Abstract
Trihexyphenidyl, a nonselective muscarinic receptor antagonist, is the small molecule drug of choice for the treatment of DYT1 dystonia, but it is poorly tolerated due to significant side effects. A better understanding of the mechanism of action of trihexyphenidyl is needed for the development of improved treatments. Because DTY1 dystonia is associated with both abnormal cholinergic neurotransmission and abnormal dopamine regulation, we tested the hypothesis that trihexyphenidyl normalizes striatal dopamine release in a mouse model of DYT1 dystonia using ex vivo fast scan cyclic voltammetry and in vivo microdialysis. Trihexyphenidyl increased striatal dopamine release and efflux as assessed by ex vivo voltammetry and in vivo microdialysis respectively. In contrast, ʟ-DOPA, which is not usually effective for the treatment of DYT1 dystonia, did not increase dopamine release in either Dyt1 or control mice. Trihexyphenidyl was less effective at enhancing dopamine release in Dyt1 mice relative to controls ex vivo (mean increase WT: 65% vs Dyt1: 35%). Trihexyphenidyl required nicotinic receptors but not glutamate receptors to increase dopamine release. Dyt1 mice were more sensitive to the dopamine release decreasing effects of nicotinic acetylcholine receptor antagonism (IC50: WT= 29.46nM, Dyt1= 12.26nM) and less sensitive to acetylcholinesterase inhibitors suggesting that nicotinic acetylcholine receptor neurotransmission is altered in Dyt1 mice, that nicotinic receptors indirectly mediate the differential effects of trihexyphenidyl in Dyt1 mice, and that nicotinic receptors may be suitable therapeutic targets for DYT1 dystonia.
Keywords: fast scan cyclic voltammetry, microdialysis, acetylcholine, nicotinic, muscarinic
INTRODUCTION
Dystonia is characterized by abnormal muscle contractions that cause debilitating abnormal postures and/or twisting movements.(J. Jankovic 1998, Albanese et al. 2013) DYT1 dystonia is one of the most prevalent forms of inherited dystonia. Most DYT1 patients carry a 3 base-pair in-frame deletion (ΔGAG) in the TOR1A gene resulting in a single glutamic acid deletion in the TorsinA protein.(Ozelius et al. 1997, J. Jankovic 1998, Ozelius et al. 1998) Despite the identification of a causal mutation, pharmacological interventions for DYT1 dystonia are limited. The preferred oral medication is the non-selective muscarinic acetylcholine receptor (mAChR) antagonist, trihexyphenidyl (THP),(Schwarz & Bressman 2009, Thenganatt & Jankovic 2014) which binds to all 5 mAChR subtypes at low nanomolar affinity (M1 = 1.6 nM, M2 = 7 nM, M3 = 6.4 nM, M4 = 2.6 nM, M5 = 15.9 nM) .(Dorje et al. 1991, Bolden et al. 1992) Although THP can be an effective treatment for DYT1 dystonia, it is often poorly tolerated due to dose-limiting side effects associated with its broad anti-muscarinic receptor activity.(Burke et al. 1986, Jabbari et al. 1989, Guthrie et al. 2000, Lumsden et al. 2016) Further, these side-effects are exacerbated by the high doses required to treat dystonia. A significant obstacle to the development of improved therapeutics is that the mechanism of action of THP is poorly understood. A better understanding of the therapeutic actions of THP could improve treatment options.
One possible mechanism of action of THP may involve the regulation of striatal dopamine (DA) neurotransmission, which is abnormal in DYT1 dystonia. Previous studies have demonstrated reductions in striatal D2 receptor density and abnormalities in DA metabolites in DYT1 patients.(Augood et al. 2002, Asanuma et al. 2005) Additionally, a significant reduction in striatal DA release is observed in several mouse models of DYT1 dystonia.(Balcioglu et al. 2007, Page et al. 2010, Song et al. 2012) Striatal DA release is regulated by acetylcholine (ACh) neurotransmission via both mAChR and nicotinic acetylcholine receptors (nAChRs).(Zhang et al. 2002, Exley & Cragg 2008, Zhang et al. 2009, Zhang et al. 2009, Threlfell & Cragg 2011) Previous studies have implicated striatal ACh in the pathophysiology of dystonia.(Bonsi et al. 2008, Bonsi et al. 2011, Maltese et al. 2014, Scarduzio et al. 2017, Bonsi et al. 2018) Cholinergic innervation in the striatum is provided by both cholinergic interneurons (ChIs)(Bolam et al. 1984) and cholinergic projections from brainstem nuclei(Dautan et al. 2016) although striatal DA release is apparently mediated by ChIs.(Brimblecombe et al. 2018) ACh released by ChIs activates nAChRs on DA terminals to enhance DA release.(Rice & Cragg 2004, Exley & Cragg 2008, Threlfell & Cragg 2011) Activation of presynaptic M2 and M4 mAChR on ChIs inhibits ACh release,(Zhang et al. 2002) thereby attenuating DA release.(Threlfell et al. 2010) Therefore, we hypothesized that THP increases DA release in the striatum by inhibiting this negative feedback mechanism. Indeed, we found that DA release was significantly reduced in Dyt1 knockin mice. THP restored DA release in Dyt1 mice through a nAChR-dependent mechanism, suggesting a mechanism of action for THP in the treatment of dystonia and identifying nAChRs as a therapeutic target.
MATERIALS AND METHODS
Animals
Dyt1 knockin mice heterozygous for the ΔE-torsinA mutation (Tor1a+/ΔE)(Goodchild et al. 2005) and normal littermates (Tor1a+/+) on a C57BL/6J background were bred at Emory University. Animals were maintained on a 12h light/dark cycle, with ad libitum access to food and water. Mice were group housed with nestlets for environmental enrichment. Male and female mice between 12–14 weeks old were used in all experiments. Mice were genotyped using PCR (forward primer, GCTATGGAAGCTCTAGTTGG; reverse primer CAGCCAGGGCTAAACAGAG). All studies were approved by the Institutional Animal Care and Use Committee at Emory University.
Brain Slice Preparation
Mice were euthanized by cervical dislocation, and the brain was sectioned at 300μm in ice-cold, oxygenated sucrose artificial cerebral spinal fluid (aCSF) containing [in mM]: sucrose [194], NaCl [20], KCl [4.4], CaCl2 [1.2], MgCl2 [1.2], NaH2PO4 [1.2], NaHCO3 [25], D-glucose [11] at pH 7.4. Slices containing the dorsolateral striatum were selected by using the presence of a fused anterior commissure (Bregma +0.26 mm) or, for the ventral striatum (nucleus accumbens; NAcc), the presence of the unfused anterior commissure (Bregma +0.98 mm) as landmarks. Brain slices were collected in a holding chamber containing oxygenated, bicarbonate-buffered aCSF containing [in mM]: NaCl [126], KCl [2.45], CaCl2 [2.4], MgCl2 [1.2], NaH2PO4 [1.2], NaHCO3 [25], D-glucose [11] and maintained at room temperature for 45–60 min before experiments began.
Fast Scan Cyclic Voltammetry
A slice was transferred to the recording chamber and perfused with oxygenated aCSF at 32°C. After 30 min, a carbon fiber electrode was inserted approximately 50 μm into the surface of the slice and a bipolar tungsten stimulating electrode was placed approximately 200 μm away. Recordings were conducted in the dorsolateral striatum or in the core of the NAcc. Dorsolateral striatum was selected because this region receives innervation from the motor cortex.(Hintiryan et al. 2016) DA release was elicited by either 1-pulse (600 μA, 4 ms pulse width) or 5-pulse 100 Hz electrical stimulation at 5 min inter-stimulus intervals to avoid rundown. The scan rate for voltammetry was 400 V/s from −0.4 V to 1.3 V to −0.4 V verses Ag/AgCl with a sampling rate of 10 Hz using a Chem-Clamp voltammeter-amperometer (Dagan Corporation, Minneapolis, MN, USA). All experiments were conducted and analyzed using Demon voltammetry software (Wake Forest University).(Yorgason et al. 2011) All drugs were diluted in aCSF and bath applied. Drugs were equilibrated in the bath for 10–20 mins before recordings commenced. For the nicotine experiment, baseline DA release was recorded, then nicotine was added to the slice for 5 mins, and then washed out for 40 mins while DA release was recorded. For the L-DOPA administration experiment, mice were injected with ʟ-DOPA plus benserazide (10 mg/kg and 5 mg/kg respectively, subcutaneous) and then euthanized 30 mins later. FSCV was then performed as described above. All electrodes were calibrated to known DA standards in aCSF using a custom-made flow cell.
In Vivo Microdialysis
Microdialysis was performed as previously described.(Song et al. 2012) Briefly, a concentric microdialysis probe that was manufactured in-house was calibrated with 100 ng/mL DA in aCSF containing [in mM]: NaCl [147], KCl [3.5], CaCl2 [1.2], MgCl2 [1.2], NaH2PO4 [1] at pH 7.0–7.4. After anesthesia with isoflurane, the probe was implanted in the dorsal striatum (anterior 0.6 mm, lateral 1.7 mm, and ventral 4.5 mm from bregma). The probe was perfused with aCSF at a flow rate of 0.6 μL/min while the mice habituated to the experimental chamber overnight. On the day of the experiment, 4 baseline samples were collected every 20 mins to establish basal monoamine concentrations. All collection tubes contained 1 μL of 6.25 mM ascorbic acid to preserve monoamines. Mice were either injected i.p. with a single 20 mg/kg dose of THP or 300 nM THP was reverse dialyzed into the striatum. Samples were collected every 20 mins after injection for 2 hrs and stored at −80 °C until high-performance liquid chromatography (HPLC) analysis. After sample collection, the probe location was verified by reverse dialysis of 3% bromophenol blue, and only mice with a probe correctly located in the dorsal striatum were included in the analysis.
Monoamine Detection
DA was detected in microdialysis samples using HPLC with electrochemical detection as previously described.(Jinnah et al. 1994) The system consisted of an ESA MD-150 × 3.2 mm column, an ESA 5020 guard cell, and an ESA 5600A Coularray detector with an ESA 6210 detector cell (ESA, Bedford MA). The guard cell potential was 475 mV; and the analytic cell potentials were set at −175, 100, 350 and 425 mV. Samples were eluted at a flow rate of 0.4 mL/min with a mobile phase composed of [in mM], [1.7] 1-octanesulfonic acid sodium, [75] NaH2PO4, 0.25% triethylamine, and 8% acetonitrile at pH 2.9. Monoamines were identified by retention time and electrochemical profile in comparison with known standards.
Acetylcholinesterase Activity Assay
Acetylcholinesterase (AChE) activity was assessed in striatal homogenates using a commercially available colorimetric AChE assay (Abcam, Cambridge, UK), according to the manufacturer’s instructions. Briefly, whole striatum was homogenized in lysis buffer by sonicating on ice. Striatal homogenates were incubated with acetylthiocholine and production of thiocholine was identified by development of DTNB-thiol adduct. The reaction was read at 410 nm using a spectrofluorometer (Molecular Devices, San Jose, CA, USA) and absorbance was calibrated to known standards of AChE (mU/mL). Activity was normalized to total protein and data were expressed as mU/mg protein.
Quantitative real-time PCR
Total RNA was extracted from mouse midbrain using Pure Link RNA mini kit (Life Technologies, Carlsbad, CA, USA). First-strand cDNA synthesis was performed using 1 μg of total RNA and SuperScript II Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed using 20ng cDNA, 450 nM primers, and 2× SYBR® Select Master Mix (Applied Biosystems, Foster City, CA, USA). PCR conditions were: 95 °C for 2 min followed by 40 cycles at 95 °C for 15s and 60 °C for 1 min. Melting curves were used to validate a single PCR product. Data was analyzed using the comparative CT method using β-actin as a control.(Livak & Schmittgen 2001) Primers used included: β-actin, forward 5’-AAGGCCAACCGTGAAAAGAT-3’ and reverse 5’-GTGGTACGACCAGAGGCATAC-3’; α4 nAChR, forward 5’-AGCGGCTCCTGAAGAGACTC-3’ and reverse 5’-ACACGTTGGTCGTCATCATC-3’; α6 nAChR, forward 5’-CGGTTTATGTCTGTGGCTATG-3’ and reverse 5’-CCCAACGCAATCTGTAGTCC-3’; α7 nAChR, forward 5’-GCAGATCATGGATGTGGATG-3’ and reverse 5’-CAAGACGTTGGTGTGGAATG-3’; β2 nAChR, forward 5’-AGATCTACAGGAGCATTAGAG-3’ and reverse 5’-CTTGGAGGGTGCGTGGATCT-3’.
Compounds
Trihexyphenidyl, atropine, nicotine, neostigmine, ʟ-DOPA, and dopamine were obtained from Sigma-Aldrich (St. Louis, MO). Dihydro-β-erythroidine (DHβE), ambenonium, and CNQX were obtained from Tocris (Minneapolis, MN).
Statistical Analysis
All data are represented as means with standard error. Dose response data were analyzed with nonlinear regression to determine IC50 or EC50. All other experiments were analyzed with either two-tailed Student’s t-test or two-way ANOVA with post hoc Sidak’s multiple comparison test. Statistical analyses were performed using Graphpad Prism 7 (https://www.graphpad.com). Statistical significance is defined as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
RESULTS
DA release is reduced in Dyt1 knockin mice
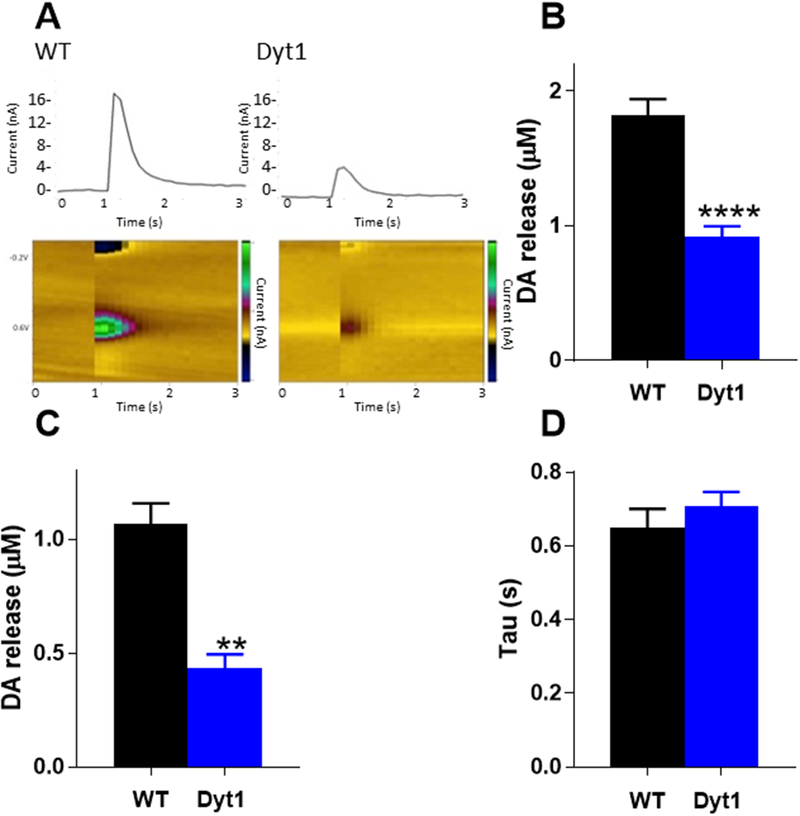
FSCV was used to measure evoked DA release in the dorsolateral striatum. DA release was stimulated with a single electrical stimulation using a perimaximal stimulus current. DA was identified by characteristic oxidation and reduction peaks at +600mV and −200mV respectively (Fig 1A). There was a significant reduction in DA release in Dyt1 mice compared to control mice (Tor1a+/+, 1.85 ± 0.12 μM, Tor1a+/ΔE,0.91 ± 0.08 μM, Student’s t test, p<0.0001) (Fig 1B). Because it is not known if the DA release deficit is mediated by region-specific microcircuits within the dorsal striatum, which is integrally involved in motor control, or if the decrement in release is a more general phenomenon, we examined DA release in the core of the NAcc. DA release was significantly reduced in the NAcc in Dyt1 mice compared to control mice (Tor1a+/+, 1.07 ± 0.09 μM, Tor1a+/ΔE, 0.44 ± 0.06 μM, Student’s t test, p<0.01) (Fig 1C). Despite the decrement in DA release in Dyt1 knockin mice, the clearance of dopamine (tau), an indirect measure of DA uptake,(Yorgason et al. 2011) was not significantly different between Dyt1 and control mice (Tor1a+/+, 0.647 ± 0.052 s, Tor1a+/ΔE, 0.707 ± 0.040 s, Student’s t test, p>0.05)(Fig 1D).
Fig 1. DA release in Dyt1 mice.
(A)Representative voltammograms and current traces of WT and Dyt1 DA release. 1-pulse electrical stimulation occurred at t=1 s. (B) Striatal DA release is significantly reduced in Dyt1 mice as assessed by FSCV (WT n=15; Dyt1=17). (C) DA release is significantly reduced in the nucleus accumbens core (n=3). (D) There is no change in DA uptake (tau) between Dyt1 and control animals (n=5). Values represent mean ± SEM.
Trihexyphenidyl enhances DA release
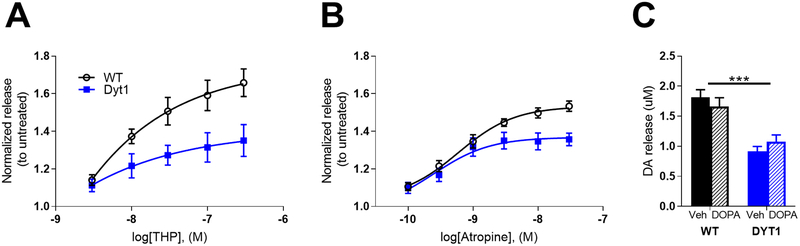
Because previous studies have shown that mAChRs regulate striatal DA release,(Zhang et al. 2002, Zhang et al. 2002, Threlfell et al. 2010, Threlfell & Cragg 2011) we tested the hypothesis that THP enhances DA release by performing FSCV in dorsolateral striatum while bath applying 3–300 nM THP. This dose range was selected based on the affinities of THP for mAChRs (2–20 nM depending on the mAChR subtype(Bolden et al. 1992)), and on known THP receptor occupancy in humans,(Shinotoh et al. 1994) which suggests that THP concentrations in brain are near-saturating for all mAChRs subtypes at the high doses used for the treatment of dystonia. A concentration of 300 nM THP achieves ~97–99% mAChR occupancy. THP dose-dependently increased DA release in both control and Dyt1 mice (Fig 2A; two-way repeated measures ANOVA drug effect, F1,10 = 14.44, p<0.01). There was also a significant genotype × dose interaction effect (two-way repeated measures ANOVA, F4,20 = 5.26, p<0.01), whereby Dyt1 mice exhibited a significantly reduced response to THP than control mice at 100nM and 300 nM (p<0.05 and p<0.01, respectively, Sidak multiple comparison test). There was no significant difference in the EC50 of THP between the genotypes (Student’s t test, p>0.05). To determine if the potentiation of DA release was an extraneous off-target effect of THP, we conducted a FSCV dose-response experiment using the prototypical mAChR antagonist atropine (Fig 2B). Atropine dose-dependently increased DA release in both genotypes (two-way repeated measures ANOVA effect of dose, F5,20 = 59.78, p<0.0001), but was significantly less effective in Dyt1 mice than control mice (two-way repeated measures ANOVA genotype × dose interaction effect F5,20 = 6.912, p<0.001) at 10 nM and 30 nM (p<0.05 and p<0.01, respectively, Sidak multiple comparison test), in agreement with the THP data. There was no significant difference in the EC50 of atropine between the genotypes (Student’s t test, p>0.05). While THP is an effective treatment in DYT1 dystonia, ʟ-DOPA and other dopaminergic agonists are seldom used.(Schwarz & Bressman 2009, Thenganatt & Jankovic 2014) We predicted that, in contrast to THP, ʟ-DOPA would not rescue the deficit in DA release given that DA synthesis and content are normal in DYT1 dystonia.(Furukawa et al. 2000) To test this, mice were pretreated with 10 mg/kg ʟ-DOPA with 5mg/kg benserazide (s.c.) and then ex vivo FSCV was performed to assess DA release (Fig 2C). 10 mg/kg ʟ-DOPA was chosen because this dose is sufficient to induce ʟ-DOPA induced dyskinesia in mice.(Fieblinger et al. 2014, Bordia et al. 2015, Lim et al. 2015, Solis et al. 2017) Consistent with this hypothesis, ʟ-DOPA had no significant effect on DA release in control or Dyt1 mice (two-way ANOVA treatment effect F1,36 = 0.0012, p>0.05).
Fig 2. Muscarinic antagonists increase DA release.
(A) Dose response curves of the effect of THP on DA release. THP dose-dependently increased DA release in WT and Dyt1 mice, but was less effective in Dyt1 (WT n=6; Dyt1 n=6). (B). Dose response curves of the effect of atropine, a prototypical mAChR antagonist, on DA release. Atropine significantly increased DA release, but was significantly less effective in Dyt1 mice (WT n=5; Dyt1 n=5). (C) Effect of L-DOPA on DA release. L-DOPA administration had no effect on DA release in Dyt1 or control mice (WT=4; Dyt1=4). Values are normalized to untreated for each genotype and represent mean ± SEM.
THP normalizes extracellular DA in Dyt1 mice
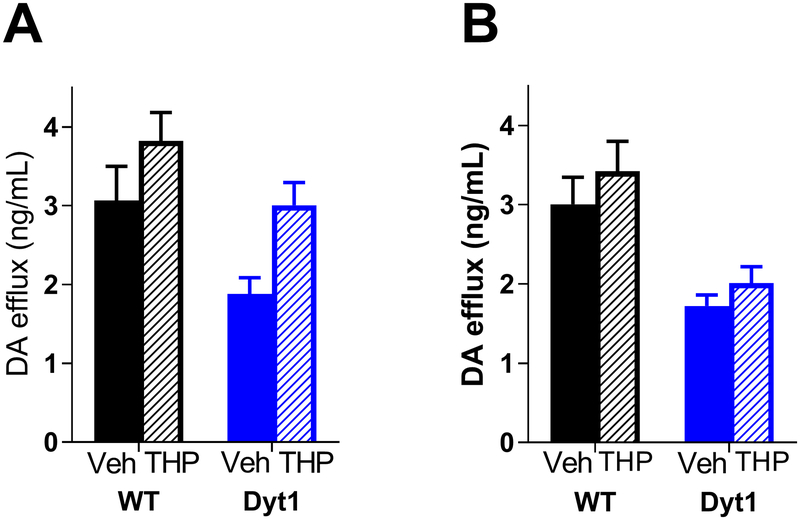
To test the DA-enhancing effects of THP in vivo, microdialysis was conducted in awake, behaving mice. THP was reverse dialyzed and extracellular DA was measured both pre- and post- treatment (Fig 3A). Extracellular DA was significantly reduced at baseline in Dyt1 mice relative to controls (Tor1a+/+, 3.07 ± 0.434 ng/mL, Tor1a+/ΔE, 1.88 ± 0.205 ng/mL; Student’s t test, p<0.05), consistent with previous results.(Song et al. 2012) THP significantly increased extracellular DA in both control and Dyt1 mice (two-way repeated measures ANOVA, main effect of treatment, F1,14 = 38.02, p<0.0001). In fact, THP increased extracellular DA in Dyt1 mice to normal WT concentrations (Tukey’s post-hoc, p>0.05). Additionally, extracellular DA was measured using microdialysis after a single peripheral administration of THP (20 mg/kg i.p.). Previous studies demonstrated that this dose reduces the severity of the dystonia in a mouse model of DOPA-responsive dystonia, but does not reduce locomotor activity.(Rose et al. 2015) Peripherally administered THP significantly increased extracellular DA (Fig 3B); (two-way repeated measures ANOVA, main effect of treatment, F1,16 = 7.003, p<0.05).
Fig 3. THP enhances DA release and extracellular DA.
(A) DA efflux after reverse dialysis of 300nM THP. THP significantly increased extracellular DA in both WT and Dyt1 mice (WT n=9; Dyt1 n=7). (B) DA efflux after i.p. injection of 20mg/kg THP. THP significantly increased DA release in both genotypes (WT n=8; Dyt1 n=10). Values represent mean ± SEM.
Functional nAChRs are required for the THP-induced increase in DA release
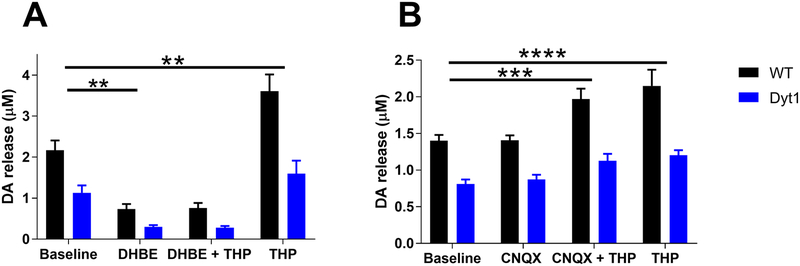
Current evidence suggests that mAChR agonists primarily influence DA release indirectly through ChIs. Activation of mAChR autoreceptors on ChIs reduces the release of ACh, thereby reducing the activation of nAChRs on DA terminals to decrease DA release.(Rice & Cragg 2004, Exley & Cragg 2008, Threlfell et al. 2010, Threlfell & Cragg 2011, Threlfell et al. 2012) Conversely, the mAChR antagonist THP may increase DA release indirectly through nAChRs. To test the hypothesis that THP requires functional nAChRs to enhance DA release, ex vivo FSCV was performed in the presence of THP (300 nM) while blocking nAChRs with the selective β2 subunit-containing nAChR antagonist DHβE (1 μM).(Harvey et al. 1996) β2 nAChR subunits are present in all nAChR on DA terminals in the dorsal striatum but absent from glutamatergic terminals in the striatum.(Wonnacott et al. 2000, Exley et al. 2008, Zhang et al. 2009) Further, DHβE is sufficient to completely block DA release induced by optogenetic stimulation of ChIs.(Fieblinger et al. 2014) DHβE alone significantly decreased DA release in both control and Dyt1 mice (Fig 4A) (two-way repeated measures ANOVA effect of treatment, F3,30 = 77.29, p<0.0001; Sidak’s posthoc test, p<0.001). DA release was significantly reduced in Dyt1 relative to control mice when nAChRs were blocked by DHβE (p<0.01, Student’s t test). The THP-induced increase in DA release was abolished in the presence of DHβE (Sidak’s post-hoc test, p>0.05). However, THP significantly increased DA release after DHβE washout (Sidak’s post-hoc test, p<0.001). These results suggest that THP requires functional nAChR to exert its DA-enhancing effects.
Fig 4. Glutamate and ACh involvement in THP mechanism of action.
(A) THP depends on nAChR to increase DA release. Blockade of nAChR with DHβE abolishes THP’s DA enhancing effects (WT n=6; Dyt1 n=6). (B) THP does not depend on glutamate signaling to increase DA release. Blockade of glutamate receptors does not alter THP’s DA-enhancing effect (WT n=4; Dyt1 n=4). Values represent mean [DA] ± SEM.
The THP-induced increase in DA release is not dependent on glutamatergic signaling
Previous studies have shown that corticostriatal glutamatergic afferents drive DA release indirectly through excitation of ChIs.(Kosillo et al. 2016) To determine if glutamate signaling is required for the THP-induced increase in DA release, we co-applied the non-specific glutamate receptor antagonist CNQX (10 μM) with THP (300 nM) (Fig 4B). CNQX alone had no effect on DA release (Sidak’s post-hoc test, p>0.05). In the presence of CNQX, THP significantly increased DA release compared to baseline (two-way repeated measures ANOVA, effect of treatment, F3,18 = 29.91, p<0.001; Sidak’s post-hoc test, p<0.001). Application of THP alone after CNQX washout significantly increased DA release compared to baseline (Sidak’s post-hoc test, p<0.0001) and this increase was not significantly different from that observed with the combined CNQX plus THP treatment (Sidak’s post-hoc test, p>0.05). These results suggest that the increase in DA release induced by THP is not dependent on glutamatergic signaling.
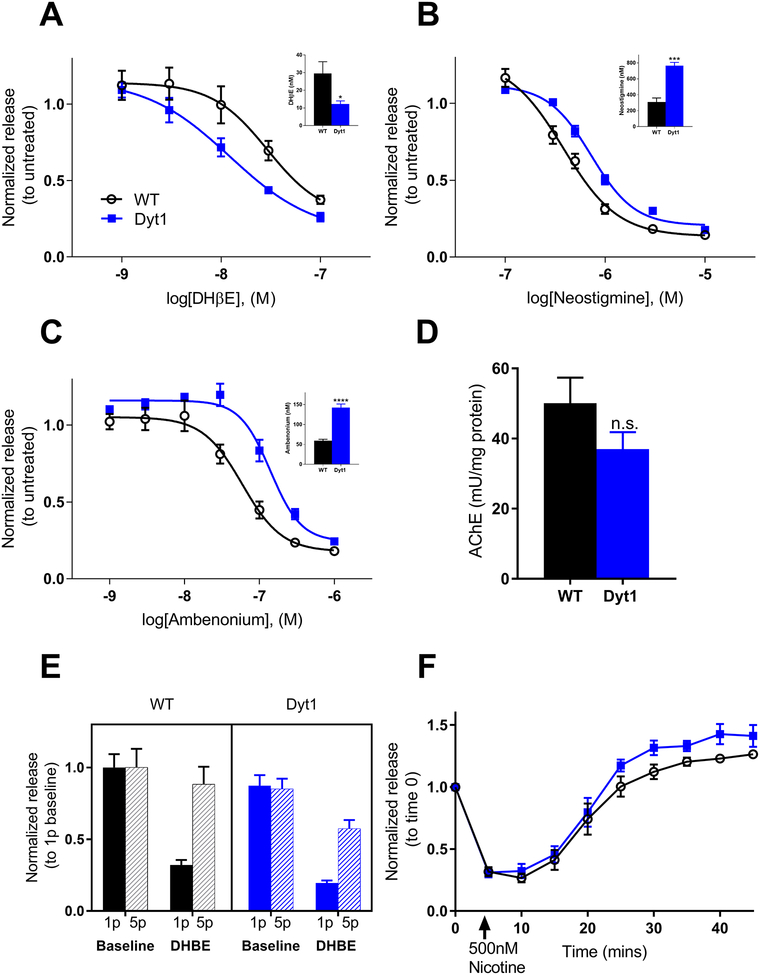
Dyt1 mice are more sensitive to nAChR antagonists
Because nAChRs are necessary for the DA release-enhancing effect of THP and this effect is attenuated in Dyt1 knockin mice, we hypothesized that nAChR neurotransmission is abnormal in Dyt1 knockin mice. Therefore, a dose-response experiment using DHβE (1–100 nM) was performed. Dyt1 mice exhibited a leftward shift in the dose-response (Fig 5A) as demonstrated by a significant reduction in the IC50 in Dyt1 mice compared to control mice (Tor1a+/+, 29.5 ± 6.5 nM, Tor1a+/ΔE, 12.3 ± 4.3 nM, Student’s t test, p<0.05) (Fig 5A inset). The leftward shift in the antagonist DHβE dose-response curve implies that neurotransmission via nAChRs is functionally attenuated in Dyt1 mice. To test this hypothesis, dose-response experiments were conducted with two different AChE inhibitors, neostigmine and ambenonium. The increased extracellular ACh induced by these compounds results in the rapid desensitization of nAChRs, thereby reducing DA release.(Zhang et al. 2004) A rightward shift in the dose-response curve for both neostigmine and ambenonium treatment was observed for Dyt1 mice compared to controls (Fig 5B & C) with a significant increase in the IC50s for both neostigmine (Tor1a+/+, 308.9 ± 50.6 nM; Tor1a+/ΔE, 764.3 ± 42.7 nM; Student’s t test, p<0.001) and ambenonium (Tor1a+/+, 59.24 ± 8.11 nM; Tor1a+/ΔE, 142.6 ± 19.9 nM; Student’s t test, p<0.0001) compared to controls. Because the attenuated response to the AChE inhibitors in Dyt1 mice could be due to differences in AChE activity in the striata of control vs Dyt1 mice, we measured AChE activity. AChE activity was not significantly different between Dyt1 and control animals (Tor1a+/+, 50.05 ± 7.32 mU/mg protein, Tor1a+/ΔE, 36.99 ± 4.86 mU/mg protein, Student’s t test, p>0.05) (Fig 5D). These results are consistent with the hypothesis that nAChR neurotransmission is attenuated in Dyt1 mice.
Fig 5. Nicotinic receptor function and ACh tone is altered in Dyt1 mice.
(A) The nAChR antagonist DHβE dose-dependently decreases DA release. The dose-response curve has a significant leftward shift for Dyt1 mice and the IC50 is significantly reduced for Dyt1 (A inset) (WT n=6; Dyt1 n=6). (B) Response to the AChE inhibitor neostigmine is altered in Dyt1 mice. There is a significant rightward shift in the dose-response curve for Dyt1 mice and the IC50 is significantly increased in Dyt1 mice (B inset) (WT n=5; Dyt1 n=5). (C) Response to the AChE inhibitor ambenonium is also altered in Dyt1 mice. There is a significant rightward shift in the dose-response curve for Dyt1 mice and the IC50 is significantly increased (C inset) (WT n=5; Dyt1 n=5). (D) AChE activity is unaltered between Dyt1 and control animals (WT n=6; Dyt1 n=6). (E) DA release in response to 1-pulse and 5-pulse electrical stimulation at baseline and after DHβE treatment. There is no significant difference in responses between genotypes (WT n=5; Dyt1 n=5). (F) Response to high concentration nicotine treatment is similar between WT and Dyt1 mice (WT n=5; Dyt1 n=5). Values are normalized to untreated for each genotype and represent mean ± SEM.
Presynaptic nAChR act as a frequency filter on DA terminals such that, when nAChR are active, tonic (1-pulse stimulation) and burst (5-pulse, 100Hz stimulation) firing yields similar levels of DA release. However, when nAChR are blocked or desensitized, DA release is significantly reduced in response to tonic stimulation compared to burst stimulation.(Zhang et al. 2009, Threlfell & Cragg 2011) Therefore, if nAChR function itself is attenuated in Dyt1 mice, the response to tonic and burst firing should resemble the blocked nAChR state. In both Dyt1 and control mice, 1-pulse stimulations evoked levels of DA release that were comparable to 5-pulse stimulations (two-way repeated measures ANOVA effect of genotype, F1,8 = 1.138, p>0.05). Blocking nAChRs with DHβE diminished DA release evoked by 1p stimulation, and the ratio between 5p and 1p evoked-release was not significantly different between Dyt1 and control mice (Student’s t test, p>0.05), suggesting that nAChR function is intact in the context of this challenge (Fig 5E). Because the attenuated nAChR function could be due to chronically desensitized receptors, DA release was assessed after exposure to a high dose of nicotine (500nM), which causes rapid desensitization of nAChRs and a subsequent decrease in DA release.(Rice & Cragg 2004) Acute nicotine exposure caused a rapid decrease in DA release followed by a return to baseline DA release after ~20 mins (Fig 5F) (two-way repeated measures drug effect F9,81 = 105.6, p<0.0001). There was no significant difference in response between Dyt1 and control mice (two-way repeated measures ANOVA genotype effect F1,9 = 3.908, p>0.05). Further, we examined midbrain nAChR mRNA expression using RT-qpCR (Fig S1). We found no significant difference in the expression of α4, α6, α7, and β2 nAChR subunits between Dyt1 knockin and control mice (two-way ANOVA genotype effect F1,40 = 3.961, p>0.05).
DISCUSSION
Here, we demonstrate that THP, one of the few small molecule drugs available for the treatment of dystonia, rescued the deficit in striatal dopamine release in Dyt1 mice. Furthermore, we demonstrate that the DA-enhancing effect of THP is dependent on nAChR neurotransmission, which is attenuated in Dyt1 mice.
Reductions in DA neurotransmission are consistently observed in a variety of Dyt1 mouse models, including transgenic Dyt1 mice(Shashidharan et al. 2005, Zhao et al. 2008, Hewett et al. 2010) and knockin Dyt1 mice.(Balcioglu et al. 2007, Song et al. 2012) Indeed, a similar diminution in striatal DA release was observed using FSCV in transgenic mice that overexpress torsinA (ΔE) specifically in TH+ neurons.(Page et al. 2010) Our results demonstrate that the deficiency in DA release is not attributable to abnormal DA reuptake or abnormal firing rates because the deficit is observed with evoked release in coronal slices, where DA neuron cell bodies are absent. Further, DA release is reduced in the NAcc, suggesting that the dysfunction is not specific to striatal microcircuits or specific to motor control regions. Interestingly, this finding may explain why DYT1 patients experience higher incidences of risk-taking behaviors than healthy controls.(Arkadir et al. 2016) Future studies are needed to determine if torsinA (ΔE) causes a global decrease in neurotransmitter release, or if abnormalities are confined to DA neurons. Indeed, a previous study demonstrated that hippocampal glutamatergic neurotransmission is reduced in Dyt1 knockin mice.(Yokoi et al. 2013)
Both THP and atropine dose-dependently increased ex vivo DA release suggesting that the DA-enhancing mechanism of action is likely at mAChRs rather than off-target effects. Importantly, direct infusion of THP into the striatum increased extracellular DA in Dyt1 mice to normal levels in vivo demonstrating that the site of action is the striatum. Further, the THP-induced increase in DA release was dependent on nAChRs suggesting THP increases DA release indirectly by modifying nAChR neurotransmission. In striatum, it is known that ACh release from ChIs is regulated by M2 and M4 mAChRs that inhibit ACh release.(Zhang et al. 2002, Threlfell et al. 2010) It is possible that THP acts through one or both of these receptor subtypes. However, we cannot exclude potential effects of other mAChR subtypes or cell types. For example, THP may act directly at M1 and/or M4 mAChRs on SPNs to enhance DA release via known retrograde endocannabinoid signaling between spiny projection neurons and DA neurons or via axon collaterals onto cholinergic interneurons,(Alcantara et al. 2001, Tepper et al. 2008, Gonzales et al. 2013, Foster et al. 2016) which could indirectly modify DA release. Additional studies using mAChR subtype-selective and cell-type selective manipulations of cholinergic neurotransmission are needed to elucidate the precise mechanism.
Despite the strong association between DA and dystonia, dopaminergic drugs are not generally used for the treatment of the dystonias. The exception is DOPA-responsive dystonia (DRD), which is caused by a reduction in DA due to mutations in genes critical for DA synthesis. In DRD patients, ʟ-DOPA, alleviates dystonia by restoring presynaptic DA concentrations and therefore activity-dependent DA release.(Ichinose & Nagatsu 1999) However, in the context of abnormal DA release despite normal striatal DA concentrations, as observed in Dyt1 knockin mice, ʟ-DOPA did not overcome the release deficit to provide significant benefit whereas THP rescued DA release. Further, unlike direct-acting DA receptor drugs that obscure presynaptic DA neuron firing patterns, THP acts indirectly on activity-dependent DA release. However, it is likely that the therapeutic effects of THP are not confined to rescuing striatal DA. Previous studies have shown that Dyt1 mice have abnormal corticostriatal LTP,(Martella et al. 2009, Martella et al. 2014, Maltese et al. 2018) and THP and the M1 mAChR selective antagonist VU0255035 restored normal plasticity,(Maltese et al. 2014) suggesting that a multipronged approach may be necessary for the treatment of dystonia.
While THP potentiated DA release, it was less effective in Dyt1 than normal mice. Because THP acts indirectly through nAChRs to increase DA release, abnormal nAChR or ChI function may underlie the reduced efficacy in Dyt1 mice. While nAChR regulation appeared normal in Dyt1 mice, as demonstrated by desensitization/resensitization to nicotine, response to tonic and phasic stimulation, and nAChR expression, Dyt1 mice exhibited an increased sensitivity to the nAChR antagonist DHβE. The simplest explanation for a leftward shift in an antagonist dose-response curve is a reduction in the competing ligand, in this case extracellular ACh. This hypothesis that extracellular ACh is reduced in Dyt1 mice is also supported by the rightward shift observed for the AChE inhibitors. In contrast, a previous study using microdialysis suggests extracellular ACh is increased in the striatum of Dyt1 mice using in vivo microdialysis. (Scarduzio et al. 2017) It is likely that the fundamental differences in the techniques particularly temporal resolution, stimulation parameters, and in vivo vs ex vivo approaches account for the discrepancies. It is also important to note that we did not directly assess surface expression of nAChR or changes in nAChR subunit composition, which are difficult to measure due to the dynamic moment-to-moment regulation of nAChRs, so we cannot exclude those factors as alternative explanations. Regardless, the reduction in DA release in Dyt1 knockin mice is not solely attributable to abnormal ACh tone because DA release in Dyt1 knockin mice was attenuated even after ACh neurotransmission was abolished by antagonists.
CONCLUSIONS
Taken together, our data suggest a potential mechanism of action for THP through the regulation of striatal DA and suggest nAChRs as a possible target for therapeutics. Indeed, two case reports found that transdermal nicotine patches or nicotine lozenges improved spastic dystonia, supporting the utility of either nAChR agonists or, more likely, nAChR positive allosteric modulators in dystonia.(Lees 1984, Vaughan et al. 1997) Future studies are needed to assess the contribution of specific mAChR subtypes to the therapeutic effects of THP to facilitate the discovery of more efficacious therapeutics with fewer side effects.
Supplementary Material
Fig S1. Nicotinic acetylcholine receptor subunit expression in midbrain. There was no significant difference in nAChR subunit mRNA expression in Dyt1 knockin versus control mice (WT n=6; Dyt1 n=6). Values are expressed as fold change over WT and represent mean ± SEM.
Acknowledgments
This work was supported by United States Department of Defense grant W81XWH-15-1-0545 and United States National Institute of Health Grants F31 NS103363 and T32 GM008602.
Abbreviations:
- mAChR
muscarinic acetylcholine receptor
- THP
trihexyphenidyl
- DA
dopamine
- ACh
acetylcholine
- nAChR
nicotinic acetylcholine receptor
- ChI
cholinergic interneuron
- NAcc
nucleus accumbens
- FSCV
fast scan cyclic voltammetry
- AChE
acetylcholinesterase
- DHβE
dihydro-β-erythroidine
Footnotes
The authors report no financial conflicts of interest concerning the research reported in this manuscript.
Literature Cited
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW and Teller JK (2013). “Phenomenology and classification of dystonia: a consensus update.” Mov Disord 28(7): 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM and Goldman-Rakic PS (2001). “Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways.” J Comp Neurol 434(4): 445–460. [DOI] [PubMed] [Google Scholar]
- Arkadir D, Radulescu A, Raymond D, Lubarr N, Bressman SB, Mazzoni P and Niv Y (2016). “DYT1 dystonia increases risk taking in humans.” Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB and Eidelberg D (2005). “Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation.” Neurology 64(2): 347–349. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO and Standaert DG (2002). “Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study.” Neurology 59(3): 445–448. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO and Standaert DG (2007). “Dopamine release is impaired in a mouse model of DYT1 dystonia.” J Neurochem 102(3): 783–788. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH and Smith AD (1984). “Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy.” Neuroscience 12(3): 711–718. [DOI] [PubMed] [Google Scholar]
- Bolden C, Cusack B and Richelson E (1992). “Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells.” J Pharmacol Exp Ther 260(2): 576–580. [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, Ponterio G and Pisani A (2011). “Centrality of striatal cholinergic transmission in Basal Ganglia function.” Front Neuroanat 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J and Pisani A (2008). “Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum.” J Neurosci 28(24): 6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Ponterio G, Vanni V, Tassone A, Sciamanna G, Migliarini S, Martella G, Meringolo M, Dehay B, Doudnikoff E, Zachariou V, Goodchild RE, Mercuri NB, D’Amelio M, Pasqualetti M, Bezard E and Pisani A (2018). “RGS9–2 rescues dopamine D2 receptor levels and signaling in DYT1 dystonia mouse models.” EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, McGregor M, McIntosh JM, Drenan RM and Quik M (2015). “Evidence for a role for alpha6(*) nAChRs in l-dopa-induced dyskinesias using Parkinsonian alpha6(*) nAChR gain-of-function mice.” Neuroscience 295: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Threlfell S, Dautan D, Kosillo P, Mena-Segovia J and Cragg SJ (2018). “Targeted Activation of Cholinergic Interneurons Accounts for the Modulation of Dopamine by Striatal Nicotinic Receptors.” eNeuro 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Fahn S and Marsden CD (1986). “Torsion dystonia: a double-blind, prospective trial of high-dosage trihexyphenidyl.” Neurology 36(2): 160–164. [DOI] [PubMed] [Google Scholar]
- Dautan D, Souza AS, Huerta-Ocampo I, Valencia M, Assous M, Witten IB, Deisseroth K, Tepper JM, Bolam JP, Gerdjikov TV and Mena-Segovia J (2016). “Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits.” Nat Neurosci 19(8): 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E and Brann MR (1991). “Antagonist binding profiles of five cloned human muscarinic receptor subtypes.” J Pharmacol Exp Ther 256(2): 727–733. [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM and Cragg SJ (2008). “Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens.” Neuropsychopharmacology 33(9): 2158–2166. [DOI] [PubMed] [Google Scholar]
- Exley R and Cragg SJ (2008). “Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission.” Br J Pharmacol 153 Suppl 1: S283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA and Surmeier DJ (2014). “Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia.” Nat Commun 5: 5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM and Conn PJ (2016). “Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release.” Neuron 91(6): 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Hornykiewicz O, Fahn S and Kish SJ (2000). “Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation.” Neurology 54(5): 1193–1195. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Pare JF, Wichmann T and Smith Y (2013). “GABAergic inputs from direct and indirect striatal projection neurons onto cholinergic interneurons in the primate putamen.” J Comp Neurol 521(11): 2502–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE and Dauer WT (2005). “Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope.” Neuron 48(6): 923–932. [DOI] [PubMed] [Google Scholar]
- Guthrie SK, Manzey L, Scott D, Giordani B and Tandon R (2000). “Comparison of central and peripheral pharmacologic effects of biperiden and trihexyphenidyl in human volunteers.” J Clin Psychopharmacol 20(1): 77–83. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN and Luetje CW (1996). “Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits.” J Neurochem 67(5): 1953–1959. [DOI] [PubMed] [Google Scholar]
- Hewett J, Johanson P, Sharma N, Standaert D and Balcioglu A (2010). “Function of dopamine transporter is compromised in DYT1 transgenic animal model in vivo.” J Neurochem 113(1): 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, Yamashita S, Bienkowski MS, Zingg B, Zhu M, Yang XW, Shih JC, Toga AW and Dong HW (2016). “The mouse cortico-striatal projectome.” Nat Neurosci 19(8): 1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H and Nagatsu T (1999). “Molecular genetics of DOPA-responsive dystonia.” Adv Neurol 80: 195–198. [PubMed] [Google Scholar]
- Jankovic J, F. S (1998). Dystonic Disorders Parkinson’s disease and movement disorders. J. J T. E Baltimore, Williams & Wilkins: 513–551. [Google Scholar]
- Jabbari B, Scherokman B, Gunderson CH, Rosenberg ML and Miller J (1989). “Treatment of movement disorders with trihexyphenidyl.” Mov Disord 4(3): 202–212. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt M, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ and Friedmann T (1994). “Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease.” J Neurosci 14(3 Pt 1): 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S and Cragg SJ (2016). “Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons.” Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ (1984). “Hemidystonia relieved by nicotine.” Lancet 2(8407): 871. [DOI] [PubMed] [Google Scholar]
- Lim SAO, Xia R, Ding Y, Won L, Ray WJ, Hitchcock SA, McGehee DS and Kang UJ (2015). “Enhanced histamine H2 excitation of striatal cholinergic interneurons in L-DOPA-induced dyskinesia.” Neurobiol Dis 76: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001). “Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.” Methods 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- Lumsden DE, Kaminska M, Tomlin S and Lin JP (2016). “Medication use in childhood dystonia.” Eur J Paediatr Neurol 20(4): 625–629. [DOI] [PubMed] [Google Scholar]
- Maltese M, Martella G, Madeo G, Fagiolo I, Tassone A, Ponterio G, Sciamanna G, Burbaud P, Conn PJ, Bonsi P and Pisani A (2014). “Anticholinergic drugs rescue synaptic plasticity in DYT1 dystonia: role of M1 muscarinic receptors.” Mov Disord 29(13): 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese M, Stanic J, Tassone A, Sciamanna G, Ponterio G, Vanni V, Martella G, Imbriani P, Bonsi P, Mercuri NB, Gardoni F and Pisani A (2018). “Early structural and functional plasticity alterations in a susceptibility period of DYT1 dystonia mouse striatum.” Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, Maltese M, Nistico R, Schirinzi T, Madeo G, Sciamanna G, Ponterio G, Tassone A, Mandolesi G, Vanni V, Pignatelli M, Bonsi P and Pisani A (2014). “Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia.” Neurobiol Dis 65: 124–132. [DOI] [PubMed] [Google Scholar]
- Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG and Pisani A (2009). “Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine.” Brain 132(Pt 9): 2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF and Breakefield XO (1997). “The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein.” Nat Genet 17(1): 40–48. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Jacoby D, Penney J, Risch NJ, Fahn S, Gusella JF and Breakefield XO (1998). “The gene (DYT1) for early-onset torsion dystonia encodes a novel protein related to the Clp protease/heat shock family.” Adv Neurol 78: 93–105. [PubMed] [Google Scholar]
- Page ME, Bao L, Andre P, Pelta-Heller J, Sluzas E, Gonzalez-Alegre P, Bogush A, Khan LE, Iacovitti L, Rice ME and Ehrlich ME (2010). “Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (DeltaE) TorsinA in transgenic mice.” Neurobiol Dis 39(3): 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME and Cragg SJ (2004). “Nicotine amplifies reward-related dopamine signals in striatum.” Nat Neurosci 7(6): 583–584. [DOI] [PubMed] [Google Scholar]
- Rose SJ, Yu XY, Heinzer AK, Harrast P, Fan X, Raike RS, Thompson VB, Pare JF, Weinshenker D, Smith Y, Jinnah HA and Hess EJ (2015). “A new knock-in mouse model of l-DOPA-responsive dystonia.” Brain 138(Pt 10): 2987–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarduzio M, Zimmerman CN, Jaunarajs KL, Wang Q, Standaert DG and McMahon LL (2017). “Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia.” Exp Neurol 295: 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CS and Bressman SB (2009). “Genetics and treatment of dystonia.” Neurol Clin 27(3): 697–718, vi. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, Weisz D, Sreenath T, Brin MF and Olanow CW (2005). “Transgenic mouse model of early-onset DYT1 dystonia.” Hum Mol Genet 14(1): 125–133. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Asahina M, Inoue O, Suhara T, Hirayama K and Tateno Y (1994). “Effects of trihexyphenidyl and L-dopa on brain muscarinic cholinergic receptor binding measured by positron emission tomography.” J Neural Transm Park Dis Dement Sect 7(1): 35–46. [DOI] [PubMed] [Google Scholar]
- Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M and Moratalla R (2017). “Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling.” Cereb Cortex 27(1): 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CH, Fan X, Exeter CJ, Hess EJ and Jinnah HA (2012). “Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia.” Neurobiol Dis 48(1): 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ and Koos T (2008). “Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons.” Brain Res Rev 58(2): 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenganatt MA and Jankovic J (2014). “Treatment of dystonia.” Neurotherapeutics 11(1): 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J and Cragg SJ (2010). “Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum.” J Neurosci 30(9): 3398–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S and Cragg SJ (2011). “Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons.” Front Syst Neurosci 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K and Cragg SJ (2012). “Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons.” Neuron 75(1): 58–64. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Delanty N, Harrington H and Murphy MB (1997). “Treatment of spastic dystonia with transdermal nicotine.” Lancet 350(9077): 565. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L and Jones IW (2000). “Presynaptic nicotinic receptors modulating dopamine release in the rat striatum.” Eur J Pharmacol 393(1–3): 51–58. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Cheetham CC, Campbell SL, Sweatt JD and Li Y (2013). “Pre-synaptic release deficits in a DYT1 dystonia mouse model.” PLoS One 8(8): e72491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA and Jones SR (2011). “Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures.” J Neurosci Methods 202(2): 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE and Dani JA (2009). “Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum.” Mol Pharmacol 76(2): 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou FM and Dani JA (2004). “Cholinergic drugs for Alzheimer’s disease enhance in vitro dopamine release.” Mol Pharmacol 66(3): 538–544. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM and Dani JA (2009). “Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine.” J Neurosci 29(13): 4035–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI and Wess J (2002). “Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice.” J Neurosci 22(5): 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS and Wess J (2002). “Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice.” J Neurosci 22(15): 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M and LeDoux MS (2008). “Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice.” Exp Neurol 210(2): 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Nicotinic acetylcholine receptor subunit expression in midbrain. There was no significant difference in nAChR subunit mRNA expression in Dyt1 knockin versus control mice (WT n=6; Dyt1 n=6). Values are expressed as fold change over WT and represent mean ± SEM.