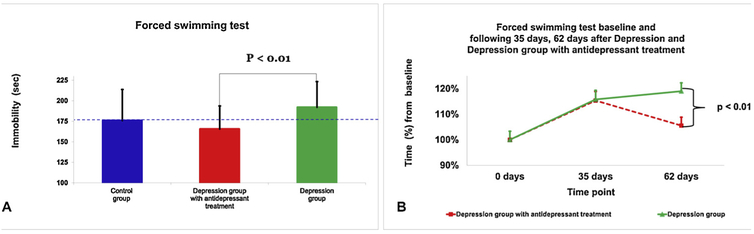
Fig. 6.
Behavior parameters in the forced swimming test. On the forced swimming test at 62 days, the immobility time was increased in the depression group compared with rats the depression group that received antidepressant therapy in mean immobility time in seconds (A) and mean percent changes in time compared to baseline (B). The data are measured as the seconds and expressed as mean ± standard deviation.
Subsequently, rats in the depression group (n = 60) were equally divided into two subgroups. One subgroup was administered antidepressant treatment with Imipramine Hydrochloride (tricyclic antidepressant) 20 mg/kg intraperitoneally once per day for a duration of 3 weeks [12–14]. The second sub-group was administered 0.9% saline (placebo) intraperitoneally once per day for a duration of 3 weeks, at the same volume as the antidepressant treatment group. Parallel to the start of drug treatment, 20 social groups of six weight-equivalent rats were constituted. Ten social groups included rats from the depression subgroup treated with placebo and control group (3 depressed and 3 control rats in each group), and another ten groups included rats from the depression subgroup treated with antidepressant therapy and control rats (3 depressed with antidepressant treatment and 3 control rats in each group).
All social groups were subjected to the complex diving-for-food situation for a duration of 21 days, in accordance with the protocol described below. Each day, rats were weighed 30 min prior to the experimental session (the weight of an animal on a given day reflects the previous day’s food consumption). Rats that lost more than 20% of its baseline weight were removed from the experiment together with their social group, and food and water were made available ad libitum. After the complex diving-for-food situation test, rats were additionally tested for clinical signs of depression and anxiety behaviors to ensure that all the rats in the depression group exhibited depressive-like behavior in the beginning and end of the experiment, and to evaluate their level of anxiety. Rats were euthanized after completing these tests.
2.4. Inducing depression by chronic unpredictable stress
The depression model consisted of the following stressors in random order: grouped housing (six rats instead of three per cage for 18 h), placement in a tilted cage (45° along the vertical axis for 3 h), food deprivation (18 h), water deprivation and exposure to an empty water bottle immediately following a period of acute water deprivation (18 h), placement in a soiled cage (300 ml of water spilled in the bedding) for 8 h, continuous lighting and reversed light/dark cycle for 48 h per week, and 5 min hot environment (40 °C). Rats were exposed to 2 of the 7 stressors daily in a random order; one in the daytime and the second at night for 5 sequential weeks [2].
2.5. Sucrose preference test
The sucrose preference test was performed as described previously [15] with minor modifications. Rats could consume 1% (w/v) by placing two bottles of sucrose solution in each cage for 24 h. Subsequently, one of the bottles was replaced with water for 24 h. Following the adaptation procedure, the rats were deprived of water and food for 1 h. The sucrose preference test was conducted at 9:00 a.m. The rats were housed in individual cages and given free access to the two bottles containing 100 ml of sucrose solution (1%, w/v) and 100 ml of water, respectively. After 4 h, the volume (ml) of both the consumed sucrose solution and water was recorded, and sucrose preference was calculated as sucrose preference (%) = sucrose consumption (ml)/(sucrose consumption (ml) + water consumption (ml)) × 100%.
2.6. Open field test
The open field test was performed as described previously [16]. The open field test task is predicated on the conflict between rodents’ innate fear for the central area of a novel or brightly lit open field and their desire to explore new environments. The open field is made of a black lusterless Perspex box (120 cm × 60 cm × 60 cm), which was divided into a 25% central zone and the surrounding border zone. Rats were placed in the corner of the open field facing the wall. The apparatus was situated in a dark room. Five percent alcohol was used to clean the apparatus prior to the introduction of each animal. The rats’ behavior (i.e., locomotor activity) was videotaped for 5 min by a Logitech HD Pro Webcam C920 camera, suspended approximately 200 cm above the open-field arena, with post-recording analysis performed using Ethovision XT software (Noldus, Wageningen, The Netherlands). The following specific parameters were analyzed: total distance travelled (cm), distance traveled in central part of the field (cm), time spent in the central part of the field (s), and the mean velocity (cm/s).
2.7. Forced swim test
The forced swim test reflects the observation that rats, when forced to swim in a restricted space in which they cannot escape, will eventually cease attempts to escape and become immobile except for small movements necessary to keep their heads above water. The forced swim test is a standard behavioral test for assessing helplessness, a hallmark of depressive-like behavior in rodents (typically rats and mice), and is often used to test the efficiency of antidepressant drugs. The test was performed as described previously [15]. Briefly, rats had an initial 15 min swimming session for training purpose with no data collection. After the first 15 min swim sessions, the rats were removed from the cylinders, dried with paper towels, and placed in heated cages for 15 min. Afterwards, they were returned to their cages. On the day of the test, a 5-min test was performed and their behavior was digitally recorded. The forced swim test was conducted by placing rats in individual glass cylinders (100 cm tall and 40 cm in diameter) containing room temperature water at a depth of 40 cm. After the 5-min test, the rats removed from the cylinders, dried with paper towels, and placed in heated cages for 15 min. Experiments were videotaped for post-recording measurements of the duration of immobility periods. As in previous tests, assessments were performed by 2 independent researchers that were blinded to the experimental groups.
2.8. Elevated plus maze test
The elevated plus maze test was performed as described previously [16]. The elevated plus maze, which consists of two open and two closed arms (each of dimensions 16 × 46 cm), was constructed from black plastic and positioned 50 cm above the floor. The closed arms, opposite to each another, had a surrounding wall of height 40 cm. The apparatus was situated in a darkened room. Experiments were recorded by a video camera suspended approximately 200 cm above the center of the plus maze. Five percent alcohol was used to clean the apparatus prior to the introduction of each animal. Rats were tested on the maze in a randomized order. Each rat was placed in the center of the plus maze facing one of the open arms, and the rat’s behavior was videotaped for 5 min for later analysis. Post-recording analysis of the total number of arm entries was accomplished, which allowed for calculating the percentage of open-arm entries and the percentage of time spent on the open arms.
2.9. Social organization test (Complex diving-for-Food task)
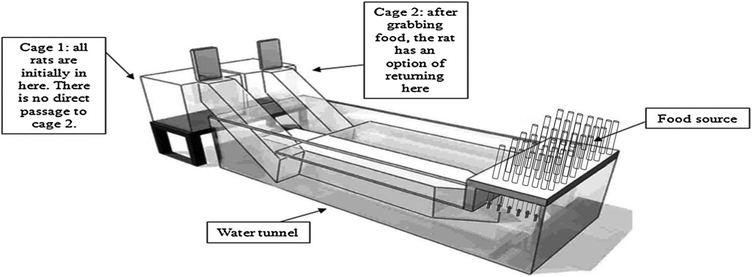
The experimental apparatus used in the test was described in previous studies [10,17,18] with minor modifications (Fig.2). The apparatus consists of 2 cages (50 × 50 × 50 cm) connected to an aquarium (130 × 35 × 50 cm) by a tunnel. The tubes with the food pellets (one food pellet in each tube) were placed at the end of the aquarium. The maximum water level in the aquarium was fixed by a transparent cover. The device was designed to oblige rats to dive and swim under water for 1 m, to prevent the possibility that the food could be consumed at the feeder, and to oblige the rats to bring the pellet of food back to the home cage or to the alternative cage. The access to the alternative cage was possible only by diving through the aquarium. The existence of an alternative cage allowed the diving rats the choice of whether to consume the food in the cage of departure or to consume the food elsewhere. Under these conditions, we propose that a rat, having fetched a food pellet from the feeder, would travel to the second cage to consume the food without a risk of having it stolen. A reduction in the accessibility of food pellets in the cage of departure should gradually oblige the rat, which would otherwise develop a habit of stealing, to dive to reach the food.
Fig. 2.
Experimental apparatus.
The aquarium was gradually filled with water, as previously described [9]. On day 1 of the experiment, rats were introduced in the experimental apparatus, empty of water, for the first time. Each group started a daily 3 h session in the same experimental cage. When the session is finished, the rats were returned to their standard cage. On days 1–3, the aquarium was empty of water to allow the animals to learn the spatial characteristics of the environment, and to learn the food location within the environment. On days 4–17, the methods of accessing the food were progressively restricted by adding water until the maximum water level was reached. From this day, the animal was obliged to dive and swim underwater to reach the feeder and return to its home cage. The total duration of this maneuver (from the cage to the feeder, and then back to the cage) should not exceed 5–6 s, and the pellet’s condition is not appreciably altered during the 2–3 s that it is underwater. The maximum water level phase lasted 17–21 days.
Throughout this experimental phase, access to the food was limited to a 3 h daily period, and rats did not have any other source of food for the remainder of the day. The two experimental cages were simultaneously videotaped during the entire feeding session (3 h) on each day of the experiment (21 days), and analyzed by two researchers blinded to the study groups. The sessions were recorded in daylight and the day before the recording, and each animal was individually marked on its coat with colored pens to allow for individual identification on videotape.
Rats that lost more than 20% of their baseline weight were removed from the experiment together with their social group, and were given ad libitum food and water. Attacks for food in all groups began at day 9–10; we therefore started to measure this parameter from day 9.
2.10. Statistical analysis
Statistical analyses were performed with the SPSS 20 package (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used, considering the number of rats in each group for deciding the appropriate test for the comparisons between the different parameters. For nonparametric data, we applied tests suitable for non-parametric data. The significance of comparisons between groups was determined using the Kruskal–Wallis followed by Mann–Whitney (for nonparametric data) or one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test or the Student’s t-test (for parametric data). Results was considered statistically significant when p < 0.05, and highly significant when p < 0.01.
3. Results
We investigated the effect of depression on social behavior and hierarchy in rats using a modified version of the complex diving-for-food situation and 4 additional behavioral tests (sucrose preference test, forced swimming test, open field test, elevated plus maze test) to evaluate the severity and duration of depression and anxiety. These behavioral tests were performed at 3 time points: at the beginning of the experiment, after 5 weeks, and at the end of the experiment (Fig. 1).
3.1. Complex diving for food situation
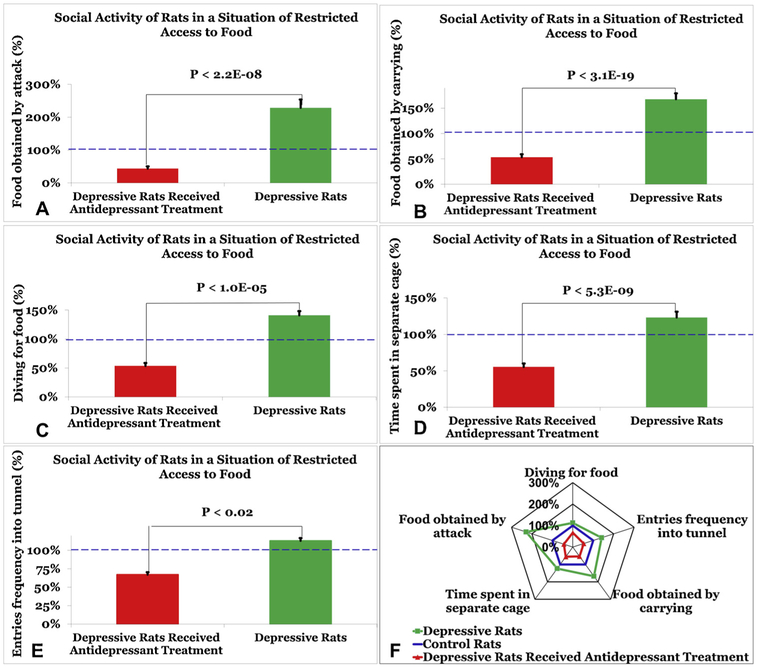
The results are presented in Fig. 3. Data is presented an average percentage ± standard error of the mean. According to a Mann-Whitney test, depressed rats showed a statistically significant increase in: 1) Food obtained by attack (232% ± 26) compared with depressed rats that received antidepressant treatment (44% ± 7, p < 0.0001, Fig. 3A) and compared with naïve rats from their social groups(1.59% ± 0.179 vs. 0.7% ± 0.152, p < 0.0001); 2) Food obtained by carrying (168% ± 12) compared with depressed rats that received antidepressant treatment (53% ± 6, p < 0.0001, Fig. 3B) and compared with naïve rats from their social groups (2.3% ± 0.16 vs.1.37% ± 0.1, p < 0.0001); 3) Diving for food (141% ± 7) compared with depressed rats that received antidepressant treatment (54% ± 5, p < 0.0001, Fig. 3C) and compared with naïve rats from their social groups (4.3% ± 0.21 vs. 3.1% ± 0.17, p < 0.0001); 4) Time spent in separate cages (123% ± 7.9) compared with depressed rats that received antidepressant treatment (55% ± 4.7, p < 0.0001, Fig. 3D), and no significant differences compared with naïve rats from their social groups (49% ± 3.1 vs. 40% ± 2.4); 5) Frequency of entries into the tunnel (113% ± 3.7) compared with depressed rats that received antidepressant treatment (67% ± 3.4, p < 0.0001, Fig. 3E) and compared with naïve rats from their social groups (31.8% ± 1.1 vs.28.1% ± 0.9, p < 0.05).
Fig. 3.
Social organization test. Results on the complex diving for food situation in the following performance parameters: food obtained by attack (A), food obtained by carrying (B), diving for food (C), time spent in separate cages (D), and frequency of entries into the tunnel (E), in control rats (blue), depressed rats (green) and depressed rats that received antidepressant treatment (red). A Radar chart (F) illustrates the performance of all three groups on each of the 5 parameters. Depressed rats were significantly more active and aggressive than control rats in 4 of 5 parameters evaluated by the social organization test (p < 0.05). Depressed rats that received antidepressant treatment demonstrated social behavior like the control group, but were less active and less aggressive than the control group (p < 0.05). The differences between the depression group and antidepressant treatment group were statistically greater than the differences between the depression group and control group in all 5 parameters of the test (p < 0.05). Data is presented an average percentage ± standard error of the mean.
3.2. Body weight changes
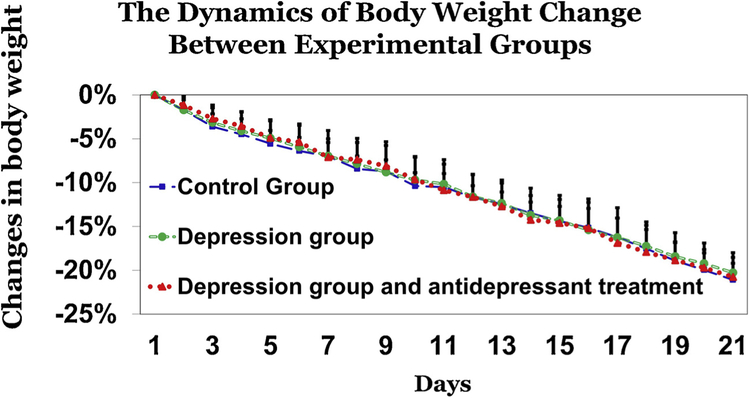
One-way ANOVA showed no statistically significant differences between 3 experimental groups for body weight change conducted for each time point experiment among 1–21dys. While there were no between group differences in body weights at the beginning of the experiment, there was an effect of day expressed as a change in body weight from day 2 to day 21 (Fig. 4). Between days 19 and 21, some of the rats lost more than 20% of their baseline weight, and were therefore removed from the experiment together with their social group, and were administered ad libitum food and water. At that time point, the social organization test in these rats was stopped.
Fig. 4.
Changes in body weight. There were no significant differences between the 3 experimental groups with respect to body weight between days 1 and 21 of the experiement. Data presented as percent change in body weight.
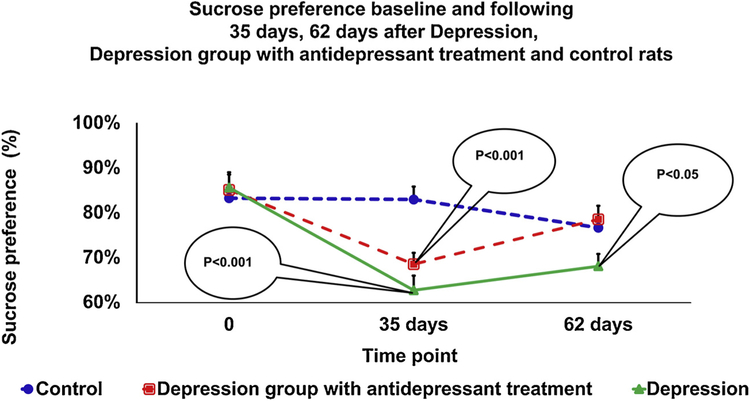
3.3. Sucrose preference test
Performance on the sucrose preference test for each group is presented in Fig. 5. Data presented as percent sucrose preference ± standard deviation. The sucrose preference data demonstrated that all rats showed similar sucrose consumption at the beginning of the experiment.
Fig. 5.
Behavior parameters in the sucrose preference test. At 35 days following experiment, the depressed rats and depressed rats that received antidepressant treatment demonstrated a significant reduction in the percent of sucrose preference compared with rats in the control group (p < 0.001 and p < 0.001, respectively). On day 62 of the experiment, there was a significant reduction in the percent of sucrose preference only in the depression group compared to the control group (p < 0.05). Data presented as percent sucrose preference ± standard deviation.
At 0 days, a one-way ANOVA showed no significant difference in the percent of sucrose preference between the depression group(85.6% ± 18.6) and depression group with antidepressant treatment(85.1% ± 18.8), compared to the control group (85.7% ± 9.9).
At 35 days, a one-way ANOVA with a Bonferroni’s post hoc test showed that both, the depression group (62.69% ± 17.7%) and the depression group treated with antidepressant therapy(68.48% ± 13.9%), had a significantly lower percent sucrose preference than the control group (84.13% ± 12.3%, p < 0.001 and p < 0.001 respectively, Fig. 5). However, post hoc analysis did not find any difference between the depression group and the depression group treated with antidepressant therapy.
At 62 days, a one-way ANOVA with a Bonferroni’s post hoc test showed that both, the depression group treated with antidepressant treatment (77% ± 16%) and the control group (78.5% ± 16%), showed significantly higher percent sucrose preference than the depression group (68% ± 15%, p < 0.05, Fig. 5). However, post hoc analysis did not find any difference between the control group and the depression group treated with antidepressant therapy.
3.4. Forced swim test
At the beginning of the experiment, a t-test showed no significant difference in immobility time between the depression group (161 s ± 33) compared with rats in the depression group that received antidepressant therapy (157 s ± 29, Fig. 6B).
At day 35 after the onset of CUS, a t-test showed no significant difference in immobility time between the depression group (187 s ± 44) compared with rats in the depression group that received antidepressant therapy (181 s ± 31). However, both the depression group and the depression group that received antidepressant therapy had a significant increase in immobility time at day 35 compared to their baseline values (p < 0.01 and p < 0.01, respectively).
At 62 days after the onset of CUS, a one-way ANOVA with Banferroni post hoc test showed that the immobility time was increased in the depression group compared with rats the depression group that received antidepressant therapy (192 s ± 31 vs. 157 s ± 29, respectively, p < 0.01, Fig. 6A). While there was a significant increase in immobility time at day 62 for the depression group compared to baseline (p < 0.001), there was no difference in immobility for the depression group that received antidepressant therapy at day 62 compared to baseline.
3.5. Open field test
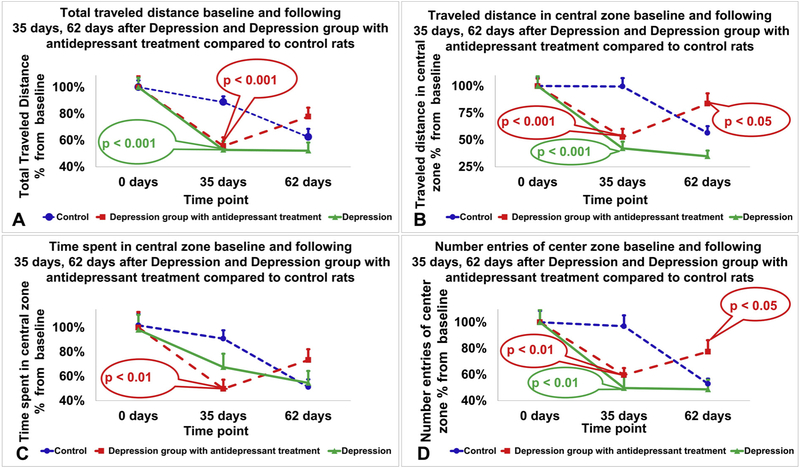
Behavior parameters in the open field test are presented in Fig. 7. Data is presented as an average percent difference from baseline ± standard error of the mean. At the onset of the experiment (first time point, day 0) a one-way ANOVA found no differences between the 3 groups with respect to total distance traveled (Fig. 7A), distance traveled in the central zone (Fig. 7B), time spent in the central zone (Fig. 7C), and number of entries in the center (Fig. 7D).
Fig. 7.
Behavior parameters in the open field test. Total traveled distance (A), traveled distance in the central zone (B), time spent in the central zone (C) and number of entries in the central zone (D) on the open field test for the control group (blue), depression group (green) and depression group with antidepressant treatment (red). At 35 days, rats in the depression group had a statistically significant reduction in the total distance traveled (p < 0.001), distance traveled in the central zone (p < 0.001), and the number of entries in the center zone (p < 0.01) compared with their baseline values. Rats in the depression group that received antidepressant treatment had a statistically significant reduction in all parameters tested at 35 days compared to control rats, including total distance traveled (p < 0.001), distance traveled in the central zone (p < 0.001), time spent in the central zone (p < 0.01) and the number of entries in the center zone (p < 0.01). At 62 days, the control group showed a similar performance on the open field test as the depression group. Conversely, the rats in the depression group treated with antidepressant therapy exhibited behavior that resembled their baseline, demonstrating increases in traveled distance in the central zone and number of entries in the center zone compared to the control group (p < 0.05 and p < 0.05, respectively). Data is presented as an average percent difference from baseline ± standard error of tror of the mean.
For total distance traveled (Fig. 7A), a one-way ANOVA followed by Bonferroni post hoc test showed that at 35 days, the depression group (52.8% ± 6.6) and depression group with antidepressant treatment (56.6% ± 6.6) were significantly decreased compared to control rats (88.8% ± 4.3, p < 0.001 and p < 0.001 respectively).
For distance traveled in the central zone (Fig. 7B), a one-way ANOVA followed by Bonferroni post hoc test showed that at 35 days, the depression group (42.2% ± 6.2) and depression group with antidepressant treatment (52.6% ± 7.7) were significantly decreased compared to control rats (99.68% ± 7.8, p < 0.001 and p < 0.001 respectively). At 62 days after the onset of CUS, a one-way ANOVA followed by Bonferroni post hoc test showed that the depression with antidepressant treatment group (83.6% ± 9.7) showed an increase in the distance traveled in the central zone compared to the control group(56.5% ± 6.3, p < 0.05).
For the time spent in the central zone (Fig. 7C), a one-way ANOVA followed by Bonferroni post hoc test showed that at 35 days, the depression group with antidepressant treatment (49.7% ± 7.4) were significantly decreased compared to control rats (90.9% ± 6.8, p < 0.01).
For the number of entries in the central zone (Fig. 7D), a one-way ANOVA followed by Bonferroni post hoc test showed that at 35 days, the depression group (49.7% ± 9.9) and depression group with antidepressant treatment (59.6% ± 5.3) were significantly decreased compared to control rats (97.0% ± 8.3, p < 0.01 and p < 0.01 respectively). At 62 days after the onset of CUS, a one-way ANOVA followed by Bonferroni post hoc test showed that the depression with antidepressant treatment group (77.5% ± 8.8) showed an increase in the number of entries in the central zone compared to the control group(53.0% ± 3.9, p < 0.05).
3.6. Elevated plus maze test
The results of the elevated plus maze test demonstrated that depression induced by CUS was not accompanied by anxiety behaviors in either depressed group. There were no statistically significant differences between the 3 experimental groups with respect to the following behavioral parameters: time spent in the open arms, time spent on the platform, number of open-arm entries, and number of platform entries before the experiment started. At the second time-point (35 days) there were no differences with respect to the following behavioral parameters: time spent in the open, time spent in platform, number of open-arm entries, and number of platform entries. Similarly, at the third time-point (62 days), there were no differences with respect to the following behavioral parameters: time spent in the open, time spent on the, number of open-arm entries, and number of platform entries.
4. Discussion
In this study, we investigated the effect of depression on social behavior and group hierarchy in rats. The main findings in this study were: 1) depressed rats were significantly more active and aggressive in all parameters of the social organization test, 2) antidepressant treatment changed the social behavior of depressed rats in a manner like the control group, and 3) depression, developed by rats in the experimental groups, was not accompanied by anxiety.
Before beginning the experiment, we anticipated that depressed rats would exhibit one of two possible behaviors. First, we expected that depressed rats would remain inactive due to a lack of motivation and decreased motor function that typically accompanies depression and would also exhibit fear and avoidant behavior that results from comorbid anxiety [19,20]. Moreover, some studies have demonstrated that many depressed patients exhibit an avoidant personality [21,22]. Second, we anticipated that depressed rats would be subordinate to naïve rats in their social groups, and would necessitate that the naïve rats dive and bring them food. This behavior was expected because empirical studies in humans have demonstrated correlations between dependence and depression [21,23,24]. Likewise, studies have demonstrated that antidepressant treatment in rodents resulted in behavioral changes from exhibiting submissive to more dominant behaviors [25–27].
Surprisingly, our results showed that depressed rats were significantly more active in all parameters of the social organization test, including aggressive behavior and attacks on other group members to obtain food. We hypothesized that a higher level of activity and aggression in depressed rats was due to a lack of fear and low anxiety level in these rats. Naturally, rats are afraid of water and do not want to enter the water and swim, even if it is to obtain food when they are very hungry. We suggest that the feeling of hunger, which is a basic instinct necessary for survival, combined with a lack of anxiety and fear, caused the depressed rats to be more active and more aggressive in obtaining food. Our hypothesis is supported by a previous study in which rats were treated with the antianxiety drug diazepam, and their social interactions were analyzed in a manner like our diving-for-food model. They demonstrated that diazepam-treated rats were more aggressive toward conspecifics than controls and compensated for their deficit of food by stealing food from other rats [28]. Further support is evident by the fact that the rate of suicide is increased in patients with depression [29,30]. Execution of suicidal behaviors in depressed patients can result from a necessary lack of fear and anxiety.
Our assumptions regarding the lack of fear and anxiety in depressed rats was supported by the results of the plus maze test, which was performed in all groups of rats at 3 different time points throughout the experiment. The plus maze test, which has been widely used to evaluate the level of anxiety in rodents, did not show any statistically significant differences between the 3 experimental groups at the 3 time points throughout the experiment. The results of this test showed that the depression that resulted from exposure to CUS was not accompanied by anxiety. Despite the high rates of comorbidity between depression and anxiety in patients, these two disorders are believed to result from differential pathogenic mechanisms [19,20]. Some research groups reported a lack of anxiogenesis [31,32], and sometimes even paradoxical anxiolytic effects, when anhedonia results from chronic mild stress exposure in animals [33]. It’s important to note that there is evidence in the literature that anxiety-like behavior may [34–40] or may not [40–43] develop following CUS. We believe this could be related to differences in the protocols of CUS, as well as rat strain, sex, age, and phenotype.
Depressed rats in our experiment were more aggressive than their social group naïve partners. Such behavior, in addition to a lack of fear and anxiety, can be also attributed to changes in the serotonergic (5HT) system that is common in depression. Serotonin seems to be implicated in the mechanisms of aggressive behavior. A reduction in serotonergic neurotransmission has been suggested as a factor underlying enhanced aggressive behavior. Highly aggressive rats have significantly lower 5-HT levels and turnover in the forebrain compared with non-aggressive rats [44,45]. Suicidal behavior, affective disorders, and a variety of other psychopathologic behaviors and syndromes have been shown to correlate with an altered serotonin system [46].
Our results also suggest that depression can be altered in the hierarchy of social groups. Although depressed rats dive for food more often than naïve rats, they were also more aggressive and obtained the food by attacking other rats in their social group. Social hierarchies guide the behavior of many species, including humans, where status plays an important role in motivation and health. Social hierarchies based on dominant–submissive relationships are common in animals and in many human societies, and is often established through aggressive behaviors. However, dominant–submissive relationships are difficult to study as they can be affected by both interactions both with others and their environment interactions [47]. Dominance hierarchy formation appears to be a more complex phenomenon than previously thought [48,49]. A hierarchy of ranks is said to be linear if the most dominant individual dominates all other group members, whereas the second most dominant individual dominates all other group members except the most dominant individual, etcetera. Alternatively, a hierarchy is said to be nonlinear if every individual dominates the same number of group mates [50]. Hierarchical status can be either fixed or changeable, and such social stratification has profound implication. In non-human and human primates, a more subordinate position in a stable social hierarchy is associated with greater stress, whereas in dynamic hierarchies, the dominant position experiences the most stress due to increased competition and instability [51] during times of reorganization, which may confer a great health risk risks [52,53].
Our results showed that depressed rats that received antidepressant treatment exhibited similar activity to naïve rats in all behavioral tests, and were significantly less active in the social behavioral test compared with control rats, which may be due to a high antidepressant dose. This may suggest that antidepressant treatment was effective in influencing both depressive-like symptoms and the social behaviors in depressed rats. However, the authors recognize that a limitation of this study was the lack of a control group treated with normal saline.
In conclusion, our results suggest that depression can alter the social behavior and hierarchy in social groups. This is an initial finding that merits further attention. Future studies should aim to test the social functioning and hierarchy in groups that consist of depressed rats and depressed rats treated with antidepressant drugs. This would help better understand how depression affects social functioning, and whether antidepressant treatment can improve social function and change the group hierarchy. Moreover, a further step would entail verifying the effect of different antidepressants on social functioning. We believe that investigations of complex social group dynamics may offer novel opportunities to study translationally-relevant mood and psychiatric disorders.
Acknowledgments
This research was partly supported by the ISRAEL SCIENCE FOUNDATION (grant No. 1490/15) awarded to Matthew Boyko and Alexander Zlotnik. The authors gratefully acknowledge Dr. Tamir Peri and Dr. Rubi Shwartz, residents in orthopedic department, Soroka Medical Center, for their help in analyzing of video records of the social organization test. Special thanks to the Y. Bykova, Applicant of the Dnipropetrovs’k regional Institute of Public Administration of National Academy of Public Administration, Office of the President of Ukraine, for helpful discussions and practical assistance in building behavior models/situation social support as a reward for access to food resources.
Footnotes
Conflict of interest statement
The authors report no conflicts of interest.
References
- [1].Rosenquist JN, Fowler JH, Christakis NA, Social network determinants of depression, Mol. Psychiatry 16 (2011) 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boyko M, Kutz R, Grinshpun J, Zvenigorodsky V, Gruenbaum SE, Gruenbaum BF, et al. , Establishment of an animal model of depression contagion, Behav. Brain Res. 281 (2015) 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zanier-Gomes PH, de Abreu Silva TE, Zanetti GC, Benati ER, Pinheiro NM, Murta BM, et al. , Depressive behavior induced by social isolation of predisposed female rats, Physiol. Behav 151 (2015) 292–297. [DOI] [PubMed] [Google Scholar]
- [4].Lang UE, Borgwardt S, Molecular mechanisms of depression: perspectives on new treatment strategies, Cell. Physiol. Biochem 31 (2013) 761–777. [DOI] [PubMed] [Google Scholar]
- [5].Hirschfeld RM, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Moller HJ, et al. , Social functioning in depression: a review, J. Clin. Psychiatry 61 (2000) 268–275. [DOI] [PubMed] [Google Scholar]
- [6].Paykel ES, Weissman M, Prusoff BA, Tonks CM, Dimensions of social adjustment in depressed women, J. Nerv. Ment. Dis 152 (1971) 158–172. [DOI] [PubMed] [Google Scholar]
- [7].Kennedy N, Foy K, Sherazi R, McDonough M, McKeon P, Long-term social functioning after depression treated by psychiatrists: a review, Bipolar Disord. 9 (2007) 25–37. [DOI] [PubMed] [Google Scholar]
- [8].Bech P, Social functioning: should it become an endpoint in trials of antidepressants? CNS Drugs 19 (2005) 313–324. [DOI] [PubMed] [Google Scholar]
- [9].Grasmuck V, Desor D, Behavioural differentiation of rats confronted to a complex diving-for-food situation, Behav. Processes 58 (2002) 67–77. [DOI] [PubMed] [Google Scholar]
- [10].Thullier F, Desor D, Mos J, Krafft B, Effect of group size on social organization in rats with restricted access to food, Physiol. Behav 52 (1992) 17–20. [DOI] [PubMed] [Google Scholar]
- [11].Krafft B, Colin C, Peignot P, Diving-for-food: a new model to assess social roles in a group of laboratory rats, Ethology 96 (1994) 11–23. [Google Scholar]
- [12].Castagné V, Moser P, Roux S, Porsolt RD, Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice, Curr. Protoc. Neurosci 55 (8) (2011) 10 A. 1–8. A. 4. [DOI] [PubMed] [Google Scholar]
- [13].Elgarf A-SA, Aboul-Fotouh S, Abd-Alkhalek HA, El Tabbal M, Hassan AN, Kassim SK, et al. , Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline, Pharmacol. Biochem. Behav 126 (2014) 152–162. [DOI] [PubMed] [Google Scholar]
- [14].Ismail B, Aboul-Fotouh S, Mansour AA, Shehata HH, Salman MI, Ibrahim EA, et al. , Behavioural, metabolic, and endothelial effects of the TNF-α suppressor thalidomide on rats subjected to chronic mild stress and fed an atherogenic diet, Can. J. Physiol. Pharmacol 92 (2014) 375–385. [DOI] [PubMed] [Google Scholar]
- [15].Boyko M, Kutz R, Gruenbaum BF, Cohen H, Kozlovsky N, Gruenbaum SE, et al. , The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion, Cogn. Affect. Behav. Neurosci 13 (2013) 847–859. [DOI] [PubMed] [Google Scholar]
- [16].Boyko M, Azab AN, Kuts R, Gruenbaum BF, Gruenbaum SE, Melamed I, et al. , The neuro-behavioral profile in rats after subarachnoid hemorrhage, Brain Res. 1491 (2013) 109–116. [DOI] [PubMed] [Google Scholar]
- [17].Helder R, Desor D, Toniolo AM, Potential stock differences in the social behavior of rats in a situation of restricted access to food, Behav. Genet 25 (1995) 483–487. [DOI] [PubMed] [Google Scholar]
- [18].Deviterne D, Peignot P, Krafft B, Behavioral profiles of adult rats in a difficult food supply social situation, related to certain early behavioral features, Dev. Psychobiol 27 (1994) 215–225. [DOI] [PubMed] [Google Scholar]
- [19].Belzer K, Schneier FR, Comorbidity of anxiety and depressive disorders: issues in conceptualization, assessment, and treatment, J. Psychiatr. Pract 10 (2004) 296–306. [DOI] [PubMed] [Google Scholar]
- [20].Schoevers RA, Van HL, Koppelmans V, Kool S, Dekker JJ, Managing the patient with co-morbid depression and an anxiety disorder, Drugs 68 (2008) 1621–1634. [DOI] [PubMed] [Google Scholar]
- [21].Sanderson WC, Wetzler S, Beck AT, Betz F, Prevalence of personality disorders in patients with major depression and dysthymia, Psychiatry Res. 42 (1992) 93–99. [DOI] [PubMed] [Google Scholar]
- [22].Corruble E, Ginestet D, Guelfi JD, Comorbidity of personality disorders and unipolar major depression: a review, J. Affect. Disord 37 (1996) 157–170. [DOI] [PubMed] [Google Scholar]
- [23].Skodol AE, Stout RL, McGlashan TH, Grilo CM, Gunderson JG, Shea MT, et al. , Co-occurrence of mood and personality disorders: a report from the Collaborative Longitudinal Personality Disorders Study (CLPS), Depress. Anxiety 10 (1999) 175–182. [PubMed] [Google Scholar]
- [24].Guelfi JD, Depression and personality disorders, Rev. Prat 58 (2008) 373–376. [PubMed] [Google Scholar]
- [25].Pinhasov A, Crooke J, Rosenthal D, Brenneman D, Malatynska E, Reduction of Submissive Behavior Model for antidepressant drug activity testing: study using a video-tracking system, Behav. Pharmacol 16 (2005) 657–664. [DOI] [PubMed] [Google Scholar]
- [26].Malatynska E, Goldenberg R, Shuck L, Haque A, Zamecki P, Crites G, et al. , Reduction of submissive behavior in rats: a test for antidepressant drug activity, Pharmacology 64 (2002) 8–17. [DOI] [PubMed] [Google Scholar]
- [27].Malatynska E, Rapp R, Harrawood D, Tunnicliff G, Submissive behavior in mice as a test for antidepressant drug activity, Pharmacol. Biochem. Behav 82 (2005) 306–313. [DOI] [PubMed] [Google Scholar]
- [28].Schroeder H, Toniolo AM, Nehlig A, Desor D, Long-term effects of early diazepam exposure on social differentiation in adult male rats subjected to the diving-for-food situation, Behav. Neurosci 112 (1998) 1209–1217. [PubMed] [Google Scholar]
- [29].Kessler RC, Borges G, Walters EE, Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey, Arch. Gen. Psychiatry 56 (1999) 617–626. [DOI] [PubMed] [Google Scholar]
- [30].Chen YW, Dilsaver SC, Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders, Biol. Psychiatry 39 (1996) 896–899. [DOI] [PubMed] [Google Scholar]
- [31].Jung YH, Hong SI, Ma SX, Hwang JY, Kim JS, Lee JH, et al. , Strain differences in the chronic mild stress animal model of depression and anxiety in mice, Biomol. Ther. (Seoul) 22 (2014) 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yoon SH, Kim BH, Ye SK, Kim MH, Chronic non-social stress affects depressive behaviors but not anxiety in mice, Korean J. Physiol. Pharmacol 18 (2014) 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schweizer MC, Henniger MS, Sillaber I, Chronic mild stress (CMS) in mice: of anhedonia,’ anomalous anxiolysis’ and activity, PLoS One 4 (2009) e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA, Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment, Neuropsychopharmacology 33 (2008) 320. [DOI] [PubMed] [Google Scholar]
- [35].Hu C, Luo Y, Wang H, Kuang S, Liang G, Yang Y, et al. , Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress, PLoS One 12 (2017) e0185129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pandey DK, Pati D, Joshi A, Mahesh R, Chronic unpredictable stress: possible animal model of comorbid depression, Int. J. Preclin. Pharm. Res 1 (2010) 54–63. [Google Scholar]
- [37].Fokos S, Panagis G, Effects of Δ9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress, J. Psychopharmacol 24 (2010) 767–777. [DOI] [PubMed] [Google Scholar]
- [38].Koprdova R, Bögi E, Belovičová K, Sedláčková N, Okuliarova M, Ujhazy E, et al. , Chronic unpredictable mild stress paradigm in male Wistar rats: effect on anxietyand depressive-like behavior, Neuro Endocrinol. Lett 37 (2016) 103–110. [PubMed] [Google Scholar]
- [39].Dönmez RA, Kaya FD, Derinöz O, Emmez ÖH, Candansayar S, Belen HB, Behavioural and neurobiological consequences of 2 different chronic stressors in rats, Turk. J. Med. Sci 44 (2014) 955–966. [DOI] [PubMed] [Google Scholar]
- [40].Paolo S, Brain P, Willner P, Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression, Physiol. Behav 56 (1994) 861–867. [DOI] [PubMed] [Google Scholar]
- [41].Willner P, Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation, Psychopharmacology 134 (1997) 319–329. [DOI] [PubMed] [Google Scholar]
- [42].Cox BM, Alsawah F, McNeill PC, Galloway MP, Perrine SA, Neurochemical, hormonal, and behavioral effects of chronic unpredictable stress in the rat, Behav. Brain Res. 220 (2011) 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vyas A, Chattarji S, Modulation of different states of anxiety-like behavior by chronic stress, Behav. Neurosci 118 (2004) 1450. [DOI] [PubMed] [Google Scholar]
- [44].Daruna JH, Kent EW, Comparison of regional serotonin levels and turnover in the brain of naturally high and low aggressive rats, Brain Res. 101 (1976) 489–501. [DOI] [PubMed] [Google Scholar]
- [45].Ferrari PF, Palanza P, Parmigiani S, de Almeida RM, Miczek KA, Serotonin and aggressive behavior in rodents and nonhuman primates: predispositions and plasticity, Eur. J. Pharmacol 526 (2005) 259–273. [DOI] [PubMed] [Google Scholar]
- [46].Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo M, et al. , Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients, Arch. Gen. Psychiatry 49 (1992) 442–446. [DOI] [PubMed] [Google Scholar]
- [47].Malatynska E, Pinhasov A, Crooke JJ, Smith-Swintosky VL, Brenneman DE, Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: technical considerations, J. Neurosci. Methods 165 (2007) 175–182. [DOI] [PubMed] [Google Scholar]
- [48].Chase ID, Tovey C, Spangler-Martin D, Manfredonia M, Individual differences versus social dynamics in the formation of animal dominance hierarchies, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 5744–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cordero MI, Sandi C, Stress amplifies memory for social hierarchy, Front. Neurosci 1 (2007) 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Beacham JL, Models of dominance hierarchy formation: effects of prior experience and intrinsic traits, Behaviour 140 (2003) 1275–1303. [Google Scholar]
- [51].Sapolsky RM, The influence of social hierarchy on primate health, Science (New York, NY) 308 (2005) 648–652. [DOI] [PubMed] [Google Scholar]
- [52].Sapolsky RM, Social status and health in humans and other animals, Annu. Rev. Anthropol 33 (2004) 393–418. [Google Scholar]
- [53].Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A, Know your place: neural processing of social hierarchy in humans, Neuron 58 (2008) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]