Abstract
Introduction
We conducted a phase Ib study () to determine whether ribociclib, an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), penetrates tumor tissue and modulates downstream signaling pathways including retinoblastoma protein (Rb) in patients with recurrent glioblastoma (GBM).
Methods
Study participants received ribociclib (600 mg QD) for 8–21 days before surgical resection of their recurrent GBM. Total and unbound concentrations of ribociclib were measured in samples of tumor tissue, plasma, and cerebrospinal fluid (CSF). We analyzed tumor specimens obtained from the first (initial/pre-study) and second (recurrent/on-study) surgery by immunohistochemistry for Rb status and downstream signaling of CDK4/6 inhibition. Participants with Rb-positive recurrent tumors continued ribociclib treatment on a 21-day-on, 7-day-off schedule after surgery, and were monitored for toxicity and disease progression.
Results
Three participants with recurrent Rb-positive GBM participated in this study. Mean unbound (pharmacologically active) ribociclib concentrations in plasma, CSF, MRI-enhancing, MRI-non-enhancing, and tumor core regions were 0.337 μM, 0.632 μM, 1.242 nmol/g, 0.484 nmol/g, and 1.526 nmol/g, respectively, which exceeded the in vitro IC50 (0.04 μM) for inhibition of CDK4/6 in cell-free assay. Modulation of pharmacodynamic markers of ribociclib CDK 4/6 inhibition in tumor tissues were inconsistent between study participants. No participants experienced serious adverse events, but all experienced early disease progression.
Conclusions
This study suggests that ribociclib penetrated recurrent GBM tissue at concentrations predicted to be therapeutically beneficial. Our study was unable to demonstrate tumor pharmacodynamic correlates of drug activity. Although well tolerated, ribociclib monotherapy seemed ineffective for the treatment of recurrent GBM.
Keywords: Glioblastoma, CDK4, CDK6, Pharmacokinetics
Introduction
Anaplastic glioma (AG; grade III) and glioblastoma (GBM; grade IV) are primary malignant brain tumor sub-types and account for the majority of high-grade gliomas. Standard treatment includes surgical resection followed by radiation and chemotherapy with temozolomide (TMZ) [1]. Despite these interventions, patients with high-grade gliomas invariably exhibit tumor progression, with a 5-year survival rate of less than 10% [2, 3]. Thus, there is an urgent need for improved treatment.
The retinoblastoma protein (Rb), which binds and inhibits E2F transcription factors in the absence of mitogens, regulates the transition from G1 to S phase of the cell cycle. Upon stimulation with growth signals, D-type cyclins increase in abundance and bind cyclin-dependent kinases 4 and 6 (CDK4/6). Cyclin D-bound CDK4 or CDK6 phosphorylates Rb, releasing E2F proteins that drive cell cycle progression [4–6]. Alterations in this CDK4/6-Rb-E2F pathway, which promotes cell proliferation, often occur in human tumors [7]. While only a small number of tumors exhibit loss or mutation of the gene encoding Rb (RB1), an increase in CDK4/6 activity functionally inactivates this protein. Glioblastomas frequently exhibit loss or mutation of CDKN2A, which encodes the p16INK4A and p14ARF upstream regulators of CDK4/6 and predicts sensitization to CDK4/6 inhibition in preclinical models [8–10]. Thus, CDK4/6 inhibition as a means of restoring Rb activity is an attractive therapeutic strategy for the treatment of high-grade gliomas.
Preclinical data demonstrate that small-molecule inhibitors of CDK4/6 induce G0 arrest of malignant cells, including GBM cells, both in vitro and pre-clinically in vivo [10–14]. The orally bioavailable small-molecule CDK4/6 inhibitor ribociclib (LEE011) has anti-tumor activity dependent upon the presence of functional Rb. Although the safety of ribociclib has been established in patients with cancer, the phase I trial excluded those with central nervous system tumors or brain metastases [15].
A particular concern for the treatment of brain tumors is the ability of systemically administered agents to cross the blood–brain barrier (BBB). A number of human studies have attempted to measure drug penetration into brain tumors with mixed results [16–25]. Additionally, the degree of tumor tissue penetration does not necessarily predict treatment efficacy [26]. While investigators often contend that most drugs do not efficiently cross the BBB, brain metastases and primary brain tumors frequently respond to systemically administered therapies [2, 27–29]. This suggests that the BBB is compromised within tumor tissue, leading to a leaky “blood-tumor barrier” that allows entry of therapeutic agents into the tumor tissue. In support of this concept, animal studies with the CDK4/6 inhibitor palbociclib demonstrated that concentrations of this drug were higher in intracerebral tumor tissue than normal brain tissue [14]. We therefore undertook a phase Ib study in patients with recurrent high-grade gliomas to assess the ability of ribociclib to penetrate brain tumor tissue and inhibit CDK4/6-Rb signaling and growth in tumor cells.
Materials and methods
Study population
Eligible study participants were 18 years or older, candidates for surgical resection following diagnosis of recurrent GBM or AG, and had available archived formalin-fixed, paraffin embedded (FFPE) or frozen tumor tissue from the initial surgery for molecular analysis. Additional eligibility criteria included participants having a Karnofsky performance scale score ≥ 70, adequate hematopoietic, renal and hepatic function, and the ability to provide written informed consent. Exclusion criteria included impaired gastro-intestinal drug absorption, a history of any cardiovascular disease, including long QT syndrome, and inability to comply with protocol procedures. Pregnant and lactating women and patients taking any strong inducers or inhibitors of CYP3A4/5 were also excluded. The clinical trial was approved by the University of Virginia Institutional Review Board () and by the FDA (IND #125168).
Study design
This single-institution (University of Virginia Health System) study was an open-label phase Ib clinical trial (Fig. 1). Baseline blood samples for ribociclib concentrations were collected prior to initiation of ribociclib treatment. Study participants were treated with ribociclib at the recommended phase II dose of 600 mg/day [15], taken once orally for 8–21 days prior to surgery. Novartis Pharmaceuticals generously provided 200 mg ribociclib capsules. Subjects received the last pre-surgical ribociclib dose 4–8 h prior to surgery to allow sufficient time for any modulation of downstream effectors of CDK4/6. We obtained blood samples within 1 h prior to surgery, and separated into plasma and buffy coat fractions. When feasible during surgery, CSF was also collected. Brain tumor core and infiltrating tumor were identified by co-localization with stealth MRI, and tissue samples of each area were obtained for FFPE or frozen (Fig. 2).
Fig. 1.
Study overview
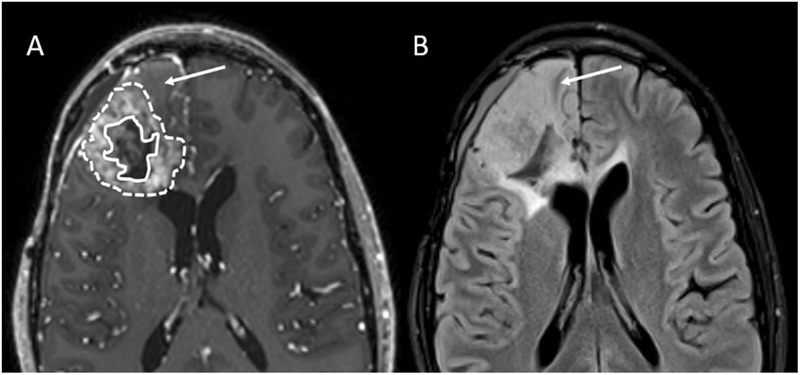
Fig. 2.
Axial contrast-enhanced T1-weighted images. Images were obtained using a 3D magnetization prepared rapid acquisition gradient recalled-echo sequence) (a), and an axial FLAIR image (b) of recurrent glioblastoma (Subject 003). In (a), enclosed within the solid line is the necrotic core; enclosed between the dashed and solid lines is the enhancing neoplasm. Arrows indicate non-enhancing neoplasm
Immunohistochemistry (IHC) for Rb was performed on recurrent GBM specimens (detailed below); participants with Rb-negative tumors were excluded from further treatment as previous work suggested that Rb-deficient tumors are unlikely to respond to CDK4/6 inhibition [10, 12]. Study participants with Rb-positive tumors continued ribociclib treatment on a 21-days-on, 7-days-off schedule of a 28-day cycle until disease progression or the development of unacceptable toxicity.
MRI assessment
All patients had a post-operative brain MRI within 72 h post-surgery to determine the extent of resection and to have as a baseline for further measurement of tumor size. Subjects with Rb-positive tumors who continued ribociclib treatment post-surgery had follow-up MRI performed (including per-fusion and diffusion-weighted images) after every two cycles of treatment. The Response Assessment in Neuro-Oncology (RANO) Working Group criteria were used to determine disease progression [30].
Safety monitoring
Complete blood counts and blood chemistry, 12-lead electrocardiogram, vital signs, Karnofsky performance status, and physical exam were monitored throughout the course of treatment. Toxicities were graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and adverse events were evaluated according to expectedness and attribution to drug treatment.
Measurement of ribociclib concentrations in tissues
The total concentrations of ribociclib in plasma, CSF, and frozen tumor tissue samples were determined using a validated liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) method as previously described [31]. The unbound fraction of ribociclib in plasma and tumor homogenate was determined using equilibrium dialysis with a 5-kDa cutoff regenerated cellulose membrane (96-well Equilibrium DIALYZER, Harvard Apparatus), as previously described [31]. Unbound ribociclib concentration in plasma or tumor tissue samples was calculated as the product of the fraction unbound and the total drug concentration. The concentration of unbound ribociclib in plasma and tumor tissue samples was calculated as the product of the unbound fraction and the total concentration.
Immunohistochemistry
Methods provided in Supplementary Information.
Results
Patient characteristics, safety, and efficacy
The planned enrollment for this study was ten evaluable study participants with Rb-positive recurrent GBM or AG. The study, however, was closed due to slow accrual after three participants completed treatment. Table 1 shows the patient demographics. All study participants received ribociclib for between 8 and 21 days, followed by surgical resection of the recurrent tumor. All enrolled participants had Rb-positive recurrent GBM and continued ribociclib treatment after surgery. All observed adverse events were grade 2 or less and were considered unlikely to be related to ribociclib treatment (Table 2). No participants withdrew from the study due to adverse events.
Table 1.
Patient demographics and study outcomes
| 001 | 002 | 003 | |
|---|---|---|---|
| Age | 81 years | 53 years | 35 years |
| Sex | Male | Male | Male |
| Date of first surgery (resection) | 9/26/2014 (subtotal) | 11/20/2015 (gross total) | 3/29/2012 (gross total) |
| Pathology | GBM | GBM | GBM |
| MGMT status | Hypermethylated | Unmethylated | Unmethylated |
| IDH-1 (R132H) | Negative | Negative | Positive |
| PFS from first surgery | 21 months | 9 months | 54 months |
| KPS at recurrence | 70 | 80 | 90 |
| Ribociclib treatment prior to surgery | 8 days | 12 days | 14 days |
| Date of second surgery (resection) | 8/16/2016 (subtotal) | 9/7/2016 (gross total) | 10/19/2016 (subtotal) |
| Pathology | Recurrent GBM | Recurrent GBM | Recurrent GBM |
| PFS from start of Ribociclib | 2 months | 5 months | 2 months |
| OS from second surgery | 10 months | 19 months | 12 months |
| OS from first surgery | 32 months | 28 months | 66 months |
GBM glioblastoma, MGMT methyl-guanine-methyl-transferase, IDH isocitrate dehydrogenase, PFS progression-free- survival, KPS Karnofsky performance scale score, OS overall survival
Table 2.
Adverse events while receiving ribociclib prior to surgery and during treatment phase
| Toxicity | Grade 1 | Grade 2 | Total |
|---|---|---|---|
| Gastrointestinal disorders | |||
| Nausea | 1 | 0 | 1 |
| General disorders and administration site conditions | |||
| Gait disturbance | 0 | 1 | 1 |
| Edema, limbs | 1 | 0 | 1 |
| Renal and urinary disorders | |||
| Creatinine increased | 0 | 1 | 1 |
| Metabolism and nutrition disorders | |||
| Hyperglycemia | 0 | 1 | 1 |
| Musculoskeletal and connective tissue disorders | |||
| Generalized muscle weakness | 0 | 1 | 1 |
| Musculoskeletal and connective tissue disorder—other: muscle pain | 1 | 0 | 1 |
| Nervous system disorders | |||
| Stroke | 1 | 0 | 1 |
| Peripheral sensory neuropathy | 1 | 0 | 1 |
| Headaches | 0 | 1 | 1 |
| Seizure | 1 | 1 | |
| Psychiatric disorders | |||
| Psychosis | 1 | 0 | 1 |
| Insomnia | 0 | 1 | 1 |
| Respiratory, thoracic and mediastinal disorders | |||
| Cough | 1 | 0 | 1 |
Study participants, 001, 002 and 003 experienced tumor progression based on MRI at 2, 5, and 2 months, respectively, following surgical resection of their recurrent tumor (Table 1). They were treated with other regimens off-study following disease progression on ribociclib. Survival times from the date of second surgery were 10, 19, and 12 months, respectively. Subject 002, who had longer progression-free and overall survival than the other study participants following the second surgery, was the only participant who had gross total resection of the recurrent tumor, which may have contributed to his improved outcome.
Ribociclib concentrations in tissues
Total and unbound (pharmacologically active) ribociclib concentrations were measured in plasma, CSF, and tumor tissue obtained 4–8 h following the last dose of ribociclib administered on the day of surgery (Table 3). Ribociclib was not detected in plasma samples obtained at baseline (prior to initiation of ribociclib treatment). Day of surgery plasma concentrations of total and unbound ribociclib ranged from 1.26–5.44 μM to 0.15–0.49 μM, respectively. The only CSF sample obtained in one patient revealed a CSF ribociclib concentration of 0.63 μM, which provided a CSF/unbound plasma concentration ratio of 1.29 (i.e., 0.63/0.49), suggesting that the CSF and unbound plasma ribociclib concentrations were similar.
Table 3.
Total and unbound ribociclib concentrations and ratios in plasma, CSF, and tumor tissue samples
| Sample | Subject ID | Fraction unbound (%) | Total ribociclib | Unbound ribociclib |
|---|---|---|---|---|
| Plasma | RIBO-001 | 10.2 | 3.65 μM | 0.37 μM |
| Plasma | RIBO-002 | 11.9 | 1.26 μM | 0.15 μM |
| Plasma | RIBO-003 | 9.0 | 5.44 μM | 0.49 μM |
| CSF | RIBO-003 | – | 0.63 μM | – |
| Enhancing tumor | RIBO-001 | 5.9 | 8.73 nmol/g | 0.52 nmol/g |
| Enhancing tumor | RIBO-001 | 3.9 | 27.22 nmol/g | 1.06 nmol/g |
| Non-enhancing tumor | RIBO-001 | 4.2 | 5.80 nmol/g | 0.25 nmol/g |
| Non-enhancing tumor | RIBO-001 | 6.1 | 11.92 nmol/g | 0.72 nmol/g |
| Tumor core | RIBO-001 | 5.0 | 16.30 nmol/g | 0.82 nmol/g |
| Enhancing tumor | RIBO-002 | 5.1 | 14.63 nmol/g | 0.74 nmol/g |
| Enhancing tumor | RIBO-002 | 6.4 | 24.14 nmol/g | 1.55 nmol/g |
| Enhancing tumor | RIBO-002 | 5.8 | 26.12 nmol/g | 1.53 nmol/g |
| Enhancing tumor | RIBO-002 | 5.4 | 34.03 nmol/g | 1.85 nmol/g |
| Tumor core | RIBO-002 | 6.4 | 17.13 nmol/g | 1.09 nmol/g |
| Tumor core | RIBO-002 | 6.4 | 48.92 nmol/g | 3.12 nmol/g |
| Enhancing tumor | RIBO-003 | 4.7 | 33.94 nmol/g | 1.61 nmol/g |
| Enhancing tumor | RIBO-003 | 4.1 | 33.48 nmol/g | 1.36 nmol/g |
| Enhancing tumor | RIBO-003 | 4.1 | 29.06 nmol/g | 1.19 nmol/g |
| Enhancing tumor | RIBO-003 | 4.3 | 23.46 nmol/g | 1.02 nmol/g |
| Tumor core | RIBO-003 | 5.7 | 21.80 nmol/g | 1.24 nmol/g |
| Tumor core | RIBO-003 | 6.3 | 21.49 nmol/g | 1.36 nmol/g |
| Subject ID | Sample | Total drug tumora | Unbound drug tumora | Total drug tumor-to-plasma ratiob | Unbound drug tumor-to-plasma ratioc |
|---|---|---|---|---|---|
| RIBO-001 | Enhancing tumor | 17.97 | 0.79 | 5.13 | 2.19 |
| Non-enhancing tumor | 8.86 | 0.48 | 2.53 | 1.35 | |
| Tumor core | 16.30 | 0.82 | 4.65 | 2.29 | |
| RIBO-002 | Enhancing tumor | 24.73 | 1.14 | 20.43 | 9.83 |
| Tumor core | 33.03 | 2.11 | 27.28 | 14.60 | |
| RIBO-003 | Enhancing tumor | 29.99 | 1.29 | 5.74 | 2.75 |
| Tumor core | 21.65 | 1.30 | 4.14 | 2.77 |
Mean concentrations of total and unbound ribociclib (in nmol/g) in tumor specimens from each subject were calculated
Total drug tumor-to-plasma ratio was calculated as the ratio of total drug concentration in tumor (converted to molarity using an estimated tissue density of 1.04 g/mL) to that in plasma
Unbound drug tumor-to-plasma ratio was calculated as the ratio of unbound drug concentration in tumor (converted to molarity using an estimated tissue density of 1.04 g/mL) to that in plasma
Ribociclib concentrations in MRI-enhancing, MRI-non-enhancing, and tumor core regions showed considerable intra- and inter-tumor variability (Table 3). The mean concentrations of total and unbound ribociclib in MRI-enhancing, non-enhancing, and tumor core are summarized in Table 3. The extent of drug-tumor penetration was assessed by the tumor-to-plasma ratio of total ribociclib (Kp) and unbound ribociclib (Kp, uu) in the three subjects. Overall, tumor tissue concentrations of total and unbound ribociclib ranged from 5.80–48.92 nmol/g (mean ± SD of 23.42 ± 10.79 nmol/g) and 0.25–3.12 nmol/g (mean ± SD of 1.24 ± 0.64 nmol/g), respectively. The unbound fraction of ribociclib in tumor specimens (3.9–6.4%) were lower than those found in plasma (9.0–11.9%), which suggested that ribociclib has higher non-specific binding to brain tumor tissue than to plasma proteins.
Drug concentrations in MRI-enhancing, MRI-non-enhancing, and tumor core specimens were similar. Ribociclib penetrated better to enhancing and tumor core as compared to non-enhancing tumor, as demonstrated by higher unbound drug concentrations and higher unbound tumor-to-plasma ratio (Table 3). This was consistent with the notion that MRI-enhancing tumor regions present a disrupted blood–brain barrier. Notably, ribociclib CSF concentration and unbound concentration in non-enhancing tumor were > fivefold of the IC50 for inhibition of CDK4/6 (Table 3), suggesting that ribociclib could penetrate and achieve potentially therapeutic concentration not only in tumor regions with disrupted blood–brain barrier but also in regions with largely intact blood–brain barrier in GBM patients.
Tumor tissue pharmacodynamic analyses
As a measure of ribociclib target engagement, we performed IHC for the CDK4/6 phosphorylation targets RbS807/811 using paired tumor tissue specimens acquired at baseline (untreated, archived, from first surgical resection) and after 8–21 days of ribociclib treatment (from second surgical resection). Two of the three subjects showed a decrease in the proportion of phospho-Rb-positive tumor cells and phospho-Rb histoscores in ribociclib-treated vs. baseline tumors, but one subject showed the opposite (Fig. S1A).
To determine if ribociclib treatment alters tumor cell fate, we measured tumor cell proliferation by Ki-67 IHC staining. Proportions of tumor cells staining positively for Ki-67 decreased in post-ribociclib-treated tumors compared to baseline in subjects 001 and 002, but in subject 003 Ki-67 staining increased compared to baseline (Fig. S1B). Surprisingly, these results directly contradict the observed changes in phospho-Rb (Fig. S1A).
Discussion
Our results suggest that ribociclib penetrates and accumulates within recurrent enhancing and non-enhancing regions of glioblastomas. In vitro studies have shown that treatment with 200 nM ribociclib (total drug) is sufficient to suppress Rb phosphorylation in cultured cells [13, 32]. Assuming a tumor tissue density of 1.04 g/mL, we observed tumor tissue concentrations of total ribociclib ranging from 6.03 to 50.88 μM (mean ± SD of 24.36 ± 11.22 μM), which exceeds those necessary for suppression of Rb phosphorylation and cell growth in vitro [GI50 = 0.276 μM]. The unbound (pharmacologically active) ribociclib intra-tumor drug concentrations ranged from 0.26 to 3.24 μM (mean ± SD of 1.29 ± 0.67 μM); although tumor drug concentrations varied widely between and within patients, all concentrations measured were predicted to cause strong target inhibition. Despite observing what appeared to be supra-pharmacological ribociclib concentrations in tumors, we found that treatment with the standard dose of ribociclib (600 mg/day) as monotherapy did not consistently inhibit tumor tissue CDK4/6 activity (assessed by phospho-Rb IHC) or cell proliferation (assessed by Ki-67 IHC) when comparing archived tumor tissue with post-ribociclib treated tumor tissue. These findings correlate with the limited clinical efficacy of CDK4/6 inhibitor monotherapy with palbociclib or ribociclib, observed herein, as well as in other types of solid tumors [33]. The wide range of ribociclib concentrations observed within tumor tissues highlights the importance of sampling multiple tissue regions in studies with primarily pharmacokinetic and pharmacodynamic endpoints.
The daily administration of ribociclib for 8 to 21 days before surgery assured enough time to achieve steady state ribociclib concentrations in plasma and meaningful tumor tissue concentrations. Our results are limited by the small number of cases, but are consistent with a prior study in which five patients with recurrent brain tumors received pre-surgical treatment with 900 mg/day of ribociclib for 5 days. In concordance with our results, unbound drug concentrations in non-enhancing and enhancing MRI tumor regions were 0.46 ± 0.24 nmol/g and 2.32 ± 1.79 nmol/g, respectively, and mean unbound ribociclib fractions were 12.7% in plasma (CV = 17.6%) and 4.6% in tumor tissues (CV = 23%) [31]. Therefore, even in non-enhancing GBM, ribociclib achieves concentrations that exceed those felt to be pharmacologically active to inhibit CDK4/6.
The pharmacodynamic endpoints to assess pathway inhibition, one of the objectives of our window-of-opportunity study, did not yield a consistent pattern. A limitation of our study is the use of paired archived baseline tumor tissue from the first surgery as a comparator to recurrent post-ribociclib tumor tissue. In a similar study, patients with recurrent GBM were treated with 7 days of the CDK4/6 inhibitor palbociclib, before surgery. In this study by Taylor et al., analysis of 4 matched samples comparing archival FFPE tumor tissue from the initial surgery and tumor obtained at the second surgery showed drug concentrations that exceeded those felt to be pharmacologically active, but did not produce significant changes in GBM cell proliferation as determined by Ki-67 IHC [34]. A caveat to the comparison of paired tumor specimens from these time points is that study participants received standard therapies (radiation, chemotherapy) after the first surgical resection, and recurrent tumors may have evolved [e.g. (epi)genetically] in the interim [35, 36]. In contrast, Tien et al. recently reported findings from a study of 12 patients with recurrent GBM (all tumors were Rb-positive with either CDKN2A deletion or amplification of CDK4/6 or CCND1/2/3) treated with 900 mg/day of ribociclib for 5 days before surgery. The authors observed a significant decrease in Rb phosphorylation and cell proliferation when comparing archived tumor samples from initial surgery to those obtained at the time of surgery after ribociclib treatment [37]. The mean concentrations of unbound ribociclib across all subjects observed by Tien et al. were similar to those reported herein in CSF (0.37 μM vs. 0.63 μM), MRI-enhancing tumor or core (2.15 nmol/g vs. 1.33 nmol/g), and MRI-non-enhancing tumor (0.56 nmol/g vs. 0.48 nmol/g). In the Tien et al. study, six patients who had a “responsive” pharmacokinetic/pharmacodynamic signal in the post-ribociclib treatment tumor continued treatment with ribociclib after surgery and achieved a median PFS of 9.7 weeks, indicating that ribociclib monotherapy has limited clinical efficacy in GBM. Interestingly, in the tumor tissue obtained from three subjects during re-resection after progression on ribociclib, they identified a rebound in phospho-Rb levels and mTOR pathway upregulation as a potential mechanism of resistance to CDK4/6 inhibition [37].
Although the optimal study would have a tumor biopsy specimen obtained at the time of recurrence, followed by the experimental treatment and then surgery to obtain tissue for relevant molecular pharmacodynamic studies (the biopsy-treat-biopsy paradigm), this is not feasible in most cases of high-grade gliomas because of the risk-benefit of an additional invasive surgical procedure. Tumor heterogeneity is another factor to consider in the interpretation of results of tumor pharmacodynamic assays because one is comparing tumor obtained at different time points and slightly different locations. However, comparison of phospho-Rb and Ki-67 measurements in primary and recurrent GBM specimens showed no significant difference between time points [37]. Furthermore, in our study, the presence of necrotic tumor precluded adequate assessment of phosphoprotein modulation in some tumor areas. In the assessment of new drugs for the treatment of gliomas, identifying appropriate pharmacodynamic biomarkers that both correlate with clinical outcomes and are amenable to serial non-invasive measurement remains a challenge.
Early disease progression in our small cohort suggested that monotherapy with ribociclib may not have meaningful clinical activity in the treatment of recurrent high-grade glioma. All of our patients had low tumor burdens after their second surgeries, and recurrent tumors were Rb-positive by IHC. In a phase II trial, 22 patients were treated with palbociclib (125 mg/day on a 21-days-on, 7-days-off cycle) for recurrent Rb-positive GBM. That study was stopped prematurely and suggested a lack of efficacy, with a median PFS of 5.1 weeks and an OS of 15.4 weeks [34]. A third CDK4/6 inhibitor (abemaciclib, 150 mg or 200 mg twice daily) showed limited efficacy in a phase I trial including 17 patients with recurrent GBM; the best response occurred in three patients experiencing stable disease for ≥ 24 weeks [38]. These results, when combined with our findings, suggest that monotherapy with current CDK4/6 inhibitors is unlikely to be of substantial benefit in the treatment of high-grade gliomas.
Enthusiasm for GBM treatment with single-agent CDK4/6 inhibition has waned following the observation of negative results from early clinical trials. Although our accrual goal was ten subjects, we closed the study following evaluation of three eligible subjects due to slow accrual, and disappointing emergent results from other trials that tested CDK4/6 inhibitor monotherapies for high-grade gliomas. Preclinical studies from our institution have suggested potential GBM resistance mechanisms to CDK4/6 inhibitors, including rapid proneural-mesenchymal transition [39], mTOR upregulation [40], and MET and TRK upregulation [41]. These reports demonstrate the synergistic potential of combining CDK4/6 inhibitors with mTOR and MET/TRK inhibitors. Along these lines, an ongoing clinical trial is defining and testing the maximum tolerated doses of the combination of ribociclib and everolimus after radiation in children with diffuse infiltrating pontine gliomas and high-grade gliomas (). Combination therapy with CDK4/6 inhibitors may thus represent a viable path forward in the treatment of GBM, and more such trials are warranted. The finding that ribociclib reaches therapeutic concentrations in GBM confirms it as a promising CDK4/6 inhibitor to be studied in such combinations.
Supplementary Material
Funding
Supported by Novartis Pharmaceuticals, which provided research grant funding and drug for the study, and the Norris Cotton Cancer Center, which provided funding to TWM. SHP has grant support from the Radiologic Society of North America, Research Scholar Grant (Grant No. RSCH1819).
Footnotes
Publisher's Disclaimer: Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest The University of Virginia (CEF) received research funding from Novartis Pharmaceuticals to conduct this clinical study. All other authors declare that they have no conflicts of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-019-03258-0) contains supplementary material, which is available to authorized users.
Ethical approval This study () was approved by the local Institutional Review Board for Health Sciences Research (IRB approval #18729), was conducted in accordance with Good Clinical Practice, and was monitored by the Data Safety and Monitoring Committee of the University of Virginia Cancer Center. The FDA (IND #125168) approved the IND application for this study. Informed consent was obtained from all individual participants included in the study, prior to performing any study related procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute ofCanada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, Treatment of Cancer Brain T,Radiation Oncology G, National Cancer Institute of Canada Clinical Trials G (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- 4.Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF (2014) Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife 3 10.7554/eLife.02872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ (1993) Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev 7:331–342 [DOI] [PubMed] [Google Scholar]
- 6.Weintraub SJ, Chow KN, Luo RX, Zhang SH, He S, Dean DC (1995) Mechanism of active transcriptional repression by the retinoblastoma protein. Nature 375:812–815. 10.1038/375812a0 [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6:353–367. 10.1158/2159-8290.CD-15-0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyer-son M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, Zhuang L, Rosenbluh J, Chen S, Xiao Y, Shapiro GI, Hahn WC, Chin L (2010) Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci USA 107:11501–11506. 10.1073/pnas.1001613107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, Yang G, Chalukya M, Wang HJ, Anderson L, Kalli KR, Finn RS, Ginther C, Jones S, Velculescu VE, Riehle D, Cliby WA, Randolph S, Koehler M, Hartmann LC, Slamon DJ (2011) Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res 17:1591–1602. 10.1158/1078-0432.CCR-10-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ, Kim S, Parasuraman S, Caponigro G, Schnepp RW, Wood AC, Pawel B, Cole KA, Maris JM (2013) Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res 19:6173–6182. 10.1158/1078-0432.CCR-13-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, Ozawa T, James CD, Waldman T (2010) Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 70:3228–3238. 10.1158/0008-5472.CAN-09-4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI (2016) A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 22:5696–5705. 10.1158/1078-0432.CCR-16-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn JG, Chang SM, Wen PY, Cloughesy TF, Greenberg H, Schiff D, Conrad C, Fink KL, Robins HI, Mehta M, DeAngelis L, Raizer J, Hess K, Lamborn KR, Dancey J, Prados MD, North American Brain Tumor C, the National Cancer I (2007) Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res 13:7401–7406. 10.1158/1078-0432.CCR-07-0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG, New Approaches to Brain Tumor Therapy C (2009) Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neuro-Oncol 91:51–58. 10.1007/s11060-008-9678-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittle IR, Malcolm G, Jodrell DI, Reid M (1999) Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br J Neurosurg 13:132–137 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MR, Kuhn J, Lamborn KR, Lieberman F, Wen PY, Mehta M, Cloughesy T, Lassman AB, Deangelis LM, Chang S, Prados M (2012) Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03–02, a phase II trial with measures of treatment delivery. J Neuro-Oncol 106:147–153. 10.1007/s11060-011-0650-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WK, Gilbert MR, Aldape KA, Wen PY, Fine HA, Mehta M, Deangelis LM, Lieberman F, Cloughesy TF, Robins HI, Dancey J, Prados MD, North American Brain Tumor C (2010) A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-Oncology 12:95–103. 10.1093/neuonc/nop015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucchetti M, Boiardi A, Silvani A, Parisi I, Piccolrovazzi S, D’Incalci M (1999) Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol 44:173–176. 10.1007/s002800050964 [DOI] [PubMed] [Google Scholar]
- 22.Green RM, Stewart DJ, Hugenholtz H, Richard MT, Thibault M, Montpetit V (1988) Human central nervous system and plasma pharmacology of mitoxantrone. J Neuro-Oncol 6:75–83 [DOI] [PubMed] [Google Scholar]
- 23.Stewart DJ, Leavens M, Maor M, Feun L, Luna M, Bonura J, Caprioli R, Loo TL, Benjamin RS (1982) Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res 42:2474–2479 [PubMed] [Google Scholar]
- 24.Stewart DJ, Lu K, Benjamin RS, Leavens ME, Luna M, Yap HY, Loo TL (1983) Concentration of vinblastine in human intracerebral tumor and other tissues. J Neuro-Oncol 1:139–144 [DOI] [PubMed] [Google Scholar]
- 25.Stewart DJ, Richard MT, Hugenholtz H, Dennery JM, Belanger R, Gerin-Lajoie J, Montpetit V, Nundy D, Prior J, Hopkins HS (1984) Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neuro-Oncol 2:133–139 [DOI] [PubMed] [Google Scholar]
- 26.Pitz MW, Desai A, Grossman SA, Blakeley JO (2011) Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neuro-Oncol 104:629–638. 10.1007/s11060-011-0564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boogerd W, Dalesio O, Bais EM, van der Sande JJ (1992) Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69:972–980 [DOI] [PubMed] [Google Scholar]
- 28.Franciosi V, Cocconi G, Michiara M, Di Costanzo F, Fosser V, Tonato M, Carlini P, Boni C, Di Sarra S (1999) Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 85:1599–1605 [PubMed] [Google Scholar]
- 29.Boogerd W, Groenveld F, Linn S, Baars JW, Brandsma D, van Tinteren H (2012) Chemotherapy as primary treatment for brain metastases from breast cancer: analysis of 115 one-year survivors. J Cancer Res 138:1395–1403. 10.1007/s00432-012-1218-y [DOI] [PubMed] [Google Scholar]
- 30.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 31.Bao X, Wu J, Sanai N, Li J (2019) Determination of total and unbound ribociclib in human plasma and brain tumor tissues using liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal 166:197–204. 10.1016/j.jpba.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eilers G, Czaplinski JT, Mayeda M, Bahri N, Tao D, Zhu M, Hornick JL, Lindeman NI, Sicinska E, Wagner AJ, Fletcher JA, Marino-Enriquez A (2015) CDKN2A/p16 loss implicates CDK4 as a therapeutic target in imatinib-resistant dermatofibrosarcoma protuberans. Mol Cancer Ther 14:1346–1353. 10.1158/1535-7163.MCT-14-0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schettini F, De Santo I, Rea CG, De Placido P, Formisano L, Giuliano M, Arpino G, De Laurentiis M, Puglisi F, De Placido S, Del Mastro L (2018) CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol 8:608 10.3389/fonc.2018.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JW, Parikh M, Phillips JJ, James CD, Molinaro AM, Butowski NA, Clarke JL, Oberheim-Bush NA, Chang SM, Berger MS, Prados M (2018) Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neurooncol 140:477–483. 10.1007/s11060-018-2977-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DI, Zairis S, Abate F, Liu Z, Elliott O, Shin YJ, Lee JK, Lee IH, Park WY, Eoli M, Blumberg AJ, Lasorella A, Nam DH, Finocchiaro G, Iavarone A, Rabadan R (2016) Clonal evolution of glioblastoma under therapy. Nat Genet 48:768–776. 10.1038/ng.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neilsen BK, Sleightholm R, McComb R, Ramkissoon SH, Ross JS, Corona RJ, Miller VA, Cooke M, Aizenberg MR (2019) Comprehensive genetic alteration profiling in primary and recurrent glioblastoma. J Neuro-Oncol 142:111–118. 10.1007/s11060-018-03070-2 [DOI] [PubMed] [Google Scholar]
- 37.Tien A-C, Li J, Bao X, DeRogatis A, Kim S, Mehta S, Sanai N (2019) A Phase 0 trial of ribociclib in recurrent glioblastoma patients incorporating a tumor pharmacodynamic- and pharmacokinetic-guided expansion cohort. Clin Cancer Res. 10.1158/1078-0432.CCR-19-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF, Nasir A, Beckmann RP, Schade AE, Fulford AD, Nguyen TS, Martinez R, Kulanthaivel P, Li LQ, Frenzel M, Cronier DM, Chan EM, Flaherty KT, Wen PY, Shapiro GI (2016) Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 6:740–753. 10.1158/2159-8290.CD-16-0095 [DOI] [PubMed] [Google Scholar]
- 39.Li M, Xiao A, Floyd D, Olmez I, Lee J, Godlewski J, Bronisz A, Bhat KPL, Sulman EP, Nakano I, Purow B (2017) CDK4/6 inhibition is more active against the glioblastoma proneural subtype. Oncotarget 8:55319–55331. 10.18632/oncotarget.19429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olmez I, Brenneman B, Xiao A, Serbulea V, Benamar M, Zhang Y, Manigat L, Abbas T, Lee J, Nakano I, Godlewski J, Bronisz A, Abounader R, Leitinger N, Purow B (2017) Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms. Clin Cancer Res 23:6958–6968. 10.1158/1078-0432.CCR-17-0803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olmez I, Zhang Y, Manigat L, Benamar M, Brenneman B, Nakano I, Godlewski J, Bronisz A, Lee J, Abbas T, Abounader R, Purow B (2018) Combined c-Met/Trk inhibition overcomes resistance to CDK4/6 inhibitors in glioblastoma. Cancer Res 78:4360–4369. 10.1158/0008-5472.CAN-17-3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.