Summary
Background
Non-clear-cell renal cell carcinomas are histologically and genetically diverse kidney cancers with variable prognoses, and their optimum initial treatment is unknown. We aimed to compare the mTOR inhibitor everolimus and the VEGF receptor inhibitor sunitinib in patients with non-clear-cell renal cell carcinoma.
Methods
We enrolled patients with metastatic papillary, chromophobe, or unclassified non-clear-cell renal cell carcinoma with no history of previous systemic treatment. Patients were randomly assigned (1:1) to receive everolimus (10 mg/day) or sunitinib (50 mg/day; 6-week cycles of 4 weeks with treatment followed by 2 weeks without treatment) administered orally until disease progression or unacceptable toxicity. Randomisation was stratified by Memorial Sloan Kettering Cancer Center risk group and papillary histology. The primary endpoint was progression-free survival in the intention-to-treat population using the RECIST 1.1 criteria. Safety was assessed in all patients who were randomly assigned to treatment. This study is registered with ClinicalTrials.gov, number .
Findings
Between Sept 23, 2010, and Oct 28, 2013, 108 patients were randomly assigned to receive either sunitinib (n=51) or everolimus (n=57). As of December, 2014, 87 progression-free survival events had occurred with two remaining active patients, and the trial was closed for the primary analysis. Sunitinib significantly increased progression-free survival compared with everolimus (8 ⋅ 3 months [80% CI 5 ⋅ 8–11 ⋅ 4] vs 5 ⋅ 6 months [5 ⋅ 5–6 ⋅ 0]; hazard ratio 1⋅41 [80% CI 1 ⋅ 03–1 ⋅ 92]; p=0 ⋅ 16), although heterogeneity of the treatment effect was noted on the basis of histological subtypes and prognostic risk groups. No unexpected toxic effects were reported, and the most common grade 3–4 adverse events were hypertension (12 [24%] of 51 patients in the sunitinib group vs one [2%] of 57 patients in the everolimus group), infection (six [12%] vs four [7%]), diarrhoea (five [10%] vs one [2%]), pneumonitis (none vs five [9%]), stomatitis (none vs five [9%]), and hand-foot syndrome (four [8%] vs none).
Interpretation
In patients with metastatic non-clear-cell renal cell carcinoma, sunitinib improved progression-free survival compared with everolimus. Future trials of novel agents should account for heterogeneity in disease outcomes based on genetic, histological, and prognostic factors.
Funding
Novartis and Pfizer.
Introduction
Non-clear-cell renal cell carcinomas are a genetically and histologically diverse set of cancers that arise from the kidney and includes types 1 and 2 papillary renal cell carcinoma, chromophobe renal cell carcinoma, translocation carcinoma, and many other rare subtypes, some of which remain histologically unclassified.1,2 Non-clear-cell renal cell carcinoma accounts for about 25% of all cases of renal cell carcinoma. However, in the metastatic setting, type 2 papillary and unclassified non-clear-cell renal cell carcinomas are the most common subtypes, given their more aggressive disease course.1,2
The optimum therapy for patients with metastatic non-clear-cell renal cell carcinomas has not been determined. Data from single-arm trials and expanded access studies of VEGF receptor tyrosine kinase inhibitors sunitinib or sorafenib suggest up to 30% of patients achieve a radiographic response. Such patients’ median progression-free survival ranges from 2–12 months, with notable heterogeneity linked to histological subtype, prognostic risk groups, and previous therapies.3–5 Data from a phase 3 trial6,7 of the mTOR inhibitor temsirolimus versus interferon alpha suggested that patients with non-clear-cell histologies had improved overall survival with mTOR inhibition. Similarly, activity of the oral mTOR inhibitor everolimus in patients with non-clear-cell renal cell carcinomas has been shown in single-arm and randomised studies.8,9 These collective data suggest that some patients with non-clear-cell renal cell carcinomas might benefit from either an initial VEGF receptor inhibitor-based approach or an mTOR inhibitor-based approach. However, no randomised trials have addressed the optimum initial approach for these patients.
Biologically, chromophobe renal cell carcinoma is often found to have activating mutations in the PI3K-mTOR pathway and preclinical sensitivity to rapamycin analogues,10,11 whereas patients with poor risk renal cell carcinoma have been shown to have improved survival with mTOR inhibitor therapy, particularly those patients with high concentrations of circulating lactate dehydrogenase.12 These findings support the rationale of targeting the mTOR pathway in patients with these disease characteristics.
We designed a randomised, investigator-initiated, international trial to compare an initial VEGF receptor inhibitor-based strategy using sunitinib with an initial mTOR inhibitor-based strategy using everolimus in patients with metastatic non-clear-cell renal cell carcinoma. The aims of this trial were to inform clinical practice about these treatments and to develop a biorepository to molecularly characterise this subset of rare cancers in the setting of prospective treatment of metastatic disease.
Methods
Study design and patients
We did this open-label, randomised trial at 17 centres in the USA, Canada, and the UK (appendix). This was an investigator-initiated study, with the Duke Cancer Institute as lead coordinating centre and biorepository. A contract research organisation, inVentiv Health Clinical, oversaw the global collection of data and safety monitoring on behalf of Duke Cancer Institute.
Eligible patients (age ≥18 years) had histologically confirmed, advanced renal cell carcinoma with non-clear-cell pathology, including unclassified subtypes, as assessed through pathological examination by a local site review. Mixtures of these non-clear-cell variants were allowed provided they consisted predominantly (ie, ≥50%) of papillary, chromophobe, or undifferentiated histology. Patients with minor clear cell components (<50%) were permitted, provided the dominant histology and presumed primary histology was non-clear-cell. Translocation carcinomas (if known) and sarcomatoid histologies were allowed irrespective of the histological mixture, provided that non-clear-cell histology was predominant and a clear cell renal cell carcinoma origin was not suspected. Additional eligibility criteria included baseline Karnofsky performance status of 60 or higher, life expectancy of at least 3 months, presence of measurable metastatic disease as per RECIST 1.1 criteria,13 and the presence of renal cell carcinoma tissue available for correlative studies from either a primary or metastatic site or both. Patients could not have received palliative radiation therapy or major surgery within 4 weeks of randomisation, and any effects of previous therapy had to have resolved to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) grade no more than 1. Patients had to have adequate bone marrow, kidney, and liver function and adequate laboratory parameters (baseline creatinine concentration ≤2 times the institutional upper limit of normal [ULN], aspartate aminotransferase and alanine aminotransferase concentration <2⋅5 times the ULN, total cholesterol concentration ≤7 ⋅ 7 mmol/dL [300 mg/dL], triglyceride concentration ≤2⋅5 times the ULN, and blood glucose concentration ≤12 ⋅ 2 mmol/dL [220 mg/dL]). Exclusion criteria included active untreated CNS metastases, previous systemic therapy for renal cell carcinoma, and collecting duct or medullary histology. Patients taking strong CYP3A4 inducers or inhibitors were excluded. Cardiovascular disorders that led to exclusion included poorly controlled hypertension (≥180/100 mm Hg), diabetes (HbAlc >10% [85 ⋅ 8 mmol/mol]), American Heart Association class 2–4 congestive heart failure, or a cardiovascular event within 6 months of randomisation. Additional exclusion criteria included the presence of non-healing wounds, active infections or second malignancies, active autoimmune disease, HIV, hepatitis infection, or recent haemorrhage. Pregnant or nursing women and patients taking drugs known to significantly prolong the corrected QT interval were all excluded.
All patients provided written informed consent under a form issued by an institutional review board. Regulatory oversight and institutional review board or ethics board approval in the USA, Canada, and the UK was maintained for this trial.
Randomisation and masking
Patients meeting eligibility criteria were randomly assigned (1:1) by inventive Health Clinical (Princeton, NJ, USA) to receive either sunitinib malate or everolimus under the supervision of staff at Duke University (Durham, NC, USA). The randomisation sequence was developed by statisticians at Duke Clinical Research Institute before the trial initiation. We used a stratified random block (two strata with six total blocks) design, with randomisation stratified for histology (papillary vs non-papillary) and the 2002 Memorial Sloan Kettering Cancer Center (MSKCC) risk group criteria, which is the most commonly used prognostic model14 (0, 1–2, 3 risk factors). Treatment was open-label and not masked, but randomisation was done under allocation concealment using a prespecified order determined by the study statisticians, in which the order of randomisation was blinded to providers and investigators.
Procedures
After randomisation, treatment with sunitinib malate (Pfizer, New York, NY, USA) was given orally at 50 mg once daily, for treatment cycles of 4 weeks on treatment and 2 weeks off treatment. Everolimus (Novartis, Cambridge, MA, USA) was given orally at 10 mg once daily. Dose modifications for sunitinib were permitted in the form of dose reductions (to 37⋅5 mg or 25 mg) or dose holds, such as alternative dosing treatment cycles of 2 weeks on treatment and 1 week off treatment, depending on the timing and severity of toxic effects. Dose holds or reductions were recommended for grade 3 toxic effects but were required for grade 4 toxic effects for each agent. Grade 4 pneumonitis or haematological toxic effects required discontinuation of everolimus. Any toxic effect that required treatment interruption for 3 weeks or more required discontinuation of study drug. Dose re-escalation was permitted in the absence of grade 2 or higher toxic effects in the previous cycle for each agent. Everolimus dose reductions to 5 mg once daily and then to 5 mg every other day were permitted.
Treatment was continued until radiographic or clinical progression as per RECIST 1.1 criteria or doctor decision in response to perceived absence of clinical benefit. Imaging, including CT and bone scans, was done at baseline and every 3 months with RECIST rereads done locally by a trained radiologist. Each site was selected as a referral centre of excellence in renal cell carcinoma with capable imaging facilities on site for RECIST 1.1 rereads. Central review of imaging was not done. Subsequent therapy at progression was at the discretion of the treating doctor without planned crossover.
Cycles were 6 weeks in length with laboratory tests (bone marrow, kidney, and liver function and calcium, phosphorus, magnesium measurements), physical examination, and toxicity assessments done on days 1, 15, and 29 in the first cycle and on days 1 and 29 of subsequent cycles. Serum lactate dehydrogenase concentrations were measured once per cycle and thyroid function tests were done once every third cycle. Quality-of-life surveys using the FACT-KSI15 were collected at baseline, on day 1 of the third and sixth cycles, and at the end of the study. Archival tumour tissue and prospective whole blood, plasma, and urine samples were collected longitudinally at baseline, day 1 of cycle 3, and at progression from all patients for exploratory correlative studies, which will be analysed and reported separately.
Outcomes
The primary endpoint was radiographic progression-free survival, defined as the time from randomisation to disease progression according to RECIST 1.1 criteria, the appearance of new primary malignancy, or death, whichever occurred first. Patients were censored at the last tumour assessment date. Prespecified secondary descriptive endpoints for this study included progression-free survival at 6 months, 12 months, and 24 months in each treatment group, duration of response, overall survival, and time to new metastasis. Other prespecified secondary endpoints included the proportion of patients achieving an overall radiographic response according to RECIST 1.1, safety and tolerability as described by the NCI Common Terminology Criteria for Adverse Events version 4.0, and quality of life using the FACT-KSI scale.15 We did exploratory outcome analyses to assess the effect of treatment on progression-free survival in subgroups of patients based on the 2002 MSKCC14 risk groups, histological subtypes, and raised lactate dehydrogenase concentration. Safety was monitored by an independent data and safety monitoring board.
Statistical analysis
The null hypothesis was that progression in patients treated with sunitinib would be comparable to that of everolimus (hazard ratio [HR] 1); the alternative hypothesis was that everolimus would cause a 60% improvement in median progression-free survival from 6 ⋅ 0 months to 9⋅6 months in the sunitinib and everolimus groups, respectively, compared with sunitinib (HR 0⋅625). Using a two-sided type I error rate of 0⋅20, we estimated that 90 progression-free survival events would allow us to detect this difference in progression with 83% power. This type I error rate was selected because we were willing to accept a higher false-positive rate in this phase 2 trial setting. We assumed that accrual would proceed at a rate of 2–3 patients per month during a 18-month enrolment period; that patients would be followed up for 24 months after study closure; and that the progression-free survival time followed an exponential distribution. We introduced an 8% increase in sample size to account for potential dropout. This final statistical analysis plan was developed and approved by the insititutional review board on Aug 15, 2014, before any planned data analysis. A high-powered trial with a two-sided type I error of 0⋅05 would have required hundreds of progression-free survival events and dozens of clinical trial sites, which, in view of the rarity of this disease, was not feasible. Therefore, the study was designed to test a hypothesis with a higher type I error rate, taking into account a realistic accrual rate within a reasonable timeframe.
The database was locked in December, 2014, and the final analysis was done once 87 events were recorded because of funding issues. As a result of this early database lock, the log-rank test had a slight reduction in the power of the trial (82%).
An intention-to-treat approach was used in the analysis for all the clinical endpoints with the exception of toxic effects. The primary analysis of the progression-free survival endpoint was based on a two-sided stratified log-rank test for treatment effect, adjusting for stratification factors. In all multivariable analyses, the reference group was sunitinib, and so an HR less than one favours everolimus and an HR greater than one favours sunitinib. The prespecified type I error rate for secondary efficacy analyses was 0⋅05. We applied the Kaplan-Meier product limit method to estimate the distributions of overall survival and progression-free survival. In addition, the proportional hazards model was used to estimate the HR of treatment effect in predicting progression-free survival, adjusting for the stratification factors. In exploratory analyses of treatment effect in subsets of patients (stratification factors and other subgroups), we did not do tests of heterogeneity of treatment group by subgroups in predicting progression-free survival because of low statistical power. Instead, we report the estimated HR and its 80% CI in the subgroups. Proportions were used to compare the number of patients with an objective response and adverse events between the two treatment groups. Summary statistics were used to compare the FACT-KSI quality-of-life results between treatment groups and the change of quality of life with time. We used SAS version 9.2 and R software for all statistical analyses.
This study is registered with ClinicalTrials.gov, number .
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
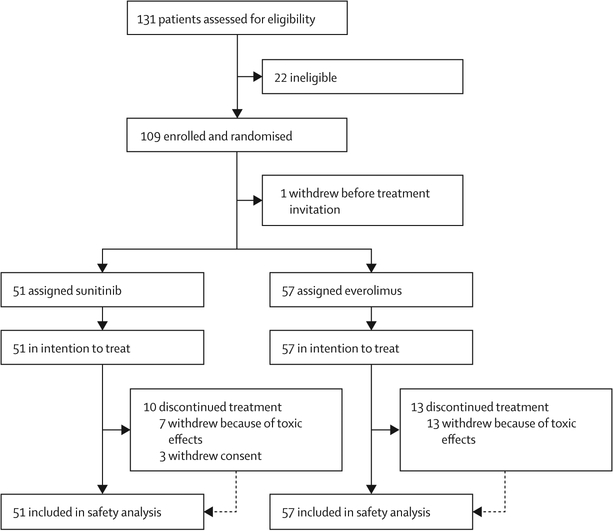
Between Sept 23, 2010, and Oct 28, 2013, 131 patients were assessed for eligibility and 109 patients were enrolled (figure 1) from 17 centres in the USA, UK, and Canada. The original intent was to enroll only 108 patients, but one participant withdrew after consent, yet before randomisation and before study drug was assigned. This patient was replaced such that, of 109 enrolled patients, 108 patients were evaluable. 51 patients were assigned sunitinib and 57 patients were assigned everolimus. Baseline demographic information for the study population is shown in table 1. Of note, only one (7%) of the 15 patients who were rated as having poor risk had chromophobe histology, whereas nine (13%) of 70 patients with papillary renal cell carcinoma and five (23%) of 22 patients with unclassified renal cell carcinoma were rated as having poor risk, indicating little overlap between risk group and histological subtype. Additionally, we found no correlation between MSKCC risk group and histological subtype: 17 (24%) of 70 patients with papillary renal cell carcinoma and 12 (32%) of 38 patients with chromophobe or unclassified renal cell carcinoma were in the good risk group, whereas 44 (63%) of 70 patients with papillary renal cell carcinoma and 20 (53%) of 38 patients with chromophobe or unclassified renal cell carcinoma were rated as being at intermediate risk.
Figure 1:
Trial profile
Table 1:
Baseline characteristics
| Sunitinib (n=51) | Everolimus (n=57) | |
|---|---|---|
| Age | 59 (24–100) | 64 (29–90) |
| Sex | ||
| Male | 37 (73%) | 44 (77%) |
| Female | 14 (27%) | 13 (23%) |
| Ethic origin | ||
| White | 42 (82%) | 52 (91%) |
| Black | 7 (14%) | 5 (9%) |
| Histological subtype | ||
| Papillary histology overall | 33 (65%) | 37 (65%) |
| Papillary histology type 1 | 4 (8%) | 2 (4%) |
| Chromophobe | 10 (20%) | 6 (10%) |
| Unclassified | 8 (16%) | 14 (25%) |
| Translocation carcinoma | 6 (12%) | 2 (4%) |
| Minor clear cell component | 5 (10%) | 8 (14%) |
| Sarcomatoid differentiation | 5 (11%) | 11 (27%) |
| Prior nephrectomy | 41 (80%) | 45 (79%) |
| Elevated lactate dehydrogenase concentration | 13 (27%) | 13 (25%) |
| Liver metastases | 16 (31%) | 15 (26%) |
| Lung metastases | 30 (59%) | 25 (44%) |
| Bone metastases | 12 (24%) | 15 (26%) |
| MSKCC risk group | ||
| 0 | 15 (29%) | 14 (25%) |
| 1–2 | 32 (63%) | 32 (56%) |
| ≥3 | 4 (8%) | 11 (19%) |
Data are median (IQR), n (%), or mean (SD). Note that percentages within histological subtypes (papillary, chromophobe, and unclassified) may add to slightly greater than 100% due to rounding. MSKCC=Memorial Sloan Kettering Cancer Center criteria.
At the time of data cutoff (Dec 8, 2014), 87 progression-free survival events were recorded (38 [75%] patients in the sunitnib group and 49 [86%] patients in the everolimus group), and 54 patients had died (23 [45%] deaths in the sunitinib group and 31 (54%) deaths in the everolimus group), with only two active participants still receiving study drug, one in each treatment group. In only ten cases did death occur before radiographic progression. Thus, most progression-free survival events were due to RECIST-defined progressive disease. Median follow-up among surviving patients was 13 months (IQR 6–22; 15 months [IQR 8–26] in the sunitinib group and 12 months [6–19] in the everolimus group). The median duration of treatment was 5 ⋅ 1 months (IQR 2 ⋅ 5–10 ⋅ 5) in the sunitinib group and 4⋅1 months (2⋅5–6⋅2) in the everolimus group. 35 (69%) patients in the sunitinib group and 39 (68%) patients in the everolimus group discontinued treatment because of progression. Seven (14%) patients received sunitinib for 12 months or more, compared with six (11%) patients who received everolimus for 12 months or more.
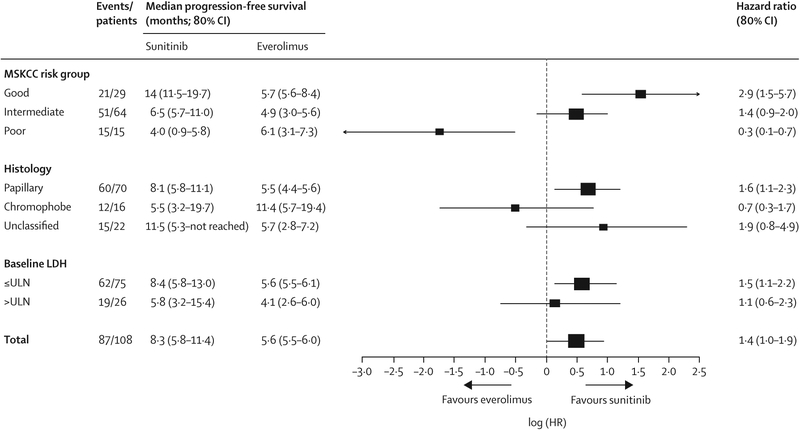
Median progression-free survival was 8⋅3 months (80% CI 5⋅8–11⋅4) for sunitinib and 5⋅6 months (5⋅5–6⋅0) for everolimus (HR 1⋅41 [80% CI 1⋅03–1⋅92]; p=0⋅16; figure 2; table 2), meeting the prespecified level of statistical significance for the study (two-sided type I error rate of 0⋅20). Progression-free survival at 6 months, 12 months, and 24 months was 40⋅3% (27⋅3–52⋅8), 17⋅0% (8⋅4–28⋅3), and 9⋅3% (3⋅0–20⋅2) for everolimus and 55⋅0% (40⋅1–67⋅7), 37⋅7% (24⋅1–51⋅2), and 22⋅8% (11⋅7–36⋅1) for sunitinib, respectively. Patients rated as being at good risk according to MSKCC criteria had a median progression-free survival of 14⋅0 months (11⋅5–19⋅7) when treated with sunitinib and a median progression-free survival of 5⋅7 months (5⋅6–8⋅4) when treated with everolimus (HR 2 ⋅ 9 [1 ⋅ 5–5 ⋅ 7]; figure 3), and patients at intermediate risk had a median progression-free survival of 6⋅5 months (5⋅7–11⋅0) for sunitinib and 4⋅9 months (3⋅0–5⋅6) for everolimus (HR 1⋅38 [0⋅96–2⋅00]). However, patients rated as being at poor risk had a median progression-free survival of 4 ⋅ 0 months (0 ⋅ 9–5 ⋅ 8) with sunitinib and 6 ⋅ 1 months (3 ⋅ 1–7 ⋅ 3) with everolimus (HR 0⋅3 [0⋅1–0⋅7]; figure 3). Exploratory outcome analyses of progression-free survival by other stratification factors are shown in figure 3. We also assessed MSKCC criteria (good vs intermediate or poor risk) for the prediction of progression-free survival, adjusting for treatment group and the stratification variable (HR 0⋅6 [0⋅4–0⋅9]; appendix).
Figure 2:
Kaplan-Meier estimates of progression-free survival according to treatment group
Table 2:
Overall response
| Proportion of patients achieving an overall partial or complete radiographic response | ||
|---|---|---|
| Sunitinib | Everolimus | |
| Overall intention-to-treat population (n=108) | 9/51 (18%) | 5/57 (9%) |
| Good risk MSKCC (n=29) | 1/15 (7%) | 1/14 (7%) |
| Intermediate risk MSKCC (n=64) | 8/32 (25%) | 3/32 (9%) |
| Poor risk MSKCC (n=15) | 0/4 | 1/11 (9%) |
| Papillary renal cell carcinoma (n=70) | 8/33 (24%) | 2/37 (5%) |
| Chromophobe renal cell carcinoma (n=16) | 1/10 (10%) | 2/6 (33%) |
| Unclassified renal cell carcinoma (n=22) | 0/8 | 1/14 (7%) |
| Lactate dehydrogenase concentration ≤ULN (n=75) | 6/35 (17%) | 5/40 (13%) |
| Lactate dehydrogenase concentration >ULN (n=26) | 2/13 (15%) | 0/13 |
Best overall radiographic response by RECIST 1.1, by treatment group overall and by histological and MSKCC risk subsets. Data are n/N (%). MSKCC=Memorial Sloan Kettering Cancer Center criteria. ULN=upper limit of normal.
Figure 3: Exploratory forest plot of progression-free survival according to subsets defined by histological subtype, MSKCC risk group, and elevated lactase dehydrogenase concentration.
MSKCC=Memorial Sloan Kettering Cancer Center. LDH=lactate dehydrogenase. HR is based on the stratified analysis with MSKCC risk group and histology where appropriate (HR>1⋅0 favours sunitinib). Note that 80% CI was used for the subgroup analysis of progression-free survival as per the statistical plan.
We estimated progression-free survival according to the prespecified stratification factor of histological subtype (figure 3). Combined, the data suggest differences in the duration of progression-free survival according to baseline risk group and histological subtype of non-clear-cell renal cell carcinoma.
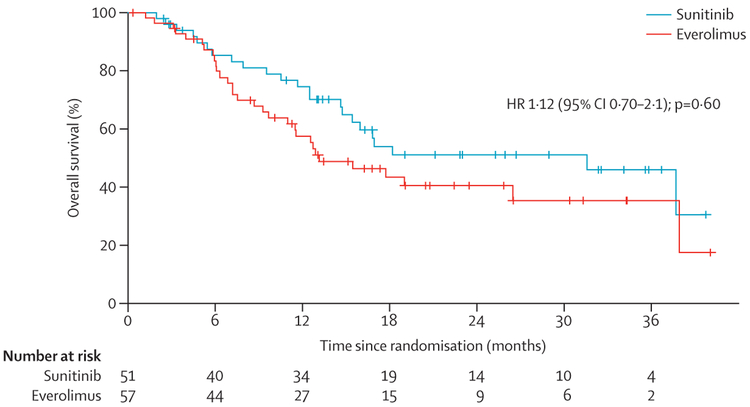
Overall survival was not different between the two treatment groups (HR 1⋅12 [95% CI 0⋅7–2⋅1]; p=0⋅60 figure 4). Median overall survival was 13⋅2 months (95% CI 9⋅7–37⋅9) in the everolimus group and 31⋅5 months (14⋅8-not reached) in the sunitinib group. No differences in overall survival were noted within subsets of patients (histology, risk group, lactate dehydrogenase concentration) according to treatment group assignment (data not shown). Subsequent therapy after progression was common, with 69 (64%) of 108 patients receiving at least one subsequent treatment for metastatic renal cell carcinoma (36 [71%] in the sunitinib group and 33 [58%] in the everolimus group). Crossover treatment from sunitinib to everolimus at time of analysis was noted in 11 (22%) of 51 patients, and crossover treatment from everolimus to sunitinib was noted in ten (18%) of 57 patients. Subsequent therapy with a VEGF inhibitor was noted in 24 (42%) of 57 patients who were initially treated with everolimus, whereas only 11 (22%) of 51 patients who were initially treated with sunitinib have been treated with an mTOR inhibitor to date. However, half of the participants were alive at the time of datalock.
Figure 4:
Kaplan-Meier estimates of overall survival according to treatment group
In further descriptive analyses, RECIST 1.1 overall radiographic responses were found in nine (18% [95% CI 7–28]) of 51 evaluable patients who were treated with sunitinib (no patients had a complete response and nine patients had a partial response) and in five (9% [1–16]) of 57 evaluable patients who were treated with everolimus (two patients had a complete response and four patients had a partial response; table 2). These responses were confirmed on subsequent scans in three (60%) of five patients treated with everolimus and three (33%) of nine patients treated with sunitinib. One of the complete responses was surgically induced in a patient who had a prolonged partial response to everolimus. The median duration of response was 8⋅3 months (95% CI 3⋅1–17⋅2) for patients in the sunitinib group and 3⋅9 months (2⋅8–9⋅1) for patients in the everolimus group. Stable disease as the best response was seen in 30 (59%) of 51 patients in the sunitinib group and in 30 (53%) of 57 patients m the everolimus group, whereas progressive disease as best response was seen in ten (20%) patients in the sunitinib group and 13 (23%) patients in the everolimus group. The composite of complete response, partial response, and stable disease lasting at least 24 weeks was seen in 19 (37%) patients in the sunitinib group and nine (16%) patients in the everolimus group (appendix).
Toxic effects were assessed in all 108 participants (table 3). 27 (53%) of 51 patients treated with sunitinib needed dose reductions, and seven (14%) patients discontinued treatment because of toxic effects. Nine (16%) of 57 patients who received everolimus needed dose reductions, and 13 (23%) participants discontinued treatment because of toxic effects. We did not record any treatment-related deaths or unexpected toxic effects from sunitinib or everolimus. Nausea, vomiting, diarrhoea, decreased appetite, hypertension, palmar-plantar erythrodysaesthesia (hand-foot syndrome), and hypothyroidism were more common in patients who had received sunitinib than in patients who received everolimus. Stomatitis, rash, grade 3 fatigue, peripheral oedema, weight loss, and pneumonitis were more common in patients treated with everolimus than in patients treated with sunitinib. Among the 51 patients who were treated with sunitinib, 12 (24%) had grade 3 or 4 hypertension, five (10%) patients had grade 3 diarrhoea, four (8%) patients had grade 3 or 4 hand-foot syndrome, and two (4%) patients had grade 3 fatigue. Among the 57 patients who were treated with everolimus, five (9%) patients had grade 3 or 4 pneumonitis, and four (7%) patients had grade 3 fatigue. Overall, 40 (78%) patients receiving sunitinib had grade 3 or worse treatment-related adverse event compared with 34 (60%) patients receiving everolimus. Serious adverse events (grade 3–5) that were felt to be at least possibly related to study treatment per protocol were reported in 34 (60%) of everolimus-treated patients and 40 (78%) of sunitinib-treated patients.
Table 3:
Summary of adverse events by NCI Common Toxicity Criteria version 4.0 and treatment
| Sunitinib (n=51) | Everolimus (n=57) | ||||||
|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | ||
| Nausea | 33 (65%) | 3 (6%) | 0 | 25 (44%) | 1 (2%) | 0 | |
| Decreased appetite | 29 (57%) | 1 (2%) | 0 | 15 (26%) | 0 | 0 | |
| Diarrhoea | 29 (57%) | 5 (10%) | 0 | 16 (28%) | 1 (2%) | 0 | |
| Fatigue | 29 (57%) | 2 (4%) | 0 | 29 (51%) | 4 (7%) | 0 | |
| Dysgeusia | 25 (49%) | 0 | 0 | 18 (32%) | 0 | 0 | |
| Infection | 25 (49%) | 6 (12%) | 0 | 24 (42%) | 4 (7%) | 0 | |
| Hand-foot syndrome | 17 (33%) | 2 (4%) | 2 (4%) | 8 (14%) | 0 | 0 | |
| Constipation | 15 (29%) | 0 | 0 | 10 (18%) | 0 | 0 | |
| Stomatitis | 14 (27%) | 0 | 0 | 22 (39%) | 5 (9%) | 0 | |
| Vomiting | 14 (27%) | 3 (6%) | 0 | 13 (23%) | 0 | 0 | |
| Anaemia | 13 (25%) | 1 (2%) | 0 | 10 (18%) | 6 (11%) | 0 | |
| Back pain | 12 (24%) | 2 (4%) | 0 | 6 (11%) | 0 | 0 | |
| Mucosal inflammation | 12 (24%) | 0 | 0 | 13 (23%) | 1 (2%) | 0 | |
| Arthralgia | 11 (22%) | 1 (2%) | 0 | 7 (12%) | 0 | 0 | |
| Dyspepsia | 11 (22%) | 0 | 0 | 0 | 0 | 0 | |
| Hypertension | 11 (22%) | 11 (22%) | 1 (2%) | 0 | 1 (2%) | 0 | |
| Aspartate aminotransferase concentration increased | 10 (20%) | 0 | 0 | 6 (11%) | 1 (2%) | 0 | |
| Rash | 10 (20%) | 1 (2%) | 0 | 17 (30%) | 1 (2%) | 0 | |
| Blood creatinine concentration increased | 9 (18%) | 0 | 0 | 6 (11%) | 0 | 0 | |
| Anxiety | 8 (16%) | 0 | 0 | 0 | 0 | 0 | |
| Cough | 8 (16%) | 0 | 0 | 24 (42%) | 1 (2%) | 0 | |
| Dyspnoea | 8 (16%) | 1 (2%) | 0 | 20 (35%) | 1 (2%) | 1 (2%) | |
| Lethargy | 8 (16%) | 2 (4%) | 0 | 8 (14%) | 1 (2%) | 0 | |
| Thrombocytopenia | 8 (16%) | 4 (8%) | 0 | 6 (11%) | 1 (2%) | 0 | |
| Abdominal pain | 7 (14%) | 1 (2%) | 0 | 8 (14%) | 1 (2%) | 0 | |
| Dry skin | 7 (14%) | 0 | 0 | 11 (19%) | 0 | 0 | |
| Gastro-oesophageal reflux disease | 7 (14%) | 0 | 0 | 0 | 0 | 0 | |
| Peripheral oedema | 7 (14%) | 1 (2%) | 0 | 14 (25%) | 1 (2%) | 0 | |
| Pruritus | 7 (14%) | 0 | 0 | 7 (12%) | 1 (2%) | 0 | |
| Weight loss | 7 (14%) | 0 | 0 | 10 (18%) | 0 | 0 | |
| White blood cell count decreased | 7 (14%) | 1 (2%) | 0 | 0 | 0 | 0 | |
| Dizziness | 6 (12%) | 0 | 0 | 0 | 0 | 0 | |
| Epistaxis | 6 (12%) | 2 (4%) | 0 | 11 (19%) | 0 | 0 | |
| Flatulence | 6 (12%) | 0 | 0 | 0 | 0 | 0 | |
| Pain in extremity | 6 (12%) | 1 (2%) | 0 | 0 | 1 (2%) | 0 | |
| Platelet count decreased | 6 (12%) | 2 (4%) | 0 | 0 | 0 | 0 | |
| Alanine aminotransferase concentration increased | 5 (10%) | 0 | 0 | 0 | 0 | 0 | |
| Dry mouth | 5 (10%) | 0 | 0 | 0 | 0 | 0 | |
| Headache | 5 (10%) | 0 | 0 | 0 | 2 (4%) | 0 | |
| Hypomagnesaemia | 5 (10%) | 0 | 0 | 0 | 0 | 0 | |
| Neutrophil count decreased | 5 (10%) | 3 (6%) | 0 | 0 | 0 | 0 | |
| Pain | 5 (10%) | 0 | 0 | 0 | 1 (2%) | 0 | |
| Hyperglycaemia | 4 (8%) | 0 | 0 | 7 (12%) | 0 | 0 | |
| Pyrexia | 4 (8%) | 0 | 0 | 8 (14%) | 0 | 0 | |
| Insomnia | 3 (6%) | 0 | 0 | 6 (11%) | 0 | 0 | |
| Musculoskeletal chest pain | 2 (4%) | 0 | 0 | 6 (11%) | 0 | 0 | |
| Maculo-papular rash | 2 (4%) | 0 | 0 | 6 (11%) | 0 | 0 | |
| Blood cholesterol concentration increased | 1 (2%) | 0 | 0 | 7 (12%) | 0 | 0 | |
| Hypertriglyceridaemia | 0 | 0 | 0 | 5 (9%) | 3 (5%) | 0 | |
| Pneumonitis | 0 | 0 | 0 | 3 (5%) | 4 (7%) | 1 (2%) | |
Adverse events with a frequency of 10% or more in eithertreatment group are presented. Patients are counted only once by the worst grade adverse event experienced. No treatment-related deaths were reported.
Laboratory test abnormalities, including thrombocytopenia, neutropenia, raised liver function test enzymes, hypothyroidism, and hypophosphataemia were more common with sunitinib than with everolimus but were usually grade 1 or 2. Hypertriglyceridaemia, hypercholesterolaemia, and hyperglycaemia were more common with everolimus than with sunitinib. Grade 3 or higher hyperglycaemia was seen in one (2%) of 57 patients who were treated with everolimus and was not seen with sunitinib treatment. Grade 3 anaemia was more common with everolimus (six [11%] of 57 patients treated with everolimus vs one [2%] of 51 patients treated with sunitinib). Point estimates for the prevalence of toxic effects and the odds ratio for a given toxic effect between treatment groups are shown in the appendix(p 4).
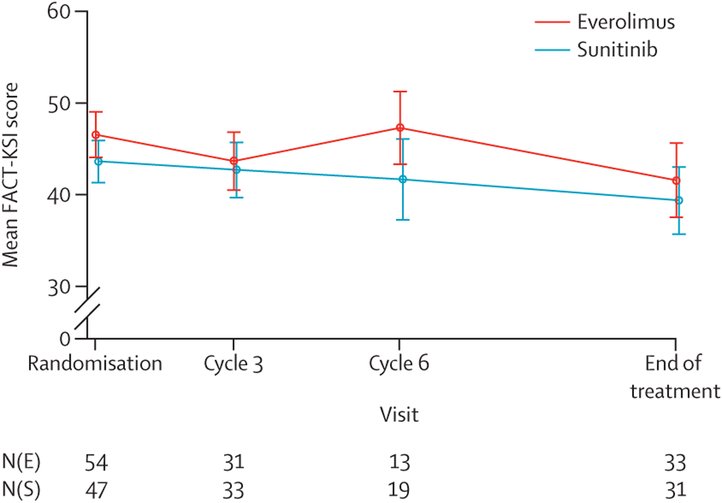
At baseline, the median FACT-KSI score was 47⋅5 (IQR 23–59) for patients in the everolimus group and 45⋅0 (27–56) in the sunitinib group (figure 5). By the third cycle, the median KSI was 44 (IQR 37–50) for everolimus (change from baseline of −4⋅5, n=31) and 43 (36–49) for sunitinib (change from baseline −1⋅0, n=33). At progression or end of treatment, median FACT-KSI score was 44 (32–50) for everolimus (change from baseline −7 ⋅ 5) and 42 (31–48) for sunitinib (change from baseline −5⋅0). Between-group differences in quality-of-life measures, including disease-related subscales, were not significant.
Figure 5: Quality of life overtime using the FACT-KSI in each treatment group.
Overall and subscales are presented. N(E) and N(S) are the number of questionnaires received at each timepoint in the everolimus group and sunitinib group, respectively.
Discussion
Our findings suggest that patients with metastatic non-clear-cell renal cell carcinoma have significantly longer progression-free survival when treated with sunitinib compared with everolimus. We also show substantial heterogeneity in outcomes by histology and prognostic risk groups. Renal cell carcinoma is a heterogeneous disease with multiple histological subtypes, defined pathologically, each with distinct molecular characteristics and clinical outcomes. Although clear cell renal cell carcinoma is the most common form of metastatic renal cell carcinoma, about 25% of metastatic cases are predominantly non-clear-cell subtypes.1,2,16 Randomised clinical trials that have led to drug approvals of VEGF receptor tyrosine kinase inhibitors, including sunitinib, by the US FDA and European Medicines Agency were performed in patients with predominantly clear cell renal cell carcinoma.17–21 As such, evidence to guide management decisions for patients with non-clear-cell renal cell carcinoma is scant. In one reported trial to date (ESPN trial22), 73 patients with non-clear-cell renal cell carcinoma and sarcomatoid clear cell renal cell carcinoma were randomly assigned to receive either everolimus or sunitinib. However, no significant differences in outcomes (progression-free survival or overall survival) were noted between the treatments overall or within histologically defined subgroups of patients. To date, regulatory agencies have been broad in offering treatment labels that include all forms of advanced renal cell carcinoma in the absence of any other treatment options. We sought to address this absence of data with, to the best of our knowledge, the largest, multinational, randomised controlled study in patients with non-clear-cell histologies in which we compare response to sunitinib with everolimus, two standards of care, orally bioavailable inhibitors of the VEGF receptor tyrosine kinase and mTOR inhibitor classes, respectively.
Our aims were to assess for a clinically significant difference (3–4-month improvement) in progression-free survival between these two standard-of-care therapies in this heterogeneous population of patients with renal cell carcinoma, explore the different patterns of clinical response between disease subgroups, and prospectively collect archival and fresh specimens of tumour and blood correlative analyses. We used a phase 2 design because of both the rarity of the disease and the absence of existing comparative data, and we explored the heterogeneity of outcomes within subgroups defined by prognosis or risk and histology. Non-clear-cell renal cell carcinoma is a compilation of several distinct and uncommon histological and genetic subtypes, and the establishment of outcomes with specific therapies in a controlled study can permit future trials in histological or prognostic risk-group-defined populations of patients with this disease.
Our clinical results reveal several informative observations. First, the difference in radiographic progression-free survival in the overall population was statistically significant in favour of sunitinib compared with everolimus. We found slight imbalances at randomisation in the number of patients with sarcomatoid renal cell carcinoma and patients who were at poor risk, favouring the sunitinib group, for example, which might have led to slight differences in outcome within small subgroups. However, our data provide evidence that initial treatment with sunitinib is reasonable and reveal populations for whom initial treatment with an mTOR inhibitor might be reasonable.
Second, clinical responses to sunitinib and everolimus varied according to predefined histological subtypes. Prognostic differences between renal cell carcinoma subtypes are well known but clinical responses to targeted therapies are not. Interestingly, we found two subtypes in which sunitinib was associated with a longer median progression-free survival than that associated with everolimus (papillary and unclassified) and one subtype (chromophobe) in which everolimus was associated with a longer median progression-free survival than that of sunitinib. Although we were unable to test for treatment group by subgroup interactions, the differences in median progression-free survival and the HRs are clinically important and warrant further confirmation and reporting in larger trials or meta-analyses of existing datasets. To some extent, these findings validate our molecular understandings of these disparate diseases, particularly in the case of chromophobe renal cell carcinoma, in which alterations in the PTEN-PI3K-AKT signal transduction pathway have been described and might predispose to downstream inhibition through mTOR.10,11,16 We will explore this disease subtype further through a comprehensive characterisation of this signal transduction pathway and others in samples collected as part of this study to develop molecular predictors of clinical efficacy. Large genomic or pathological series have been done in the past, but none to date have prospective clinical annotation in a multicentre setting to assess treatment response.
The third key clinical observation was that clinical outcomes on treatment varied by risk stratification. We found that median progression-free survival was longer with first-line sunitinib than with everolimus in patients with non-clear-cell renal cell carcinoma who had been rated as good or intermediate risk according to MSKCC criteria, whereas median progression-free survival was longer for patients rated as poor risk treated with everolimus than with sunitinib. This finding is consistent with previous work, suggesting a clinical benefit with mTOR inhibition in poor risk renal cell carcinoma, irrespective of histological subtype.6,7 Results of a previous phase 3 study6,7 of the intravenously administered mTOR inhibitor temsirolimus showed an overall survival advantage in untreated patients at poor risk compared with interferon alpha, and a subgroup analysis of patients with non-clear-cell renal cell carcinoma included in this study suggested an even greater benefit in this population. Although our subgroup results are not adequately powered to show a significant difference between these agents, the present data are in line with previous findings, particularly around everolimus,8,9 and might help in the design of future trials. In particular, the median progression-free survival for everolimus across this population of patients at good risk, intermediate risk, and poor risk suggest PI3K-mTOR pathway activity might be increased preferentially in more aggressive phenotypes. This same result was found in an earlier study of temsirolimus for renal cell carcinoma23 and was one of the factors that affected the study population of the above-mentioned phase 3 study of temsirolimus versus interferon. Future survival updates will be able to assess these and novel risk factors for overall survival in this population.
Although a larger confirmatory study is needed to estimate the treatment effect with a tighter CI, these results offer the most definitive evidence to date supporting not only the heterogeneity of this disease and outcomes, but also that responses and outcomes to either an mTOR inhibitor-based or VEGF tyrosine kinase inhibitor-based approach depend on the specific groups.
Several limitations to our findings must be highlighted. First, our subgroup analyses suggest that histological subtypes are indeed a mixed population of patients and are essentially distinct diseases. However, because of the low statistical power, we were unable to test for treatment group by subgroup interaction in predicting progression-free survival. In the future, we would recommend investigation of these populations in either prespecified subgroups, or in distinct study populations, ideally driven by molecular predictors linked to the benefit of a specific systemic therapy. For example, patients with papillary renal cell carcinoma and c-MET activation could be selected for targeted c-MET inhibition,24 or patients with PD-L1 biomarker expression could be selected for PD-1 or PD-L1 immune checkpoint inhibition.25 Second, we have relied on individual clinical pathology assessments rather than a central pathology interpretation. However, over 90% of patients contributed banked tissues for future correlative analyses, which will include pathological assessment, and we anticipate future associative analyses of biomarkers, histology, and outcomes from this trial. We minimised the potential for misclassification through the selection of renal cell carcinoma tertiary academic referral centres, each of which had access to a trained genitourinary pathologist who reviewed each case at the local site. Our results therefore provide generalisability for clinical practice. Central reads of our research specimens are limited by the known heterogeneity of renal cell carcinoma and risk misclassification.26,27
An additional limitation was that the final analysis was based on 87 events of progression-free survival, which was lower than the target of 90 events. Thus, this reduced the power for the log-rank test from the original design of 83% to 82%. We do not believe that this reduced power changes the conclusions that can be drawn from this trial. We did note some imbalances in the proportion of certain subsets of patients at baseline due to chance (sarcomatoid and translocation carcinoma), but in view of the rarity of these subtypes, we could not have stratified for these in advance. Finally, we relied on investigator-assessed radiographic RECIST response and determinations of progression-free survival. Central radiology assessments in real time would have been cost prohibitive, and how meaningful a central read is in extrapolating clinical trial data to the real world setting, which was our intent of this study, is unclear.
In summary, our clinical results shed important insights for clinical management of this patient population. These data will also support both the design of future correlative and clinical studies of standard-of-care agents and novel approaches to improve the outcomes of this understudied population of patients with renal cell carcinoma.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed and American Society of Clinical Oncology abstracts using the search terms “non-clear cell renal cell carcinoma”, “RCC”, “papillary RCC”, or “chromophobe RCC”, with specific attention to prospective therapeutic trials of mTOR inhibitors (everolimus, temsirolimus) or VEGF inhibitors (sunitinib, sorafenib, bevacizumab, axitinib, pazopanib). We included open access datasets and retrospective analyses of datasets published between 1990 and 2015, and key historical data are included in the references cited. The only controlled study reported in abstract form in this population is the ESPN trial, which did not show superiority for progression-free survival with sunitinib or everolimus in a population of patients with non-clear-cell renal cell carcinoma, although this study was smaller (n=73) than our study and permitted patients with clear cell renal cell carcinoma who showed sarcomatoid features; the complete study is yet to be published. Our study thus represents the largest controlled trial assessing front-line therapy in patients with non-clear-cell renal cell carcinoma and provides evidence to suggest heterogeneity of clinical benefit in these patients according to prognostic risk group and histological subtype.
Added value of this study
Several key findings are of immediate clinical importance. First, sunitinib significantly improved progression-free survival compared with everolimus. Second, heterogeneity of outcomes was notable, particularly with respect to histological subtype and prognostic risk group. In exploratory, non-powered analyses, sunitinib was more effective in prolonging progression-free survival in patients rated as being at good or intermediate risk according to Memorial Sloan Kettering Cancer Center criteria and in patients with papillary or unclassified histologies, whereas everolimus was more effective in prolonging progression-free survival in patients at poor risk or with chromophobe histology. However, the treatment group by subgroups interactions were not tested because of small sample sizes, but the present data provide reasonable estimates of the median and the hazard ratio for effect sizes in these subgroups. Finally, we found no increase in unexpected toxic effects or differential quality of life with these agents in patients with non-clear-cell renal cell carcinoma.
Implications of all the available evidence
Based on the present study and previous clinical studies, decisions on therapeutic choice between sunitinib and everolimus for patients with metastatic non-clear-cell renal cell carcinoma should be based on prognostic risk criteria, histological subtype, and the known, expected side-effects. Future clinical trials in these patients should also consider this heterogeneity of outcome when assessing novel agents.
Acknowledgments
We thank the trial coordinators and their staff for their support in the conduct of this trial and the patients and their families for their dedication to this research. We thank Omar Din at Cancer Clinical Trial Centre, Weston Park Hospital, Sheffield, UK, and Mary MacKenzie at London Health Sciences Center, London, ON, Canada, for their support of this study at their centres. We thank the Duke Center for Human Genetics biorepository and David Layfield for their support. We thank the staff at the Cancer Research UK Clinical Trials Unit, Glasgow, UK, who were instrumental in delivering the UK sites for this study. We thank both Novartis and Pfizer for their financial support of this investigator-initiated trial and both inVentiv Health Clinical and Ergomed for their monitoring and data collection support across all centres.
Footnotes
Declaration of interests
AJA and DJG reports grants from Novartis and Pfizer during the conduct of the study; grants and personal fees from Dendreon, Sanofi-Aventis, Bayer, Medivation/Astellas, and Janssen, outside the submitted work. DJG also reports grants from Innocrin and Exelixis and personal fees from BMS and Janssen. TE is an employee of AstraZeneca and reports grants from AstraZeneca, personal fees from Novartis, Roche, BMS, and AVEO, grants from Bayer, grants and personal fees from Pfizer, GSK, personal fees and grant to institution from Astellas, outside the submitted work. JAG reports grants and personal fees from Pfizer and Novartis, during the conduct of the study; grants and personal fees from Bayer and Medivation/Astellas, and personal fees from Sanofi-Aventis, outside the submitted work. TFL reports grants from Novartis and Pfizer, during the conduct of the study; grants from Abbott, Abraxis, Acceleron, Amgen, AstraZeneca, Biovex, and Cerulean, Eisai, Eli Lilly grants and personal fees from Argos and Aveo, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffman-La Roche, Immatics, Merck, Roche, Synta, Threshold, Tracon, EMD Serono, Millennium, and Schering-Plough, personal fees from Genentech, and grants and personal fees from Novartis, Pfizer, Prometheus, and Wyeth, outside the submitted work. CKK reports personal fees from Pfizer, Novartis, BMS, and Sanofi-Aventis, outside the submitted work. UNV reports grants and personal fees from Novartis and Pfizer, outside the submitted work. CWR reports personal fees from Pfizer and Genentech, research grant to institution from Onyx, outside the submitted work. RJJ reports grants from Pfizer and Novartis, during the conduct of the study; grants and personal fees from Pfizer, and grants, personal fees, and non-financial support from Novartis and GSK, outside the submitted work. WMS reports grants and personal fees from Pfizer, outside the submitted work. LMP reports personal fees from Pfizer and Novartis. SH, SB, JP, REH, JDH, IP, AP, CML, and SO declare no competing interests.
References
- 1.Bitting RL, Madden J, Armstrong AJ. Therapy for non-clear cell histologies in renal cancer. Curr Clin Pharmacol 2011; 6:169–80. [DOI] [PubMed] [Google Scholar]
- 2.Shuch B, Amin A, Armstrong AJ, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol 2015; 67: 85–97. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008; 26:127–31. [DOI] [PubMed] [Google Scholar]
- 4.Gore ME, Porta C, Oudard S, et al. Sunitinib in metastatic renal cell carcinoma (mRCC): preliminary assessment of toxicity in an expanded access trial with subpopulation analysis. Proc Am Soc Clin Oncol 2007; 25 (suppl 18): abstr 5010. [Google Scholar]
- 5.Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol 2012; 23: 2108–14. [DOI] [PubMed] [Google Scholar]
- 6.Dutcher JP, Szczylik C, Tannir N, et al. Correlation of survival with tumor histology, age, and prognostic risk group for previously untreated patients with advanced renal cell carcinoma (adv RCC) receiving temsirolimus (TEMSR) or interferon-alpha (IFN). Proc Am Soc Clin Oncol 2007; 25 (suppl 18): abstr 5033. [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356: 2271–81. [DOI] [PubMed] [Google Scholar]
- 8.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol 2013; 24:1026–31. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014; 32: 2765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaux A, Albadine R, Schultz L, et al. Dysregulation of the mammalian target of rapamycin pathway in chromophobe renal cell carcinomas. Hum Pathol 2013; 44: 2323–30. [DOI] [PubMed] [Google Scholar]
- 11.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014; 26: 319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 2012; 30: 3402–07. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002; 20: 289–96. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Yount S, Du H, et al. Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) J Support Oncol 2006; 4:191–99. [PubMed] [Google Scholar]
- 16.Linehan WM, Pinto PA, Srinivasan R, et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res 2007; 13: 671s–79s. [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009; 27: 3312–18. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356:115–24. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295: 2516–24. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378:1931–39. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010; 28: 2137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol 2015; published online November 25 DOI: 10.1016/j.eururo.2015.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 2004; 22: 909–18. [DOI] [PubMed] [Google Scholar]
- 24.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013; 31:181–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Fay AP, Gray KP, et al. PD-Ll expression in nonclear-cell renal cell carcinoma. Ann Oncol 2014; 25: 2178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 2014; 46: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.