Abstract
Background
Present‐centered therapy (PCT) is a non‐trauma, manualized psychotherapy for adults with post‐traumatic stress disorder (PTSD). PCT was originally designed as a treatment comparator in trials evaluating the effectiveness of trauma‐focused cognitive‐behavioral therapy (TF‐CBT). Recent trials have indicated that PCT may be an effective treatment option for PTSD and that patients may drop out of PCT at lower rates relative to TF‐CBT.
Objectives
To assess the effects of PCT for adults with PTSD. Specifically, we sought to determine whether (1) PCT is more effective in alleviating symptoms relative to control conditions, (2) PCT results in similar alleviation of symptoms compared to TF‐CBT, based on an a priori minimally important differences on a semi‐structured interview of PTSD symptoms, and (3) PCT is associated with lower treatment dropout as compared to TF‐CBT.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register, the Cochrane Library, Ovid MEDLINE, Embase, PsycINFO, PubMed, and PTSDpubs (previously called the Published International Literature on Traumatic Stress (PILOTS) database) (all years to 15 February 2019 search). We also searched the World Health Organization (WHO) trials portal (ICTRP) and ClinicalTrials.gov to identify unpublished and ongoing trials. Reference lists of included studies and relevant systematic reviews were checked. Grey literature searches were also conducted to identify dissertations and theses, clinical guidelines, and regulatory agency reports.
Selection criteria
We selected all randomized clinical trials (RCTs) that recruited adults diagnosed with PTSD to evaluate PCT compared to TF‐CBT or a control condition. Both individual and group PCT modalities were included. The primary outcomes of interest included reduced PTSD severity as determined by a clinician‐administered measure and treatment dropout rates.
Data collection and analysis
We complied with the Cochrane recommended standards for data screening and collection. Two review authors independently screened articles for inclusion and extracted relevant data from eligible studies, including the assessment of trial quality. Random‐effects meta‐analyses, subgroup analyses, and sensitivity analyses were conducted using mean differences (MD) and standardized mean differences (SMD) for continuous data or risk ratios (RR) and risk differences (RD) for dichotomous data. To conclude that PCT resulted in similar reductions in PTSD symptoms relative to TF‐CBT, we required a MD of less than 10 points (to include the 95% confidence interval) on the Clinician‐Administered PTSD Scale (CAPS). Five members of the review team convened to rate the quality of evidence across the primary outcomes. Any disagreements were resolved through discussion. Review authors who were investigators on any of the included trials were not involved in the qualitative or quantitative syntheses.
Main results
We included 12 studies (n = 1837), of which, three compared PCT to a wait‐list/minimal attention (WL/MA) group and 11 compared PCT to TF‐CBT. PCT was more effective than WL/MA in reducing PTSD symptom severity (SMD ‐0.84, 95% CI ‐1.10 to ‐0.59; participants = 290; studies = 3; I² = 0%). We assessed the quality of this evidence as moderate. The results of the non‐inferiority analysis comparing PCT to TF‐CBT did not support PCT non‐inferiority, with the 95% confidence interval surpassing the clinically meaningful cut‐off (MD 6.83, 95% CI 1.90 to 11.76; 6 studies, n = 607; I² = 42%). We assessed this quality of evidence as low. CAPS differences between PCT and TF‐CBT attenuated at 6‐month (MD 1.59, 95% CI ‐0.46 to 3.63; participants = 906; studies = 6; I² = 0%) and 12‐month (MD 1.22, 95% CI ‐2.17 to 4.61; participants = 485; studies = 3; I² = 0%) follow‐up periods. To confirm the direction of the treatment effect using all eligible trials, we also evaluated PTSD SMD differences. These results were consistent with the primary MD outcomes, with meaningful effect size differences between PCT and TF‐CBT at post‐treatment (SMD 0.32, 95% CI 0.08 to 0.56; participants = 1129; studies = 9), but smaller effect size differences at six months (SMD 0.17, 95% CI 0.05 to 0.29; participants = 1339; studies = 9) and 12 months (SMD 0.17, 95% CI 0.03 to 0.31; participants = 728; studies = 5). PCT had approximately 14% lower treatment dropout rates compared to TF‐CBT (RD ‐0.14, 95% CI ‐0.18 to ‐0.10; participants = 1542; studies = 10). We assessed the quality of this evidence as moderate. There was no evidence of meaningful differences on self‐reported PTSD (MD 4.50, 95% CI 3.09 to 5.90; participants = 983; studies = 7) or depression symptoms (MD 1.78, 95% CI ‐0.23 to 3.78; participants = 705; studies = 5) post‐treatment.
Authors' conclusions
Moderate‐quality evidence indicates that PCT is more effective in reducing PTSD severity compared to control conditions. Low quality of evidence did not support PCT as a non‐inferior treatment compared to TF‐CBT on clinician‐rated post‐treatment PTSD severity. The treatment effect differences between PCT and TF‐CBT may attenuate over time. PCT participants drop out of treatment at lower rates relative to TF‐CBT participants. Of note, all of the included studies were primarily designed to test the effectiveness of TF‐CBT which may bias results away from PCT non‐inferiority.The current systematic review provides the most rigorous evaluation to date to determine whether PCT is comparably as effective as TF‐CBT. Findings are generally consistent with current clinical practice guidelines that suggest that PCT may be offered as a treatment for PTSD when TF‐CBT is not available.
Plain language summary
Present‐centered therapy (PCT) for post‐traumatic stress disorder (PTSD) in adults
Review Question
Is present‐centered therapy (PCT) an effective treatment option for adults with post‐traumatic stress disorder (PTSD) as compared to the recommended trauma‐focused cognitive‐behavioral therapies (TF‐CBT)?
Background
PTSD is a psychiatric disorder that can develop in individuals who are exposed to a traumatic event. Although most trauma survivors experience gradual diminishment of symptoms and recover from the trauma exposure, some will go on to develop PTSD and experience persistent symptoms that disrupt biological, psychological, and social functioning.
TF‐CBT is considered one of the most effective treatments for PTSD. Trauma‐focused therapies require patients to think about and/or talk about their prior traumas, which may prevent some patients from accessing or engaging in these treatments. PCT is a non‐trauma based treatment that incorporates common psychotherapeutic components, and which may appeal to patients reluctant to engage in trauma‐focused treatments. Although originally developed to be a treatment comparator in TF‐CBT trials, PCT has performed well in these trials and may be associated with lower treatment dropout rates. If PCT is deemed to be comparably as effective as TF‐CBT and also has lower treatment dropout rates, then it may be a preferred treatment option for those who do not want to participate in trauma‐focused treatments. This systematic review seeks to determine whether PCT is an effective treatment option compared to TF‐CBT for adults with PTSD.
Study Characteristics
This review included 12 studies that comprised a total of 1837 participants. Eleven studies that included 1826 participants contributed to the quantitative syntheses. Participants were all adults, but ranged in demographics and trauma types. All studies recruited participants in the United States and there was a predominance of studies conducted on military veterans.
Key Results
PCT does not appear to be as effective as trauma focused treatments in reducing PTSD severity at post‐treatment. However, PCT is associated with reduced treatment dropout rates compared to TF‐CBT.
Quality of the Evidence
Several of the TF‐CBT trials included in this review were well designed and executed. However, we assessed the overall quality of evidence for our primary outcome (post‐treatment PTSD severity) as low based on inconsistent outcomes and some imprecision in the results. We rated the quality of the evidence on differential treatment dropout as moderate.
Summary of findings
Background
Description of the condition
Post‐traumatic stress disorder (PTSD) is a psychiatric condition that can develop in individuals following exposure to a traumatic event. Common post‐traumatic symptoms include re‐experiencing of the traumatic event (e.g. nightmares, flashbacks), avoidance of people or situations that trigger memories of the traumatic event, negative beliefs and feelings, and hyperarousal symptoms such as difficulty sleeping and hypervigilance (APA 2013). Although most trauma survivors experience a gradual diminishment of symptoms and recover from the traumatic exposure (Morina 2014; Sayed 2015), some individuals develop PTSD and experience persistent post‐trauma symptoms that disrupt biological, psychological, and social functioning (APA 2013).
Over 80% of the general population may experience a traumatic event, with over one out of two of these individuals exposed to multiple traumatic events in their lifetime (Benjet 2016). The World Health Organization (WHO) estimates that lifetime PTSD prevalence rates range from 0.3% in China to 6.1% in New Zealand, although methodological differences limit direct interpretation of differences in prevalence rates between countries (Kessler 2008). Approximately 5% of the general USA population may currently have PTSD, with close to 6% experiencing PTSD at some point in their lifetime (Goldstein 2017). Lifetime PTSD prevalence rates of USA military veterans are consistent with these estimates (Smith 2016). From a societal perspective, mental illness is costly (Whiteford 2013). A 2008 report estimated that the economic impact of PTSD among USA military personnel ranged between USD 4 billion and USD 6 billion over two years (Tanielian 2008).
Description of the intervention
Present‐centered therapy (PCT) was originally developed as a strong comparator treatment that captured many of the effective components of 'good psychotherapy', to test whether trauma‐focused cognitive‐behavioral therapy (TF‐CBT) demonstrated effects beyond nonspecific psychotherapeutic benefits (Schnurr 2001; Schnurr 2005; Schnurr 2007b; Shea 2018). The nonspecific therapeutic components of PCT include the establishment of positive interpersonal connections through the therapeutic relationship(s), normalization of symptoms, validation of experiences, provision of emotional support, and increasing a sense of mastery and self‐confidence in dealing with problems (Schnurr 2005; Schnurr 2007b; Shea 2018). As PCT was developed as a treatment comparator for TF‐CBT, treatment components exclude trauma exposure, cognitive restructuring, or behavioral activation. PCT has elements of supportive therapy, but is a more structured approach that follows a manual and includes the use of a diary to record problems throughout the week. In clinical trials, PCT is typically modified to mirror the active treatment under investigation in terms of length, number of sessions, and modality (group versus individual).
How the intervention might work
The goals of PCT are to improve patients’ insight into their current symptoms, enhance interpersonal connectedness, and promote a greater sense of mastery via use of effective approaches to solving problems. In treatment, patients gain increased insight into how current behaviours are influenced by PTSD symptoms, explore adaptive solutions to these problems, and are encouraged to implement some of these chosen solutions. Through the application and practice of more effective solutions to daily stressors, patients experience enhanced psychosocial functioning and decreased symptoms. Additional mechanisms underlying PCT may rely on the therapeutic benefits that emerge from a caring relationship, including instillation of hope and optimism, shared goal setting, and increased positive self‐regard (Schnurr 2001; Schnurr 2007a; Schnurr 2007b; Shea 2018). As patients learn and practice more adaptive approaches to dealing with problems, they develop a greater sense of mastery over their environment and experience improved functioning and alleviated symptoms (Shea 2018).
Why it is important to do this review
Several psychological therapies to treat PTSD have been developed and tested to include TF‐CBT, non‐trauma‐focused CBT, eye movement desensitization and reprocessing (EMDR), acceptance and commitment therapy (ACT), and psychodynamic psychotherapy. These active treatments have typically been compared to PCT, supportive therapies, or wait‐list control conditions. Several previous systematic reviews have evaluated the effectiveness of the different active PTSD treatments (Bisson 2005; Bisson 2007; Bisson 2013; Lee 2016; Watts 2013). The most recent Cochrane systematic review concluded that TF‐CBT is more effective than other therapies, although TF‐CBT was also associated with higher treatment dropout rates (Bisson 2013). Notably, in this systematic review, PCT was categorized with "other therapies" that included supportive counselling, hypnotherapy, and psychodynamic therapy. However, PCT is distinct from these other therapies, and a growing body of literature suggests that PCT is an effective treatment for patients with PTSD. Several TF‐CBT trials using PCT as the comparator treatment failed to detect any post‐treatment differences on clinician‐rated PTSD symptoms (Foa 2018; Resick 2015; Schnurr 2003). PCT is also associated with lower treatment dropout rates relative to TF‐CBT across several trials (Imel 2013). Patients express high satisfaction and confidence in PCT as an effective PTSD treatment (Schnurr 2007b), and its has been deemed a well‐established treatment with promising research support (APA 2016). These recent findings raise questions on whether PCT, a non‐trauma based treatment, is comparably as effective as TF‐CBT and potentially more acceptable for patients based on lower treatment dropout rates. Although a previous meta‐analysis concluded PCT was as efficacious as TF‐CBT (Frost 2014), the review only included the five trials available at the time and did not apply a strict non‐inferiority framework to compare the treatments (AHRQ 2012). Applying a non‐inferiority analysis in such circumstances is needed as it evaluates whether a new treatment (i.e. PCT), which may have lower treatment dropout rates, is comparably as effective as the standard recommended treatment using established thresholds (AHRQ 2012). To date, no systematic reviews using Cochrane standards have been conducted to explicitly evaluate PCT in comparison to TF‐CBT. This systematic review provides the most rigorous evaluation of PCT to date by applying a non‐inferiority framework to determine whether PCT demonstrates comparable effectiveness to TF‐CBT and lower treatment dropout rates.
Objectives
To assess the effects of PCT for adults with PTSD. Specifically, we sought to determine whether PCT (1) is more effective in alleviating symptoms relative to control conditions (i.e. wait list, standard care, or other minimal attention groups); (2) results in similar reduction of PTSD severity as compared to TF‐CBT as based on clinician‐rated PTSD symptoms; and (3) is associated with lower treatment dropout rates when compared to TF‐CBT.
Methods
Criteria for considering studies for this review
Types of studies
We included any RCTs that evaluated PCT compared to TF‐CBT or a control condition. We did not use setting, sample size, or publication status to determine study inclusion.
Types of participants
Participant characteristics
This review included trials with a study population consisting of adults of any gender, aged 18 years and over.
Diagnosis
Any individual diagnosed with PTSD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) (APA 2000) or Fifth Edition (DSM‐V) (APA 2013), or the International Classification of Diseases, Tenth Edition (ICD‐10) (WHO 1992), as determined by a structured interview or clinician diagnosis. At least 70% of participants were required to have a PTSD diagnosis. A minimum of one month must have passed since the trauma occurred. We applied no restrictions based on severity of PTSD symptoms or type of traumatic event.
Comorbidities
We applied no restrictions based on the absence or presence of comorbid conditions, although PTSD was required to be the primary diagnosis.
Setting
We applied no restrictions based on study setting.
Types of interventions
Experimental intervention
PCT (see previous description): Present‐centered therapy is a non‐trauma focused, time‐limited treatment for adults with PTSD. Nonspecific therapeutic factors, such as therapist support, are considered part of the treatment. Introductory sessions involve education about PTSD; later sessions involve discussion of daily difficulties and assistance for patients in managing current symptoms through the acquisition of effective coping strategies. We did not include interventions with an active exposure component, or any that emphasized cognitive restructuring. We included therapies given in both group and individual settings. As PCT is typically modified to mirror the active treatment under investigation, we did not limit the number and length of sessions of PCT. When review authors had serious doubts about whether a trial treatment qualified as PCT, we attempted to obtain the treatment manual. In these cases, treatment protocols were reviewed by two authors (BB, EB) to determine whether main features of the treatment were consistent with PCT. A third expert in PCT (TS) reviewed the ratings and made a final decision about whether the treatment under investigation should be categorized as PCT.
Comparator interventions
Control conditions: wait list, standard care, minimal attention, repeated assessment, or other minimal attention groups
Trauma‐focused CBT: refers to a category of evidence‐based psychological treatments for PTSD that incorporate CBT techniques as a primary component, trauma exposure and/or trauma processing, and psychoeducation.
Types of outcome measures
We included studies that met the above inclusion criteria, regardless of whether they reported on the following outcomes.
Primary outcomes
-
Efficacy outcomes
Reduced severity of PTSD symptoms as determined by a clinician‐administered standardized measure (e.g. the Clinician Administered PTSD Symptom Scale (CAPS; Weathers 2001)).
-
Non‐inferiority outcomes: the goal of non‐inferiority research is to determine whether a new treatment has comparable efficacy to an existing treatment, such that the new treatment results in differences that are no worse than a prespecified margin. Conclusions of non‐inferiority are determined based on whether the confidence interval (CI) exceeds this prespecified minimally important difference (MID).
Reduced PTSD severity as assessed by the CAPS, with the 95% CI excluding the MID value. We also calculated standardized mean differences to include studies that did not use the CAPS to confirm the direction of the effect and provide an estimate of the effect size. Based on existing guidelines (Berliner 2019), any effect size < 0.2 was considered not clinically meaningful.
-
Adverse events outcomes
Rates of dropout at post‐treatment for any reason
Secondary outcomes
Reduced severity of PTSD symptoms as determined by a standardized self‐report measure (e.g., the PTSD Checklist (Weathers 1993), the Post‐traumatic Diagnostic Scale (Foa 1995))
Loss of PTSD diagnosis
Reduced severity of depression symptoms as determined by a standardized self‐report measure (e.g., the Beck Depression Inventory (Beck 1961), the Quick Inventory of Depressive Symptomatology (Rush 2003))
Reduced severity of anxiety symptoms as determined by a standardized self‐report measure (e.g. the Spielberger State Trait Anxiety Inventory (Spielberger 1983))
Reduced severity of dissociative symptoms as determined by a standardized self‐report measure (e.g. the Dissociative Experiences Scale (Bernstein 1986))
Timing of outcome assessment
Meta‐analyses took into account the timing of outcome assessments, using data from completion of the intervention, shorter‐term follow‐up (one to six months), and long‐term follow‐up (longer than six months). All data in the shorter‐term follow‐up ended up being between five to seven months, and all studies with longer‐term assessments were at 12 months. Therefore, we decided to focus on post‐treatment outcomes as our primary time point, and to evaluate shorter‐term follow‐up that was five to seven months post‐treatment (labelled as six‐month post‐treatment follow‐up, for convenience), and longer‐term follow‐up (labelled as 12‐month follow‐up, for accuracy).
Hierarchy of outcome measures
When several measures were included for a single outcome, we selected measures in the order laid out for each outcome, as above, and any other validated scales after those. We prioritised clinician‐administered scales over self‐reported scales.
Search methods for identification of studies
Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders Group retains a specialized register of RCTs ‐ the CCMD‐CTR. This Register contains over 40,000 reference records (reports of RCTs) for anxiety and depressive disorders, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to about 12,500 individually PICO‐coded study records. Reports of trials for inclusion in the Register are collated after (weekly) generic searches of MEDLINE (1950‐), Embase (1974‐), and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, key journals (upon handsearching), conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group website; an example of the core MEDLINE search is displayed in Appendix 1. The register is current to June 2016 only.
Electronic searches
CCMD's Information Specialist searched the CCMD‐CTR to 1 June 2016, as follows:
Cross‐search of the studies and reference registers using the following terms to identify relevant reports on RCTs: (present centred or present centered or present focused or present focussed).
As the CCMDCTR was only current to June 2016, CCMD's Information Specialist ran additional searches on the following databases in February 2018 and February 2019 (Appendix 2):
Ovid MEDLINE (1946 to 15 February 2019);
Ovid Embase (1974 to 2019 Week 07);
Ovid PsycINFO (1806 to February Week 1 2019);
Cochrane Central Register of Controlled Trials (CENTRAL) (all years to Issue 2, February 2019);
WHO ICTRP (all years to 15 February 2019);
Clinicaltrials.gov (all years to 15 February 2019);
ProQuest PTSDpubs (all years to 15 February 2019);
ProQuest Dissertations & Theses Global (all years to 15 February 2019).
Two review authors screened all references to check for eligibility. When appropriate, we tagged reports of the same trial together to ensure that no trial was counted twice.
We applied no restrictions based on date, language, or publication status to the searches.
We also searched international trial registries via the trials portal of the World Health Organization (ICTRP) and ClinicalTrials.gov, to identify unpublished and ongoing studies.
We searched reference lists of included studies for additional relevant studies and screened other systematic reviews of psychological interventions for PTSD to identify additional studies not retrieved by our search.
Searching other resources
Grey literature
We searched the grey literature for dissertations and theses, clinical guidelines, and regulatory agency reports (when appropriate), using the following sources.
Digital Access to Research Theses (DART)‐Europe E‐theses Portal (http://www.dart‐europe.eu/).
Electronic Theses Online System (EThOS) ‐ service of the British Libraries (http://ethos.bl.uk/).
Open Access Theses and Dissertations (https://oatd.org).
National Guideline Clearing House (http://guideline.gov/).
Open Grey (http://www.opengrey.eu/).
Correspondence
We contacted trialists and subject experts for information on unpublished and ongoing studies, and to request additional trial data.
Data collection and analysis
Selection of studies
Two review authors (BB, EB) independently screened titles and abstracts for potential inclusion of all studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved full‐text study reports/publications, and two review authors (BB, EB) independently screened full texts to identify studies for inclusion, and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author (DE). We identified and excluded duplicate records and collated multiple reports that related to the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form that was piloted on at least one study in the review to extract study characteristics and outcome data. Two review authors (BB, EB) extracted the following study characteristics and outcome data from included studies.
Methods: study design, study duration, study setting, recruitment, number of study centers and locations, withdrawals, and dates of study.
Participants: N, mean age, age range, gender, severity of condition, trauma type, duration of time since trauma, comorbid conditions, diagnostic criteria, inclusion criteria, and exclusion criteria.
Interventions: interventions and comparisons.
Outcomes: primary and secondary outcomes specified and collected, method of collection, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by consultation with a third review author (DE). One review author (EB) transferred data into the Review Manager (RevMan 2014) file. A second review author (BB) double‐checked that data were entered correctly by comparing data presented in the systematic review with data provided in the study reports.
Main planned comparisons
PCT versus control conditions (standard care, wait list, minimal attention, or repeated assessment)
PCT versus TF‐CBT
Assessment of risk of bias in included studies
Two review authors (BB, EB) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (DS). We assessed risk of bias according to the following domains. If information was not reported in the trial publications, then authors were contacted and this information was requested.
Random sequence generation: describes the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We considered this domain to be at 'low' risk of bias if investigators described a process by which each participant had an equal chance of being randomized to each group; at 'high' risk of bias if investigators describe a non‐random component in the sequence generation process; and at 'unclear' risk of bias if information was insufficient for a judgment of high or low risk of bias.
Allocation concealment: describes the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. We considered this domain to be at 'low' risk of bias if there was no chance of investigators foreseeing participant assignment; at 'high' risk of bias if investigators could possibly foresee assignments, such as allocation based on alternation or rotation, or an open random allocation schedule (e.g. a list of random numbers); and at 'unclear' risk of bias if information was insufficient for a judgment of high or low risk of bias.
Blinding of participants and personnel: describes all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. When administering psychological interventions, it is not feasible to blind participants or personnel administering the intervention.
Blinding of outcome assessment: describes all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We considered lack of blinding separately for patient‐reported and clinician‐rated outcomes. We considered this domain to be at 'low' risk of bias for an outcome if outcome assessors were blinded; at 'high' risk of bias for an outcome if outcome assessors were not blinded; and at 'unclear' risk of bias for an outcome when information was insufficient for a judgment of high or low risk of bias. For self‐report measures, we rated all outcomes as 'high'.
Incomplete outcome data: describes the completeness of outcome data for each main outcome, including attrition and exclusions from analysis. We considered this domain to be at 'low' risk of bias for an outcome if no data were missing, or if data were imputed appropriately; at 'high' risk of bias for an outcome if missing data were likely related to true outcomes, if analyses considered only the data of treatment completers, or if missing data were imputed inappropriately; and at 'unclear' risk of bias for an outcome when information was insufficient for a judgment of high or low risk of bias. In sum, we considered the effect of incomplete outcome data separately for each outcome and took into account whether reasons for missing data were acceptable, whether trial authors conducted an intention‐to‐treat (ITT) analysis, and the potential impact of missing data on the particular outcome.
Selective outcome reporting: describes how the possibility of selective outcome reporting was examined by the review authors, and what they found. We considered this domain to be at 'low' risk of bias if the study protocol was available and all prespecified outcomes were reported, or if a study protocol was not available but it is clear that published reports included all expected outcomes; at 'high' risk of bias if not all of the study's prespecified outcomes were reported, if they were reported incompletely, or if primary outcomes were not prespecified or outcomes of interest were reported incompletely; and at 'unclear' risk of bias if information was insufficient for a judgment of high or low risk of bias.
Other bias: describes any important concerns about bias not addressed by the other domains in the tool but arising during our assessment process. We judged other risks of bias as 'low' or 'high' based on their threats to validity. We considered this domain to be at 'unclear' risk of bias if information was insufficient for a judgment of high or low risk of bias.
Measures of treatment effect
Continuous data
PCT vs control conditions:
We calculated SMDs and the 95% confidence intervals to combine information across studies. The SMDs were calculated using the baseline standard deviation in each study consistent with recommendations of Feingold (Feingold 2009).
PCT vs TF‐CBT non‐inferiority analysis:
We used CAPS unstandardized MD and 95% confidence intervals (CIs) as the target measure from each study. Regression coefficients from longitudinal models for change or direct comparisons of the amount of change between the study groups were extracted, when reported. For studies that only provided data on pre and post‐treatment means, we calculated a difference score and used a correlation estimate of 0.50 to calculate a standard error for the difference score. Meta‐analysis was measure‐specific to retain the unstandardized scale of each target measure. Measures of summary differences and associated 95% confidence intervals were compared with the minimal important difference (MID) for each outcome based on the following anchors, which indicate clinically important changes: ≥ 10‐point MD on the Clinician‐Administered PTSD Scale (CAPS) (Schnurr 2001); ≥ 10‐point MD on the PTSD Checklist (PCL) (Monson 2008); and ≥ 5‐point MD on the Beck Depression Inventory (BDI) (Beck 1993). To conclude that PCT resulted in symptom reductions no worse than those observed for the TF‐CBT groups, the 95% CI had to exclude the MID value for that particular outcome. To incorporate studies that did not use the CAPS, we also calculated SMD and 95% confidence intervals to combine information across studies that used different measurement instruments to assess the overall direction of any association. An effect size of > 0.2 was considered a clinically important difference as based on existing guidelines (Berliner 2019). The SMDs were calculated using the baseline standard deviation in each study consistent with recommendations of Feingold (Feingold 2009).
Dichotomous data
We analyzed dichotomous data as both the absolute difference and the risk ratio in terms of the proportion experiencing the outcome of interest between two treatment groups. We calculated 95% CIs for both measures.
Unit of analysis issues
Cluster‐randomized trials
Application of meta‐analysis is conventionally based on the assumption that the primary unit of randomization is the individual study participant. However, many clinical RCTs have primarily randomized intact social units of individuals to intervention groups. If clustering was incorporated in some of the studies in this review, we planned to adjust for the clustering effect by dividing clusters by a 'design effect.' Operationally, the design effect in cluster‐randomized trials is the ratio of the variance estimate with clustering to the variance estimate derived from simple random sampling (Kish 1995). In the proposed research, we planned to calculate the design effect (DE) by using a standard formula for cluster sampling (Kish 1995). The equation is DE = 1 + (n ‐ 1) × ICC, where n is the mean number of participants per cluster and ICC is the intraclass correlation coefficient. If the ICC was not reported, we planned to borrow an estimate from a similar study. After taking design effects into account for cluster‐randomized trials, we planned to derive the summary effect size estimate by using the weighted average approach, with weight for each study operationally defined as the inverse variance of the effect size estimator for that study (Borenstein 2009).
Studies with multiple treatment groups
For trials with three or more arms, we planned to first consider conducting pairwise meta‐analysis, with each pair of arms serving as a preliminary analysis. For dichotomous data, we planned to also compare differences in multiple proportions by using a Chi2 approach, as proposed by Cohen (Cohen 1977). After careful examination through these preliminary analyses, we planned to consider combining data from arms with similar intervention effects.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). We documented all correspondence with trialists and reported which trialists responded.
We did not use data from an outcome measure when more than 50% of data were missing. For continuous outcomes, we calculated missing standard deviations from other available data, such as confidence intervals, standard errors, and P, T, or F values. As we used only summary measures for the analysis, we assumed that missing data for each study were randomly distributed and thus did not influence the quality of estimates.
Assessment of heterogeneity
Studies brought together in a systematic review will inevitably present various types of heterogeneity, conventionally classified as clinical, methodological, and statistical heterogeneity in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In assessing clinical heterogeneity, we closely examined differences between factors associated with intervention or participant characteristics across studies included in the meta‐analysis. When inspecting methodological heterogeneity, we identified differences between methodological factors across studies that could result in substantial diversity in outcome measurements. We paid particular attention to whether outcome variables were defined in the same fashion; whether they were measured by the same quantity and scale; and whether, if differences did exist, methodological diversity affected the quality of the summary effect size estimate. When clinical and methodological heterogeneity does occur in meta‐analysis, statistical heterogeneity becomes inevitable. We measured the degree of inconsistency in findings across studies by using the I2 statistic (Higgins 2003). Specifically, the I2 statistic indicates the percentage of observed variation that is attributed to true differences across studies; accordingly, we interpreted the I2 score by adhering to the following criteria, as proposed in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
For all its advantages and strengths, meta‐analysis is a rough statistical approach used to estimate a summary effect size, and it is subject to several limitations. Perhaps most notably, much of the meta‐analysis literature relies on results from publications and other accessible sources, resulting in strong selection bias in a weighted average. In the fields of medicine and psychology, study results displaying negative results or insignificant findings are much less likely to be accepted by scientific journals for publication. As many study results failing to show significance presumably still lie in researchers' file drawers, this publication bias is often referred to as the 'file drawer' problem (Rosenthal 1979). To date, no sufficiently satisfactory solution has been found for correcting this type of bias. If the studies included in a meta‐analysis are not randomly selected, which we believe is generally the case, the distribution of effect sizes tends to be skewed, resulting in a biased weighted average of effect sizes.
In our analyses, we viewed the summary effect size estimate from meta‐analysis as representing the statistic for a sample of studies that tends to be skewed. Statistically, the distribution of effect size estimates for selected studies will likely be truncated, leading to non‐normally distributed data. Because studies not accessible for meta‐analysis are usually those with low or even reversed effect sizes, the summary effect size from meta‐analysis tends to be overestimated. We prepared a funnel plot and examined it for asymmetry.
Data synthesis
We applied the random‐effects meta‐analysis to incorporate heterogeneity across studies. Through this statistical approach, the summary effect size estimate measures the mean of systematically different effects in different studies, while its confidence interval describes uncertainty in the estimate. We calculated between‐study variance to measure such uncertainty; its square root was the estimated standard deviation of study‐specific effects from which the 95% confidence interval can be readily derived.
Subgroup analysis and investigation of heterogeneity
To investigate heterogenous results and to evaluate whether PCT had different effects based on treatment modality and type we carried out the following subgroup analysis for the primary outcomes. (1) As group and individual TF‐CBT tend to have differential effects, we were interested to see whether PCT performed differently across these modalities. (2) Given that PE and CPT are two of the most common TF‐CBT treatments used, we also explored whether PCT had differential effects based on these specific trauma treatments.
Treatment modality (individual or group). PCT is administered in both individual and group formats; this variable may affect heterogeneity and treatment outcomes.
Treatment type (PE or CPT). PCT may have differential efficacy based on the comparator treatment.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes, focusing on those trials with lowest risk of bias as based on the following criteria: outcome masking, appropriate handling of missing data (ITT; mixed‐model analysis), adequate power, and low levels (< 40%) of post‐randomization treatment loss.
'Summary of findings' table
We used the GRADE approach to summarize and interpret findings, and we used the GRADE profiler to import data from RevMan 2014 to create 'Summary of findings' tables. We assessed the quality of evidence by examining the following.
Limitations in study design and implementation.
Indirectness of evidence.
Unexplained heterogeneity or inconsistency of results.
Imprecision of effect estimates.
Potential publication bias.
For each outcome, we graded the quality of evidence according to the following categories.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
We downgraded evidence from 'high quality' by one level for serious study limitations, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias, and by two levels for 'very serious' concerns. We included the primary outcomes of PTSD severity and dropout rates.
Results
Description of studies
See the Characteristics of included studies and the Characteristics of excluded studies tables.
Results of the search
The search was originally conducted in February 2018 and was run again in February 2019. The search identified 850 records via electronic database searches and 27 additional records through complementary searches of the grey literature, and backwards and forwards citation chasing of included studies and relevant systematic reviews. After removing duplicates, we screened 416 titles and abstracts, excluding 340 records. Two studies (three references) are still awaiting full‐text review as they were completed but not yet published and no data were posted on clinical trial registries or could be obtained from the authors (Characteristics of studies awaiting classification). We identified one ongoing study (Characteristics of ongoing studies). We reviewed 72 full‐text reports and included 35 references, representing 12 unique studies (Figure 1).
1.
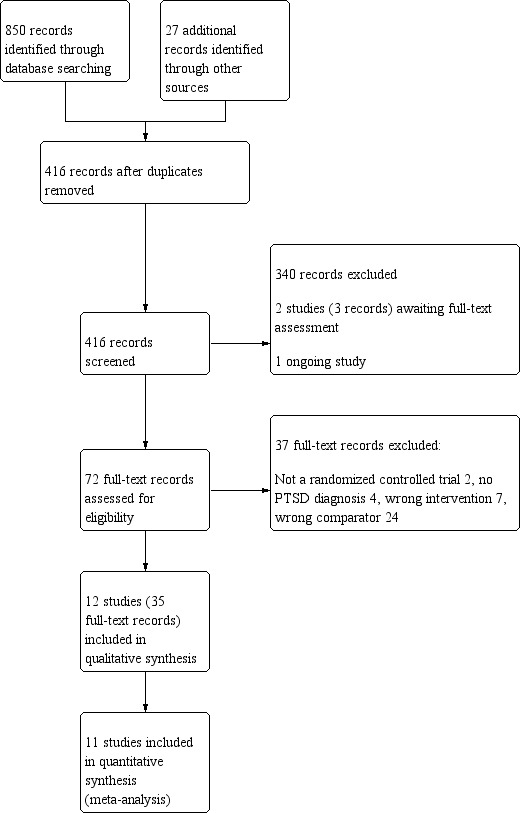
Study flow diagram.
We contacted authors of the following studies to obtain clarification about study eligibility: Classen 2011; Ford 2011; NCT00607815; Ready 2010; Ready 2018; Schnurr 2003; Schnurr 2007; Suris 2013. All authors provided the requested information.
Included studies
Design
All studies were randomized controlled trials with a parallel group design.
Sample size
The 12 studies randomized a total of 1837 participants. Two of the studies were small, with fewer than 50 participants recruited (Rauch 2015; Ready 2010). Three of the studies were large, with greater than 200 participants recruited (Foa 2018; Schnurr 2003; Schnurr 2007).
Setting
All studies were conducted in outpatient mental health settings in the USA.
Participants
All studies recruited adult participants. Five of the studies included only male participants (NCT00607815; Ready 2010; Ready 2018; Schnurr 2003; Sloan 2018), and three of the studies included only female participants (Ford 2011; McDonagh 2005; Schnurr 2007). The study population in seven of the studies consisted of veterans only (NCT00607815; Rauch 2015; Ready 2010; Ready 2018; Schnurr 2003; Sloan 2018; Suris 2013), while one study included only active duty USA Army soldiers (Resick 2015), and two studies included both veterans and active duty military (Foa 2018; Schnurr 2007). The remaining study populations were mothers or primary caregivers of young children (Ford 2011) and women who experienced childhood sexual abuse (McDonagh 2005). All studies used a structured clinical interview based on the DSM‐IV or DSM‐V to confirm PTSD diagnosis, and all but one study required that participants meet criteria for full PTSD. One study included participants with 'partial PTSD' (Ford 2011). In this study, at least 70% of participants met DSM‐IV criteria for PTSD, as specified in our inclusion criteria.
Interventions
In the majority of included studies, PCT was based on the original manual used in Schnurr 2003 for group present‐centered treatment (GPCT) and Schnurr 2007 for individual PCT. Two studies used a different version of PCT (Ford 2011; McDonagh 2005) that was deemed to be consistent with the original PCT manuals. Eight studies conducted PCT in an individual format (Foa 2018; Ford 2011; McDonagh 2005; NCT00607815; Rauch 2015; Ready 2010; Schnurr 2007; Suris 2013), and the remaining four studies conducted PCT in a group format (Ready 2018; Resick 2015; Schnurr 2003; Sloan 2018). The number of PCT sessions for all of the TF‐CBT trials was between 10 and 14, except for two trials in which there were approximately 30 sessions (Ready 2018; Schnurr 2003).
Comparisons
Present‐centered therapy was compared to a control condition (wait list or minimal contact) in three studies (Foa 2018; Ford 2011; McDonagh 2005); PCT was compared to a TF‐CBT in eleven studies. Trauma‐focused treatments included CPT (NCT00607815; Resick 2015; Suris 2013), prolonged exposure (Foa 2018; Rauch 2015; Schnurr 2007), group‐based exposure therapy (Ready 2018), virtual reality exposure therapy (Ready 2010), cognitive behavioral therapy (McDonagh 2005), group cognitive behavioral therapy (Sloan 2018), and trauma‐focused group therapy (Schnurr 2003). One study comparing PCT to a control condition included an additional treatment arm, "trauma affect regulation: guide for education and therapy" (TARGET; Ford 2011). This treatment arm was not included in any analyses because it was not trauma‐focused (Ford 2011).
Outcomes
Primary outcomes were reduction in severity of clinician‐rated PTSD symptoms and treatment dropout rates. Two studies used the CAPS to compare PCT to a wait‐list/minimal attention group (Ford 2011; McDonagh 2005) and one trial used the Posttraumatic Symptom Scale‐Interview (PSS‐I; Foa 2018). Six studies used the CAPS to compare post‐treatment PTSD scores between PCT and TF‐CBT groups (NCT00607815; McDonagh 2005; Rauch 2015; Ready 2018; Schnurr 2007; Suris 2013). Two trials used the PSS‐I to compare post‐treatment PTSD scores (Foa 2018; Resick 2015). One trial used the CAPS‐5 to compare post‐treatment PTSD scores (Sloan 2018). Definition of dropout varied across trials (see Table 3).
1. Treatment dropout definitions across TF‐CBT trials.
| Trial | Dropout Definition |
| Chard 2018 | Dropout numbers were obtained from results provided on the study’s clinicaltrials.gov trial registration, which includes the number of participants who started the treatment, completed the treatment, and did not complete the treatment for each group. We considered participants who did not complete the treatment to be dropouts. |
| Foa 2018 | Manuscript provided the number of participants that did and did not receive the 'full intervention' in each group, with reasons provided. 'Full intervention' was not explicitly defined. We considered participants who did not receive the 'full intervention' to be dropouts. |
| Ford 2011 | Dropout rates were provided based on the following definition in the manuscript: “…stringent criterion of attending fewer than half of the 12 treatment sessions and not completing a posttherapy or follow‐up assessment.” |
| McDonagh 2005 | Definition of dropout was not explicitly defined in the manuscript, but appeared to be defined as participants who did not complete treatment, based on the description of the dropout analysis. |
| Rauch 2015 | The manuscript defined treatment completers as those who received at least seven sessions and a mid‐ or post‐treatment assessment. To obtain dropout numbers, we subtracted the number provided for treatment completers from the number randomized for each group. |
| Ready 2010 | The manuscript stated that two participants did not complete treatment, with reasons, but did not provide an explicit definition. We considered those participants described as not completing the treatment to be dropouts. |
| Ready 2018 | The manuscript included the number of dropouts during treatment, but did not provide an explicit definition. |
| Resick 2015 | The manuscript included the number of participants who completed the intervention, for each treatment group, with reasons, but did not provide an explicit definition. To obtain dropout numbers, we subtracted the number provided for treatment completers from the number randomized for each group. |
| Schnurr 2003 | The manuscript provided the number of participants who dropped out of either active treatment or booster sessions. |
| Schnurr 2007 | The manuscript provided the numbers of participants who completed treatment, received some treatment, and did not receive any treatment. We considered participants who did not complete the treatment to be dropouts. |
| Sloan 2018 | Treatment completers were defined as participants who completed at least ten treatment sessions. To obtain dropout numbers, we subtracted the number provided for treatment completers from the number randomized for each group. |
| Suris 2013 | The manuscript provided the number of participants who did and did not complete treatment in each group. Treatment completers were defined as those completing all 12 sessions of therapy. We considered participants who did not complete the treatment to be dropouts. |
Excluded studies
We excluded 37 records representing 24 studies for reasons listed in the Characteristics of excluded studies table. Reasons for exclusion were: study was not an RCT (Grant 2005; Resick 2009); participants did not meet criteria for PTSD (Classen 2011; Hong 2013; NCT03760731; Rosner 2018); study did not meet PCT intervention criteria (Classen 2001; Foa 1991; NCT00607412; NCT01274741; NCT02081417); and PCT was not compared to a TF‐CBT or control condition (Bormann 2018; Bremner 2017; Davis 2019; Harris 2018; Haynes 2012; King 2016; Lang 2017; NCT02233517; NCT02398227; NCT03056157; NCT03429166; NCT03764033; Polusny 2015). Specifically, two trials were excluded (Classen 2011; Foa 1991) because the manual was deemed to be inconsistent with PCT as defined for this systematic review. The PCT in the Classen 2011 trial appeared to place a greater emphasis on group processes and cognitive restructuring, whereas the supportive care intervention in the Foa 1991 trial lacked the structure and active components of PCT.
Two studies are awaiting classification (Characteristics of studies awaiting classification) and one is ongoing (Characteristics of ongoing studies); these will be added to the update of this review, as appropriate.
Risk of bias in included studies
Details of the risk of bias for included studies are available in the Characteristics of included studies table, and a graphical representation of the risk of bias ratings for each domain across the included studies is available in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed sequence generation and allocation concealment separately. When the study report did not provide adequate information for a judgment, we contacted the study authors. Additional information about sequence generation and/or allocation concealment was requested by study authors for seven studies (McDonagh 2005; NCT00607815; Rauch 2015; Ready 2010; Ready 2018; Resick 2015; Schnurr 2003), and adequate information was provided by study authors for six studies (NCT00607815; Rauch 2015; Ready 2010; Ready 2018; Resick 2015; Schnurr 2003). Ten studies reported an appropriate method of sequence generation and were judged to be at low risk of bias (Foa 2018; Ford 2011; NCT00607815; Rauch 2015; Ready 2010; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013). We judged sequence generation to be at high risk of bias in two studies (McDonagh 2005; Ready 2018). Ten studies reported adequate allocation concealment, and were judged to be at low risk of bias (Foa 2018; Ford 2011; NCT00607815; Rauch 2015; Ready 2010; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013). Allocation was determined to be absent or inadequate in one study (Ready 2018). The remaining study did not report any methods of allocation concealment, and was judged to be at unclear risk of bias (McDonagh 2005).
Blinding
Blinding of participants and personnel is not feasible in studies of psychological interventions, and all included studies were judged to be at high risk of bias for this domain. We judged blinding of outcome assessors separately for patient‐reported and observer‐rated symptoms, since participants were aware of group assignment in all trials. All studies were judged to be at high risk of bias for blinding of outcome assessors for patient‐reported symptoms. For clinician‐rated symptoms, one trial reported that, due to technical difficulties, assessors were not blind to condition, and was judged to be at high risk of bias (Ford 2011). The remaining studies were judged to be at low risk of bias for blinding of outcome assessors for observer‐rated symptoms.
Incomplete outcome data
Analyses were conducted on treatment completers only in two studies (Rauch 2015; Ready 2010) or excluded a large proportion of randomized patients from the analyses in one study (Suris 2013). These studies were judged to be at high risk of bias for incomplete outcome data. One study was judged to be at unclear risk of bias because it was unpublished and study authors did not respond to requests to confirm the numbers provided in clinicaltrials.gov (NCT00607815). Several studies were deemed as unclear risk of bias due to differential treatment dropout rates between comparison arms (Foa 2018; McDonagh 2005; Schnurr 2007; Sloan 2018). The remainder of the studies were judged to be low risk of bias (Ford 2011; Ready 2018; Resick 2015; Schnurr 2003).
Selective reporting
Two studies were judged to be at high risk of bias for selective reporting because their clinical trial registrations included outcome measures that were not reported in study publications (Ford 2011; Ready 2010). One study was judged to be at unclear risk of bias for selective reporting because no protocol was available, and the study publication did not include a self‐report measure of PTSD (McDonagh 2005). The remaining studies either reported on all of the outcomes specified in their protocols or clinical trial registrations (Foa 2018; NCT00607815; Rauch 2015; Ready 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018), or, for those studies without protocols or clinical trial registrations, reported on all outcomes expected to be included in RCTs of adults with PTSD (Suris 2013), and were judged to be at low risk of bias for selective reporting.
Other potential sources of bias
Studies that included investigators who developed the experimental treatment under investigation (Foa 2018; Ford 2011; NCT00607815; Rauch 2015; Ready 2010; Ready 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013) were considered at unclear risk of bias, given potential concerns with allegiance to the treatment under study. We had additional concerns about potential bias in one study due to the possibility of significant differences between groups at baseline (Ready 2010).
Effects of interventions
Summary of findings for the main comparison. Present‐centered therapy compared to control conditions for post‐traumatic stress disorder (PTSD) in adults.
| Present‐centered therapy compared to control conditions for post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: post‐traumatic stress disorder (PTSD) in adults Setting: Intervention: present‐centered therapy Comparison: control conditions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control conditions | Risk with present‐centered therapy | |||||
| PTSD severity (post‐treatment) ‐ standardized difference | SMD 0.84 SD lower (1.1 lower to 0.59 lower) | ‐ | 290 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | This corresponds to a clinically meaningful effect as based on current guidelines (Berliner 2019). | |
| Dropout | Study population | RR 1.30 (0.51 to 3.29) | 290 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | ||
| 120 per 1,000 | 156 per 1,000 (61 to 396) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 2 trials were judged to pose a higher risk of bias.
2 Dropout defined differently across trials
3 OIS was not met for the event of interest across studies (total sample of 2876 needed based on a RR of 1.30 to indicate a meaningful difference).
Summary of findings 2. Present‐centered therapy compared to trauma‐focused cognitive behavioral therapy for post‐traumatic stress disorder (PTSD) in adults.
| Present‐centered therapy compared to trauma‐focused cognitive behavioral therapy for post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: post‐traumatic stress disorder (PTSD) in adults Setting: Intervention: present‐centered therapy Comparison: trauma‐focused cognitive behavioral therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with trauma‐focused cognitive behavioral therapy | Risk with present‐centered therapy | |||||
| CAPS PTSD severity (post‐treatment) ‐ mean difference | Median post‐treatment CAPS = 53 (range: 30 to 72) | MD 6.83 higher (1.9 higher to 11.76 higher) | ‐ | 607 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | |
| PTSD severity (post‐treatment) ‐ standardized difference | SMD 0.32 SD higher (0.08 higher to 0.56 higher) | ‐ | 1129 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 | This corresponds to a clinically meaningful effect as based on current guidelines (Berliner 2019). | |
| Treatment dropout | Study population | RR 0.58 (0.49 to 0.69) | 1542 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ||
| 341 per 1,000 | 198 per 1,000 (167 to 235) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Statistical heterogeneity was moderate to high (I2 = 42% and 69%, respectively). Point estimates varied across meaningful thresholds as defined in the methods section.
2 Confidence interval overlapped meaningful difference as defined in the methods section.
3 3 trials used completer analysis only; raised concerns given differential dropout between groups.
5 Dropout defined differently across trials
All comparisons and outcomes are reported below. Eleven studies including 1826 participants contributed to these comparisons. One trial was not included in any analyses because it was the only trial to include an alternate treatment modality (virtual reality) and was deemed too clinically heterogeneous (Ready 2010). Results were reported for all available outcome measures specified in the methodology.
Comparison 1. PCT versus wait list/minimal attention, superiority analyses
Three studies included three trial arms consisting of the experimental PTSD intervention under study, PCT (placebo treatment), and a wait list/minimal attention (WL/MA) comparison group (Foa 2018; Ford 2011; McDonagh 2005). We used these three studies to compare PCT to WL/MA. For all of these comparisons, data were only available at post‐treatment.
Primary Outcomes
1.1 Clinician‐rated PTSD severity (post‐treatment)
Two studies used the CAPS (Ford 2011; McDonagh 2005) and one study used the PSS‐I (Foa 2018) to compare PCT to a WL/MA group on post‐treatment PTSD severity. Meta‐analysis on PTSD SMD indicated that PCT had a greater reduction in PTSD severity at post‐treatment compared to the WL/MA group (SMD ‐0.84, 95% CI ‐1.10 to ‐0.59; participants = 290; studies = 3; I² = 0%; Analysis 1.1; Figure 4).
1.1. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 1 Clinician‐administered PTSD, standardized difference.
4.
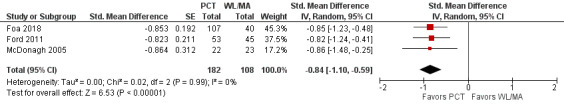
Forest plot of comparison: PCT vs WL/MA
Outcome: Clinician‐administered PTSD severity, post‐treatment ‐ Standardized Mean Difference
1.2. Treatment Dropout
Three studies comparing PCT to a WL/MA condition recorded whether individuals left the study early for any reason. No differences were detected in treatment dropout rates between PCT and the WL/MA groups (RD 0.07, 95% CI ‐0.02 to 0.16; RR 1.30, 95% CI 0.51 to 3.29; participants = 290; studies = 3; I2 = 33%; Analysis 1.3; Figure 5; RR 1.30, 95% CI 0.51 to 3.29; Analysis 1.2).
1.3. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 3 Dropout, post‐treatment ‐ Risk Difference.
5.
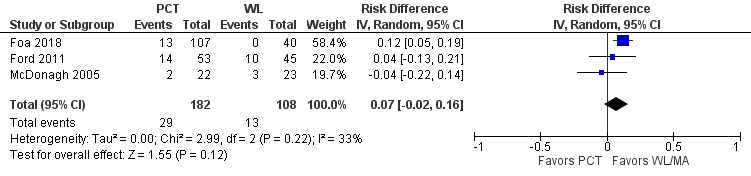
Forest plot of comparison: PCT vs WL/MA
Outcome: Treatment dropout ‐ Risk Difference
1.2. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 2 Dropout, post‐treatment ‐ Risk Ratio.
Secondary outcomes
1.3 Self‐reported PTSD symptoms (post‐treatment)
Only one study used a self‐report PTSD measure (PCL) to compare PCT to WL/MA at post‐treatment (Foa 2018). Evidence from this study indicated that PCT was more effective than WL/MA in reducing post‐treatment PTSD symptoms (MD ‐7.52, 95% CI ‐10.99 to ‐4.05; Analysis 1.4).
1.4. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 4 PTSD Checklist, post‐treatment.
1.4 Loss of PTSD diagnosis (post‐treatment)
Three studies contributed to this comparison (Foa 2018; Ford 2011; McDonagh 2005). Loss of PTSD diagnosis rates were higher in PCT compared to the WL/MA group (RD ‐0.23, 95% CI ‐0.33 to ‐0.12; Analysis 1.6; Figure 6; RR 0.45, 95% CI 0.30 to 0.67; participants = 290; studies = 3; Analysis 1.5).
1.6. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 6 Loss of PTSD diagnosis, post‐treatment ‐ Risk Difference.
6.
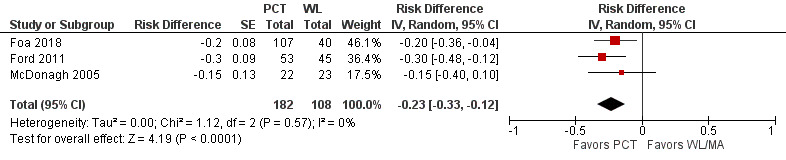
Forest plot of comparison: PCT vs WL/MA
Outcome: Loss of PTSD diagnosis, post‐treatment ‐ Risk Difference
1.5. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 5 Loss of PTSD diagnosis, post‐treatment ‐ Risk Ratio.
1.5 Self‐reported depression symptoms (post‐treatment)
Two studies with a total of 143 participants used a self‐report depression measure (BDI) to compare post‐treatment depression symptoms between PCT and the WL/MA condition (Ford 2011; McDonagh 2005). PCT was associated with a greater overall reduction in depression symptoms relative to the WL/MA group at post‐treatment (MD ‐5.06, 95% CI ‐8.60 to ‐1.52; participants = 143; studies = 2; I² = 0% Analysis 1.7, Figure 7).
1.7. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 7 BDI, post‐treatment.
7.
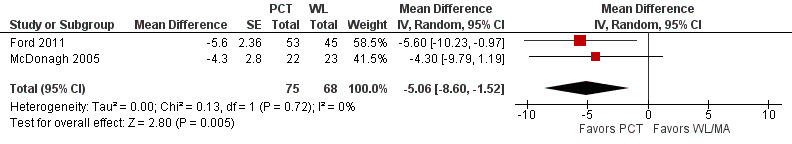
Forest plot of comparison: PCT vs WL/MA
Outcome: BDI, post‐treatment ‐ Mean Difference
1.6 Self‐reported anxiety symptoms (post‐treatment)
One study compared PCT to WL/MA using a self‐report anxiety measure (McDonagh 2005). There was a lack of precision in this estimate to determine whether there was a difference between the PCT and WL/MA on post‐treatment anxiety severity (MD ‐5.10, 95% CI ‐11.56 to 1.36, Analysis 1.8).
1.8. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 8 STAI, post‐treatment.
1.7 Self‐reported dissociation symptoms (post‐treatment)
One study compared PCT to WL/MA on post‐treatment dissociation symptoms (McDonagh 2005). The PCT intervention did better than the WL/MA group at post‐treatment although there was a lack of precision on this outcome (MD ‐13.30, 95% CI ‐21.26 to ‐5.34; Analysis 1.9).
1.9. Analysis.
Comparison 1 PCT versus WL/MA, Outcome 9 DES, post‐treatment.
2. PCT versus TF‐CBT, non‐inferiority analyses
Ten studies including 1221 participants contributed to these comparisons (Foa 2018; McDonagh 2005; NCT00607815; Rauch 2015; Ready 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013).
Primary outcomes
2.1 Clinician‐rated PTSD severity (post‐treatment)
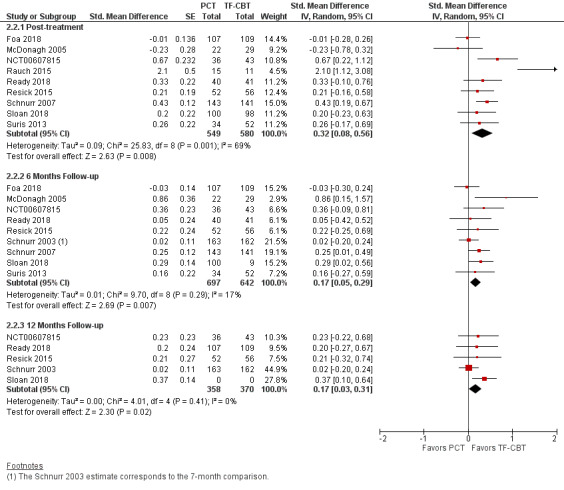
Six trials used the CAPS to compare PTSD severity at post‐treatment (McDonagh 2005; NCT00607815; Rauch 2015; Ready 2018; Schnurr 2003; Schnurr 2007; Suris 2013). The primary non‐inferiority analysis excluded Schnurr 2003 as based on the heterogeneity of the treatment length and post‐treatment assessment timing (six‐month treatment as compared to most other TF‐CBT trials that were three months). There was moderate heterogeneity among the included studies (I² = 42%). At post‐treatment, TF‐CBT participants reported lower PTSD severity compared to the PCT group (MD 6.83, 95% CI 1.90 to 11.76; participants = 607; studies = 6; Analysis 2.1). The upper bound of the 95% confidence interval extended past the MID threshold of 10 points and did not support PCT as a non‐inferior treatment to TF‐CBT (Figure 8). Three additional studies, that used a different clinician‐administered PTSD assessment, were included in a subsequent analysis to compare SMDs on post‐treatment PTSD severity (Foa 2018; Resick 2015; Sloan 2018). The TF‐CBT group did better than the PCT group on post‐treatment PTSD severity with an effect size > 0.20 indicating a clinically meaningful difference (SMD 0.32, 95% CI 0.08 to 0.56; participants = 1129; studies = 9; I² = 69%; Analysis 2.2; Figure 9).
2.1. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 1 CAPS.
8.

Forest plot of comparison: PCT vs TF‐CBT
Outcome: CAPS PTSD severity scores ‐ Mean Differences
2.2. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 2 Clinican‐administered PTSD, standardized difference.
9.
Forest plot of comparison: PCT vs TF‐CBT
Outcome: Clinician‐administered PTSD severity ‐ Standardized Mean Differences
2.2 Clinician‐rated PTSD severity (six months follow‐up)
Six studies compared CAPS scores around six months post‐treatment follow‐up (McDonagh 2005; NCT00607815; Ready 2018; Schnurr 2003; Schnurr 2007; Suris 2013). There was no evidence of PTSD severity differences at this time point between PCT and TF‐CBT (MD 1.59, 95% CI ‐0.46 to 3.63; participants = 906; studies = 6; I² = 0%; Analysis 2.1; Figure 8). Three additional studies, that used a different clinician‐administered PTSD assessment, were included in a subsequent analysis to compare SMDs at six months follow‐up (Foa 2018; Resick 2015; Sloan 2018). TF‐CBT was associated with a small effect size difference that was not clinically meaningful (SMD 0.17, 95% CI 0.05 to 0.29; participants = 1339; studies = 9; I² = 17%;) Analysis 2.2; Figure 9).
2.3 Clinician‐rated PTSD severity (12 months follow‐up)
Three studies compared CAPS scores at 12 months follow‐up (NCT00607815; Ready 2018; Schnurr 2003). There was no evidence of PTSD severity differences at 12 months follow‐up between PCT and TF‐CBT (MD 1.22, 95% CI ‐2.17 to 4.61; participants = 485; studies = 3; I² = 0%; Analysis 2.1; Figure 8). Two additional studies, that used a different clinician‐administered PTSD assessment, were included in a subsequent analysis to compare SMDs at 12 months follow‐up (Resick 2015; Sloan 2018). TF‐CBT was associated with a small effect size difference that was not clinically meaningful (SMD 0.17, 95% CI 0.03 to 0.31; participants = 728; studies = 5; I² = 0%; Analysis 2.2; Figure 9).
2.4 Treatment Dropout
Ten studies recorded whether individuals left the study early for any reason across groups (Foa 2018; McDonagh 2005; NCT00607815; Rauch 2015; Ready 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013). PCT dropout rates were approximately 14% lower compared to TF‐CBT dropout rates (RD ‐0.14, 95% CI ‐0.18 to ‐0.10; RR 0.58, 95% CI 0.49 to 0.69; participants = 1542; studies = 10; I² = 0%; Analysis 2.3; Analysis 2.4; Figure 10).
2.3. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 3 Dropout ‐ Risk Ratio.
2.4. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 4 Dropout ‐ Risk Difference.
10.
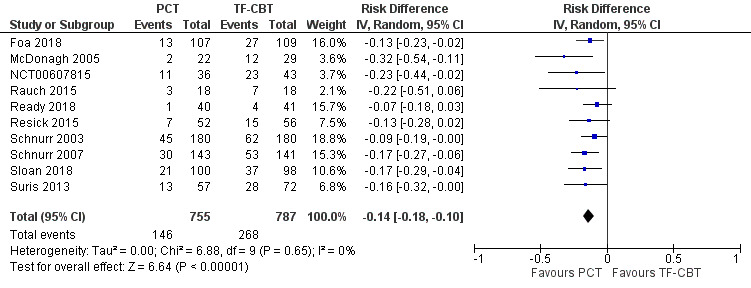
Forest plot of comparison: PCT vs TF‐CBT
Outcome: Dropout ‐ Risk Difference
Secondary outcomes
2.6 Self‐reported PTSD symptoms
The PCL was the only self‐report PTSD measure used to compare PTSD severity differences at post‐treatment (7 studies: Foa 2018; NCT00607815; Ready 2018; Resick 2015; Schnurr 2007; Sloan 2018; Suris 2013), six‐month follow‐up (8 studies: Foa 2018; NCT00607815; Ready 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018; Suris 2013), and 12‐month follow‐up (5 studies: NCT00607815; Ready 2018; Resick 2015; Schnurr 2003; Sloan 2018). At post‐treatment, TF‐CBT scores were approximately 5 points lower than PCT scores and did not meet the MID criteria for a clinically meaningful difference (MD 4.50, 95% CI 3.09 to 5.90; participants = 983; studies = 7; I² = 3%; Analysis 2.5; Figure 11). At six‐month follow‐up, TF‐CBT scores were approximately 3 points lower than PCT scores which was not considered clinically meaningful (MD 3.44, 95% CI 1.86 to 5.02; participants = 1181; studies = 8; I² = 0%), and there was no evidence of differences on PCL scores at 12‐month follow‐up (MD 1.60, 95% CI ‐0.17 to 3.37; participants = 791; studies = 5; I² = 0%).
2.5. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 5 PCL.
11.
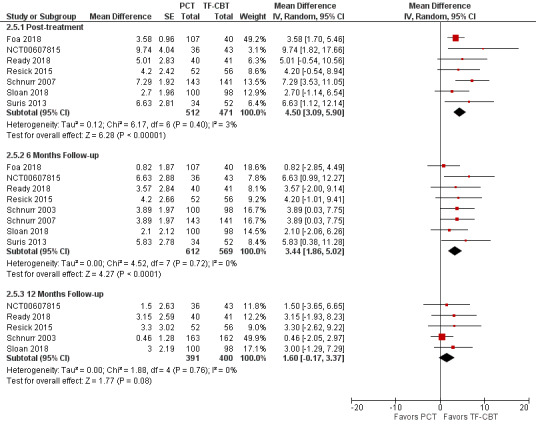
Forest plot of comparison: PCT vs TF‐CBT
Outcome 2.6: PCL ‐ Mean Differences
2.7 Loss of PTSD diagnosis (post‐treatment)
Four studies contributed to this comparison (Foa 2018; McDonagh 2005; Schnurr 2007; Sloan 2018). Loss of PTSD diagnosis rates were higher in TF‐CBT compared to PCT (RD 0.11, 95% CI 0.04 to 0.19; participants = 749; studies = 4; I² = 38%; Analysis 2.7; Figure 12; RR 1.36, 95% CI 1.03 to 1.81; participants = 749; studies = 4; I² = 38%; Analysis 2.6 ).
2.7. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 7 Loss of PTSD diagnosis ‐ Risk Difference.
12.
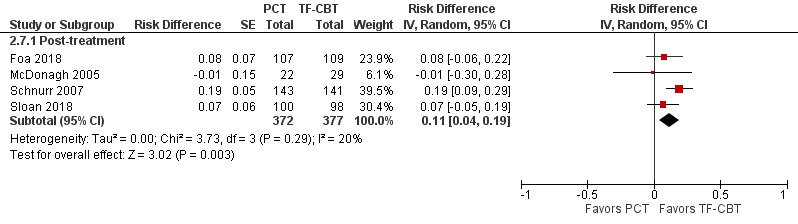
Forest plot of comparison: PCT vs TF‐CBT
Outcome: Loss of PTSD diagnosis, post‐treatment ‐ Risk Difference
2.6. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 6 Loss of PTSD diagnosis ‐ Risk Ratio.
2.8 Self‐reported depression symptoms (post‐treatment)
In the five trials that used the BDI as the self‐report depression measure (McDonagh 2005; NCT00607815; Resick 2015; Schnurr 2007; Sloan 2018), there was no evidence of PCT inferiority on post‐treatment depression severity as based on a MID of 5 points (MD 1.78, 95% CI ‐0.23 to 3.78; participants = 705; studies = 5; Analysis 2.8). On standardized self‐report depression scores, seven studies were included (McDonagh 2005; NCT00607815; Ready 2018; Resick 2015; Schnurr 2007; Sloan 2018; Suris 2013). The effect size difference between treatments was < 0.20 which was not considered clinically meaningful (SMD 0.19, 95% CI 0.04 to 0.33; participants = 887; studies = 7; I² = 13%; Analysis 2.9; Figure 13).
2.8. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 8 BDI.
2.9. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 9 Depression, standardized difference.
13.
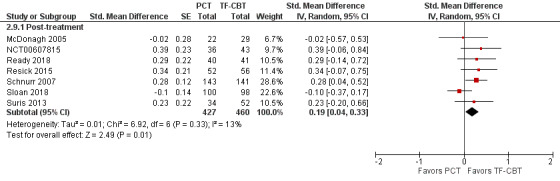
Forest plot of comparison: PCT vs TF‐CBT
Outcome: Depression Severity, post‐treatment ‐ Standardized Mean Differences
2.9 Self‐reported anxiety symptoms (post‐treatment)
Four studies contributed to this analysis (McDonagh 2005; NCT00607815; Schnurr 2007; Sloan 2018). There was no evidence of differences on anxiety symptoms at post‐treatment between PCT and TF‐CBT (SMD 0.32, 95% CI ‐0.08 to 0.71; participants = 612; studies = 4; I² = 80%; Analysis 2.10; Figure 14). However, there was a lack of precision in this estimate.
2.10. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 10 Anxiety, standardized difference.
14.
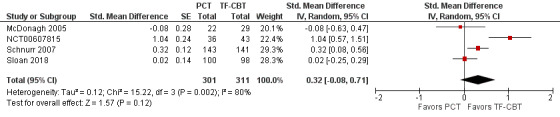
Forest plot of comparison: PCT vs TF‐CBT
Outcome: Anxiety Severity, post‐treatment ‐ Standardized Mean Differences
2.10 Self‐reported dissociation symptoms (post‐treatment)
One study compared PCT to TF‐CBT on post‐treatment dissociation symptoms (McDonagh 2005). There was no evidence of differences on dissociation severity at post‐treatment (MD 4.00, 95% CI ‐3.51 to 11.51; participants = 51; studies = 1; Analysis 2.11).
2.11. Analysis.
Comparison 2 PCT versus TF‐CBT, Outcome 11 DES.
3. Subgroup analyses: Treatment modality and TF‐CBT intervention type
To investigate heterogeneity and whether treatment modality influenced the primary outcomes, we conducted subgroup analyses on: individual versus group treatment format, and trauma treatment type (PE versus CPT). There were not enough trials to justify subgroup analyses on control condition comparisons.
3.1: Treatment modality: Individual vs group treatment
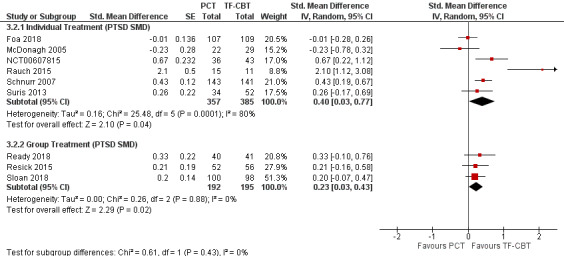
Five studies used the CAPS to compare individual PCT to individual TF‐CBT (McDonagh 2005; NCT00607815; Rauch 2015; Schnurr 2007; Suris 2013) and only one trial used the CAPS to compare group PCT (GPCT) to group TF‐CBT (Ready 2018). The test for subgroup differences was not significant (Chi² = 0.21, df = 1 (P = 0.64), I² = 0%; Analysis 3.1; Figure 15). Subgroup analyses evaluating PTSD SMD among individual (SMD 0.40, 95% CI 0.03 to 0.77; participants = 742; studies = 6) and group treatments (SMD 0.23, 95% CI 0.03 to 0.43; participants = 387; studies = 3) were consistent, with no significant subgroup differences (Chi² = 0.61, df = 1 (P = 0.43), I² = 0%; Analysis 3.2; Figure 16).
3.1. Analysis.
Comparison 3 PCT versus TF‐CBT Subgroup Analyses, Outcome 1 Treatment Modality: CAPS Mean Difference.
15.
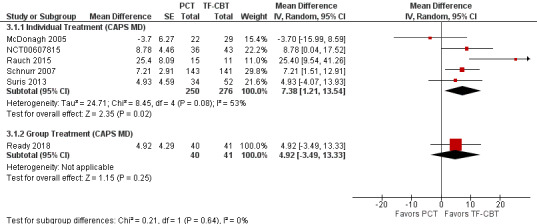
Forest plot of comparison: 3 PCT vs TF‐CBT Subgroup Analyses, outcome: 3.1 Treatment Modality: CAPS Mean Difference
3.2. Analysis.
Comparison 3 PCT versus TF‐CBT Subgroup Analyses, Outcome 2 Treatment Modality: PTSD SMD.
16.
Forest plot of comparison: 3 PCT vs TF‐CBT Subgroup Analyses, outcome: 3.2 Treatment Modality: PTSD SMD
3.2: TF‐CBT intervention: Prolonged Exposure versus Cognitive Processing Therapy
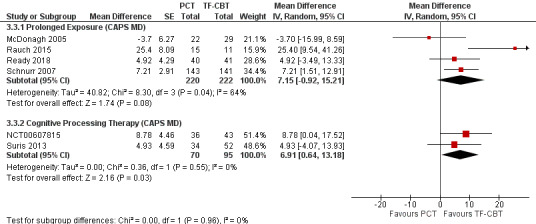
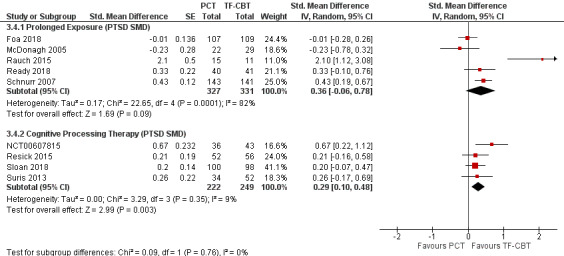
Four studies were characterized to align most closely with CPT (NCT00607815; Resick 2015; Sloan 2018; Suris 2013) and five studies to align with PE (Foa 2018; McDonagh 2005; Rauch 2015; Ready 2018; Schnurr 2007). The test for subgroup differences was not significant (Chi² = 0.00, df = 1 (P = 0.96), I² = 0%; Analysis 3.3; Figure 17). In evaluating SMDs, the results comparing CPT and PE subgroups were also not significant (Chi² = 0.09, df = 1 (P = 0.76), I² = 0%; Analysis 3.4; Figure 18).
3.3. Analysis.
Comparison 3 PCT versus TF‐CBT Subgroup Analyses, Outcome 3 Trauma Treatment: CAPS Mean Difference.
17.
Forest plot of comparison: 3 PCT vs TF‐CBT Subgroup Analyses, outcome: 3.3 Trauma Treatment: CAPS Mean Difference
3.4. Analysis.
Comparison 3 PCT versus TF‐CBT Subgroup Analyses, Outcome 4 Trauma Treatment: PTSD SMD.
18.
Forest plot of comparison: 3 PCT vs TF‐CBT Subgroup Analyses, outcome: 3.4 Trauma Treatment: PTSD SMD
4. Sensitivity analyses
To explore whether trial quality had any effect on the primary outcomes, we conducted sensitivity analyses on post‐treatment CAPS scores including only those trials with the lowest risk of bias as based on: (a) outcome masking, (b) appropriate handling of missing data (ITT; mixed‐model analysis), (c) adequate power, and (d) low levels (< 40%) of post‐randomization treatment loss. Six studies were identified (Foa 2018; Resick 2015; Schnurr 2003; Schnurr 2007; Sloan 2018). The sensitivity analyses excluded Schnurr 2003 since the timing of the post‐treatment assessment was not comparable to the other five trials.
4.1 Clinician‐rated PTSD severity (post‐treatment)
Only one study, rated as higher quality, used the CAPS to assess PTSD severity at post‐treatment (Schnurr 2007). The results from this trial did not support PCT non‐inferiority (MD 7.21, 95% CI 1.51 to 12.91; participants = 284; studies = 1; Analysis 4.1). All four higher‐quality studies were included to evaluate PTSD SMD differences between PCT and TF‐CBT. There was moderate heterogeneity across trials (I² = 50%). The results indicated that TF‐CBT had lower post‐treatment PTSD severity compared to PCT with an effect size > 20 (SMD 0.21, 95% CI 0.02 to 0.41; participants = 806; studies = 4; Analysis 4.2).
4.1. Analysis.
Comparison 4 Sensitivity Analyses: Higher‐Quality Studies, Outcome 1 CAPS Mean Difference.
4.2. Analysis.
Comparison 4 Sensitivity Analyses: Higher‐Quality Studies, Outcome 2 PTSD SMD.
4.2 Treatment Dropout
Treatment dropout results were consistent with the primary analyses, indicating that dropout rates were lower in PCT as compared to TF‐CBT (RD ‐0.13, 95% CI ‐0.18 to ‐0.08; RR 0.60, 95% CI 0.49 to 0.74; participants = 1166; studies = 5; I2 = 0%; Analysis 4.3; Analysis 4.4).
4.3. Analysis.
Comparison 4 Sensitivity Analyses: Higher‐Quality Studies, Outcome 3 Treatment Dropout: Risk Ratio.
4.4. Analysis.
Comparison 4 Sensitivity Analyses: Higher‐Quality Studies, Outcome 4 Treatment Dropout: Risk Difference.
5. Publication bias
We explored the potential effects of publication bias using funnel plots. We constructed two funnel plots using data from the PCT versus TF‐CBT comparison, with one involving continuous data on the CAPS and the second involving dichotomous data on dropouts. The first funnel plot examined the measure of clinician‐rated PTSD symptoms (Figure 19) and was roughly symmetrical. As the studies became less precise, the results of the studies tended be more variable and scattered to either side of the more precise larger studies. The second funnel plot also used data from the PCT versus TF‐CBT comparisons to examine the dichotomous measure of treatment dropout (Figure 20; Figure 21). Although this funnel plot was slightly less symmetrical as the studies became less precise, we would not expect that there would be publication bias in favor of our treatment under investigation, given that the studies included in the review were primarily evaluating the comparator treatments. The few studies included in this review also limits the conclusions that can be drawn from these funnel plots.
19.
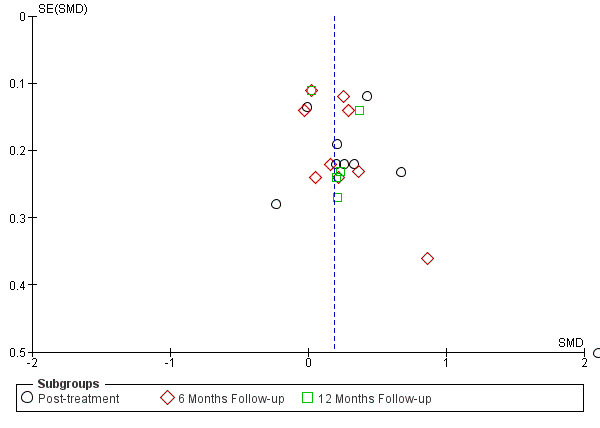
Funnel plot of comparison: PCT vs TF‐CBT, outcome: 2.2 Clinican‐administered PTSD, standardized difference
20.
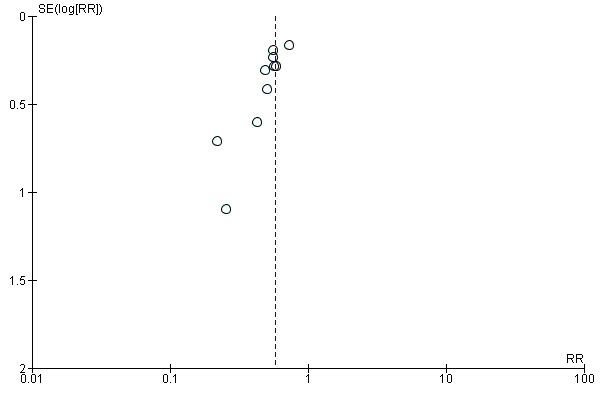
Funnel plot of PCT vs TF‐CBT studies on dropout at post‐treatment
21.
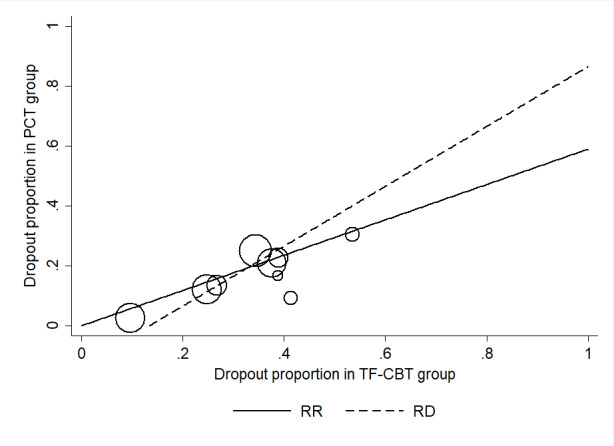
Labbe plot of dropout for PCT vs TF‐CBT
Discussion
Summary of main results
We included 12 studies of 1837 participants in the current systematic review to determine whether (1) PCT is more effective than wait list/minimal attention (WL/MA) control groups in reducing PTSD symptoms, (2) PCT is non‐inferior to TF‐CBT based on a preset MID on a semi‐structured interview of PTSD severity, and (3) PCT is associated with lower treatment dropout rates as compared to TF‐CBT.
PCT is more effective than WL/MA conditions in reducing PTSD severity measured at post‐treatment, with a moderate to large effect size. There were no differences detected in treatment dropout rates between PCT and the WL/MA conditions. PCT is more effective than the WL/MA conditions in reducing the severity of self‐reported PTSD and depression symptoms, and in reducing the number of people with a PTSD diagnosis at post‐treatment. One study compared self‐reported anxiety symptoms between groups and there was a lack of precision to determine whether there was a meaningful difference post‐treatment. One study found that PCT did better than the WL/MA group at reducing dissociation symptoms post‐treatment.
The results from the non‐inferiority analysis comparing PCT to TF‐CBT suggest that PCT is likely not as effective as TF‐CBT in reducing post‐treatment PTSD severity. The PTSD SMD results were consistent with this finding and suggest an effect size difference that was clinically meaningful in favor of TF‐CBT. Treatment differences between PCT and TF‐CBT appeared to attenuate at six‐month and 12‐month follow‐up periods. Over 10% more people may drop out of TF‐CBT compared to PCT. Secondary outcomes comparing PCT and TF‐CBT showed that TF‐CBT was more effective in reducing the number of people with a PTSD diagnosis at post‐treatment. Results of the self‐report PTSD and depression outcomes suggest that PCT may result in a similar reduction in symptoms as compared to TF‐CBT. There was a lack of precision to interpret differences on post‐treatment self‐reported anxiety symptoms. There was also a lack of precision in the one study to compare self‐reported dissociation symptoms between groups.
Subgroup Analysis: Individual and group therapy modalities
The results from the subgroup analyses evaluating (1) individual and group therapy trials, and (2) PE and CPT interventions compared to PCT were largely consistent with the primary results, and did not support PCT non‐inferiority in reducing post‐treatment PTSD severity.
Sensitivity Analyses
The results from the sensitivity analyses that included only the higher‐quality studies were also consistent with the primary results and did not support PCT non‐inferiority in reducing post‐treatment PTSD severity. The results also supported the finding that PCT has lower treatment dropout rates compared to TF‐CBT.
Overall completeness and applicability of evidence
The current systematic review provides the most comprehensive synthesis of the available literature on PCT for PTSD to date, applying a rigorous evaluation of the non‐inferiority of PCT compared to TF‐CBT. The studies included in this review directly addressed the primary review questions. We reached out to all authors, as needed, to request any missing information, and most authors responded to these requests. Participants were all adults, but ranged in demographics and trauma types. All studies recruited participants in the United States and there was a predominance of studies conducted on military veterans: nine studies were conducted on military veterans recruited in the USA Veterans Health Affairs, three studies recruited USA active duty military service members, one study recruited USA mothers or primary caregivers of young children, and one study recruited USA women with a history of childhood sexual abuse. Thus, there may be some concerns that the results do not generalize as well to non‐military or non‐USA populations. In most trials, PCT was based on the original PCT manual. When this was not the case, we ensured that the PCT manual was consistent with the criteria established in the protocol and consulted with experts to make a final determination. The comparator treatments included the primary front‐line trauma treatments (PE and CPT), and we were able to conduct subgroup analyses to determine whether PCT had differential effects based on the trauma‐focused treatment comparison. Studies included both individual and group treatment modalities and we were able to conduct subgroup analyses comparing these different modalities. Several trials included three trial arms, permitting us to compare PCT with a control condition to evaluate its effectiveness in reducing post‐trauma symptoms. There were several trials using the CAPS which allowed us to test our non‐inferiority hypotheses using mean differences and a well‐established MID threshold. We also calculated PTSD SMDs using all available trials which supported our primary findings. There was differential dropout between PCT and TF‐CBT which may affect our assumptions of missing at random (MAR) and be a potential limitation to the analyses.
Quality of the evidence
There was evidence of clinical and statistical heterogeneity in the included studies. Subgroup and sensitivity analyses did not explain this heterogeneity and were consistent with our main findings. Notably, all of the included studies were primarily designed to test the effectiveness of TF‐CBT which could bias results of the PCT non‐inferiority. The quality of evidence for our non‐inferiority analyses was low based on methodological limitations in a few of the trials and inconsistent results. Although PCT was deemed to be less effective than TF‐CBT, the differential effects between treatments ranged across the MID (i.e. low precision which affected the quality of evidence). Given the low quality of evidence and the potential for bias toward the experimental treatments, future trials may influence our understanding of how effective PCT is compared to TF‐CBT. The quality of evidence comparing treatment dropout between PCT and TF‐CBT was rated as moderate. Future research that standardizes how dropout is defined will increase our understanding of the differential dropout rates between trauma‐focused and non‐trauma‐focused treatments.
Potential biases in the review process
This review followed the Cochrane Collaboration Guidelines and every effort was made to minimise bias in the review process. Nevertheless, the potential risk of missing trials cannot be completely eliminated. We performed comprehensive searches of all relevant databases with minimal restrictions. The data screening and extraction process was strictly adhered to based on the Cochrane recommended procedures and standards. We consulted with content experts throughout the review process. Two of the review authors were investigators on some of the included trials and helped develop PCT. However, neither investigator was involved in any of the qualitative or quantitative syntheses to minimize any potential bias. .
Agreements and disagreements with other studies or reviews
The overall findings suggest that PCT is an effective treatment for PTSD when compared to control conditions. However, PCT may be less effective than TF‐CBT in reducing post‐treatment PTSD severity. The findings also indicate that fewer patients drop out of PCT compared to TF‐CBT. These results are somewhat inconsistent with a previous review that only included five trials and that concluded that PCT was as efficacious as TF‐CBT (Frost 2014). The current review differs from this previous review by including more studies comparing PCT to TF‐CBT and applying a stricter non‐inferiority framework in which the range of treatment differences had to be within a prespecific MID range (AHRQ 2012). The 95% CI in our meta‐analyses exceeded this MID threshold, and thus our results did not support PCT non‐inferiority. However, consistent with that review and other meta‐analyses (Frost 2014; Imel 2013), results did show that treatment dropout rates were lower in PCT compared to TF‐CBT. Our findings are consistent with current clinical practice guidelines that suggest that PCT may be offered as a treatment for PTSD when individual trauma‐focused psychotherapy is not readily available or not preferred (Berliner 2019; VA/DoD 2017), although some guidelines have yet to include an official recommendation on PCT (APA 2017; NICE 2018).The current review can help substantiate and inform the recommendations laid out in future clinical guidelines by providing a comprehensive, rigorous, and transparent evaluation of PCT.
Authors' conclusions
Implications for practice.
1. PCT may not be as effective as TF‐CBT in reducing post‐treatment PTSD severity among adults with PTSD.
2. PCT has lower treatment dropout rates compared to TF‐CBT.
3. The differential effects of PCT versus TF‐CBT on PTSD severity may attenuate over longer time periods.
Implications for research.
1. Research evaluating how to best match patients to the most effective PTSD treatments that takes into account patient preferences, scientific evidence, and clinical judgment will advance our approaches to treating adults with PTSD.
2. Additional effectiveness trials comparing PCT to TF‐CBT across different subgroups of trauma survivors to examine the true uptake of the different interventions (with standardized definitions of dropout), across longer‐term outcomes, will inform the tradeoffs between efficacy and attrition.
3. PCT was originally designed as a placebo treatment. Future research is warranted that evaluates an augmented version of PCT to determine whether PCT is as effective as TF‐CBT in treating patients with PTSD.
Notes
The views expressed in this presentation are those of the authors and do not necessarily represent the official policy or position of the Defense Health Agency, Department of Defense, or Veteran Affairs Health System.
Acknowledgements
We wish to thank Sarah Dawson and Chris Cooper of the Cochrane Common Mental Disorders Group for developing the search strategy. We also wish to thank all of the study investigators and their commitment to improving the treatment of adults with PTSD who allowed this systematic review to be possible. Thank you for your responsiveness to our requests for additional information.
The authors and the Cochrane Common Mental Disorders Editorial Team, are also grateful to the following peer reviewers for their time and comments: Philippa Davies, Neil Roberts, Lindsay Robertson and Myfanwy Williams. They would also like to thank copy editor, Anne Lethaby.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the UK National Health Service, the NIHR, or the UK Department of Health and Social Care.
Appendices
Appendix 1. OVID MEDLINE: core search strategy of CCMD used to inform the specialized register
A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record.
Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, as appropriate to each resource.
A quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL) is conducted c/o the Cochrane Register of Studies Online (CRSO).
Appendix 2. Other database searches
Search‐1 February/March 2018
MEDLINE (46)
Embase (70)
PsycINFO (50)
CENTRAL (59)
ProQuest PTSDpubs (44)
Total 269 Duplicates 175 Unique references to screen = 94
WHO ICTRP 24
Clinicaltrials.gov 113
CCMD Register 46
Total: 183 Duplicates: 69
Total register references to screen = 114
ProQuest Dissertations & Theses Global, n = 62
Search strategies
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) Host: OVID Data Parameters: 1946 to February 21, 2018 Date Searched: Monday, February 26, 2018 Searched by: Chris PRESS checked by: Erin Hits: 46 Search strategy: 1 (present adj (centred or centered or focused or focussed)).ti,ab,kw,ot. 116 2 randomised controlled trial.pt. 454274 3 controlled clinical trial.pt. 92178 4 randomized.ab. 403825 5 placebo.ab. 186674 6 clinical trials as topic.sh. 182669 7 randomly.ab. 285626 8 trial.ti. 178411 9 (2 or 3 or 4 or 5 or 6 or 7 or 8) 1135022 10 (1 and 9) 46 Notes: N/A File: UO2 MEDLINE n46 Embase Host: OVID Data Parameters: 1974 to 2018 February 23 Date searched: Monday, February 26th 2018 Searched by: Chris Cooper PRESS checked by: Erin Hits: 70 Search strategy: 1 (present adj (centred or centered or focused or focussed)).ti,ab,kw,ot. 145 2 randomised controlled trial.de. 488351 3 randomization.de. 77066 4 placebo.de. 319947 5 placebo?.ti,ab. 268183 6 (randomised or randomised).ti,ab. 730215 7 randomly.ab. 370505 8 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. 270431 9 factorial$.ti,ab. 32095 10 allocat$.ti,ab. 124228 11 assign$.ti,ab. 330561 12 volunteer$.ti,ab. 229070 13 crossover procedure.de. 54404 14 (crossover$ or cross over$).ti,ab. 92366 15 (quasi adj (experimental or random$)).mp. 17305 16 (control$ adj3 (trial$ or study or studies or group$)).ti,ab. 1129574 17 (2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16) 2514303 18 (1 and 17) 70 Notes: N/A File: UO2 Embase n70 PsycINFO Host: OVID Data Parameters: 1806 to February Week 3 2018 Date searched: Monday, February 26th 2018 Searched by: Chris Cooper PRESS checked by: Erin Hits: 50 Search strategy: 1 (present adj (centred or centered or focused or focussed)).ti,ab,ot. 243 2 (randomised or randomised).ti,ab. 69832 3 placebo.ab. 36417 4 randomly.ab. 65035 5 trial.ti. 25551 6 (2 or 3 or 4 or 5) 153550 7 (1 and 6) 50 Notes: N/A File: UO2 PsycINFO n50 Cochrane Central Register of Controlled Trials (CENTRAL) Host: Wiley Data Parameters: Issue 1 of 12, January 2018 Date searched: Monday, February 26th 2018 Searched by: Chris Cooper PRESS checked by: Erin Hits: 59 Search strategy: (present near/1 (centred or centered or focused or focussed)):ti,ab,kw (Word variations have been searched) Notes: N/A File: UO2 CENTRAL n=50
PILOTS: Published International Literature On Traumatic Stress Host: Pro Quest Data Parameters: Issue 1871‐Current Date searched: Tuesday March 6, 2018 Searched by: Chris Cooper Hits: 44
Search strategy: Set#: S1 Searched for: ti((present NEAR/2 (centred or centered or focused or focussed))) OR ab((present NEAR/2 (centred or centered or focused or focussed))) Results: 100 Set#: S2 Searched for: MAINSUBJECT.EXACT("Randomized Clinical Trial") Results: 1210 Set#: S3 Searched for: ab((randomised or randomised or placebo or randomly)) Results: 2931 Set#: S4 Searched for: ti(trial) Results: 784 Set#: S5 Searched for: S2 or S3 or S4 Results: 3226 Set#: S6 Searched for: S1 and S5 Results: 44
ProQuest Dissertations & Theses Global
ti(("present centred" or "present centered" or "present focused" or "present focussed")) OR ab(("present centred" or "present centered" or "present focused" or "present focussed")) n=62
WHO ICTRP Searched via: http://www.who.int/ictrp/en/ Date Searched: February 22, 2018 Searched by: Erin Hits: 24 Search strategy: (present centred or present centered or present focused or present focussed)
Clinical Trials.gov Searched via: https://clinicaltrials.gov/ct2/home Date Searched: February 22, 2018 Searched by: Erin Hits: 113 Search strategy: present centred present centered present focused present focussed
CCMDCTR Register Searched via: Cochrane CRS Date searched: Monday, February 26, 2018 [Register current to June 2016 only] Searched by: Chris Cooper Hits: 46 Search Strategy: (present centred or present centered or present focused or present focussed) Notes: N/A File: UO2 CCDAM n = 46
*********************************************************************************
Search‐2 February 2019
MEDLINE (1946 to 15 February 2019) (58)
Embase (2018 to 2019 Week 07) (20)
PsycINFO (1806 to February Week 1 2019) (81)
CENTRAL (2018 to Issue 2, February 2019) (27)
WHO ICTRP (2018 to 15 February 2019) (3)
Clinicaltrials.gov (all years to 15 February 2019) (30)
CCMD Register (not searched, only current to June 2016)
ProQuest PTSDpubs (all years to 15 February 2019) (107)
ProQuest Dissertations & Theses Global (all years to 15 February 2019) (10)
Searched by: Sarah Dawson Total = 336 Duplicates removed within this batch, n = 150 Duplicates removed against earlier search results (sent by CC), n = 71 Records to screen, n = 115
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to February 15, 2019> Search Strategy: 1 (present adj (centred or centered or focused or focussed)).ti,ab,kw,ot. (134) 2 randomised controlled trial.pt. (476303) 3 controlled clinical trial.pt. (92914) 4 (randomised or randomised).ti,ab,kw. (558846) 5 randomly.ab. (305364) 6 placebo.ab. (195371) 7 clinical trials as topic.sh. (186040) 8 trial.ti. (194121) 9 or/2‐8 (1236634) 10 (1 and 9) (58)
Ovid Embase <2018 to 2019 Week 07> Search Strategy: 1 (present adj (centred or centered or focused or focussed)).ti,ab,kw,ot. (165) 2 randomised controlled trial.de. (536016) 3 randomization.de. (81197) 4 placebo.de. (330442) 5 placebo?.ti,ab. (284480) 6 (randomised or randomised).ti,ab. (797176) 7 randomly.ab. (400977) 8 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. (285584) 9 factorial$.ti,ab. (34478) 10 allocat$.ti,ab. (136069) 11 assign$.ti,ab. (356785) 12 volunteer$.ti,ab. (240727) 13 crossover procedure.de. (58243) 14 (crossover$ or cross over$).ti,ab. (97979) 15 (quasi adj (experimental or random$)).mp. (19487) 16 (control$ adj3 (trial$ or study or studies or group$)).ti,ab. (1224543) 17 or/2‐16 (2689918) 18 1 and 17 (83) 19 (2018* or 2019*).dc,dd,dp,yr. (2088349) 20 (18 and 19) (20)
Ovid PsycINFO 1806 to February Week 1 2019 1 (present adj (centred or centered or focused or focussed)).ti,ab,id,ot. (268) 2 posttraumatic stress disorder/ or complex ptsd/ or desnos/ (30247) 3 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab,id. (42801) 4 (2 or 3) (44002) 5 (1 and 4) (81)
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 2. 2019 Advanced search strategy: (present near/1 (centred or centered or focused or focussed)):ti,ab,kw (Word variations not searched) Date limited: 1/2/2018 to 15/2/2019 n = 27
WHO ICTRP Searched via: http://www.who.int/ictrp/en/ Date Searched: February 22, 2018 to February 15, 2019 (present centred or present centered or present focused or present focussed) n = 3
Clinical Trials.gov Searched via: https://clinicaltrials.gov/ct2/home Date Searched: February 15, 2019 "present centred" OR "present centered" OR "present focused" OR "present focussed" | PTSD n = 30 PTSD synonyms (automatically searched): Post‐traumatic stress disorder or combat fatigue or combat neurosses or post traumatic stress syndrome or post‐traumatic neuroses or traumatic neurosis Proquest PTSDpubs (previously Published International Literature on Traumatic Stress (PILOTS) (all years to 15‐Feb‐2018) noft((present NEAR/1 (centred OR centered OR focused OR focussed))) n = 107 ProQuest Dissertations & Theses Global (all years to 15 Feb 2019) ti(("present centred" or "present centered" or "present focused" or "present focussed")) OR ab(("present centred" or "present centered" or "present focused" or "present focussed")) AND ti((PTSD or postrauma* or post‐trauma* or "post trauma*" or desnos or "acute stress disorder*" or "combat disorder*" or "war neuros*")) OR ab((PTSD or posttrauma* or post‐trauma* or "post trauma*" or desnos or "acute stress disorder*" or "combat disorder*" or "war neuros*")) n = 10
Data and analyses
Comparison 1. PCT versus WL/MA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinician‐administered PTSD, standardized difference | 3 | 290 | Std. Mean Difference (Random, 95% CI) | ‐0.84 [‐1.10, ‐0.59] |
| 2 Dropout, post‐treatment ‐ Risk Ratio | 3 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.51, 3.29] |
| 3 Dropout, post‐treatment ‐ Risk Difference | 3 | 290 | Risk Difference (IV, Random, 95% CI) | 0.07 [‐0.02, 0.16] |
| 4 PTSD Checklist, post‐treatment | 1 | 147 | Mean Difference (Random, 95% CI) | ‐7.52 [‐10.99, ‐4.05] |
| 5 Loss of PTSD diagnosis, post‐treatment ‐ Risk Ratio | 3 | 290 | Risk Ratio (Random, 95% CI) | 0.45 [0.30, 0.67] |
| 6 Loss of PTSD diagnosis, post‐treatment ‐ Risk Difference | 3 | 290 | Risk Difference (Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 7 BDI, post‐treatment | 2 | 143 | Mean Difference (Random, 95% CI) | ‐5.06 [‐8.60, ‐1.52] |
| 8 STAI, post‐treatment | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐5.10 [‐11.56, 1.36] |
| 9 DES, post‐treatment | 1 | 45 | Mean Difference (Random, 95% CI) | ‐13.30 [‐21.26, ‐5.34] |
Comparison 2. PCT versus TF‐CBT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CAPS | 7 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Post‐treatment | 6 | 607 | Mean Difference (Random, 95% CI) | 6.83 [1.90, 11.76] |
| 1.2 6 Months Follow‐up | 6 | 906 | Mean Difference (Random, 95% CI) | 1.59 [‐0.46, 3.63] |
| 1.3 12 Months Follow‐up | 3 | 485 | Mean Difference (Random, 95% CI) | 1.22 [‐2.17, 4.61] |
| 2 Clinican‐administered PTSD, standardized difference | 10 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Post‐treatment | 9 | 1129 | Std. Mean Difference (Random, 95% CI) | 0.32 [0.08, 0.56] |
| 2.2 6 Months Follow‐up | 9 | 1339 | Std. Mean Difference (Random, 95% CI) | 0.17 [0.05, 0.29] |
| 2.3 12 Months Follow‐up | 5 | 728 | Std. Mean Difference (Random, 95% CI) | 0.17 [0.03, 0.31] |
| 3 Dropout ‐ Risk Ratio | 10 | 1542 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.49, 0.69] |
| 4 Dropout ‐ Risk Difference | 10 | 1542 | Risk Difference (IV, Random, 95% CI) | ‐0.14 [‐0.18, ‐0.10] |
| 5 PCL | 8 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 Post‐treatment | 7 | 983 | Mean Difference (Random, 95% CI) | 4.50 [3.09, 5.90] |
| 5.2 6 Months Follow‐up | 8 | 1181 | Mean Difference (Random, 95% CI) | 3.44 [1.86, 5.02] |
| 5.3 12 Months Follow‐up | 5 | 791 | Mean Difference (Random, 95% CI) | 1.60 [‐0.17, 3.37] |
| 6 Loss of PTSD diagnosis ‐ Risk Ratio | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Post‐treatment | 4 | 749 | Risk Ratio (Random, 95% CI) | 1.36 [1.03, 1.81] |
| 7 Loss of PTSD diagnosis ‐ Risk Difference | 4 | Risk Difference (Random, 95% CI) | Subtotals only | |
| 7.1 Post‐treatment | 4 | 749 | Risk Difference (Random, 95% CI) | 0.11 [0.04, 0.19] |
| 8 BDI | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 8.1 Post‐treatment | 5 | 705 | Mean Difference (Random, 95% CI) | 1.78 [‐0.23, 3.78] |
| 9 Depression, standardized difference | 7 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 9.1 Post‐treatment | 7 | 887 | Std. Mean Difference (Random, 95% CI) | 0.19 [0.04, 0.33] |
| 10 Anxiety, standardized difference | 4 | 612 | Std. Mean Difference (Random, 95% CI) | 0.32 [‐0.08, 0.71] |
| 11 DES | 1 | 51 | Mean Difference (Random, 95% CI) | 4.0 [‐3.51, 11.51] |
Comparison 3. PCT versus TF‐CBT Subgroup Analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment Modality: CAPS Mean Difference | 6 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Individual Treatment (CAPS MD) | 5 | 526 | Mean Difference (Random, 95% CI) | 7.38 [1.21, 13.54] |
| 1.2 Group Treatment (CAPS MD) | 1 | 81 | Mean Difference (Random, 95% CI) | 4.92 [‐3.49, 13.33] |
| 2 Treatment Modality: PTSD SMD | 9 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Individual Treatment (PTSD SMD) | 6 | 742 | Std. Mean Difference (Random, 95% CI) | 0.40 [0.03, 0.77] |
| 2.2 Group Treatment (PTSD SMD) | 3 | 387 | Std. Mean Difference (Random, 95% CI) | 0.23 [0.03, 0.43] |
| 3 Trauma Treatment: CAPS Mean Difference | 6 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Prolonged Exposure (CAPS MD) | 4 | 442 | Mean Difference (Random, 95% CI) | 7.15 [‐0.92, 15.21] |
| 3.2 Cognitive Processing Therapy (CAPS MD) | 2 | 165 | Mean Difference (Random, 95% CI) | 6.91 [0.64, 13.18] |
| 4 Trauma Treatment: PTSD SMD | 9 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 Prolonged Exposure (PTSD SMD) | 5 | 658 | Std. Mean Difference (Random, 95% CI) | 0.36 [‐0.06, 0.78] |
| 4.2 Cognitive Processing Therapy (PTSD SMD) | 4 | 471 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.10, 0.48] |
Comparison 4. Sensitivity Analyses: Higher‐Quality Studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CAPS Mean Difference | 1 | 284 | Mean Difference (Random, 95% CI) | 7.21 [1.51, 12.91] |
| 1.1 Post‐treatment PTSD | 1 | 284 | Mean Difference (Random, 95% CI) | 7.21 [1.51, 12.91] |
| 2 PTSD SMD | 4 | 806 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.02, 0.41] |
| 2.1 Post‐treatment PTSD | 4 | 806 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.02, 0.41] |
| 3 Treatment Dropout: Risk Ratio | 5 | 1166 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.49, 0.74] |
| 4 Treatment Dropout: Risk Difference | 5 | 1166 | Risk Difference (IV, Random, 95% CI) | ‐0.13 [‐0.18, ‐0.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Foa 2018.
| Methods |
Design: parallel group RCT Dates of study: January 2011 ‐ July 2016 Number of study centers and locations: one, Ft. Hood, Texas Recruitment: service members who screened out from other STRONG STAR studies were offered recruitment. Potential participants were also identified through referrals from various providers. Potential participants could also self‐refer in response to flyers and pamphlets. Study duration: six months |
|
| Participants |
Participants: 370 Age: mean (SD) 32.7 (7.5) for massed PE group, 32.9 (7.1) for spaced PE group, 32.5 (7.5) for PCT group, 32.7 (7.7) for MCC group Sex: 85.5% male for massed PE group, 90.8% male for spaced PE group, 85.0% male for PCT group, 95.0% male for MCC group Baseline PSS‐I score: mean (CI) 25.20 (23.81 to 26.59) for massed PE group, 25.31 (23.95 to 26.66) for spaced PE group, 25.96 (24.69 to 27.23) for PCT group, 24.83 (23.00 to 26.66) for MCC group Trauma type: exposure to a DSM‐IV‐TR criterion A combat‐related traumatic event (PTSD could be indexed to a noncombat‐related event) Duration of time since trauma: not reported Comorbid conditions: not reported Diagnostic criteria: DSM‐IV‐TR criteria, assessed with the PSS‐I Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: massed prolonged exposure therapy (PE) Description: a manualized CBT consisting of imaginal exposure followed by processing thoughts and feelings related to the imaginal experience; in‐vivo exposure, psychoeducation about PTSD, and controlled breathing training Delivered by: therapists were 2 credentialed psychologists and 1 credentialed social worker who had completed a 4‐day PE workshop, a 2‐day PCT workshop, and 2 supervised cases of PE and PCT Number of sessions: daily sessions were administered on 10 consecutive weekdays over a 2‐week period Format: individual Group II: spaced prolonged exposure therapy (PE) Description: a manualized CBT consisting of imaginal exposure followed by processing thoughts and feelings related to the imaginal experience; in‐vivo exposure, psychoeducation about PTSD, and controlled breathing training Delivered by: therapists were 2 credentialed psychologists and 1 credentialed social worker who had completed a 4‐day PE workshop, a 2‐day PCT workshop, and 2 supervised cases of PE and PCT Number of sessions: 10 sessions were delivered over 8 weeks: 6 once weekly, and 2 twice weekly during the first and last weeks Format: individual Group III: PCT Manual/Model: CSP 494 version Delivered by: therapists were 2 credentialed psychologists and 1 credentialed social worker who had completed a 4‐day PE workshop, a 2‐day PCT workshop, and 2 supervised cases of PE and PCT Number of sessions: ten 90‐minute sessions were scheduled similarly to spaced therapy Format: individual Group IV: minimal contact control (MCC) Description: participants were asked about their well‐being, offered support as needed, and received contact information in case symptoms worsened Delivered by: therapists were 2 credentialed psychologists and 1 credentialed social worker who had completed a 4‐day PE workshop, a 2‐day PCT workshop, and 2 supervised cases of PE and PCT Number of sessions: 10‐ to 15‐minute therapist telephone calls once weekly for 4 weeks Format: individual |
|
| Outcomes |
PTSD:
|
|
| Notes | Out of 110 randomized to massed PE, 17 did not complete post‐treatment follow‐up, 25 did not complete 2‐week follow‐up, 44 did not complete 12‐week follow‐up, and 48 did not complete 6‐month follow‐up. Out of 110 randomized to spaced PE, 31 did not complete post‐treatment follow‐up, 34 did not complete 2‐week follow‐up, 46 did not complete 12‐week follow‐up, and 54 did not complete 6‐month follow‐up. Out of 110 randomized to PCT, 22 did not complete post‐treatment follow‐up, 29 did not complete 2‐week follow‐up, 39 did not complete 12‐week follow‐up, and 50 did not complete 6‐month follow‐up. Out of 10 randomized to MCC, 0 did not complete post‐treatment follow‐up, and 1 did not complete 2‐week follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was entered by a study statistician into a secure, web‐based application using SAS version 9.4 (SAS Institute Inc), which was accessed by the project coordinator on enrolment of each participant." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization sequence was entered by a study statistician into a secure, web‐based application using SAS version 9.4 (SAS Institute Inc), which was accessed by the project coordinator on enrolment of each participant." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | PTSD symptom severity was assessed by independent evaluators blinded to treatment condition, before and after treatment, and at 2‐week, 12‐week, and 6‐month follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four randomized participants were not included in analyses. Investigators stated that, Quote: "...pattern mixture modelling found no significant differences in the change in outcome over time between participants with missing data and those without missing data." Higher dropout rates in the TF‐CBT arm relative to PCT |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's protocol were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; principal investigator is a developer of treatment under investigation (PE). |
Ford 2011.
| Methods |
Design: parallel group RCT Dates of study: enrolment began February 4, 2005, and the last follow‐up was November 6, 2007 Number of study centers and locations: 2 medical centers in Connecticut Recruitment: health clinics, family service centers, community centers, and residential treatment centers in the Hartford, Connecticut, area Study duration: six months |
|
| Participants |
Participants: 146 Age: mean (SD) 30.7 (6.9) overall, age range 18‐45 Sex: 0% male Baseline CAPS score: mean (SD) 62.3 (18.1) for TARGET group, 61.9 (21.3) for PCT group, 68.7 (17.0) for wait‐list group Trauma type: not reported Duration of time since trauma: not reported Comorbid conditions: 72% of participants met SCID criteria for a current Axis I disorder other than PTSD. 61% met criteria for anxiety disorders, 34% met criteria for depressive disorders, 8% met criteria for bipolar disorders, and 9% met criteria for psychotic disorders. 43% met criteria for past substance dependence or abuse and 11% met criteria for substance abuse or dependence in the past year. Diagnostic criteria: CAPS‐IV, full and partial (meets Criterion B and Criterion C or D) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: TARGET (trauma affect regulation: guide for education and therapy) Description: a cognitive behavioral therapy that aims to enhance affect regulation without trauma memory processing. Because this treatment is not trauma‐focused, this treatment arm was not included in analyses for this review. Delivered by: eight experienced female therapists with doctoral degrees in clinical psychology, psychiatry or master's degrees in social work, counseling, or marriage and family therapy. Five of these therapists conducted TARGET. Number of sessions: 12 50‐minute sessions Format: individual Group II: PCT Manual/Model: McDonagh 2005 version Delivered by: eight experienced female therapists with doctoral degrees in clinical psychology, psychiatry or master's degrees in social work, counseling, or marriage and family therapy. Three of these therapists conducted PCT. Number of sessions: 12 sessions Format: individual Group III: wait‐list |
|
| Outcomes |
PTSD:
Depression:
Anxiety:
|
|
| Notes | Out of 48 randomized to TARGET, 14 did not complete the post‐treatment interview, 17 did not complete 3‐month follow‐up, and 17 did not complete 6‐month follow‐up. Out of 53 randomized to PCT, 18 did not complete the post‐treatment interview, 19 did not complete 3‐month follow‐up, and 21 did not complete 6‐month follow‐up. Out of 45 randomized to the control group, 10 did not complete the post‐wait‐list interview. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Experimental condition was assigned at the end of the baseline interview via an Excel‐generated standard sequence‐concealed number". |
| Allocation concealment (selection bias) | Low risk | Quote: "Experimental condition was assigned at the end of the baseline interview via an Excel‐generated standard sequence‐concealed number". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | High risk | Quote: "All interviewers were blind to the experimental condition in baseline interviews, but due to technical difficulties they were not blind to experimental condition at posttherapy or follow‐up interviews". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | High risk | The clinicaltrials.gov trial registration for this study included the outcome measure "Multiscale Dissociation Inventory," and this outcome was not reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; principal investigator is a developer of treatment under investigation (TARGET). |
McDonagh 2005.
| Methods |
Design: parallel group RCT Dates of study: not reported Number of study centers and locations: one, location not reported Recruitment: not reported Study duration: six months |
|
| Participants |
Participants: 74 Age: mean (SD) 39.8 (9.9) for CBT group, 39.6 (9.6) for the PCT group, 42.0 (9.8) for the wait‐list group Sex: 0% male Baseline CAPS score: mean (SD) 69.9 (16.8) for the CBT group, 67.7 (14.6) for the PCT group, 72.0 (17.6) for the wait‐list group Trauma type: CSA Duration of time since trauma: not reported Comorbid conditions: 10.8% of the sample met criteria for borderline personality disorder Diagnostic criteria: DSM‐IV criteria for PTSD as assessed by the CAPS Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: cognitive behavioral therapy (CBT) Description: primary components were PE, in vivo exposure, and cognitive restructuring. Therapists also provided psychoeducation, both about PTSD and the rationale for therapy techniques, and taught the use of breathing retraining. Homework involved each of the components of treatment following their introduction during sessions. Delivered by: female clinicians experienced in conducting therapy with trauma survivors. Three psychologists, all of whom had prior training in CBT, received training on implementation of the CBT manual. Number of sessions: 14 Format: individual Group II: PCT Manual/Model: McDonagh 2005 version Delivered by: female clinicians experienced in conducting therapy with trauma survivors. Three clinical social workers with master’s degrees received PCT training from the authors of that manual. Number of sessions: 14 Format: individual Group III: wait‐list Description: participants assigned to the WL were told that they could receive their choice of the two treatments in about 14 weeks, after completing the post‐WL assessment. |
|
| Outcomes |
PTSD:
Depression:
Anxiety:
Dissociation:
|
|
| Notes | Withdrawals not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The random assignment process was changed partway through the study to increase the chance of assignment to CBT. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Interviews were conducted by clinicians blind to treatment condition and who had no other role in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conducted both intention‐to‐treat and treatment completer analyses. Higher dropout rates in the TF‐CBT arm relative to PCT |
| Selective reporting (reporting bias) | Unclear risk | A study protocol was not available, and there was no self‐report measure of PTSD. |
| Other bias | Low risk | None detected |
NCT00607815.
| Methods |
Design: parallel group RCT Dates of study: June 15, 2009 ‐ December 27, 2015 Number of study centers and locations: one VA medical center (Cincinnati, Ohio) Recruitment: not reported Study duration: 1 year |
|
| Participants |
Participants: 79 Age: mean (SD) 30.9 (7.6) overall, 29.5 (7.1) for the CPT group, 32.1 (7.9) for the PCT group Sex: 100% male Baseline CAPS score: mean (SD) 61.8 (13.1) overall, 60.1 (11.6) for the CPT group, 62.4 (14.8) for the PCT group Trauma type: not reported Duration of time since trauma: not reported Comorbid conditions: not reported Diagnostic criteria: SCID and CAPS Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: cognitive processing therapy (CPT) Description: clients learn the skills of recognizing and challenging dysfunctional cognitions related to post‐traumatic beliefs. Delivered by: not reported Number of sessions: 12 weekly sessions Format: individual Group II: PCT Manual/Model: CSP 494 version Delivered by: not reported Number of sessions: not reported Format: individual |
|
| Outcomes |
PTSD:
Depression:
Anxiety:
|
|
| Notes | Unpublished trial. Data was obtained from clinicaltrials.gov and author contact. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study statistician used a random number generator program (information received via email correspondence with the lead investigator). |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was not known prior to randomization (information received via email correspondence with the lead investigator). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trial |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation was not feasible. |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Protocol stated that outcomes assessors would be blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data have not been published and study authors did not respond to requests to confirm numbers given in clinicaltrials.gov. Higher dropout rates in the TF‐CBT arm relative to PCT |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's clinicaltrials.gov trial registration were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; principal investigator is a developer of treatment under investigation (CPT). |
Rauch 2015.
| Methods |
Design: parallel group RCT Dates of study: January 2008 ‐ July 2010 Number of study centers and locations: one VA medical center (Ann Arbor, Michigan) Recruitment: veterans who presented to the medical center clinical team Study duration: 12 weeks |
|
| Participants |
Participants: 30 Age: mean (SD) 31.9 (7.6) Sex: 92% male Baseline CAPS score: not reported Trauma type: not reported Duration of time since trauma: not reported Comorbid conditions: 57% had depression or dysthymia, 10% had alcohol abuse, 29% met criteria for another anxiety disorder Diagnostic criteria: CAPS score greater than or equal to 50 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: prolonged exposure therapy (PE) Description: includes psychoeducation, exposure to trauma memories (imaginal exposure), in vivo exposure to trauma‐related avoided situations (in vivo exposure), and emotional processing Delivered by: an experienced PE provider Number of sessions: 10 to 12, 80‐min sessions Format: individual Group II: PCT Manual/Model: CSP 494 version Delivered by: the first author was the study therapist Number of sessions: 10 to 12, 80‐min sessions Format: individual |
|
| Outcomes |
PTSD:
|
|
| Notes | No data on 6 participants (not clear which groups), 11 of 18 completed PE, 15 of 18 completed PCT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized to condition via random number allocation (information received via email correspondence with the lead investigator). |
| Allocation concealment (selection bias) | Low risk | Investigators did not have knowledge of which group participants would be assigned to (information received via email correspondence with the lead investigator). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Interviews conducted by independent evaluators |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses done on treatment completers |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's clinicaltrials.gov trial registration were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; member of study team (Rothbaum) had authored a book on the treatment under investigation (PE). |
Ready 2010.
| Methods |
Design: parallel group RCT Dates of study: not reported Number of study centers and locations: one VA medical center (Atlanta, Georgia) Recruitment: presentations to mental health staff within medical center, flyers in the mental health clinic, advertising on medical center–wide TV, advertisements in local free weekly newspapers, and announcing the study in ongoing PTSD groups Study duration: six months |
|
| Participants |
Participants: 11 Age: mean (SD) 57.0 (3.0) for the VRET group, 58 (3.1) for the PCT group Sex: 100% male Baseline CAPS score: 87.8 (15.4) for the VRET group, 101.0 (9.5) for the PCT group Trauma type: not reported Duration of time since trauma: not reported Comorbid conditions: not reported Diagnostic criteria: CAPS score of greater than 60 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: virtual reality exposure therapy (VRET) Description: participants engage in exposure exercises via virtual reality technologies. Delivered by: not reported Number of sessions: ten 90‐minute sessions Format: individual Group II: PCT Manual/Model: CSP 494 version Delivered by: not reported Number of sessions: ten 90‐minute sessions Format: individual |
|
| Outcomes |
PTSD:
Depression:
|
|
| Notes | Of the five veterans in PCT, four completed the measures at post‐treatment and four completed the measures at follow‐up. Of the six veterans in the VRET condition, five completed the measures at post‐treatment and four completed the measures at follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (information received via email correspondence with the lead investigator). |
| Allocation concealment (selection bias) | Low risk | Investigators were not aware of which treatment group patients would be assigned to (information received via email correspondence with the lead investigator). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Interviews were conducted by a licensed clinical psychologist blind to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses included treatment completers only |
| Selective reporting (reporting bias) | High risk | The clinicaltrials.gov trial registration for this study included the outcome measure "Mississippi Scale for Combat‐Related PTSD," and this outcome was not reported in the publication. |
| Other bias | Unclear risk | Possible significant differences between groups at baseline. A study author (Rothbaum) is a consultant to and owns equity in a company developing products related to the research conducted for this study. |
Ready 2018.
| Methods |
Design: parallel group RCT Dates of study: July 2007 ‐ August 2012 Number of study centers and locations: one VA medical center (Decatur, Georgia) Recruitment: individuals referred to an outpatient PTSD program were first screened and informed about the study Study duration: one year |
|
| Participants |
Participants: 81 Age: mean 61.4 overall, 61.4 for the GBET group, 61.3 for the GPCT group Sex: 100% male Baseline CAPS score: mean (SD) 82.4 (15.1) for the GBET group, 81.2 (14.3) for the GPCT group Trauma type: combat Duration of time since trauma: not reported Comorbid conditions: not reported Diagnostic criteria: war‐related PTSD as assessed by the CAPS Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: group based exposure therapy (GBET) Description: patients undergo exposure by making war trauma presentations to the group, listening to recordings of their presentations, and hearing the presentations of other group members. Patients learn about PTSD symptoms, sleep hygiene, specific stress/anger management techniques, and ways to cognitively restructure trauma‐related thinking. Delivered by: four psychotherapists without prior GBET experience Number of sessions: twice a week for 16 weeks for three hours per day Format: group Group II: GPCT Manual/Model: CSP 420 version Delivered by: not reported Number of sessions: twice a week for 16 weeks for 90 minutes per session Format: group |
|
| Outcomes |
PTSD:
|
|
| Notes | 6 out of 41 in GBET group did not complete 12‐month CAPS, 7 out of 40 in PCGT did not complete 12‐month CAPS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomized by block. The treatment condition of the first block was determined by a coin flip, and alternated thereafter (information received via email correspondence with the lead investigator). |
| Allocation concealment (selection bias) | High risk | Groups alternated and so it was predictable, subsequent to the first group, in which group participants would be assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Assessors were blind to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent to treat analysis used |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's clinicaltrials.gov trial registration were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; principal investigator is a developer of treatment under investigation (GBET). |
Resick 2015.
| Methods |
Design: parallel group RCT Dates of study: February 2011 ‐ June 2013 Number of study centers and locations: one, military base in Ft. Hood, Texas Recruitment: direct referrals from military providers or advertisements Study duration: one year |
|
| Participants |
Participants: 108 Age: mean (SD) 31.8 (7.3) for the CPT‐C group, 32.4 (7.9) for the GPCT group Sex: 93% male for the CPT‐C group, 92% male for the GPCT group Baseline PSS‐I score: 27.7 (7.4) for the CPT‐C group, 27.1 (7.0) for the GPCT group Trauma type: required experience of a Criterion A traumatic event that occurred during military deployment. However, the diagnosis of PTSD may have been based on another, worse Criterion A event at anytime in their lives. Duration of time since trauma: not reported Comorbid conditions: not reported Diagnostic criteria: DSM–IV–TR criteria assessed by the PSS‐I. After the items were assessed, there was one item added to determine whether the symptoms had been present for the past month in order to establish the time frame necessary for PTSD diagnosis. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: cognitive processing therapy ‐ cognitive only (CPT‐C) Description: a cognitive therapy that focuses on why patients believe the index event occurred, how that event affected their beliefs about self and others, and how to differentiate thoughts from facts. Patients then learn to label events, thoughts, and subsequent emotions, while the therapist helps them examine the facts and context of the trauma through Socratic questioning. Using progressive worksheets, patients are taught to examine their own thoughts and emotions and develop new, more balanced thinking about traumatic events. Delivered by: five female civilian therapists with limited CPT‐C experience prior to the trial were trained with an official 3‐day CPT workshop and a 1‐day PCT workshop. Number of sessions: 12 bi‐weekly Format: group Group II: GPCT Manual/Model: CSP 420 version Delivered by: civilian therapists Number of sessions: 12 bi‐weekly Format: group |
|
| Outcomes |
PTSD:
Depression:
|
|
| Notes | In CPT‐C group, 11 did not complete post‐treatment, 20 did not complete 6‐month follow‐up, and 28 did not complete 12‐month follow‐up. In the GPCT group, 3 did not complete post‐treatment, 14 did not complete 6‐month follow‐up, and 24 did not complete 12‐month follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer program to create randomized block sizes (information received via email correspondence with a member of the study team). |
| Allocation concealment (selection bias) | Low risk | A custom web‐based application masked the randomization process (information received via email correspondence with a member of the study team). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Administered by independent evaluators blind to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mixed‐effects model used that accounted for missing data |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's clinicaltrials.gov trial registration were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; principal investigator is a developer of treatment under investigation (CPT‐C). |
Schnurr 2003.
| Methods |
Design: parallel group RCT Dates of study: not reported Number of study centers and locations: 10 VA medical centers Recruitment: participants were referred by clinical staff at the ten study sites Study duration: 12 months for all participants, 18 and 24 months for a subset of participants |
|
| Participants |
Participants: 360 Age: mean (SD) 50.7 (3.7) overall, 50.6 (3.7) for TFGT group, 50.8 (3.8) for GPCT group Sex: 100% male Baseline CAPS score: mean (SD) 80.4 (1.5) for the TFGT group, 82.0 (1.4) for the GPCT group Trauma type: combat Duration of time since trauma: not reported Comorbid conditions: 56% had a current mood disorder, 31.7% had a current anxiety disorder, and 4.6% had current substance abuse Diagnostic criteria: combat‐related PTSD according to DSM‐IV criteria as measured by the CAPS Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: trauma‐focused group therapy (TFGT) Description: treatment components entail psychoeducation about PTSD, coping resource assessment, and self‐management of symptoms, pre‐military autobiographies, war zone scene identification, exposure, and cognitive restructuring, relapse prevention. Delivered by: two master’s‐ or doctoral‐level clinicians with previous experience in treating PTSD led each group. They were not required to have formal training in exposure techniques or cognitive‐behavioral therapy. They provided only 1 of the 2 treatments and were randomly assigned to the treatment they provided. Number of sessions: weekly for 30 weeks, then 5 monthly booster sessions Format: group Group II: GPCT Manual/Model: CSP 420 version Delivered by: two master’s‐ or doctoral‐level clinicians with previous experience in treating PTSD Number of sessions: weekly for 30 weeks, then 5 monthly booster sessions Format: group |
|
| Outcomes |
PTSD:
|
|
| Notes | Out of 180 participants assigned to TFGT, 18 did not participate in measurement. Out of 180 participants assigned to PCGT, 17 did not participate in measurement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using permuted blocks to ensure balance in treatment groups by CAPS score. |
| Allocation concealment (selection bias) | Low risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Assessors were not aware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat analysis used |
| Selective reporting (reporting bias) | Low risk | A study protocol was not available, but all expected study outcomes were reported. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; a co‐investigator is the developer of treatment under investigation (Trauma‐Focused Group Therapy). |
Schnurr 2007.
| Methods |
Design: parallel group RCT Dates of study: August 2002 ‐ October 2005 Number of study centers and locations: nine VA medical centers, two VA re‐adjustment counseling centers, and one military hospital Recruitment: clinician referrals Study duration: six months |
|
| Participants |
Participants: 277 Age: mean (CI) 44.6 (43.1 to 46.2) for the PE group, 44.9 (43.4 to 46.5) for the PCT group Sex: 0% male Baseline CAPS score: mean (CI) 77.6 (74.8 to 80.4) for the PE group, 77.9 (75.1 to 80.6) for the PCT group Trauma type: sexual trauma (68.3%), physical assault (15.8%), and war‐zone exposure (5.6%) Duration of time since trauma: average of 23.0 years in PE group, 22.8 years in PCT group Comorbid conditions: in the PE group, 75.2% had any current comorbid psychiatric disorder, 61.7% had a current comorbid mood disorder, 48.9% had a current comorbid anxiety disorder, and 2.1% had current comorbid substance abuse. In the PCT group, 80.4% had any current comorbid psychiatric disorder, 65.7% had a current comorbid mood disorder, 46.9% had a current comorbid anxiety disorder, and 2.1% had current comorbid substance abuse. Diagnostic criteria: overall CAPS score of 45 or higher, and symptoms occurred at least monthly with moderate intensity Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: prolonged exposure therapy (PE) Description: "...included education about common reactions to trauma; breathing retraining; prolonged (repeated) recounting (imaginal exposure) of trauma memories during sessions; homework (listening to a recording of the recounting made during the therapy session and repeated in vivo exposure to safe situations the patient avoids because of trauma‐related fear); and discussion of thoughts and feelings related to exposure exercises. Sessions 1 and 2 were introductory and included provision of the treatment rationale and education about PTSD. Imaginal exposure occurred in sessions 3 through 10." Delivered by: 52 female therapists who were master’s‐ or doctoral‐level clinicians experienced in treating women with PTSD Number of sessions: 10 weekly 90‐minute sessions Format: individual Group II: PCT Manual/Model: CSP 494 version Delivered by: 52 female therapists who were master’s‐ or doctoral‐level clinicians experienced in treating women with PTSD Number of sessions: 10 weekly 90‐minute sessions Format: individual |
|
| Outcomes |
PTSD:
Depression:
Anxiety:
|
|
| Notes | 21 lost to follow‐up out of 141 in PE group, 17 lost to follow‐up out of 142 in PCT group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using permuted blocks with random block sizes of 4 or 6. |
| Allocation concealment (selection bias) | Low risk | Study staff called a computerized voice information system at the study coordinating center to obtain the treatment assignment for participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Assessors blinded to treatment assignment performed assessments before and after treatment and at 3‐ and 6‐month follow‐up appointments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis for all outcomes. Higher dropout rates in the TF‐CBT arm relative to PCT |
| Selective reporting (reporting bias) | Low risk | No published protocol, but all outcomes specified in design paper were reported in study publications. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; study team member (Foa) is a developer of treatment under investigation (PE). |
Sloan 2018.
| Methods |
Design: parallel group RCT Dates of study: November 2012 ‐ August 2017 Number of study centers and locations: two ‐ VA Boston Healthcare System and Providence VA Medical Center Recruitment: clinic referrals and flyer announcements Study duration: 12 months |
|
| Participants |
Participants: 198 Age: mean (SD) 55.82 (12.05) overall, 54.4 (11.44) for the GCBT group, 57.22 (12.51) for the GPCT group Sex: 100% male Baseline CAPS‐5 score: mean (SD) 39.84 (9.84) for the GCBT group, 39.37 (9.52) for the GPCT group Trauma type: 69.7% combat, 7.1% death of or trauma to friend or loved one, 3% adult sexual assault, 4% adult nonsexual assault, 0.5% childhood sexual assault, 3.5% childhood nonsexual assault, 8.6% accident, 3.5% other Duration of time since trauma: average 334 months Comorbid conditions: in the GCBT group, 55.1% had MDD, 21.4% had GAD, 12.2% had panic disorder, 9.2% had binge eating disorder, 7.1% had social anxiety disorder, 5.1% had specific phobia, 3.06% had OCD, 3.06% had cannabis abuse, 1.02% had alcohol abuse. In the GPCT group, 57% had MDD, 18% had GAD, 14% had panic disorder, 6% had binge eating disorder, 10% had social anxiety disorder, 3% had specific phobia, 3% had OCD, 2% had cannabis abuse, 3% had alcohol abuse. Diagnostic criteria: CAPS‐5 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: group cognitive‐behavioral treatment (GCBT) Description: "...focuses on nurturing group cohesion while introducing cognitive‐behavioral interventions that focus on trauma. Considerable emphasis is placed on between‐session practice (homework) in an effort to foster acquisition and generalization of skills. Interventions include psychoeducation, in vivo and written exposure, progressive muscle relaxation, cognitive restructuring of post‐trauma dysfunctional thoughts, assertion training, behavioral activation, and prevention of symptom recurrence." Delivered by: two therapists, drawn from existing mental health providers, led each group. Number of sessions: 14 two‐hour sessions scheduled across 16 weeks Format: group Group II: GPCT Manual/Model: CSP 420 version Delivered by: two therapists, drawn from existing mental health providers, led each group. Number of sessions: 14 two‐hour sessions scheduled across 16 weeks Format: group |
|
| Outcomes |
PTSD:
Depression:
|
|
| Notes | Out of 98 randomized to GCBT, 11 did not complete the mid‐treatment assessment, 24 did not complete the 1‐month assessment, 20 did not complete the 3‐month assessment, 32 did not complete the 6‐month assessment. Out of 100 randomized to GPCT, 6 did not complete the mid‐treatment assessment, 12 did not complete the 1‐month assessment, 14 did not complete the 3‐month assessment, 18 did not complete the 6‐month assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study statistician generated randomization using the sealed envelope program. |
| Allocation concealment (selection bias) | Low risk | After all participants in a group cohort completed baseline assessments, sealed envelopes were opened to reveal treatment assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Assessments were conducted by assessors unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses were conducted using all data points for participants who were randomized (i.e., intent to treat). Higher dropout rates in the TF‐CBT arm relative to PCT |
| Selective reporting (reporting bias) | Low risk | All outcome measures listed in the study's clinicaltrials.gov trial registration were reported in the publication. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; study team member (Beck) is a developer of treatment protocol under investigation (GCBT). |
Suris 2013.
| Methods |
Design: parallel group RCT Dates of study: February 2007 ‐ August 2009 Number of study centers and locations: one VA medical center (Dallas, Texas) Recruitment: letters describing the study, posted advertisements, and promotion of the study in therapy groups, staff meetings, and at veterans’ centers Study duration: six months |
|
| Participants |
Participants: 129 Age: mean (SD) 46.1 (9.8) overall, 44.6 (10.5) for the CPT group, 48.4 (8.2) for the PCT group Sex: 15% male Baseline CAPS score: mean (SD) 85.1 (2.7) for the CPT group, 83.8 (3.3) for the PCT group Trauma type: MST Duration of time since trauma: ≥ 3 months prior to study entry Comorbid conditions: not reported Diagnostic criteria: an overall severity cut‐off score of 45 on the CAPS and the “1–2” rule of scoring Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group I: Cognitive processing therapy (CPT) Description: "The first seven sessions address education, examination of thoughts through Socratic dialogue, and skill building; the remaining five sessions challenge beliefs surrounding themes of safety, trust, power,self‐esteem, and intimacy." Delivered by: trained masters‐ or doctoral‐level mental health providers trained in CPT Number of sessions: 12 Format: individual Group II: PCT Manual/Model: CSP 494 version Delivered by: trained masters‐ or doctoral‐level mental health providers trained in PCT Number of sessions: 12 Format: individual |
|
| Outcomes |
PTSD:
Depression:
|
|
| Notes | 40 did not complete post‐treatment assessment; 40 did not complete 2‐month assessment; 40 did not complete 4‐month assessment; 39 did not complete 6‐month assessment. The baseline CAPS re‐experiencing subscale (CAPS B) was significantly higher for the CPT than for the PCT group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence was used to assign people into intervention and comparator groups using block randomization. |
| Allocation concealment (selection bias) | Low risk | Condition was only revealed after participant's pin number was entered into the excel spreadsheet. Did not seem likely that staff knew ahead of time which arm participants would be assigned to |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible in psychotherapy trials |
| Blinding of outcome assessment (detection bias) Patient reported symptoms | High risk | Blinding of participants to treatment allocation not feasible |
| Blinding of outcome assessment (detection bias) Observer rated symptoms | Low risk | Assessors were blind to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to treatment fidelity issues, 43 subjects were removed from the analysis. |
| Selective reporting (reporting bias) | Low risk | A study protocol was not available, but study publications included expected outcomes. |
| Other bias | Unclear risk | Potential concerns of bias due to investigator allegiance; study team member (Chard) is a developer of treatment protocol under investigation (CPT). |
BAI: Beck Anxiety Inventory BDI: Beck Depression Inventory BDI‐II: Beck Depression Inventory‐II CAPS: Clinician Administered PTSD Scale CAPS‐IV:Clinician Administered PTSD Scale for DSM‐IV CAPS‐5: Clinician Administered PTSD Scale for DSM‐5 CAPS‐B: Clinician Administered PTSD Scale re‐experiencing subscale CBT: cognitive behavioral therapy CI: confidence interval CPT: cognitive processing therapy CPT‐C: cognitive processing therapy ‐ cognitive only CSA: childhood sexual abuse CSP: VA Cooperative Studies Program DES: Dissociative Experiences Scale DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision GAD: generalized anxiety disorder GCBT: group cognitive behavioral therapy GBET: group‐based exposure therapy GPCT: group present‐centered therapy HPA: hypothalamic‐pituitary‐adrenal MCC: minimal contact control MDD: major depressive disorder MST: military sexual trauma OCD: obsessive‐compulsive disorder OEF: Operation Enduring Freedom OIF: Operation Iraqi Freedom OND: Operation New Dawn PCL: PTSD Checklist PCL‐5: PTSD Checklist for DSM‐5 PCL‐S: PTSD Checklist ‐ Specific Version PCT: present‐centered therapy PE: prolonged exposure PSS‐I: PTSD Symptom Scale‐Interview PTSD: post‐traumatic stress disorder QIDS: Quick Inventory of Depressive Symptomatology RCT: randomized controlled trial SCID: Structured Clinical Interview for DSM‐IV SD: standard deviation STAI: The State‐Trait Anxiety Inventory STRONG STAR: South Texas Research Organizational Network Guiding Studies on Trauma and Resilience TARGET: Trauma Affect Regulation: Guide for Education and Therapy TF‐CBT: trauma‐focused cognitive behavioral therapy TFGT: trauma‐focused group therapy VA: Veterans Affairs VRET: virtual reality exposure therapy WL: wait list
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bormann 2018 | PCT was not compared to TF‐CBT or a control condition. |
| Bremner 2017 | PCT was not compared to TF‐CBT or a control condition. |
| Classen 2001 | Present‐focused group therapy was not consistent with our definition of PCT. |
| Classen 2011 | Participants were not required to have a diagnosis of PTSD. |
| Davis 2019 | PCT was not compared to TF‐CBT or a control condition. |
| Foa 1991 | Present‐focused group therapy was not consistent with our definition of PCT. |
| Grant 2005 | Not an RCT |
| Harris 2018 | PCT was not compared to TF‐CBT or a control condition. |
| Haynes 2012 | PCT was not compared to TF‐CBT or a control condition. |
| Hong 2013 | Participants were required to have experienced or witnessed DSM‐IV‐TR Criterion A traumatic event, but were not required to have clinically significant levels of PTSD. |
| King 2016 | PCT was not compared to TF‐CBT or a control condition. |
| Lang 2017 | PCT was not compared to TF‐CBT or a control condition. |
| NCT00607412 | Present‐centered therapy was not an intervention. |
| NCT01274741 | Present‐centered therapy was not an intervention. |
| NCT02081417 | Present‐centered therapy was not an intervention. |
| NCT02233517 | PCT was not compared to TF‐CBT or a control condition. |
| NCT02398227 | PCT was not compared to TF‐CBT or a control condition. |
| NCT03056157 | PCT was not compared to TF‐CBT or a control condition. |
| NCT03429166 | PCT was not compared to TF‐CBT or a control condition. |
| NCT03760731 | Participants were not required to have a diagnosis of PTSD. |
| NCT03764033 | PCT was not compared to TF‐CBT or a control condition. |
| Polusny 2015 | PCT was not compared to TF‐CBT or a control condition. |
| Resick 2009 | Conference presentation that did not present the results of an RCT |
| Rosner 2018 | Participants were not required to have a diagnosis of PTSD. |
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision PCT: present‐centered therapy PTSD: post‐traumatic stress disorder RCT: randomized controlled trial TF‐CBT: trauma‐focused cognitive‐behavioral therapy
Characteristics of studies awaiting assessment [ordered by study ID]
Lely 2017.
| Methods | RCT |
| Participants | Age 55 or older; PTSD according to DSM‐IV criteria |
| Interventions | Narrative Exposure Therapy vs PCT |
| Outcomes | Primary outcomes:
|
| Notes | No full text, manuscript in preparation and data not provided by study investigators |
NCT00057629.
| Methods | RCT |
| Participants | 18 to 67 years old; PTSD according to DSM‐IV criteria resulting from sexual assault, and at least 12 weeks after sexual assault |
| Interventions | Prolonged exposure vs supportive counseling vs treatment‐as‐usual group therapy |
| Outcomes | Primary outcomes:
|
| Notes | No full text, manuscript in preparation and data not provided by study investigators |
CAPS: Clinician Administered PTSD Scale DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition HTQ‐16: Harvard Trauma Questionnaire PCT: present‐centered therapy PTSD: post‐traumatic stress disorder RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02556645.
| Trial name or title | A comparison of web‐prolonged exposure (Web‐PE) and present‐centered therapy (PCT) for PTSD among active‐duty military personnel |
| Methods | RCT |
| Participants | 18 to 65 years old; adult male and female active duty military personnel who had deployed since 9/11; seeking treatment for PTSD; PTSD according to DSM‐5; DSM‐5 Criterion A event is combat‐related or an operational experience that occurred during deployment |
| Interventions | Web‐PE vs PCT |
| Outcomes | Primary outcomes:
|
| Starting date | 05/2016 |
| Contact information | Dr Carmen McLean Center for the Treatment and Study of Anxiety University of Pennsylvania Philadelphia, Pennsylvania |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02556645 |
CAPS: Clinician Administered PTSD Scale DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition PCT: present‐centered therapy PTSD: post‐traumatic stress disorder RCT: randomized controlled trial Web‐PE: web‐prolonged exposure
Differences between protocol and review
(1) The exclusion criteria in our protocol did not state that trials that compared PCT with active treatments other then TF‐CBT would be excluded. These trials were not included in the review, as they were not relevant to the original study objectives. In the review, an additional exclusion criterion was added to address this omission: comparisons other than TF‐CBT or control conditions.
(2) We were more explicit about the non‐inferiority hypothesis and the use of mean differences (as the primary analysis) and standardized mean differences (as supplemental analyses). Mean differences are more precise, despite the loss of potential eligible studies, which is important for non‐inferiority analyses. Furthermore, we still confirmed these primary analyses by evaluating SMD between treatments using guidance from ISTSS (Berliner 2019).
(3) Subgroup analyses focused on just TF‐CBT and PCT comparisons and were limited to treatment modality (group versus individual) and TF‐CBT intervention type (CPT versus PE).
(4) We simplified our sensitivity analyses to focus on just those studies deemed higher quality based on outcome masking, appropriate handling of missing data (ITT; mixed‐model analysis), adequate power, and low levels (< 40%) of post‐randomization treatment loss.
Contributions of authors
Bradley Belsher and Daniel Evatt had the initial idea for undertaking the review. Bradley Belsher, Erin Beech, and Xian Liu wrote the initial draft of the protocol. Bradley Belsher, Erin Beech, and Derek Smolenski wrote the draft of the review. Daniel Evatt, Jean Otto, Paula Schnurr, Tracie Shea, and Craig Rosen reviewed the review and provided comments. All review authors reviewed and approved the final draft.
Declarations of interest
BB: none known. EB: none known. DE: none known. CSR: none known. XL: none known. JO: none known. PPS: I have received grant funding from the Department of Veterans Affairs and the Department of Defense to conduct research on treatments for PTSD that include Present‐Centered Therapy. I also served as a VA Co‐Champion of the workgroup that developed the 2017 VA/DoD PTSD Practice Guideline. In addition, I have received payment from Noblis Therapeutics for consulting on the design of a research study on PTSD treatment. TS: I have received grant funding from the Department of Veterans Affairs to conduct research on treatments for PTSD that include Present‐Centered Therapy, and have conducted training for research studies using Present‐Centered Therapy. I am contributing a chapter to a book in progress on Present‐Centered Therapy.
New
References
References to studies included in this review
Foa 2018 {published data only}
- Brown LA, Clapp JD, Kemp JJ, Yarvis JS, Dondanville KA, Litz BT, et al. The pattern of symptom change during prolonged exposure therapy and present‐centered therapy for PTSD in active duty military personnel. Psychological Medicine 2018;49(12):1‐10. [DOI] [PubMed] [Google Scholar]
- Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present‐centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA 2018;319(4):354‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2011 {published data only}
- Ford JD, Steinberg KL, Zhang W. A randomized clinical trial comparing affect regulation and social problem‐solving psychotherapies for mothers with victimization‐related PTSD. Behavior Therapy 2011;42(4):560‐78. [DOI] [PubMed] [Google Scholar]
McDonagh 2005 {published data only}
- McDonagh A, Friedman M, McHugo G, Ford J, Sengupta A, Mueser K, et al. Randomized trial of cognitive‐behavioral therapy for chronic posttraumatic stress disorder in adult female survivors of childhood sexual abuse. Journal of Consulting and Clinical Psychology 2005;73(3):515‐24. [DOI] [PubMed] [Google Scholar]
NCT00607815 {unpublished data only}
- NCT00607815. A comparison of cognitive processing therapy (CPT) versus present centered therapy (PCT) for veterans. Clinicaltrials.gov/ct2/show/NCT00607815 (first received 6 February 2008).
Rauch 2015 {published data only}
- NCT00475241. Exposure therapy for chronic PTSD: efficacy and mechanisms. Clinicaltrials.gov/ct2/show/NCT00475241 (first received 21 May 2007).
- Rauch S, Sripada R, King A, Abelson J, Rothbaum B, Liberzon I. Extinction and change in cognitions and cortisol activity in posttraumatic stress disorder treatment. Neuropsychopharmacology 2014;39(13):S356‐7. [Google Scholar]
- Rauch SAM, King AP, Abelson J, Tuerk PW, Smith E, Rothbaum BO, et al. Biological and symptom changes in posttraumatic stress disorder treatment: a randomized clinical trial. Depression and Anxiety 2015;32(3):204‐12. [DOI] [PubMed] [Google Scholar]
- Sripada R, King A, Liberzon I, Rauch S. Within‐session salivary cortisol reactivity during psychotherapy is associated with treatment outcome for post‐traumatic stress disorder. American College of Neuropsychopharmacology 54th Annual Meeting, Hollywood FL. 2015:S111‐2.
- Sripada RK, Rauch SAM, Tuerk PW, Smith E, Defever AM, Mayer RA, et al. Mild traumatic brain injury and treatment response in prolonged exposure for PTSD. Journal of Traumatic Stress 2013;26(3):369‐75. [DOI] [PubMed] [Google Scholar]
Ready 2010 {published data only}
- NCT00167804. Comparing virtual reality therapy to usual treatment for PTSD. Clinicaltrials.gov/ct2/show/NCT00167804 (first received 14 September 2005).
- Ready DJ, Gerardi RJ, Backscheider AG, Mascaro N, Rothbaum BO. Comparing virtual reality exposure therapy to present‐centered therapy with 11 U.S. Vietnam veterans with PTSD. Cyberpsychology, Behavior and Social Networking 2010;13(1):49‐54. [DOI] [PubMed] [Google Scholar]
Ready 2018 {published data only}
- NCT00535223. Group based exposure therapy for combat‐related PTSD. Clinicaltrials.gov/ct2/show/NCT00535223 (first received 26 September 2007).
- Ready DJ, Mascaro N, Wattenberg MS, Sylvers P, Worley V, Bradley‐Davino B. A controlled study of group‐based exposure therapy with Vietnam‐era veterans. Journal of Loss and Trauma 2018;23(6):439‐57. [Google Scholar]
Resick 2015 {published data only}
- Bryan CJ, Clemans TA, Hernandez AM, Mintz J, Peterson AL, Yarvis JS, et al. Evaluating potential iatrogenic suicide risk in trauma‐focused group cognitive behavioral therapy for the treatment of PTSD in active duty military personnel. Depression and Anxiety 2016;33(6):549‐57. [DOI] [PubMed] [Google Scholar]
- NCT01286415. Group cognitive processing therapy for combat‐related posttraumatic stress disorder (PTSD). Clinicaltrials.gov/ct2/show/NCT01286415 (first received 31 January 2011).
- Pruiksma KE, Taylor DJ, Schuster Wachen J, Mintz J, Young‐McCaughan S, Peterson AL, et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychological Trauma: Theory, Research, Practice, and Policy 2016;8(6):697‐701. [DOI] [PubMed] [Google Scholar]
- Resick PA, Shuster Wachen J, Mintz J, Young‐McCaughan S, Roache JD, Borah AM, et al. A randomized clinical trial of group cognitive processing therapy compared with group present‐centered therapy for PTSD among active duty military personnel. Journal of Consulting and Clinical Psychology 2015;83(6):1058‐68. [DOI] [PubMed] [Google Scholar]
Schnurr 2003 {published data only}
- Monson CM, Gradus JL, Young‐Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree?. Psychological Assessment 2008;20(2):131‐8. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Foy DW, Shea MT, Hsieh FY, Lavori PW, et al. Randomized trial of trauma‐focused group therapy for posttraumatic stress disorder: results from a Department of Veterans Affairs cooperative study. Archives of General Psychiatry 2003;60(5):481‐9. [DOI] [PubMed] [Google Scholar]
Schnurr 2007 {published data only}
- Rosen CS, Greenbaum MA, Schnurr PP, Holmes TH, Brennan, PL, Friedman MJ. Do benzodiazepines reduce the effectiveness of exposure therapy for posttraumatic stress disorder?. Journal of Clinical Psychiatry 2013;74(12):1241‐8. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 2007;297(8):820‐30. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Lunney CA. Differential effects of prolonged exposure on posttraumatic stress disorder symptoms in female veterans. Journal of Consulting and Clinical Psychology 2015;83(6):1154‐60. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Lunney CA. Residual symptoms following prolonged exposure and present‐centered therapy for PTSD in female veterans and soldiers. Depression & Anxiety 2019;36(2):162‐9. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Lunney CA. Work‐related outcomes among female veterans and service members after treatment of posttraumatic stress disorder. Psychiatric Services 2012;63(11):1072‐9. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Lunney CA, Forshay E, Thurston VL, Chow BK, Resick PA, et al. Sexual function outcomes in women treated for posttraumatic stress disorder. Journal of Women's Health 2009;18(10):1549‐57. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Lunney CA, Schnurr PP. The influence of the dissociative subtype of posttraumatic stress disorder on treatment efficacy in female veterans and active duty service members. Journal of Consulting and Clinical Psychology 2016;84(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sloan 2018 {published data only}
- Sloan DM, Unger W, Lee DJ, Beck JG. A randomized controlled trial of group cognitive behavioral treatment for veterans diagnosed with chronic posttraumatic stress disorder. Journal of Traumatic Stress 2018;31:886‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Hollands J, Litwack SD, Ryabchenko KA, Niles BL, Beck JG, Unger W, et al. Alliance across group treatment for veterans with posttraumatic stress disorder: the role of interpersonal trauma and treatment type. Group Dynamics: Theory, Research, and Practice 2018;22(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suris 2013 {published data only}
- Azimipour S. Expectancy, Adherence, and Depression as Predictors of Therapeutic Outcome as Measured by PTSD Symptoms in Veterans with MST [Thesis]. University of Texas Southwestern Medical Center, 2012. [Google Scholar]
- Fekadu RA. The Impact of Cognitive Processing Therapy on Quality of Life and Healthcare Use in Veterans with Posttraumatic Stress Disorder due to Military Sexual Trauma [Thesis]. University of Texas Southwestern Medical Center, 2013. [Google Scholar]
- Holliday R, Link‐Malcolm J, Morris EE, Suris A. Effects of cognitive processing therapy on PTSD‐related negative cognitions in veterans with military sexual trauma. Military Medicine 2014;179(10):1077‐82. [DOI] [PubMed] [Google Scholar]
- Holliday R, Williams R, Bird J, Mullen K, Suris A. The role of cognitive processing therapy in improving psychosocial functioning, health, and quality of life in veterans with military sexual trauma‐related posttraumatic stress disorder. Psychological Services 2015;12(4):428‐34. [DOI] [PubMed] [Google Scholar]
- Mullen K, Holliday R, Morris E, Raja A, Suris A. Cognitive processing therapy for male veterans with military sexual trauma‐related posttraumatic stress disorder. Journal of Anxiety Disorders 2014;28(8):761‐4. [DOI] [PubMed] [Google Scholar]
- Suris A, Link‐Malcolm J, Chard K, Ahn C, North C. A randomized clinical trial of cognitive processing therapy for veterans with PTSD related to military sexual trauma. Journal of Traumatic Stress 2013;26(1):28‐37. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bormann 2018 {published data only}
- Bormann JE, Plumb DN, Beck DJ, Glickman M, Zhao S, Osei‐Bonsu PE, et al. Portable meditation‐based mantram program reduces PTSD symptoms in veterans: a randomized controlled trial. International Society for Traumatic Stress Studies Annual Symposium, Miami FL. 2014.
- Bormann JE, Thorp SR, Smith E, Glickman M, Beck D, Plumb D, et al. Individual treatment of posttraumatic stress disorder using mantram repetition: a randomized clinical trial. American Journal of Psychiatry 2018;175(10):979‐88. [DOI] [PubMed] [Google Scholar]
- NCT01506323. Mantram repetition meditation for veterans with PTSD. Clinicaltrials.gov/ct2/show/NCT01506323 (first received 2 September 2015).
- Plumb D, Bormann J, Beck D, Glickman M, Zhao S, Osei‐Bonsu, P, et al. Meditation‐based mantram repetition program for veterans with PTSD: a randomized controlled trial in the VA healthcare system. Journal of Alternative and Complementary Medicine 2014;20(5):A10. [Google Scholar]
Bremner 2017 {published data only}
- Bremner JD, Mishra S, Campanella C, Shah M, Kasher N, Evans S, et al. A pilot study of the effects of mindfulness‐based stress reduction on post‐traumatic stress disorder symptoms and brain response to traumatic reminders of combat in Operation Enduring Freedom/ Operation Iraqi Freedom combat veterans with post‐traumatic stress disorder. Frontiers in Psychiatry 2017;8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Classen 2001 {published data only}
- Classen C, Koopman C, Nevill‐Manning K, Spiegel D. A preliminary report comparing trauma‐focused and present‐focused group therapy against a wait‐listed condition among childhood sexual abuse survivors with PTSD. Journal of Aggression, Maltreatment & Trauma 2001;4(2):265‐88. [Google Scholar]
- Ginzburg K, Butler LD, Giese‐Davis J, Cavanaugh CE, Neri E, Koopman C, et al. Shame, guilt, and posttraumatic stress disorder in adult survivors of childhood sexual abuse at risk for human immunodeficiency virus. Journal of Nervous and Mental Disease 2009;197:536‐42. [DOI] [PubMed] [Google Scholar]
Classen 2011 {published data only}
- Classen CC, Palesh OG, Cavanaugh CE, Koopman C, Kaupp JW, Kraemer HC, et al. A comparison of trauma‐focused and present‐focused group therapy for survivors of childhood sexual abuse: a randomized controlled trial. Psychological Trauma: Theory, Research, Practice, and Policy 2011;3(1):84‐93. [Google Scholar]
- NCT00220597. Group therapies for reducing HIV‐risk behavior in women who have survived childhood sexual abuse. Clinicaltrials.gov/ct2/show/nct00220597 (first received 22 September 2005).
Davis 2019 {published data only}
- Davis LL, Whetsell C, Hamner MB, Carmody J, Rothbaum BO, Allen RS, et al. A multisite randomized controlled trial of mindfulness‐based stress reduction in the treatment of posttraumatic stress disorder. Psychiatric Research & Clinical Practice 2019;1:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01532999. A multisite randomized controlled trial of mindfulness meditation therapy for PTSD. Clinicaltrials.gov/ct2/show/NCT01532999 (first received 15 February 2012).
Foa 1991 {published data only}
- Foa EB, Rothbaum BO, Riggs DS, Murdock TB. Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive‐behavioral procedures and counseling. Journal of Consulting and Clinical Psychology 1991;59(5):715‐23. [DOI] [PubMed] [Google Scholar]
Grant 2005 {published data only}
- Grant DH. Managing posttraumatic stress disorder: a present‐centered approach. Federal Practitioner 2005;22(3):39‐46. [Google Scholar]
Harris 2018 {published data only}
- Harris JI, Usset T, Voecks C, Thuras P, Currier J, Erbes C. Spiritually integrated care for PTSD: a randomized controlled trial of "Building Spiritual Strength". Psychiatry Research 2018;267:420‐8. [DOI] [PubMed] [Google Scholar]
Haynes 2012 {published data only}
- Haynes P, Kelly MR, Parthasarathy S, Bootzin R. A randomized controlled trial of cognitive behavioral social rhythm group therapy (CBSRT) for male veterans with PTSD, major depressive disorder, and sleep problems. Sleep 2012;35:A338. [Google Scholar]
- Kelly MR, Haynes PL, Parthasarathy S, Bootzin RR, Perkins S. Negative mood regulation expectancies and trauma symptoms following a randomized controlled trial of cognitive behavioral social rhythm group therapy for male veterans with PTSD, major depressive disorder, and sleep problems. Sleep 2016;39:A299. [Google Scholar]
- NCT0098469. A trial of group psychotherapy for veterans and military personnel With post traumatic stress disorder (PTSD). Clinicaltrials.gov/ct2/show/NCT00984698 (first received 25 September 25 2009).
Hong 2013 {published data only}
- Hong MJ. The Role of Prolonged Exposure and Present Centered Therapy on Optimism in Trauma‐Exposed Adults [Master's thesis]. Long Beach (CAL): Californian State University, 2013. [Google Scholar]
King 2016 {published data only}
- King A, Giardino N, Rauch S, Sripada R, Hu JY, Liberzon I. Pilot study of mindfulness‐based exposure therapy for PTSD in OEF/OIF veterans: preliminary clinical outcomes and pre‐post fMRI neuroimaging. Neuropsychopharmacology 2011;36(13):S162. [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. Altered default mode network (DMN) resting state functional connectivity following a mindfulness‐based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety 2016;33(4):289‐99. [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch SAM, Porter KE, Favorite TK, et al. A pilot study of mindfulness‐based exposure therapy in OEF/OIF combat veterans with PTSD: altered medial frontal cortex and amygdala responses in social‐emotional processing. Frontiers in Psychiatry 2016;7(154):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01347749. Mindfulness and present centered therapies for PTSD: efficacy and mechanisms. Clinicaltrials.gov/ct2/show/NCT01347749 (first received 4 May 2011).
Lang 2017 {published data only}
- Lang AJ, Schnurr PP, Jain S, He F, Walser RD, Bolton E, et al. Randomized controlled trial of acceptance and commitment therapy for distress and impairment in OEF/OIF/OND veterans. Psychological Trauma: Theory, Research, Practice and Policy 2017;9(Suppl 1):74‐84. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Schnurr PP, Jain S, Raman R, Walser R, Bolton E, et al. Evaluating transdiagnostic treatment for distress and impairment in veterans: a multi‐site randomized controlled trial of Acceptance and Commitment Therapy. Contemporary Clinical Trials 2012;33(1):116‐23. [DOI] [PubMed] [Google Scholar]
- NCT0125304. Acceptance and commitment therapy (ACT) for OEF/OIF veterans. Clinicaltrials.gov/ct2/show/NCT01253044 (first received 3 December 2010).
NCT00607412 {published data only}
- NCT00607412. Alcohol use disorders (AUDs) and post‐traumatic stress disorder (PTSD) treatment for victims of partner violence. Clinicaltrials.gov/ct2/show/NCT00607412 (first received 05 February 2008).
NCT01274741 {published data only}
- NCT01274741. Study of treatment for posttraumatic stress disorder and substance use. Clinicaltrials.gov/ct2/show/NCT01274741 (first received 11 January 2011).
NCT02081417 {published data only}
- NCT02081417. Patient‐centered trauma treatment (PaCTT). Clinicaltrials.gov/ct2/show/NCT02081417 (first received 7 March 2014).
NCT02233517 {published data only}
- NCT02233517. CBT for aggression in veterans (CBT‐A). Clinicaltrials.gov/ct2/show/NCT02233517 (first received 8 September 2014).
NCT02398227 {unpublished data only}
- NCT02398227. Treatment of PTSD in residents of battered women's shelters (HOPE). Clinicaltrials.gov/ct2/show/NCT02398227 (first received 25 March 2015).
NCT03056157 {published data only}
- NCT03056157. Psychosocial rehabilitation after moral injury and loss with adaptive disclosure. Clinicaltrials.gov/ct2/show/NCT03056157 (first received 17 February 2017).
NCT03429166 {published data only}
- NCT03429166. Connecting women to care: home‐based psychotherapy for women with MST living in rural areas (CWC). Clinicaltrials.gov/ct2/show/NCT03429166 (first received 12 February 2018).
NCT03760731 {published data only}
- NCT03760731. Thriving in the midst of moral pain: the acceptability and feasibility of acceptance and commitment therapy for moral injury (ACT‐MI) among warzone veterans. Clinicaltrials.gov/ct2/show/NCT03760731 (first received 30 November 2018).
NCT03764033 {published data only}
- NCT03764033. A novel posttraumatic stress disorder treatment for veterans with moral injury (IOK). Clinicaltrials.gov/ct2/show/NCT03764033 (first received 4 December 2018).
Polusny 2015 {published data only}
- Polusny MA, Erbes CR, Thuras P, Moran A, Lamberty GJ, Collins RC, et al. Mindfulness‐based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA 2015;314(5):456‐65. [DOI] [PubMed] [Google Scholar]
Resick 2009 {published data only}
- Resick PA, Schuster Jl. Cognitive processing therapy for combat‐related post‐traumatic stress disorder. Military Health Research Forum, Kansas City MO. 2009.
Rosner 2018 {published data only}
- Rosner R, Rimane E, Vogel A, Rau J, Hagl M. Treating prolonged grief disorder with prolonged grief‐specific cognitive behavioral therapy: study protocol for a randomized controlled trial. Trials 2018;19(241):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lely 2017 {published data only}
- Lely J, Bout J, Ter Heide JJ, Aa N, Knipscheer JW, Kleber RJ. Narrative exposure therapy vs. present‐centered therapy with older adults: results from an RCT. Innovation in Aging 2017;1(Suppl 1):58. [Google Scholar]
- NTR3987. NET with older adults [NOVO: NET Onderzoek Voor ouderen]. Trialregister.nl/trial/3820 (first received 7 May 2013).
NCT00057629 {published data only}
- NCT00057629. Prolonged exposure therapy for post traumatic stress disorder following sexual assault. Clinicaltrials.gov/ct2/show/NCT00057629 (first received 7 April 2003).
References to ongoing studies
NCT02556645 {published data only}
- NCT02556645. A comparison of web‐prolonged exposure (Web‐PE) and present‐centered therapy (PCT) for PTSD among active‐duty military personnel. Clinicaltrials.gov/ct2/show/NCT02556645 (first received 22 September 2015).
Additional references
AHRQ 2012
- Treadwell J, Uhl S, Tipton K, Singh S, Santaguida L, Sun X, et al. Assessing equivalence and noninferiority. Methods Research Report. AHRQ publication no 12‐EHCO45‐EF. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/equivalence_research.pdf (accessed prior to 29 October 2019).
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC, USA: American Psychiatric Publishing, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA, USA: American Psychiatric Publishing, 2013. [Google Scholar]
APA 2016
- American Psychological Association. Present‐centered therapy for post‐traumatic stress disorder. www.div12.org/psychological‐treatments/treatments/present‐centered‐therapy‐for‐post‐traumatic‐stress‐disorder/. (accessed April 25, 2016).
APA 2017
- American Psychological Association. Clinical practice guideline for the treatment of PTSD. https://www.apa.org/ptsd‐guideline/ (accessed prior to 29 October 2019).
Beck 1961
- Beck AT, Ward CH. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1993
- Beck A, Steer RA. Beck Depression Inventory (BDI) Manual. San Antonio, TX, USA: Psychological Corporation, 1993. [Google Scholar]
Benjet 2016
- Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychological Medicine 2016;46(2):327‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berliner 2019
- Berliner L, Bisson J, Cloitre M, Forbes D, Goldbeck L, Jensen T, et al. ISTSS PTSD prevention and treatment guidelines: methodology and recommendations. www.istss.org/getattachment/Treating‐Trauma/New‐ISTSS‐Prevention‐and‐Treatment‐Guidelines/ISTSS_PreventionTreatmentGuidelines_FNL.pdf.aspx (accessed prior to 5 November 2019).
Bernstein 1986
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease 1986;174(12):727‐35. [DOI] [PubMed] [Google Scholar]
Bisson 2005
- Bisson J, Andrew M. Psychological treatment of post‐traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003388.pub2] [DOI] [PubMed] [Google Scholar]
Bisson 2007
- Bisson J, Andrew M. Psychological treatment of post‐traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003388.pub3] [DOI] [PubMed] [Google Scholar]
Bisson 2013
- Bisson J, Roberts NP, Andrew M, Cooper R, Lewis C. Psychological therapies for chronic post‐traumatic stress disorder (PTSD) in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003388.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. John Wiley & Sons, Ltd, 2009. [Google Scholar]
Cohen 1977
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ, USA: Lawrence Erlbaum Associates, 1977. [Google Scholar]
Feingold 2009
- Feingold A. Effect sizes for growth‐modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods 2009;14(1):43‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foa 1995
- Foa E. The Posttraumatic Diagnostic Scale (PDS) Manual. Minneapolis, MN, USA: National Computer Systems, 1995. [Google Scholar]
Frost 2014
- Frost ND, Laska KM, Wampold BE. The evidence for present‐centered therapy as a treatment for posttraumatic stress disorder. Journal of Traumatic Stress 2014;27(1):1‐8. [DOI] [PubMed] [Google Scholar]
Goldstein 2017
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, et al. The epidemiology of DSM‐5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions‐III. Social Psychiatry and Psychiatric Epidemiology 2016;51(8):1137‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imel 2013
- Imel ZE, Laska K, Jakcupcak M, Simpson TL. Meta‐analysis of dropout in treatments for post‐traumatic stress disorder. Journal of Consulting and Clinical Psychology 2013;81(3):394‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2008
- Kessler RC, Ustun TB, editors. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York, NY, USA: Cambridge University Press, 2008. [Google Scholar]
Kish 1995
- Kish L. Survey Sampling. New York, NY, USA: Wiley, 1995. [Google Scholar]
Lee 2016
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmussen AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta‐analyses to determine first‐line treatments. Depression and Anxiety 2016;33(9):792‐806. [DOI] [PubMed] [Google Scholar]
Monson 2008
- Monson CM, Gradus JL, Young‐Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree?. Psychological Assessment 2008;20(2):131‐8. [DOI] [PubMed] [Google Scholar]
Morina 2014
- Morina N, Wicherts JW, Lobbrecht J, Priebe S. Remission from post‐traumatic stress disorder in adults: a systematicreview and meta‐analysis of long term outcome studies. Clinical Psychology Review 2014;34:249‐55. [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Post‐traumatic stress disorder. NG116. www.nice.org.uk/guidance/ng116/chapter/Recommendations (accessed prior to 29 October 2019).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenthal 1979
- Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin 1979;86(3):638‐41. [Google Scholar]
Rush 2003
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16‐item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS‐C), and self‐report (QIDS‐SR): a psychometric evaluation in patients with chronic depression. Biological Psychiatry 2003;54(5):573‐83. [DOI] [PubMed] [Google Scholar]
Sayed 2015
- Sayed S, Iacoviello BM, Charney DS. Risk factors for the development of psychopathology following trauma. Current Psychiatry Reports 2015;17(8):70. [DOI] [PubMed] [Google Scholar]
Schnurr 2001
- Schnurr PP, Friedman MJ, Lavori PW, Hsieh FY. Design of Department of Veterans Affairs cooperative study no. 420: group treatment of posttraumatic stress disorder. Controlled Clinical Trials 2001;22(1):74‐88. [DOI] [PubMed] [Google Scholar]
Schnurr 2005
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Resick PM, et al. Issues in the design of multisite clinical trials of psychotherapy: VA Cooperative Study No. 494 as an example. Contemporary Clinical Trials 2005;26:626‐36. [DOI] [PubMed] [Google Scholar]
Schnurr 2007a
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. Journal of the American Medical Association 2007;297(8):820‐30. [DOI] [PubMed] [Google Scholar]
Schnurr 2007b
- Schnurr PP, Shea MT, Friedman MJ, Engel CC. Posttraumatic stress disorder and cognitive behavioral therapy ‐ in reply. JAMA 2007;297(24):2965. [DOI] [PubMed] [Google Scholar]
Shea 2018
- Shea TM. Present centered therapy for PTSD: origins, theoretical basis, and key components. Symposium of the International Society for the Study of Traumatic Stress; 2018 November; Washington DC. 2018.
Smith 2016
- Smith SM, Goldstein RB, Grant BF. The association between post‐traumatic stress disorder and lifetime DSM‐5 psychiatric disorders among veterans: data from the National Epidemiologic Survey on Alcohol and Related Conditions‐III (NESARC‐III). Journal of Psychiatric Research 2016;82:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA, USA: Consulting Psychologists Press, 1983. [Google Scholar]
Tanielian 2008
- Tanielian TL, Jaycox LH. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Washington, DC, USA: RAND Corporation, 2008. [Google Scholar]
VA/DoD 2017
- Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder 2017. www.ptsd.va.gov/professional/treat/txessentials/cpg_ptsd_management.asp (accessed prior to 29 October 2019).
Watts 2013
- Watts BV, Schnurr PP, Mayo L, Young‐Xu Y, Weeks WB, Friedman MJ. Meta‐analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry 2013;74(6):e541‐50. [DOI] [PubMed] [Google Scholar]
Weathers 1993
- Weathers FW, Litz BT, Herman JA, Huska JA, Keane TM. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Annual Convention of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX, USA. 1993.
Weathers 2001
- Weathers FW, Keane TM, Davidson JRT. Clinician‐administered PTSD scale: a review of the first ten years of research. Depression and Anxiety 2001;13(3):132‐56. [DOI] [PubMed] [Google Scholar]
Whiteford 2013
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382:1575‐86. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization (WHO). The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva, Switzerland: WHO, 1992. [Google Scholar]
References to other published versions of this review
Belsher 2017
- Belsher B, Beech E, Evatt D, Rosen CS, Liu X, Otto J, et al. Present‐centered therapy (PCT) for post‐traumatic stress disorder (PTSD) in adults. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD012898] [DOI] [PMC free article] [PubMed] [Google Scholar]


