Abstract
Introduction.
Although black patients with endometrial cancer (EC) have worse survival compared with white patients, the interaction between age/race has not been examined. The primary objective was to evaluate the impact of age at diagnosis on racial disparities in disease presentation and outcome in EC.
Methods.
We evaluated women diagnosed with EC between 1991 and 2010 from the Surveillance, Epidemiology, and End Results. Mutation status for TP53 or PTEN, or with the aggressive integrative, transcript-based, or somatic copy number alteration-based molecular subtype were acquired from the Cancer Genome Atlas. Logistic regression model was used to estimate the interaction between age and race on histology. Cox regression model was used to estimate the interaction between age and race on survival.
Results.
78,184 white and 8518 black patients with EC were analyzed. Median age at diagnosis was 3-years younger for black vs. white patients with serous cancer and carcinosarcoma (P < 0.0001). The increased presentation of non-endometrioid histology with age was larger in black vs. white patients (P < 0.0001). The racial disparity in survival and cancer-related mortality was more prevalent in black vs. white patients, and in younger vs. older patients (P < 0.0001). Mutations in TP53, PTEN and the three aggressive molecular subtypes each varied by race, age and histology.
Conclusions.
Aggressive histology and molecular features were more common in black patients and older age, with greater impact of age on poor tumor characteristics in black vs. white patients. Racial disparities in outcome were larger in younger patients. Intervention at early ages may mitigate racial disparities in EC.
Keywords: Endometrial cancer, Race, Disparity, SEER, Age, Survival
GRAPHICAL ABSTRACT
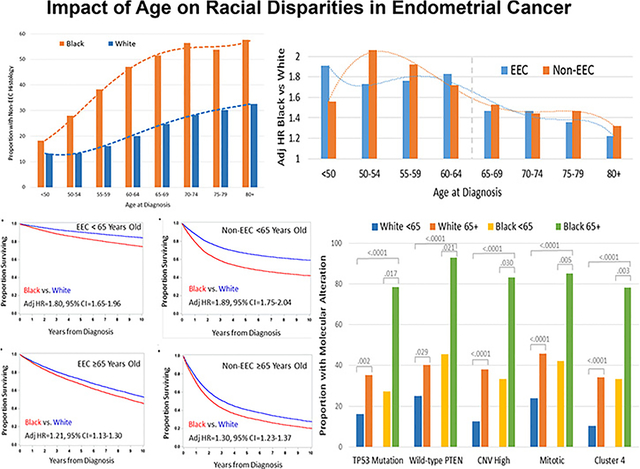
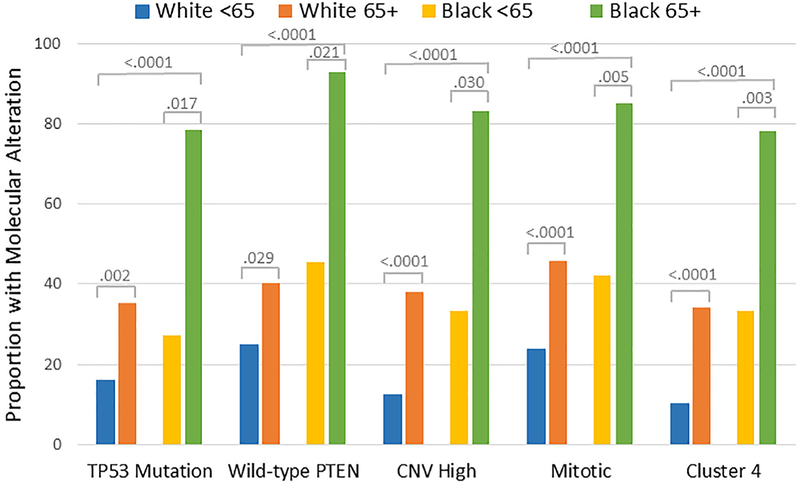
Impact of age of diagnosis on the proportion of the high-risk histology (upper left), adjusted risk of death (upper right), survival (lower left), and the proportion with a TP53 mutation, wildtype PTEN, copy number variant (CNV) high subtype, mitotic transcript-based subtype and the cluster 4 subtype in black compared with white endometrial cancer patients.
1. Introduction
Endometrial cancer (EC) is the most common cancer of female reproductive organs in the United States [1]. The American Cancer Society estimates that 61,380 new cases of EC will be diagnosed in 2017 and 10,920 women will succumb to their disease [1]. The incidence for EC is similar for black and white women, but black women are 2.5 times more likely to die from EC [2]. Compared with white women, black women are diagnosed with higher stage and grade, higher-risk histology, and have worse survival [3,4]. Unfortunately, this disparity continues to grow at a rate that exceeds that of other racial or ethnic groups [5]. Racial disparities in EC are multifactorial with varying response to treatment, comorbidities, and genetic mutations being often cited reasons [4,6–10]. Racial differences have also been reported in prevalence of prognostic transcripts including PSPHL, SERPINA4, ITGA3, BET1L, FAM228B and HEATR6 [11–13], the aggressive copy number variant (CNV) high, somatic copy number alteration (SCNA) cluster 4 and transcript-based mitotic molecular subtypes [14], mutations in TP53 and PTEN [9,15], oncoproteins such as HER2 [8,16,17] or copy number alterations in 1q23 [18] in EC. Additionally, socioeconomic factors and access to care limitations contribute to racial variations seen in EC [19–21].
Age is an important prognostic factor and may provide insight on the racial disparities seen in EC, both from biologic and patient-care views [22]. Compared with younger women, older women tend to be diagnosed with higher stage and grade, more aggressive histology, greater depth of myometrial invasion, and have worse recurrence-free survival [19, 23–25]. Among black women these poor prognostic factors are more pronounced leading to inferior survival [19]. Previous studies examining race and age have discriminated young and old based on a single age threshold but did not evaluate the interaction between them [19,23,26]. Careful examination of the impact of increasing age at time of diagnosis may uncover reasons for racial disparities in EC, supporting the evaluation of alternate treatments and changes in practice guidelines.
Using data from the Surveillance, Epidemiology, and End Results (SEER) program, we investigated the impact of increasing age at diagnosis on racial disparity in EC between black and white women. To further investigate whether a difference in access to care may be a cause for the racial disparity seen in EC, we examined survival for black and white women when comparing women <65 and ≥65 years as Medicare is the primary health insurer for 97% of Americans ≥65 years [27]. Lastly, we sought to determine whether prevalence in mutations and aggressive molecular subtypes varied by age at diagnosis and race in EC patients.
2. Methods
This research investigation utilized public data from SEER and the Cancer Genome Atlas (TCGA). An institutional review board (IRB) waiver was obtained from Western IRB in accordance with use of publicly available de-identified data (14–1679).
2.1. SEER cohort
Data for this study were obtained from the 18 geographic region SEER program representing 26% of the United States population [28]. Patients with common EC histologic subtypes between 1991 and 2010 were selected using the following International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [29] codes [S1 in the Supplement]. Eligible cases were dichotomized as endometrioid endometrial cancer (EEC) or non-EEC (serous, mixed, clear cell, and carcinosarcoma). EEC tumors were further classified based on grade (G) of the tumor (G1, G2, G3). Stage of disease was classified as local (stage IA or IB), regional (stage II-III), distant (stage IV), and unstaged. Patients diagnosed at advanced stage had regional or distant disease. Patients with in situ disease were excluded. Races other than black or white were also excluded. Patients with missing follow up time from diagnosis, vital status, stage or grade (EEC only) were removed from survival analyses. Patients with multiple primary malignancies were not excluded. Overall survival was the primary outcome. Cancer-related mortality and non-cancer mortality were also analyzed.
2.2. TCGA cohort
Clinical, mutation, molecular subtype and RNA sequencing data were obtained from TCGA. Mutation data for TP53 and PTEN for uterine corpus endometrial cancers (UCEC) were downloaded from Broad Institute Firehose data standardization run on Nov. 2015 [30]. TCGA UCEC provisional clinical data, and clinical data with molecular subtype annotation published in Nature [31] were extracted from cgdsr (version 1.2.5) package in R (version 3.1.2) on Aug.18, 2016. This cohort included 291 eligible white patients (207 non-Hispanic white, 3 Hispanic white and 81 with unknown ethnicity) and 46 eligible black patients (22 non-Hispanic black, 1 Hispanic black and 23 with unknown ethnicity). Mutation status for TP53 or PTEN was available in 219 patients. Molecular classification data were available for CNV high subtype in 204 patients, mitotic subtype in 300 patients, and SCNA cluster 4 subtype in 327 patients.
2.3. Statistical methods
Impact of age at diagnosis and race on histologic subtype was evaluated using a logistic regression model, with odds ratio (OR) favoring non-EEC per 10-year increase in age or for black vs. white patients to indicate the strength of effect. The relationship between age and log(OR) of the risk of non-EEC was evaluated using a graphic method proposed by Hosmer and Lemeshow [32], with the assessment generally supporting a linear relationship. The model was also extended by adding an interaction term between age and race to determine whether impact of increasing age on subtype was consistent between racial groups. Distribution by histology was also calculated by 8 age groups (<50, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79 and ≥80 years) and the proportion of non-EEC against age was illustrated graphically. An initial age threshold of 50 years was selected as a surrogate for menopausal status whereas the threshold of 65 years was utilized as a surrogate for improved access to care given Medicare eligibility [27]. A similar approach was applied to test associations across age, race and stage. Differences between categorical variables were evaluated using Chi-square test. The difference in age at diagnosis between white and black EC patients and by race and histology was examined using an analysis of variance test.
Survival analyses were performed to evaluate impact of age at diagnosis, at 10-year increments, in the 8 age groups listed above or dichotomized at 50 or 65 years of age, on racial disparity in outcome in EEC or non-EEC while controlling other available prognostic factors (S2 in the Supplement). Cox proportional hazards model was used as the primary outcome analysis to evaluate the risk of death from any cause. Secondary analyses of outcome were performed using Fine and Gray’s sub-distribution hazards model to evaluate the association between age/race and cumulative incidence of cancer-related and non-cancer deaths. To estimate the adjusted hazard ratio (HR) and 95% confidence interval (CI) for each age group, all survival analyses were performed stratified by stage, tumor grade and year of diagnosis for patients with EEC or by stage and year of diagnosis for patients with non-EEC. Patients with multiple primary malignancies were excluded from competing risk analyses. Adjusted survival functions were also obtained based on Cox model [33], with difference between groups displayed graphically.
TCGA data were analyzed to determine if the odds of presenting with a mutation in TP53 or PTEN, or with an aggressive molecular subtype varied by race and/or age of diagnosis. For these analyses, age was classified into two groups (<65 and ≥65 years). Molecular subtypes were combined as “non-aggressive” vs “aggressive;” with the latter defined as either copy number variant (CNV) high integrative molecular subtype, the mitotic transcript-based molecular subtype, or somatic copy number alteration (SCNA) cluster 4 molecular subtype as defined previously [31]. Differences in mutations or the aggressive molecular subtypes between age groups were compared using Fisher’s exact test, with odds ratio (OR) for age < 65 vs ≥65 estimated from logistic regression modeling, using profile-likelihood method. Analyses were performed separately for black and white patients, but interaction between age and race was also pre-checked.
3. Results
There were 86,702 eligible women with EC in the SEER cohort including 75.7% with EEC and 25.3% with non-EEC (Table 1). Overall, 90.2% were white and 9.8% were black. The median age of the cohort was 64 years. Black women with EEC, serous or carcinosarcoma were diagnosed three years younger than white women (P < 0.0001). Median age at diagnosis (interquartile range [IQR]) for black vs. white women with uterine serous cancer was 67 (61–73) vs. 70 (63–77) and 67 (61–74) vs. 70 (61–74) for those with uterine carcinosarcoma, respectively. Median age at diagnosis and IQR, however, did not appear to vary significantly for black vs. white women with a mixed epithelial (P = 0.148) or clear cell (P = 0.143) carcinoma of the uterus.
Table 1.
Clinical characteristics in white and black patients from SEER and diagnosed between 1991 and 2010 with endometrial cancer, endometrioid endometrial cancer or non-endometrioid endometrial cancer.
| Histologic Cell Type | ||||||
|---|---|---|---|---|---|---|
| All Histologic Cell Typesa,d | Endometrioidb,d | Non-endometrioidc,d | ||||
| White (N = 78,184) | Black (N = 8518) | White (N = 54,423) | Black (N = 4163) | White (N = 16,727) | Black (N = 3626) | |
| Age (years) | ||||||
| Median (IQR) | 64 (56–73) | 64 (57–72) | 62(55–72) | 61 (54–69) | 68 (60–77) | 66(60–73) |
| < 50 | 8469 (10.8) | 920 (10.8) | 6573 (12.1) | 689 (16.6) | 1103 (6.6) | 161 (4.4) |
| 50–54 | 8665 (11.1) | 707 (8.3) | 6753 (12.4) | 455 (10.9) | 1132 (6.8) | 193 (5.3) |
| 55–59 | 12,012 (15.4) | 1158 (13.6) | 9017 (16.6) | 639 (15.4) | 1898 (11.4) | 428 (11.8) |
| 60–64 | 11,956 (15.3) | 1627 (19.1) | 8603 (15.8) | 771 (18.5) | 2330 (13.9) | 742 (20.5) |
| 65–69 | 10,639 (13.6) | 1437 (16.9) | 7208 (13.2) | 604 (14.5) | 2596 (15.5) | 709 (19.6) |
| 70–74 | 9068 (11.6) | 1133 (13.3) | 5842 (10.7) | 435 (10.5) | 2504 (15.0) | 620 (17.1) |
| 75–79 | 7696 (9.8) | 822 (9.7) | 4839 (8.9) | 322 (7.7) | 2243 (13.4) | 411 (11.3) |
| ≥ 80 | 9679 (12.4) | 714 (8.4) | 5588 (10.3) | 248 (6.0) | 2921 (17.5) | 362 (10.0) |
| Cell Type | ||||||
| Endometrioid | 60,899 (77.9) | 4710 (55.3) | 54,423 (100) | 4163 (100) | - | - |
| Grade 1 | 26,850 (49.3) | 1614 (38.8) | - | - | ||
| Grade 2 | 19,234 (35.3) | 1488 (35.7) | - | - | ||
| Grade 3 | 8339 (15.3) | 1061 (25.5) | - | - | ||
| Serous | 5951 (7.6) | 1536 (18.0) | - | - | 5742 (34.3) | 1471 (40.6) |
| Mixed | 5190 (6.6) | 519 (6.1) | - | - | 5099 (30.5) | 501 (13.8) |
| Clear Cell | 1401 (1.8) | 315 (3.7) | - | - | 1331 (8.0) | 299 (8.3) |
| Carcinosarcoma | 4743 (6.1) | 1438 (16.9) | - | - | 4555 (27.2) | 1355 (37.4) |
| Stage | ||||||
| Local | 55,595 (71.1) | 4651 (54.6) | 43,373 (79.7) | 3032 (72.8) | 8278 (49.5) | 1366 (37.7) |
| Regional | 14,332 (18.3) | 2174(25.5) | 8666 (15.9) | 827 (19.9) | 4819 (28.8) | 1263 (34.8) |
| Distant | 6305 (8.1) | 1336 (15.7) | 2384 (4.4) | 304 (7.3) | 3630 (21.7) | 997 (27.5) |
| Unstaged | 1952 (2.5) | 357 (4.2) | - | - | - | - |
| Year of Diagnosis | ||||||
| 1991–2000 | 16,899 (21.6) | 1642 (19.3) | 11,119 (20.4) | 662 (15.9) | 4682 (28.0) | 828 (22.8) |
| 2001–2010 | 61,285 (78.4) | 6876 (80.7) | 43,304 (79.6) | 3501 (84.1) | 12,045 (72.0) | 2798 (77.2) |
Bolding was used to indicate the values that were the most significantly different in black compared with white patients.
Patients with missing data in tumor grade or stage included for analysis of distribution in histologic subtypes.
Patients with missing data in tumor grade or stage were excluded.
Patients with missing data in stage were excluded.
Median age with interquartile range (IQR) at diagnosis for white vs black patients was examined using an analysis of variance test.
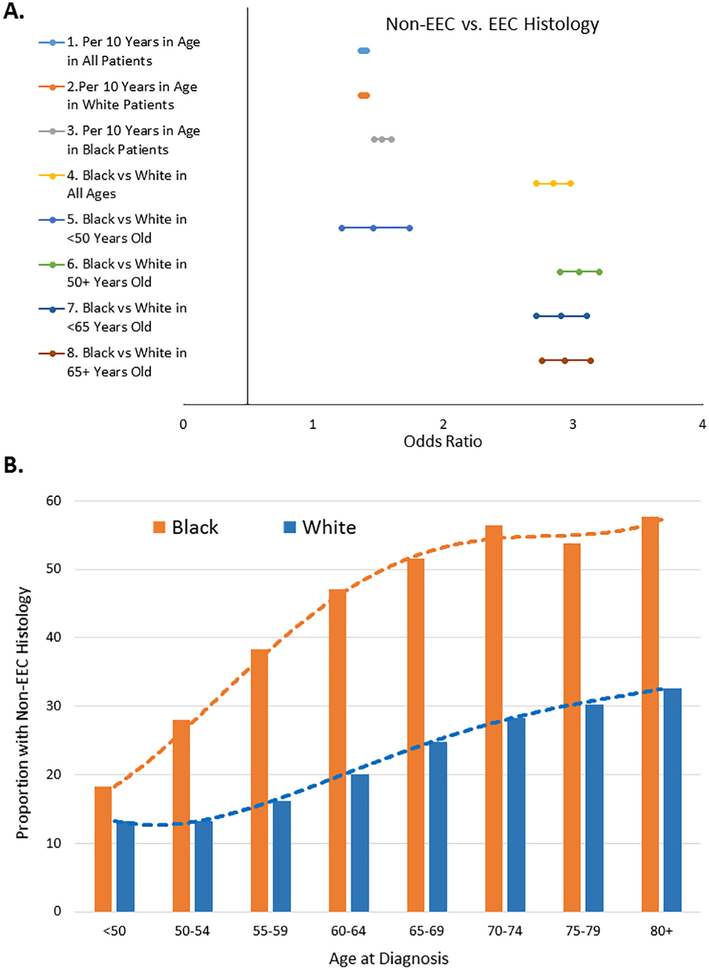
Older women were more likely to present with the higher-risk, non-EEC histology whether age at diagnosis was evaluated at 10-year increments or dichotomized at either age 50 or 65 (Fig. 1A). Fig. 1B displays the dynamic incremental shift in prevalence of non-EEC histology for each 10-year increase in age at diagnosis. The most dramatic shifts in prevalence included reductions in EEC histology and increases in both uterine serous cancer and uterine carcinosarcoma (Fig. 1C). The impact of age was particularly profound in black EC patients (P < 0.0001 for interaction). Black EC patients were also more likely to present with advanced stage (Fig. 1D) but the relationship between age and stage was similar in black vs. white patients with EEC or non-EEC (P > 0.05 for interaction).
Fig. 1.
Impact of age on histology and stage among white and black endometrial cancer patients. A, This Forest plot displays odds ratio and 95% confidence intervals from logistic regression model of the effect of age at diagnosis and race on the odds of presenting with non-endometrioid endometrial cancer (non-EEC) vs. endometrioid endometrial cancer (EEC). Older white and black women in all subgroups were more likely to present with non-EEC than EEC histology. B, This bar chart illustrates the proportion of black vs. white patients with non-EEC histology for each 10-year increase in age of diagnosis. There was a dynamic incremental shift in prevalence of non-EEC for each 10-year increase in age at diagnosis. C, The proportion of EEC, uterine serous cancer (USC), mixed epithelial endometrial cancer (mixed), uterine clear cell cancer (UCC) and uterine carcinosarcoma (UCS) is displayed in pie charts for the subset of white or black patients diagnosed at <50 or at ≥50 years of age. D, This bar chart displays the proportion of black vs. white patients with advanced stage EEC or advanced stage non-EEC across a continuum of intervals of age at diagnosis including <50, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80+ years old. There was an incremental higher prevalence of advanced stage disease with old age at diagnosis in non-EEC than EEC histology among black vs. white patients.
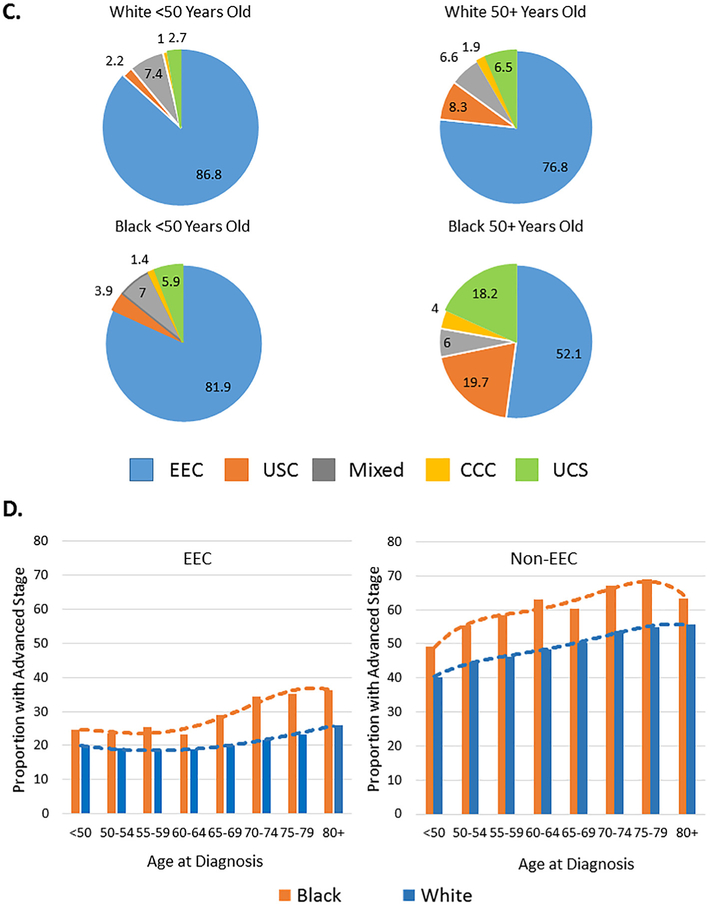
As of this analysis, 35% of white and 53% black EC patients had died, and the median follow-up time for the survivors was 7.6 years and 6.8 years, respectively. Black EC patients had worse survival than white women. The disparity in survival was greatest in women diagnosed <65 years old. The impact of race and age on survival was evident in patients with both EEC and non-EEC (Fig. 2). Racial disparity in survival in the younger age patients persisted after adjusting for other prognostic factors (Fig. 2B and E). In addition, race remained an independent predictor of worse survival in older age patients after controlling for age, stage and grade (Fig. 2C and F).
Fig. 2.
Racial disparities in survival in black vs. white endometrial cancer patients. Adjusted survival distributions were obtained using Hosmer and Lemeshow’s method and compared for black vs. white patients with EEC (A–C) or non-EEC (D–F) histology and diagnosed at any age (A, D), <65 years old (B, E) or ≥65 years old (C, F). Stratified Cox regression modeling was performed, and the adjusted hazard ratio (Adj HR) and 95% confidence interval (CI) for the specific subset of black vs. white patients were inserted into the appropriate panel. The multivariate Cox models included adjustments for stage, tumor grade and year of diagnosis for patients with EEC (A–C) or by stage and year of diagnosis for patients with non-EEC (D–F). The multivariate Cox modeling results incorporated into panels A and D also included an adjustment for age group.
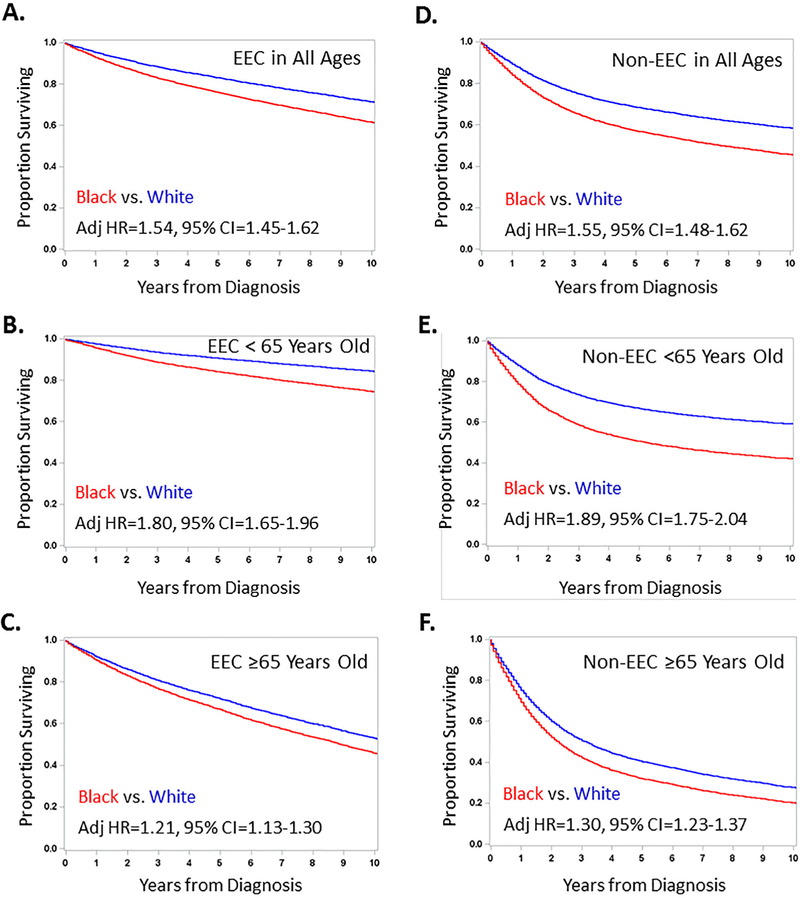
Fig. 3A illustrates the association between age at diagnosis on racial disparities, and the attenuated survival disparities in patients with both EEC and non-EEC. It was noted that the HR for black vs. white patients dropped below 1.5 around 65 years of age. The Forest plots in Fig. 3B show the larger adjusted HR for death in women diagnosed at <65 vs. ≥65 years old with EEC (1.80 vs. 1.21, P < 0.0001 interaction test) or the high-risk histology (1.89 vs. 1.30, P < 0.0001 interaction test).
Fig. 3.
Adjusted risk of all-cause mortality (overall survival), cancer-related mortality and non-cancer mortality for black vs white endometrial cancer patients. A, This bar chart displays the adjusted hazard ratio (Adj HR) for black vs. white endometrial cancer patients with endometrioid endometrial cancer (EEC) or non-EEC histology who were <50, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79 or 80+ years of age at diagnosis. This illustrates the dynamic inverse impact of age of diagnosis on racial disparities in risk of death in patients with both EEC and non-EEC. B, These Forest plots illustrate the adjusted hazard ratio and 95% confidence interval from Cox proportional hazards modeling† for overall survival in subsets 1–12 or sub-distribution hazards modeling of competing risks for cumulative cancer-related deaths (cancer mortality)¥ in subsets 13–14 or cumulative non-cancer deaths (non-cancer mortality)¥ in subsets 15–16. Analyses performed in patients with EEC are displayed in the left Forest plot whereas those in patients with non-EEC are shown in the right Forest plot. Cox modeling and competing risk analyses were stratified by age, stage, tumor grade and/or year of diagnosis for patients with EEC or by age, stage and/or year of diagnosis for patients with non-EEC. Differences in risk of death† or cumulative cancer-related deaths¥ were compared and P-values <0.05 for interaction tests were incorporated into panel B.
The racial disparity in survival was larger in EEC patients with G1/G2 vs. G3 disease (Fig. 3B). The adjusted HR for black vs. white patients with local, regional or distant EEC was, however, very similar (P > 0.05 for interaction). In contrast, the racial disparity in survival was worse in non-EEC patients diagnosed with local or regional vs. distant disease (P = 0.017 for interaction).
Adjusted competing risk analyses illustrated that the racial association with cumulative incidence in cancer-related deaths in both EEC and non-EEC was more dramatic in patients diagnosed at <65 compared with ≥65 years old (Fig. 3B). The racial association with cumulative incidence in non-cancer deaths was seen in patients diagnosed <65 years old, but not in those diagnosed ≥65 years old (Fig. 3B).
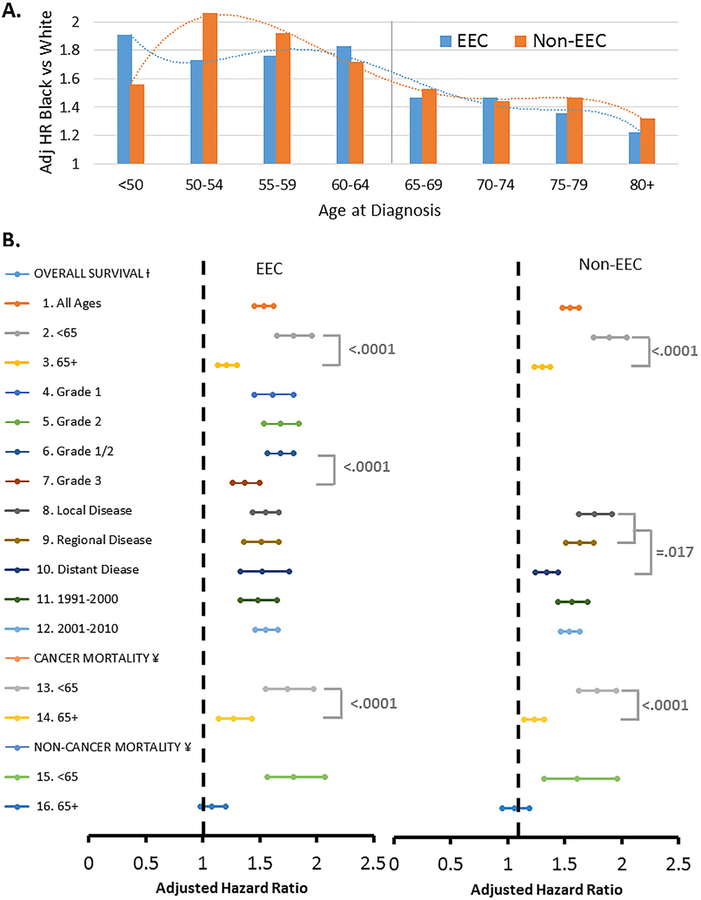
Relationships between age at diagnosis and race on the presentation of aggressive genomic alterations were analyzed in black vs white EC patients from TCGA. Mutations in TP53, wild-type PTEN and the three aggressive molecular subtypes have previously been shown to be associated with the high-risk histology [23]. Each of these aggressive molecular features were more common in black vs. white patients, and in women diagnosed at ≥65 vs. <65 years old (Fig. 4). There was a suggestion from logistic regression analysis that the impact of age on mutations or molecular subtypes was greater in black than white patients. The odds ratio for presenting with the following aggressive molecular features in black vs. white patients diagnosed at ≥65 and <65 years old was 9.8 vs. 2.9 for a TP53 mutation, 15.6 vs. 2.0 for wild-type PTEN, 10.0 vs. 4.3 for CNV high subtype, 7.8 vs. 2.7 for the mitotic subtype, and 7.2 vs. 4.6 for the SNCA cluster 4 subtype, but all the interaction tests did not reach the level of statistical significance (P > 0.05 for all).
Fig. 4.
Molecular alterations that vary by race and age in endometrial cancer. Endometrial cancer patients with clinical data including race and age at diagnosis, and mutation status for TP53 or PTEN (N = 219), or one of the aggressive molecular subtypes including the integrative copy number (CNV) high subtype (N = 204), transcript-based mitotic subtype (N = 300), and somatic copy number alteration (SCNA)-based cluster 4 subtype (N = 327) from the Cancer Genome Atlas Research Network were evaluable for analysis. Fisher’s exact test was used to compare proportions between groups. The bar chart displays the proportion of white patients diagnosed at < 65 years of age, white patients diagnosed at 65+ years of age, black patients diagnosed at < 65 years of age, or black patients diagnosed at 65+ years of age with a TP53 mutation, wild-type PTEN, CNV High subtype, Mitotic subtype or Cluster 4 subtype. Mutations in TP53, wild-type PTEN and the three aggressive molecular subtypes were significantly more common in black vs. white endometrial cancer patients and in patients diagnosed at 65+ vs. <65 years of age.
4. Discussion
This study demonstrates multiple important findings on the impact of age at diagnosis on the racial disparities seen in EC starting with differences in disease presentation and culminating in differences in survival. High-risk histology was more likely to be diagnosed at older ages and this association was stronger in black patients. Histology was similar in women diagnosed <50 years, but diverged in those diagnosed at ≥50 years of age. Worse survival was observed across all ages in black vs. white EC patients. The associated differences in survival and cancer-related mortality, however, decreased as age increased, especially among those ≥65 years. There was no difference in non-cancer mortality after age 65, but the disparity in cancer-related mortality persisted.
EECs are related to unopposed estrogen and have a favorable prognosis while the poorly differentiated non-EECs have worse prognosis [16]. Eleven percent of women developed EC <50 years old. In this subset, histology did not vary by race. However, black EC patients diagnosed >50 years had greater than a 50% chance of having a high-risk histology vs. about 25% among white women. Nonetheless, literature regarding the increased prevalence of non-EECs in black vs. white patients has not described this risk as a function of increasing age [3,5, 34–37].
Molecular alterations may also explain at least some of the disparities reported between older vs. younger black and white EC patients. Prior studies have documented molecular alterations between black and white EC patients including racial differences in prevalence of prognostic transcripts [11–13], molecular subtypes [14], mutations [9,15], proteins [8,16,17] or copy number alterations [18]. For example, TP53 mutations and the aggressive molecular subtypes defined by TCGA [31] were more common in black vs. white EC patients [14] and associated with poor outcomes [8,14,15,17]. In contrast, PTEN mutations and certain transcripts were more common in white vs. black patients and associated with a more favorable outcome [9,12]. None of these studies evaluated whether any of these molecular alterations varied by age of diagnosis and race. We not only confirmed that mutations in TP53 and wild-type PTEN were significantly more common in black vs. white EC patients, but also showed that they were also more common in older vs. younger patients regardless of race.
Lastly, we recently reported that the integrative CNV high, mitotic transcript-based and SCNA cluster 4 subtypes were more common in black vs. white EC patients and were associated with worse outcome [14]. Herein, we extend those findings to demonstrate that all three aggressive molecular subtypes were more common in black vs. white patients regardless of age. In addition, we observed a higher prevalence of these subtypes in patients diagnosed ≥65 vs. <65 years old. These findings are consistent with our SEER analysis, supporting an interactive biologic relationship between age at diagnosis and race in EC and suggesting that aggressive tumor and molecular characteristics may explain the earlier development of the high-risk EC in black patients.
Several studies examining the impact of age on diagnosis of EC are consistent with our findings [24,25,38,39]. Evaluation of race and categorized age showed black women diagnosed with EC >60 years had worse prognostic factors and worse survival [19]. In a previous analysis by our group, we evaluated EC patients treated in a military healthcare system, where patients have equal access irrespective of race, ethnicity, or socioeconomic status. In this analysis, black women >50 years old were more likely to present with non-EEC tumors, non-localized tumors, and poorly differentiated tumors. This study, however, did not evaluate whether racial disparities in EC in an equal access military healthcare system may be related to variation in age-related cancer outcome [23].
Our current findings involving population-based data show that although black women are at a greater risk for having non-EEC histology with older age, the negative impact of race on survival was reduced after age 65. Limited finances and lack of insurance are more often obstacles for black women seeking medical care, likely leading to delays in diagnosis and treatment [4,40]. We hypothesize that our results showing decreased racial disparities in survival after 65 may reflect, at least in part, improved access to care given Medicare eligibility. We were unable to evaluate the impact of socioeconomic factors, comorbidities, and timing and type of treatment implementation in this investigation. Finally, we acknowledge there are multiple other factors in place that lead to the racial disparities seen in EC as an analysis of four Gynecologic Oncology Group trials of patients with advanced or recurrent EC showed black patients were 26% more likely to die from disease than white patients even after adjusting for known prognostic factors and treatment [7,41].
Our data diverges from some reports on impact of racial disparities and age in EC. A pan-cancer analysis using SEER showed that black women were diagnosed with cancers of the uterine corpus an average of two years later than white women [38]; nonetheless, our analysis showed racial differences in age of diagnosis that were dependent on histology. Previous studies examining impact of age on racial disparities in EC have selected a single age threshold to delineate young vs old [19,23]. However, we examined each 5-year increase in age with respect to histologic cell type, stage, and survival among women >50 years to illustrate impact of increasing age on racial disparity dynamically and precisely. Furthermore, when evaluating survival, we also dichotomized age at 65 years old given Medicare eligibility, which allows us to hypothesize that the survival differences were associated with access to care. This is important given the interesting findings of less disparity in survival among black women ≥65 compared with <65 years old in those diagnosed with both EEC and non-EEC histologic cell types, giving weight to the argument that socioeconomic factors contribute to outcome.
Analysis of SEER data allows for assessment of a large sample of women who were treated for EC. Previous review of SEER data also confirms the quality of racial data obtained is excellent [42]. However, pathologic review of SEER cases could not be independently confirmed. Currently, SEER does not provide detailed information on adjuvant chemotherapy, surgical complexity, socioeconomic factors, access to care, disease progression or molecular alterations. As previously mentioned, this analysis did not account for comorbidities, an important determinant of overall survival in women with EC [39]. Nevertheless, we evaluated TCGA data to determine which molecular alterations varied by age at diagnosis and race, but inability to adjust for known prognostic factors absent from these datasets limits our ability to draw definitive conclusions. Another limitation reflects the possibility of chance findings given the number of statistical tests performed. Census data from 2010 indicates that 13.0% of the total resident population of the United States self-designated to be black or African American [43], which suggests that SEER and TCGA are fairly representative. The low proportion of black patients in SEER (10%) and TCGA (13.6%), however, represents a weakness in this and other racial disparities research. Race was either self-designated or provider-designated race, which may impact interpretation of racial disparity research.
SEER and TCGA data illustrate important findings on the racial disparities seen in EC and how age is associated with these disparities. The high-risk non-EEC is more likely diagnosed at an older age and this association was larger in black patients. Black patients consistently had worse survival and cancer-specific mortality even after adjusting for prognostic clinical variables, but the relative difference becomes smaller with increasing age. TP53 mutation, wild-type PTEN, and the aggressive molecular subtypes (CNV high, mitotic and SCNA cluster 4) were significantly more common in black patients, though increased in all patients diagnosed at ≥65. Additional research is needed to determine mechanisms of action and causality. Perhaps most importantly, this study supports the development of an investigation to determine if increasing access to care among younger black women may allow for earlier interventions and improvements in EC-related outcome. Nevertheless, racial disparities seen in EC remain multifactorial, and the significant survival differences observed emphasizes need for continued research on this topic.
Supplementary Material
HIGHLIGHTS.
Racial differences in age at diagnosis for uterine serous cancer and carcinosarcoma.
Increased presentation of type II tumors with age was larger in black vs. white patients.
Survival differences between white and black patients decreases with increasing age.
Mutations in TP53 and PTEN, and molecular subtypes vary by age of diagnosis and race.
Acknowledgment
The authors acknowledge funding and support from the National Cancer Institute for the SEER program and TCGA Research Network.
Funding Funding for this project was provided from an award from the Uniformed Services University of the Health Sciences from the Defense Health Program (HU0001-16-2-0006) to the Gynecologic Cancer Center of Excellence, PI: Chad A. Hamilton, Co-PI: G. Larry Maxwell, and administered by the Henry M Jackson Foundation for the Advancement of Military Medicine (HJF).
Footnotes
Declaration of interests
The authors report no conflict of interest.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Presentations
Abstracts for this work have been submitted for consideration for presentation at the Armed Forces District Meeting of the American Congress of Obstetricians and Gynecologists on September 24–27, 2017 in San Antonio TX and at the American Association for Cancer Research Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved on September 25–28, 2017 in Atlanta, GA.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2017.07.145.
References
- [1].American Cancer Society, Cancer facts & figures. Atlanta: American Cancer Society Available from https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html 2017.
- [2].Jemal A, Siegel R, Xu J, Ward E, Cancer statistics, 2010, CA Cancer J. Clin 60 (5) (2010) 277–300. [DOI] [PubMed] [Google Scholar]
- [3].Long B, Liu FW, Bristow RE, Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States, Gynecol. Oncol 130 (3) (2013) 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farley J, Risinger JI, Rose GS, Maxwell GL, Racial disparities in blacks with gynecologic cancers, Cancer 110 (2) (2007) 234–243. [DOI] [PubMed] [Google Scholar]
- [5].Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R, The growing burden of endometrial cancer: a major racial disparity affecting black women, Cancer Epidemiol. Biomark. Prev 24 (9) (2015) 1407–1415. [DOI] [PubMed] [Google Scholar]
- [6].Randall TC, Armstrong K, Differences in treatment and outcome between African-American and white women with endometrial cancer, J. Clin. Oncol 21 (22) (2003) 4200–4206. [DOI] [PubMed] [Google Scholar]
- [7].Maxwell GL, Tian C, Risinger J, Brown CL, Rose GS, Thigpen JT, et al. , Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study, Cancer 107 (9) (2006) 2197–2205. [DOI] [PubMed] [Google Scholar]
- [8].Santin AD, Bellone S, Siegel ER, Palmieri M, Thomas M, Cannon MJ, et al. , Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in uterine serous papillary cancer, Am. J. Obstet. Gynecol 192 (3) (2005) 813–818. [DOI] [PubMed] [Google Scholar]
- [9].Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, et al. , Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers, Clin. Cancer Res 6 (8) (2000) 2999–3005. [PubMed] [Google Scholar]
- [10].Elshaikh MA, Munkarah AR, Robbins JR, Laser BS, Bhatt N, Cogan C, et al. , The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma, Gynecol. Oncol 128 (2) (2013) 171–174. [DOI] [PubMed] [Google Scholar]
- [11].Allard JE, Chandramouli GV, Stagliano K, Hood BL, Litzi T, Shoji Y, et al. , Analysis of PSPHL as a candidate gene influencing the racial disparity in endometrial cancer, Front. Oncol 2 (2012) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bateman NW, Dubil E, Wang G, Hood BL, Litzi T, Oliver J, Darcy KM, Hamilton CA, Conrads TP, Maxwell GL, Proteome and transcriptome alterations in black endometrial cancer patients correlate with poor disease outcome (ACCR abstract #5277), Proc. Amer. Assoc. Cancer Res 58 (2017) 1351–1352. [Google Scholar]
- [13].Bateman NW, Dubil EA, Wang G, Hood BL, Oliver JM, Litzi TA, Gist GD, Mitchell DA, Blanton B, Phippen NT, Tian C, Zahn CM, Cohn DE, Havrilesky LJ, Berchuck A, Shriver CD, Darcy KM, Hamilton CA, Conrads TP, Maxwell GL, Race-specific molecular alterations correlate with differential outcomes for black and white endometrioid endometrial cancer patients, Cancer (2017 Jun 27) 10.1002/cncr.30813 (Epub ahead of print)28654152. [DOI] [PubMed] [Google Scholar]
- [14].Dubil EA, Wang TG, Bateman NW, Levine DA, Conrads TP, Hamilton CA, Maxwell GL, Darcy KM, Racial differences in molecular subtypes between black and white patients with endometrial cancer (SGO abstract #8291), Gynecol. Oncol 145 (S1) (2017) 17. [Google Scholar]
- [15].Kohler MF, Berchuck A, Davidoff AM, Humphrey PA, Dodge RK, Iglehart JD, et al. , Overexpression and mutation of p53 in endometrial carcinoma, Cancer Res. 52 (6) (1992) 1622–1627. [PubMed] [Google Scholar]
- [16].Deligdisch L, Holinka CF, Endometrial carcinoma: two diseases? Cancer Detect. Prev 10 (3–4) (1987) 237–246. [PubMed] [Google Scholar]
- [17].Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, et al. , HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients, J. Clin. Oncol 24 (15) (2006) 2376–2385. [DOI] [PubMed] [Google Scholar]
- [18].Morrison C, Miecznikowski J, Darcy KM, Dolce JM, Kandel E, Erwin DO, et al. , A GOG 210 aCGH study of gain at 1q23 in endometrioid endometrial cancer in the context of racial disparity and outcome, Genes Chromosomes Cancer. 49 (9) (2010) 791–802. [DOI] [PubMed] [Google Scholar]
- [19].Aziz H, Hussain F, Edelman S, Cirrone J, Aral I, Fruchter R, et al. , Age and race as prognostic factors in endometrial carcinoma, Am. J. Clin. Oncol 19 (6) (1996) 595–600. [DOI] [PubMed] [Google Scholar]
- [20].Liu JR, Conaway M, Rodriguez GC, Soper JT, Clarke-Pearson DL, Berchuck A, Relationship between race and interval to treatment in endometrial cancer, Obstet. Gynecol 86 (4 Pt 1) (1995) 486–490. [DOI] [PubMed] [Google Scholar]
- [21].Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB, Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival, Am. J. Public Health 94 (12) (2004) 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Persson I, Adami HO, Malker B, Pettersson B, Long-term survival in endometrial cancer with special reference to age as a prognostic factor, Ups. J. Med. Sci 89 (2) (1984) 159–170. [DOI] [PubMed] [Google Scholar]
- [23].Oliver KE, Enewold LR, Zhu K, Conrads TP, Rose GS, Maxwell GL, et al. , Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment, Gynecol. Oncol 123 (1) (2011) 76–81. [DOI] [PubMed] [Google Scholar]
- [24].Gayar OH, Robbins JR, Parikh K, Lu M, Buekers T, Munkarah A, et al. , Hysterectomy for uterine adenocarcinoma in the elderly: tumor characteristics, and long-term outcome, Gynecol. Oncol 123 (1) (2011) 71–75. [DOI] [PubMed] [Google Scholar]
- [25].Vance S, Yechieli R, Cogan C, Hanna R, Munkarah A, Elshaikh MA, The prognostic significance of age in surgically staged patients with type II endometrial carcinoma, Gynecol. Oncol 126 (1) (2012) 16–19. [DOI] [PubMed] [Google Scholar]
- [26].Aziz H, Rotman M, Hussain F, Smith G, Chan E, Choi K, et al. , Poor survival of black patients in carcinoma of the endometrium, Int. J. Radiat. Oncol. Biol. Phys 27 (2) (1993) 293–301. [DOI] [PubMed] [Google Scholar]
- [27].Olson SH, Atoria CL, Cote ML, Cook LS, Rastogi R, Soslow RA, et al. , The impact of race and comorbidity on survival in endometrial cancer, Cancer Epidemiol. Biomark. Prev 21 (5) (2012) 753–760. [DOI] [PubMed] [Google Scholar]
- [28].National Cancer Institute Department of Health and Human Services, SEER as a research resource Available from https://seer.cancer.gov/about/factsheets/SEER_Research_Brochure.pdf 2016.
- [29].Fritz AG, International Classification of Diseases for oncology: ICD-O, 3rd ed.vii, World Health Organization, Geneva, 2000. (240 pp). [Google Scholar]
- [30].Broad Institute TCGA Genome Data Analysis Center, Analysis-ready standardized TCGA data from Broad GDAC Firehose stddata__2015_11_01 run, Broad Institute of MIT and Harvard, 10.7908/C1571BB1 2016. [DOI] [Google Scholar]
- [31].N. Cancer Genome Atlas Research, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. , Integrated genomic characterization of endometrial carcinoma, Nature 497 (7447) (2013) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hosmer DW, Lemeshow S, Applied Logistic Regression, Wiley-Interscience, Hoboken, N.J, 2005. [Google Scholar]
- [33].Hosmer DW, Lemeshow S, May S, Applied Survival Analysis: Regression Modeling of Time-to-event Data, Wiley-Interscience, Hoboken, N.J, 2008. [Google Scholar]
- [34].Cote ML, Ruterbusch JJ, Ahmed Q, Bandyopadhyay S, Alosh B, Abdulfatah E, et al. , Endometrial cancer in morbidly obese women: do racial disparities affect surgical or survival outcomes? Gynecol. Oncol 133 (1) (2014) 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. , Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time, Cancer 115 (6) (2009) 1276–1285. [DOI] [PubMed] [Google Scholar]
- [36].Sherman ME, Devesa SS, Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus, Cancer 98 (1) (2003) 176–186. [DOI] [PubMed] [Google Scholar]
- [37].Plaxe SC, Saltzstein SL, Impact of ethnicity on the incidence of high-risk endometrial carcinoma, Gynecol. Oncol 65 (1) (1997) 8–12. [DOI] [PubMed] [Google Scholar]
- [38].Robbins HA, Engels EA, Pfeiffer RM, Shiels MS, Age at cancer diagnosis for blacks compared with whites in the United States, J Natl Cancer Inst. 107 (3) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA, Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer, Gynecol. Oncol 131 (3) (2013) 593–597. [DOI] [PubMed] [Google Scholar]
- [40].Allard JE, Maxwell GL, Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer, Cancer Control 16 (1) (2009) 53–56. [DOI] [PubMed] [Google Scholar]
- [41].Maxwell GL, Tian C, Risinger JI, Hamilton CA, Barakat RR, Gynecologic Oncology Group S, Racial disparities in recurrence among patients with early-stage endometrial cancer: is recurrence increased in black patients who receive estrogen replacement therapy? Cancer 113 (6) (2008) 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. , Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies, Cancer Causes Control 18 (2) (2007) 177–187. [DOI] [PubMed] [Google Scholar]
- [43].American Community Survey, US Census Bureau Available from https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.