Abstract
Adolescents and young adults (AYA) surviving classical Hodgkin lymphoma (cHL) risk long term fatal treatment-related toxicities. We utilized the Surveillance, Epidemiology and End Results (SEER) program to compare excess mortality rate (EMR-observed minus expected mortality) for 10-year survivors of AYA cHL diagnosed in 1973–1992 and 1993–2003 eras. The 15-year EMR reduced from 4.88% to 2.19% while the 20-year EMR reduced from 9.46% to 4.07% between eras. Survivors of stages 1–2 had lower EMR than survivors of stages 3–4 cHL in the 1993–2003 but not in the 1973–1992 era. There was an overall decline in risk of death between 10 and 15 years from diagnosis, driven mostly by second neoplasms and cardiovascular mortality. Despite reduction in fatal second neoplasms and cardiovascular disease with more current therapy, long term survivors of AYA cHL still have a higher risk of death than the general population highlighting the need for safer therapies.
1 |. INTRODUCTION
Adolescents and young adults (AYA) constitute the majority of patients with classical Hodgkin lymphoma (cHL), and most of those patients will be cured with multi-agent chemotherapy or combined used of chemotherapy and radiation therapy.1 However, this therapeutic success has been hindered by the development of significant treatment-related late toxicities.2,3 In addition to having a high risk of developing second malignant neoplasms (SMN), cHL survivors face a variety of chronic medical conditions linked to cancer therapy.4 Treatment-induced chronic illness and SMN contribute to premature deaths among cHL survivors.5
Successive generations of cHL therapy over the past several decades have modified the long term risk and toxicity profile of survivors. The early 1990s marks the transition between use of higher dose, extended-field radiation, and MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) chemotherapy6 to lower doses, involved-field radiation, and ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) regimen as more common approaches to treat patients with cHL.7 More recently, both pediatric and adult studies have focused on early risk stratification and response-adapted therapy to tailor treatment strategies.8
Those approaches have led to further improvement in survival rates and risk reduction of therapy related long-term toxicities as exemplified in a large cohort of pediatric cHL survivors.9 The increased risk of death in long-term survivors and the temporal trends in such risk have not been well characterized at the population level, particularly in young adults. In this study, we utilized a large population-based data set focusing on AYA cHL survivors to better understand and compare excess mortality in 2 consecutive eras. We aimed to evaluate possible factors associated with late excess mortality, and described and compared causes of death among 10-year survivors of AYA cH L across different time periods.
2 |. METHODS
Using data from the National Cancer Institute (NCI) Surveillance Epidemiology and End Results (SEER) program, we analyzed the excess mortality rate (EMR) of 10-year cHL AYA survivors treated in 2 distinct eras based on the year of diagnosis, 1973–1992 and 1993–2003, reflecting the development of risk-adapted response-based treatment approach and treatment de-intensification.6 For cHL, SEER collects data, when available, on histological subtype, Ann-Arbor staging (available since 1983) and the use of radiation therapy, but not on the use of systemic therapy or hematopoietic cell transplantation. Additionally, SEER collects survival status, occurrence of subsequent malignancies and cause of death.
Participating registries in SEER-18 comprise the Alaska Native, Atlanta, Connecticut, Detroit, Greater California, Greater Georgia, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Jersey, New Mexico, San Francisco-Oakland, San Jose-Monterey, Rural Georgia, Seattle-Puget Sound, and the Utah Tumor Registry, corresponding to approximately 28% of the US population (based on the 2000 census). We included all patients between the ages of 15–39 years at the time of cHL diagnosis. We identified cHL cases using the third edition of the International Classification of Disease for Oncology (ICD-O-3) histology codes 9650/3–9655/3, and 9661/3–9667/3 (cHL) diagnosed between 1973–1992 or 1993–2003 as a first malignant neoplasm. The age range was chosen to match the NCI definition of “adolescent and young adult”. Only patients known to be alive at 10 years from diagnosis were included in the analysis. Follow-up was updated to the end of 2013 (November 2015 submission), so patients in the most recent cohort had at least 10 years of follow-up.
2.1 |. Data analysis and statistics
We described the population included utilizing the case listing session function of SEER*Stat Version 8.3.2. We obtained actual and expected survival at time points of interest utilizing the survival function in SEER*Stat using the US census of 2000 as reference. Since the study addresses exclusively 10-year survivors, actual and expected mortality were adjusted to 0% at 10 years for the cohorts being studied. The main parameter studied was EMR, which is, the difference between the observed mortality rate in the cohort being studied and the expected mortality rate in a contemporaneous population of similar age, gender, and race/ethnicity structure. Along with relative survival, EMR is a possible approach to compare observed and expected survival (or mortality) of a cohort. EMR is defined as “the death rate in the general population due to the excess risk imposed by specific disease”.10 We chose to use EMR as it provides a more direct and intuitive way to measure the impact of the condition being studied (in this case being a 10-year survivor of AYA cHL) on the risk of death. For example, an EMR of 5% detected between years 10 and 15 from diagnosis mean that patients who are 10-year cHL survivors have a 5% higher absolute risk of dying during the 5 subsequent years than individuals of similar age, gender, and race/ethnicity in the population who are not cHL survivors.
We compared EMR in 10-year cHL survivors diagnosed between time periods of 1973–1992 and 1993–2003. We subsequently explored the impact of gender, stage, and use of radiation treatment (for patients with localized stage) on EMR across the 2 eras. We intended to compare cause of death (COD) among the 10-year AYA cHL survivors between the 2 eras. However, the 2 cohorts inherently have a different duration of follow-up and contain survivors who reach very distinct ages. For instance, a 25 year-old patient diagnosed at the onset of the 1973–1992 era would have a follow-up until the age of 65, while a 25-year-old patient diagnosed at the onset of the 1993–2003 era would have a follow-up until the age of 45, encompassing periods of life where risk of death in the population is very dissimilar. We corrected this discrepancy by analyzing COD in a short time-period of 5 years (beginning 10 to 15 years from diagnosis) in the subsets of cohorts with at least 15 years of follow-up. Therefore, in this study, COD is described and compared for patients diagnosed in two 5-year time-periods, 1983–1987 and 1993–1997, respectively. Of note, 1983–1987 was chosen as the first time-period given the fact that staging for cHL was only reported in SEER on and after 1983. The cause-specific risk of death during the 5-year period is described in deaths/1000 person-year at risk.
Comparisons between rates were performed using Z-test. We performed statistical analysis using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY). Statistical tests were 2-sided and considered significant when P<.05.
3 |. RESULTS
3.1 |. Population
Table 1 delineates the characteristics of the patients diagnosed during the time periods of 1973–1992 (N = 6480) and 1993–2003 (N = 5870). Median follow-up was 312 months (range 120–491) for the 1973–1992 and 162 months (range 120–251 months) for the 1993–2003 cohort. In similar order, the median age of patients was 26 and 27 years respectively. While the majority of patients had localized stage cHL in both cohorts, a higher proportion of patients in the 1973–1992 cohort received radiation therapy. Age at diagnosis, gender, and race/ethnicity distributions were similar between the 2 eras.
TABLE 1.
Characteristics of 10-year AYA cHL survivors in the 2 consecutive eras
| 1973–1992 (N = 5870) |
1993–2003 (N = 6480) |
P | |
|---|---|---|---|
| Follow up in months, median (range) | 312 (120–491) | 162 (120–251) | N/A |
| Age in years, median (range) | 26 (15–39) | 27 (15–39) | <0.001 |
| Male gender | 3008 (51.2%) | 3172 (48.9%) | 0.01 |
| Race/ethnicity | <0.001 | ||
| Non-hispanic black | 364 (6.2%) | 615 (9.5%) | |
| Non-hispanic white | 5104 (87.0%) | 4783 (73.8%) | |
| Hispanic | 259 (4.4%) | 761 (11.7%) | |
| Other | 115 (2.0%) | 276 (4.3%) | |
| Unknown | 28 (0.5%) | 45 (0.7%) | |
| Stage (%) | <0.001 | ||
| 1 and 2 | 2171 (37.0%) | 4405 (68%) | |
| 3 and 4 | 992 (18.6%) | 1750 (27%) | |
| Unknown | 2607 (44.4%) | 325 (5%) | |
| Radiation | <0.001 | ||
| Yes | 3762 (64.1%) | 3194 (49.3%) | |
| No | 1940 (33.0%) | 3074 (47.4%) | |
| Unknown | 168 (2.9%) | 212 (3.3%) | |
| Histologic subtype (%) | <0.001 | ||
| Nodular sclerosis | 4102 (69.9%) | 5068 (78.2%) | |
| Mixed-cellularity | 965 (16.4%) | 616 (9.5%) | |
| Classical NOS | 513 (8.7%) | 606 (9.4%) | |
| Lymphocyte-rich | 228 (3.9%) | 153 (2.4%) | |
| Lymphocyte-depleted | 62 (1.1%) | 37 (0.6%) |
3.2 |. Excess mortality
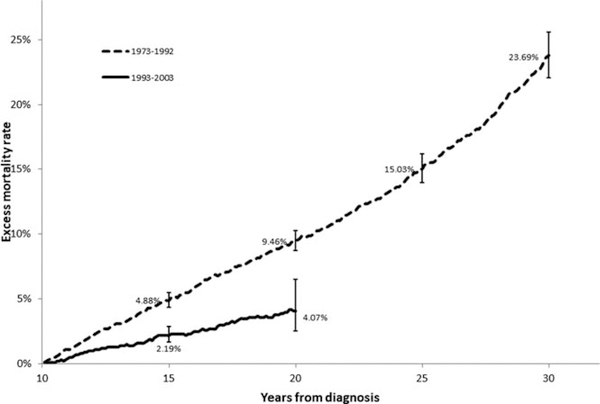
The 15-year, 20-year, 25-year, and 30-year EMR for 10-year AYA cHL survivors diagnosed during 1973–1993 was 4.88% (95% C.I. 4.36%−5.48%); 9.46% (95% C.I. 8.72%−10.26%); 15.03% (95% C.I. 13.95–16.18%); and 23.69% (95% C.I. 22.08%−25.58%), respectively. In the more recent cohort, there was a substantial reduction in the 15-year and 20-year EMR to 2.19% (95% C.I, 1.69%−2.86%, P<.0001) and 4.07% (95% C.I. 2.53%−6.52%, P<.0001), respectively (Figure 1).
FIGURE 1.
Comparative 15- and 20-year excess mortality rates for 10-year survivors of adolescents and young adults with classical Hodgkin lymphoma diagnosed between 1973–1992 and 1993–2003
3.3 |. Factors associated with increased excess mortality
When analyzing patients in the 1973–1992 cohort, we found no difference in EMR between patients with localized (stages 1–2) and advanced (stages 3–4) cHL at any of the time points (Figure 2A). Male survivors had higher EMR at 15, 20, and 25 years from diagnosis than female survivors (Figure 2B).
FIGURE 2.
Comparative 15- and 20-year excess mortality rates for 10-year survivors of adolescent and young adult with classical Hodgkin lymphoma displaying A, localized stage vs. advanced stage; B, male vs. female; and C, radiation vs. no radiation treatment of patients with localized stage for patients diagnosed between 1973–1992; and D, localized stage vs. advanced stage; E, male vs. female; and F, radiation vs. no radiation treatment of patients with localized stage for patients diagnosed between 1993–2003
In the most recent cohort (1993–2003), we found that the 15-and 20-year EMR for 10-year AYA survivors with advanced stage cHL was 3.48% (95% C.I. 2.40%−5.24%), and 6.46% (95% C.I. 3.23%−13.1%), respectively. These results were found to be significantly higher in comparison to survivors of localized stage cHL at 15-(1.69%, 95% C.I 1.19%−2.44%, P = .007) and at 20-year (2.57%, 95% C.I. 1.81%−4.09%, P = .03) EMR treated in the same era (Figure 2D). Similarly, for patients treated in the most recent era, there was higher EMR among male survivors in comparison to females at 15-years post-treatment (2.98%, 95% C.I. 2.15%−4.08% for males vs. 1.59%, 95% C.I. 1.05%−2.47% for females, P = .02) (Figure 2E). We found no significant difference in EMR between localized-stage patients treated with or without radiation therapy in the 1993–2003 cohort (Figure 2F).
3.4 |. Causes of death
Although it is not possible to attribute the cause of excess mortality without information on expected COD in the general population, we analyzed overall COD at years 10 and 15 from diagnosis as a window into the factors possibly implicated in excess risk of death. There were 98 deaths among the 1598 patients followed for a total of 7703.2 person-years in the sub-cohort diagnosed between 1983 and 1987. For the 1993–1997 sub-cohort, there were 67 deaths among 2196 patients followed for a total of 10 615.6 person-years.
The SMN was the leading cause of death among patients in the 1983–1987 sub-cohort, while cHL was the leading cause of the death in the more recent sub-cohort (Table 2). There was an overall decline in risk of death between the 2 eras, driven mostly by a reduction in risk of death from SMN and cardiovascular causes. The reduction in death from cHL among 10-year survivors did not reach statistical significance.
TABLE 2.
Causes of death among 10-year AYA cHL survivors in the 2 consecutive eras
| 1983–1987 |
1993–1997 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender |
Stageb |
Gender |
Stageb |
||||||||
| Cause of Death | Total N = 1598 |
Male (N = 840) |
Female (N = 785) |
Stage 1/2 (N = 971) |
Stage 3/4 (N = 527) |
Total N = 2196 |
Male (N = 1093) |
Female (N = 1103) |
Stage 1/2 (N = 1476) |
Stage 3/4 (N = 628) |
Pc |
| Person-year | 7703.2 | 4049.2 | 3784.1 | 4680.7 | 2540.4 | 10615.6 | 5283.6 | 5331.9 | 7135.0 | 3035.7 | |
| All causes | 98 | 60 | 38 | 55 | 31 | 67 | 45 | 22 | 32 | 31 | |
| Riska (95% C.l.) | 12.7 (10.4–15.5) | 14.8 (11.5–19) | 10.0 (7.3–13.7) | 11.7 (9.1–15.3) | 12.2 (8.6–17.3) | 6.3 (5–8) | 8.5 (6.4–11.4) | 4.1 (2.7–6.2) | 4.9 (3.2–6.3) | 10.2 (7.2–14.4) | <0.0001 |
| Hodgkin lymphoma | 22 (22.5%) | 12 (20%) | 10 (26.3%) | 10 (18.2%) | 9 (29%) | 17 (25.4%) | 12 (26.7%) | 5 (22.7%) | 8 (25%) | 8 (25.8%) | |
| Riska (95% C.l.) | 2.9 (1.9–4.4) | 3.0 (1.7–5.2) | 2.6 (1.4–4.8) | 2.1 (1.1–3.9) | 3.5 (1.8–6.7) | 1.6 (1–2.6) | 2.3 (1.3–4) | 0.9 (0.4–2.1) | 1.1 (0.6–2.2) | 2.6 (1.3–5.1) | 0.07 |
| Second Neoplasm | 26 (26.5%) | 10 (16.7%) | 16 (42.1%) | 15 (27.3%) | 8 (25.8%) | 15 (22.4%) | 8 (17.8%) | 7 (31.9%) | 8 (25%) | 6 (19.4%) | |
| Riska (95% C.l.) | 3.4 (2.3–5) | 2.5 (1.4–4.6) | 4.2 (2.6–6.S) | 3.2 (1.9–5.3) | 3.1 (1.6–6.1) | 1.4 (0.8–2.3) | 1.5 (0.8–3) | 1.3 (0.6–2.7) | 1.1 (0.6–2.2) | 2 (0.9–4.3) | 0.006 |
| NHL | 7 | 4 | 3 | 5 | 1 | 3 | 2 | 1 | 3 | - | |
| Acute leukemia | 5 | 1 | 4 | 2 | 3 | 1 | - | 1 | 1 | - | |
| Breast | 3 | - | 3 | 2 | 1 | 1 | - | 1 | - | - | |
| Lung | 4 | 3 | 1 | 3 | - | - | - | - | - | - | |
| Other cancer | 7 | 2 | 5 | 3 | 3 | 10 | 6 | 4 | 4 | 6 | |
| Cardiovascular | 22 (22.4%) | 17 (28.3%) | 5 (13.2%) | 13 (23.7%) | 8 (25.8%) | 13 (19.4%) | 10 (22.2%) | 3 (13.7%) | 3 (9.4%) | 9 (29%) | |
| Riska (95% C.I.) | 2.6 (1.9–44) | 4.2 (2.6–6.7) | 1.3 (0.6–3.1) | 2.8 (1.6–4.8) | 3.1 (1.6–6.1) | 1.2 (0.7–2.1) | 1.9 (1–3.5) | 0.6 (0.2–1.7) | 0.4 (0.1–1.2) | 3 (1.6–5.7) | 0.01 |
| Infection | 6 (6.1%) | 4 (6.7%) | 2 (5.3%) | 3 (5.5%) | 2 (6.5%) | 7 (10.4%) | 5 (11.1%) | 2 (9.1%) | 5 (15.6%) | 1 (3.2%) | |
| Riska (95% C.I.) | 0.8 (0.4–1.7) | 0.9 (0.4–2.6) | 0.5 (0.1–1.9) | 0.6 (0.2–1.8) | 0.8 (0.2–2.9) | 0.6 (0.3–1.4) | 0.9 (0.4–2.1) | 0.4 (0.1–1.4) | 0.7 (0.3–1.6) | 0.3 (0–1.8) | 0.76 |
| Accident/Suicide | 7 (7.1%) | 7 (11.2%) | 0 | 4 (7.3%) | 2 (6.5%) | 5 (16.1%) | 4 (6%) | 1 (4.5%) | 1 (3.1%) | 4 (13%) | |
| Riska (95% C.I.) | 0.9 (0.4–1.9) | 1.7 (0.8–3.5) | 0 (0–0.1) | 0.85 (0.4–2.3) | 0.79 (0.2–2.9) | 0.5 (0.2–1.1) | 0.76 (0.2–1.1) | 0.19 (0–11) | 0.14 (0–0.7) | 1.3 (0.5–3.4) | 0.25 |
| Other/unavailable | 15 (15.3%) | 10 (16.7%) | 5 (13.2%) | 13 (23.6% | 2 (6.5%) | 10 (15%) | 6 (13.3%) | 4 (18.2%) | 7 (21.9%) | 3 (9.7%) | |
| Riska (95% C.I.) | 1.9 (1.1–3.2) | 2.5 (1.4–4.6) | 1.3 (0.6–3.1) | 2.8 (1.6–4.8) | 0.8 (0.2–2.9) | 0.9 (0.5–1.7) | 1.1 (0.5–2.4) | 0.8 (0.3–2) | 1 (0.5–2) | 1 (0.3–2.9) | 0.07 |
Death per 1000 person-year.
The total of the 2 stage categories is less than the total number of patients due to missing stage in some cases.
Comparisons apply to all individuals in 1983–1987 and 1993–1997 cohorts.
The reduction in risk of death in years 10–15 for 10-year survivors seems to span across both genders and it was far more pronounced among patients with localized cHL where there was substantial reduction in risk of death from any cause (11.7 to 4.5 deaths/1000 person-year, P< .0001), from SMN (3.2 to 1.1 deaths/1000 person-year, P = .012) and from cardiovascular causes (2.8 to 0.4 deaths/1000 person-year, P< .0001), but not from cHL (2.1 to 1.1 deaths/1000 person-year, P= .16), as shown in Table 2. In addition, the reduction in risk of death in years 10–15-year in females was driven primarily by reduction in death from SMN (4.2 to 1.3 deaths/1000 person-year, P= .006), while in males the more pronounced risk reduction was in death from cardiovascular diseases (4.2 to 1.9 deaths/1000 person-year, P = .04). For 10-year survivors of advanced stage AYA cHL, there was no significant reduction in the overall risk of death in the 5 subsequent years (12.2 to 10.2 deaths/1000 person-year, P = .47).
4. |. DISCUSSION
Using a large population-based data set, the present study verified that despite significant improvements in treatment and cure rates in the most recent years, long-term survivors of AYA cHL continue to have a higher risk of death than matched population. Our findings are of crucial importance since cHL predominantly affects AYA, individuals who otherwise would have a long life expectancy. As the cohort ages, some deaths are expected, as in the matched population, making it important to use the expected mortality in the population as reference. Therefore, using EMR allows us to determine the impact of having cHL and/or receiving cHL therapy on the long-term risk of death.
The ideal “cure” scenario would result in long-term survivors having the same risk of death as individuals of the same age, gender, and race/ethnicity, resulting in EMR of zero. Unfortunately, this is not the case despite recent advances in therapy as demonstrated in our study, wherein patients in the 1973–1992 cohort, continued to have steady increase in EMR beyond 20 years from diagnosis reaching 23.69% at 30 years from diagnosis (20-years follow-up of 10-year survivors) (Figure 1). This is consistent with previous large cohorts of pediatric and adult cHL patients diagnosed up to the mid-1990s showing significantly increased risk of death even after 25 years post cancer therapy.11–15
We detected a reduction in EMR in the more recent cohort. AYA diagnosed between 1993–2003 and surviving 10 years after cHL therapy had a 4% EMR after 10 years of additional follow-up (Figure 1). That translates into 1 in 25, 10-year survivor of AYA cHL dying during the next 10 years because they had cHL and received therapy for cHL. The reduction in EMR across eras was driven mostly by patients with localized disease (Figure 2A,D). This is possibly linked to de-intensification of therapy with less toxic chemotherapy regimens, shorter courses of chemotherapy and employment of lower doses and smaller fields of radiation.16–19 Although the database utilized lacks details of therapy, this possibility is further supported by our observation of a substantial reduction in risk of late mortality from SMN and cardiovascular diseases in localized cHL survivors, 2 complications intrinsically linked to therapy.11,20 Conversely, we did not observe a substantial reduction in late EMR among 10-year survivors of advanced cHL (Figure 2A,D) as survivors remained at high risk of death from cHL, SMN, and cardiovascular disease in both eras (Table 2). This concerning observation highlights the need to improve both the efficacy and safety of therapies utilized in AYA with advanced cHL. The development of novel therapies for advanced cHL needs to continue to emphasize not only short-term efficacy but also long-term safety.
Recently, the Childhood Cancer Survivor Study (CCSS) showed significant reductions in the rate of death (from 5.3% to 2.6%, P = .006 from any health-related cause 15 years from diagnosis across treatment eras (1970–1999) among 4332 survivors of cHL diagnosed before the age of 21.9 The CCSS investigators also demonstrated temporal reductions in chest radiotherapy and anthracycline exposure over time. Interestingly, the study did not show that adjustments in therapy impacted the relative rates of death among long-term childhood cHL survivors, suggesting that other factors, such as better screening methods and supportive care, may have played a role in reducing late mortality other than adjustments in treatment exposure.9 Continued focus on early preventative medicine and on improving supportive care to all survivors may be an effective way to decrease mortality among cHL survivors diagnosed in the most recent years.
We found that SMNs are still the second most common cause of death in long-term survivors of cHL in AYA. Reduction in risk of SMN was seen in large cohort of pediatric cHL survivors treated over a time span of several decades and followed for at least 15 years.2 In that study, the authors speculated that the reduction in standardized incidence ratio for SMN likely resulted from decreasing use of radiation therapy.2 Another study, however, found no appreciable impact in the risk of SMN in a large cohort of cHL survivors treated between 1965 and 2000. This may be likely due to the fact that current radiation therapy practices, such as lower doses or smaller radiation therapy fields, were broadly used only after 2000.21 In addition to modifications in radiation therapy fields and doses, reduction in risk of SMN could have resulted from minimum exposure or complete elimination of nitrogen mustard from HL treatment regimens in the 1990s.22,23
We found reduction in deaths from SMN among survivors of localized cHL but not among survivors of advanced cHL (Table 2), reinforcing the hypothesis that de-intensification of therapy in localized cHL is linked to reduction in risk of fatal SMN.
We observed a decline in the risk of death due to cardiovascular diseases between the 2 eras (Table 2). We also found higher late EMR among male survivors in comparison to females across eras. It is possible that this finding correlates in part with the fact that cardiovascular diseases appear to disproportionally affect late mortality among males. Mechanisms of cardiovascular toxicity among cHL survivors are complex, multifactorial, and persist throughout life.24–27 Hypertension, smoking, hypercholesterolemia, and diabetes are also more prevalent among men. These factors seem to further accentuate the risk of cardiovascular complications among cHL survivors that is usually driven by radiation therapy and anthracycline exposure.20,28–30 It is possible that reduction in deaths from cardiovascular disease reflects both improved safety of therapy and better control of risk factors, including smoking cessation.
Our study is met by some limitations. It is believed that the vast majority of patients included in this cohort would have received chemotherapy or combined modality therapy. This information, however, is not available in the SEER registry. Therefore, the association between therapeutic interventions and possible outcomes should be interpreted as hypothesis generating. Additionally, since we do not have information on COD in the general population using the same criteria utilized for COD assignment in SEER, it is not possible to estimate which components of late mortality are attributable to cHL and its treatment. However, it is reasonable to assume that the majority of the deaths, due to SMN, for example, were linked to prior cHL therapy in an otherwise young patient cohort.
In conclusion, long-term survivors of AYA cHL continue to have a higher risk of death in comparison to matched population despite improvements in treatment and cure rates. Although advances in the management of localized cHL have likely resulted in reduced long-term mortality, improvements are still needed, particularly for patients with advanced disease. Future management of cHL in AYA needs therapies that combine improved anti-lymphoma efficacy, short and long-term safety and are paired with a comprehensive survivorship program aimed at reducing cardiovascular risk-factors and intervening early to prevent long-term complications.
Funding information
The authors report no funding sources associated with this manuscript
Footnotes
CONFLICT OF INTEREST
LJC received research support from Genentech. ACX, NE, and JWT declare no competing financial interests.
REFERENCES
- [1].Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood. 2008;111(6):2977–2983. [DOI] [PubMed] [Google Scholar]
- [2].Turcotte LM, Liu Q, Yasui Y. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017;317(8):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matasar MJ, Ford JS, Riedel ER, Salz T, Oeffinger KC, Straus DJ. Late morbidity and mortality in patients with Hodgkin’s lymphoma treated during adulthood. J Natl Cancer Inst 2015;107(4):djv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ng AK. Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood. 2014;124(23): 3373–3379. [DOI] [PubMed] [Google Scholar]
- [5].Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the childhood cancer survivor study. Blood. 2011;117(6):1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tubiana M, Henry-Amar M, Carde P, et al. Toward comprehensive management tailored to prognostic factors of patients with clinical stages I and II in Hodgkin’s disease. The EORTC Lymphoma Group controlled clinical trials: 1964–1987. Blood. 1989;73(1):47–56. [PubMed] [Google Scholar]
- [7].Richardson SE, McNamara C. The management of classical Hodgkin’s lymphoma: past, present, and future. Adv Hematol 2011; 2011:865870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15(5):608–638. [DOI] [PubMed] [Google Scholar]
- [9].Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016; 374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lenner P. The excess mortality rate. A useful concept in cancer epidemiology. Acta Oncol 1990;29(5):573–576. [DOI] [PubMed] [Google Scholar]
- [11].Aleman BMP, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE . Long-term cause-specific mortality of patients treated for Hodgkin’s disease. JCO. 2003;21 (18):3431–3439. [DOI] [PubMed] [Google Scholar]
- [12].Henry-Amar M, Hayat M, Meerwaldt JH, et al. Causes of death after therapy for early stage Hodgkin’s disease entered on EORTC protocols. EORTC Lymphoma Cooperative Group. Int J Radiat Oncol Biol Phys 1990;19(5):1155–1157. [DOI] [PubMed] [Google Scholar]
- [13].Hoppe RT. Hodgkin’s disease: complications of therapy and excess mortality. Ann Oncol 1997;8(Suppl1):115–118. [PubMed] [Google Scholar]
- [14].Aviles A, Neri N, Cuadra I, Alvarado I, Cleto S. Second lethal events associated with treatment for Hodgkin’s disease: a review of 2980 patients treated in a single Mexican institute. Leuk Lymphoma 2000;39(3–4):311–319. [DOI] [PubMed] [Google Scholar]
- [15].Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol 2002;20(8): 2101–2108. [DOI] [PubMed] [Google Scholar]
- [16].Ferme C, Thomas J, Brice P, et al. ABVD or BEACOPPbaseline along with involved-field radiotherapy in early-stage Hodgkin lymphoma with risk factors: results of the European Organisation for Research and Treatment of Cancer (EORTC)-Groupe d’Etude des Lymphomes de l’Adulte (GELA) H9-U intergroup randomised trial. Eur J Cancer. 2017;81:45–55. [DOI] [PubMed] [Google Scholar]
- [17].Thomas J, Ferme C, Noordijk EM, et al. Results of the EORTC-GELA H9 randomized trials: the H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin’s lymphoma (HL). Haematologica 2007;92(S5):27.17229632 [Google Scholar]
- [18].Zittoun R, Audebert A, Hoerni B, et al. Extended versus involved fields irradiation combined with MOPP chemotherapy in early clinical stages of Hodgkin’s disease. JCO. 1985;3(2):207–214. [DOI] [PubMed] [Google Scholar]
- [19].Santoro A, Bonadonna G, Valagussa P, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. JCO. 1987;5(1):27–37. [DOI] [PubMed] [Google Scholar]
- [20].Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007; 109(5):1878–1886. [DOI] [PubMed] [Google Scholar]
- [21].Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 2015;373(26):2499–2511. [DOI] [PubMed] [Google Scholar]
- [22].Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. JCO. 2013;31(5): 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Delwail V, Jais JP, Colonna P, Andrieu JM. Fifteen-year secondary leukaemia risk observed in 761 patients with Hodgkin’s disease prospectively treated by MOPP or ABVD chemotherapy plus high-dose irradiation. Br J Haematol 2002;118(1):189–194. [DOI] [PubMed] [Google Scholar]
- [24].van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- [26].Fidler MM, Reulen RC, Henson K, et al. Population-based long-term cardiac-specific mortality among 34 489 five-year survivors of childhood cancer in Great Britain. Circulation. 2017;135(10):951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med 2016;164(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. JCO. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995. [DOI] [PubMed] [Google Scholar]