Abstract
Accumulating research indicates oxytocin plays a significant role in regulating the behavioral and neurobiological responses to stress. Evidence from preclinical models suggests the effect of oxytocin on stress-responsivity appears to be dependent on individual characteristics, including sex. Although the interaction between oxytocinergic and stress systems has been widely studied in rodents, recent efforts have been made to examine the interface between these two systems in humans. This brief review examines how administration of oxytocin can influence the neuroendocrine, behavioral, and neural responses to stress, explores how sex may impact these effects, and provides considerations for future work.
Introduction
Over the last 20 years, oxytocin research has largely focused on the neuropeptide’s role in social behaviors; however, a history of work dating back to the 1950s has investigated the interaction between oxytocin and stress systems [1]. Oxytocin is released peripherally and centrally in response to a variety of psychogenic and physical stressors and is an influential modulator of the neuroendocrine stress response. Consequently, there has been a concerted research effort to explore oxytocin and its relationship to psychiatric conditions characterized by stress-system dysregulation such as addiction. Accumulating evidence indicates oxytocinergic regulation of the stress response is shaped as a function of individual characteristics and context [2]. In particular, the effects of oxytocin appear to be sexually dimorphic. The aim of this brief review is to highlight some of the current work being done to understand how oxytocin can affect the neurobiological and behavioral responses to stress and how sex differences may impact these processes.
Oxytocin-induced changes in stress-reactivity: rodents
Hypothalamic and neuroendocrine effects
The stress response consists of a series of neural events that stimulate the hypothalamic–pituitary–adrenal (HPA) axis. Activation of the HPA axis stimulates a neuroendocrine cascade beginning with the release of corticotropin-releasing factor (CRF) from the paraventricular nucleus (PVN) of the hypothalamus. CRF promotes secretion of adrenocorticotropin (ACTH) from the anterior pituitary which in turn stimulates the production and release of glucocorticoids from the adrenal glands (Figure 1). Accompanying this ‘classic’ neuroendocrine response to stress is the secretion of oxytocin from the posterior pituitary terminals [3]. Synthesized within magnocellular neurons located within the PVN, supraoptic nucleus (SON), and accessory magnocellular nuclei (AN) of the hypothalamus, oxytocin is released into circulation from neurohypophysial terminals in response to a diverse set stressors (e.g., [4,5]). The mechanisms underlying oxytocin’s regulatory influence on HPA reactivity are still being uncovered, however, endogenous and exogenous elevations in oxytocin are associated with reductions in CRF, ACTH, and Cortisol.
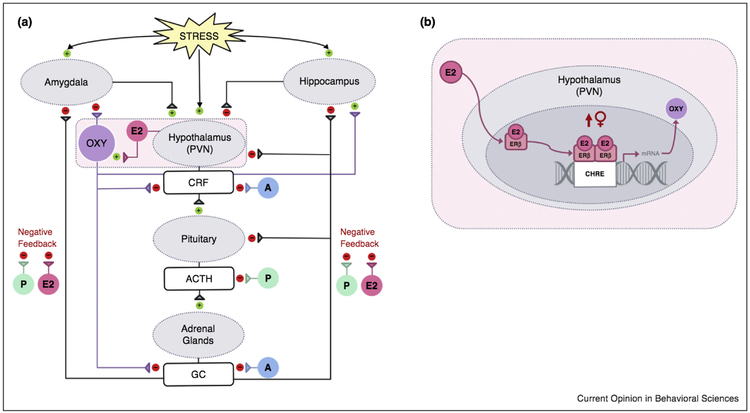
Figure 1.
(a) Model of hypothesized interactions between oxytocin, gonadal steroids, and stress systems. In response to acute stress, a neuroendocrine cascade is triggered which promotes CRF secretion within the PVN of the hypothalamus that leads to the release of ACTH from the anterior pituitary which ultimately promotes the production and release of glucocorticoids (GC) from the cortices of the adrenal glands. Centrally, exposure to stress recruits areas within stress-sensitive neural pathways including the amygdala and hippocampus, both of which regulate neuroendocrine responses to stress. Oxytocin administration prior to stress inhibits this neuroendocrine response, reducing the secretion of CRF and glucocorticoids. In the CNS, oxytocin administration attenuates stress-related activity within the amygdala and appears to protect against stress-induced alterations in hippocampal synaptic plasticity. Circulating gonadal hormones including androgens (A), estrogens (E2), and progesterone (P) have the potential to affect HPA axis activity and modulate oxytocin neuron functioning which may contribute to sex differences in stress responsiveness. In general, androgens including testosterone reduce stress-induced glucocorticoid release, in part via androgen inhibition of CRF expression. Ovarian steroid effects on HPA axis activity are more complex. In rodents, estradiol and progesterone inhibit glucocorticoid negative feedback that may impair downregulation of the stress response. Conversely, estradiol can decrease neuroendocrine responses to stress via its actions on estrogen receptor beta (ERβ). (b) Estrogen receptor beta regulation of oxytocin mRNA expression. Within the PVN, estradiol binds to nuclear ERβ which rapidly dimerizes and binds to the composite hormone response element (CHRE) of the oxytocin gene. This enhances oxytocin transcript expression and increases oxytocin peptide levels which can ultimately lead to overall reductions in stress-related HPA axis activity. Although the mechanisms are not yet understood, it appears that females may be more sensitive to these effects.
Adapted from Acevedo-Rodriguez et al. [24].
Oxytocin administration can inhibit ACTH and subsequent glucocorticoid secretion in response to stress [6,7]. Several lines of evidence suggest downstream reductions in HPA axis reactivity may result from oxytocinergic suppression of CRF neuron activity (Figure 1). Interactions between oxytocin and CRF systems are well-documented. For example, some PVN neurons co-express CRF and OT [8,9]. Further, within the PVN small subpopulations of CRF neurons express oxytocin receptor mRNA and, reciprocally, clusters of oxytocin neurons contain CRF2 receptors [8]. Central administration of oxytocin reduces hypothalamic CRF neuron excitability [10], inhibits spontaneous excitatory transmission onto CRF neurons [10], reduces CRF mRNA expression [4,11•], attenuates stress-induced c-fos expression, a marker of neuronal activity, in CRF neurons and concomitantly reduces neuroendocrine responses to restraint stress [7].
During times of stress, glucocorticoids can also modulate oxytocin neurotransmission. For example, recent work by Torner and colleagues demonstrated adrenalectomized rats display heightened stress-induced plasma oxytocin secretion but blunted intra-PVN release of oxytocin following forced swimming [12]. Intravenous infusion of corticosterone prior to forced swimming rescued the stress-induced intra-PVN increase of oxytocin and slightly enhanced peripheral plasma concentrations of oxytocin, indicating stress-induced release of oxytocin within the brain and periphery are both modulated, albeit independently, by corticosterone [12].
Extrahypothalamic effects
Oxytocinergic modulation of neuronal activity at sites beyond the hypothalamus reduce behavioral and molecular responses to stress. Oxytocin receptors are widely expressed throughout the brain including in stress-sensitive brain regions like the hippocampus and amygdala [13]. Exogenous oxytocin administration is associated with reductions in stress-related activity within these regions. For example, Windle and colleagues have reported intra-cerebroventricular infusion of oxytocin reduced restraint stress-induced ACTH and corticosterone release and attenuated c-fos expression within all subfields of the dorsal hippocampus [7]. Oxytocin administration can also block stress-induced impairment of hippocampal-dependent memory and reduce stress-induced alterations in hippocampal synaptic plasticity. Lee and colleagues demonstrated rodents treated with intranasal oxytocin prior to being exposed to a restraint-tail shock stress procedure exhibited less impaired spatial memory and enhanced maintenance of long-term potentiation and reduced long-term depression within the CA1 of the hippocampus, compared to their vehicle treated counterparts; an effect that could be blocked by pretreatment with an oxytocin receptor antagonist [14].
Oxytocin can also influence activity within the central nucleus of the amygdala (CeA), a region mediating behavioral responses to fearful stimuli and critical to the formation of anxiety-like behavior. Acute stressors,like forced swimming, can trigger oxytocin release within the CeA [15]. Further, stimulated oxytocin release or infusion of oxytocin into the CeA, reduces CeA signaling via enhancement of local GABAergic interneuron activity, and is associated with an attenuation of stress, fear, and aggressive behaviors [15-19].
Sex differences
Sex differences in oxytocin system parameters have been noted, though appears to be species-specific [20•]. However, when sex differences have been noted, oxytocin levels tend to be higher in females compared to males [20•]. In general, oxytocin inhibits HPA axis reactivity in both females and males [20•,21], though, oxytocinergic effects on stress system reactivity is influenced by the activational effects of sex steroids and is dependent on the reproductive state [22]. For example, modulation of estrogen receptor beta (ERβ) activity by gonadal steroids within the PVN facilitates oxytocin mRNA expression and is associated with reductions in anxiety-like behaviors and attenuation of ACTH and corticosterone responses to stress [23,24]. ERβ receptors are highly expressed in oxytocin neurons within PVN and are associated with decreases stress reactivity when activated [24]. The oxytocin promoter contains a composite response element through which ERβ can modulate oxytocin gene expression. Specifically, actions of estradiol or 3β-diol, a testosterone metabolite, on ERβ receptors stimulate oxytocin promoter activity, which gives rise to increases oxytocin expression and, subsequently, oxytocin neurotransmission [23,25]. Indeed, it appears that reductions in stress reactivity by ERβ activation is mediated by oxytocinergic activity [26-28]. Kudwa and colleagues demonstrated treatment with the ERβ-selective agonist R-diarylpropionitrile reduced corticosterone responses to restraint stress and attenuated anxiety-like behavior in the elevated plus maze test in gonadectomized male and female rats [28]. Intracer-ebroventricular pretreatment with an oxytocin receptor antagonist blocked these effects demonstrating an interaction between ERβ and oxytocin systems to modulate behavioral and HPA responses to stress [28]. Some studies suggest females may be more sensitive to some of ERβ effects. For instance, female ERβ knockout mice exhibit increased anxiety behaviors but not their male counterparts [29] and treatment with R-diarylpropionitrile reduces neuroendocrine responses to stress and anxiety-like behaviors more effectively in females compared to males [28].
Beyond the PVN, sex-specific oxytocin effects have been noted within other areas of the brain including the medial prefrontal cortex (mPFC) and the bed nucleus of the stria terminals (BNST). Recently, Li and colleagues described the presence of an oxytocin-sensitive, sexually dimorphic mPFC circuit [30••]. Specifically, Li describes the presence of oxytocinergic interneurons within the mPFC that when stimulated results in the release of GABA and corticotropin releasing hormone binding protein (CRHBP), a protein which inhibits the activity of CRF. In male animals, upon stimulation of these oxytocinergic interneurons, CRHBP is released, CRF activity is inhibited, and this promotes a reduction in anxiety; however, in females, perhaps due to higher levels of CRF within the PVN, CRHBP is unable to effectively reduce CRF activity and, as such, does not result in an anxiolytic effect. Similar sex-specific oxytocin effects have been noted in the BNST. Steinman and colleagues demonstrated that exposure to social defeat enhances the activity of oxytocin neurons within the PVN in both males and females and induces increases in oxytocin neuron activity and oxytocin mRNA expression in the BNST for up to 10 weeks, but only in females. Further, treatment with intranasal oxytocin reduced stress-related behavior and increased social interaction among males, but failed to alter social interaction behavior in stressed females and produced a context-sensitive reduction in stress-related behaviors, specifically inducing a greater anxiolytic response when females tested in a familiar environment compared to an unfamiliar environment [31•]. In sum, these data indicate that oxytocinergic modulation of stress-reactivity can vary as a result of sexually dimorphic circuits within the brain.
Oxytocin-induced changes in stress-reactivity: humans
Owing to the methodological limitations of studying humans, systemic pharmacological challenges have been typically employed to examine the influence of oxytocin on stress reactivity. Although multiple meta-analyses confirm exogenous oxytocin has a measurable effect on behavior and neural activity (e.g., [32-34]), the mechanisms by which exogenous oxytocin exerts its impact are still being investigated. Central mechanisms are suspected. A recent study by Lee and colleagues [35•] indicates exogenous oxytocin administered by either intravenous or intranasal routes is detectable within the cerebrospinal fluid (CSF) in non-human primates. However, this does not rule out the possibility that peripheral mechanisms, possibly involving oxytocin activity at the adrenal gland, may also contribute [36].
Hypothalamic and neuroendocrine effects
Similar to what is observed in rodent models, oxytocin administration can attenuate neuroendocrine responses to stress in humans. A recent meta-analysis indicated that a single acute dose of intranasal oxytocin can produce reductions in Cortisol within the context of studies which robustly activate the HPA axis, like intrapersonal stress [34]. Paradigms utilizing social-evaluative threat (e.g., Trier Stress Test, TSST), elicit strong Cortisol responses in humans and demonstrate concomitant rises in circulating oxytocin [37]. Stimulated oxytocin release or treatment with intranasal oxytocin prior to intrapersonal stress is associated with attenuated plasma and salivary Cortisol responses to stress [38-40, but see 41] and reductions in reported feelings of fear, anxiety, and perceived stress [38,40-42]. Oxytocin administration also appears to enhance the buffering effect of social support on stress. Although social support in and of itself can suppress salivary Cortisol responses to stress (e.g., TSST, [38]), intranasal oxytocin can improve the effectiveness of the stress-buffering effect of social support, enhancing the attenuation of stress-induced Cortisol release [38]. Although less is known about oxytocin’s effect on other aspects of the HPA response, early studies indicated that systemic oxytocin administration could reduce plasma ACTH responses to a pharmacological stress challenge (e.g., CRF, [43]) and following social isolation in non-human primates [6].
Extrahypothalamic effects
Functional magnetic resonance imaging (fMRI) experiments reveal that intranasal oxytocin can also impact brain activity elicited in response to stress, negative affect, and fear. Increases in amygdala activity are typically observed in response to viewing negative, fearful or otherwise threatening stimuli (e.g., [44-46]). Intranasal oxytocin modulates activity within this area, reducing amygdala activation in response to fear [44-46], anger [45], and in response to breaches of trust in men [47]. A recent meta-analysis examining intranasal oxytocin fMRI studies indicates that oxytocin administration reduces amygdala reactivity to negatively-valenced stimuli but may enhance amygdala activity in response to positive stimuli [32]. In addition to valence, the effects of oxytocin on amygdala activity may also depend on the subregion examined. For instance, Gamer and colleagues demonstrated that oxytocin specifically attenuated activity within the anterior amygdala in response to fearful faces but enhanced activity in response to happy faces; whereas, oxytocin enhanced activity within the posterior amygdala in response to gaze changes towards the eyes of faces, irrespective of valence [48]. These data collectively suggest oxytocin may attenuate reactivity of the amygdala towards negative stimuli but enhance activity relevant to the processing of socially relevant information.
Sex differences
Until recently, relatively few studies examining oxytocin in the context of stress have incorporated female participants into their design or examined sex as a factor of interest within their data analyses. Although the data is still relatively limited, intranasal oxytocin can produce anxiolytic effects in both men [38,42,49] and women [42,50]; though, oxytocin may have distinct sex-specific effects depending on the challenge employed. Perhaps the most consistently reported example of such an effect is the impact of oxytocin on amygdala responses to negatively-valenced stimuli. As previously described, oxytocin reduces amygdala activity in response to negative faces [44,45], threatening scenes [44] and negative social interactions in men [47]. However, women treated with oxytocin exhibit greater amygdala responses when viewing angry faces [51,52] and threatening scenes [53]. This differential modulation of neural activity by oxytocin may serve to promote detection of socially relevant and potentially threatening stimuli in females, while reducing threat sensitivity in males [53]. Outside the amygdala, oxytocin can impact activity within the dorsal anterior cingulate, inferior frontal gyrus, and anterior insula in response to negatively-valenced face stimuli in a sex-specific manner. Specifically, oxytocin treatment results in reductions in activity across these regions in men and increases in women [54]. Women also display reduced coupling of the amygdala to the anterior cingulate and inferior frontal gyrus during the processing of subliminally presented fearful and angry faces, which may represent an alteration in prefrontal regulatory control over amygdala activity in women [54].
Conclusions
Mounting evidence suggests oxytocin can impact stress, anxiety, and the processing of negative emotional stimuli in a sexually dimorphic manner. As oxytocin is currently being investigated for use as a treatment option for psychiatric disorders characterized by dysregulation within brain stress systems, it will be important to examine whether oxytocin may be differentially effective in reducing some stress-related features in men and women. For example, in addictive disorders, oxytocin has been observed to impact activity within circuits associated with the negative reinforcing effects of substances of abuse [55•]. Oxytocin administration is hypothesized to offset some of the neuroadaptations in brain stress systems which result from repeated administration and withdrawal from drugs of abuse which are believed to contribute to the elevated stress responses, negative emotional states, and increased anxiety associated with acute and protracted drug withdrawal [56]. As such, oxytocin could counteract the negative motivational state which drives negative reinforcement to subsequently reduce compulsive drug seeking [55•,57]. Indeed, oxytocin administration can attenuate withdrawal symptoms, reduce anxiety-like behaviors, and prevent stress-induced reinstatement of drug self-administration in humans and animals [55•]. Whether oxytocin has the same impact on these behaviors in females and males is not currently known. Given that oxytocin can influence stress-related activity differently in males and females, and, like many other aspects of addiction, negative reinforcement effects may be different in males and females [58,59], it is important that future studies examine the extent to which oxytocin can reduce the negative motivational states associated with addiction and whether these effects differ according to sex.
Much more work is needed to understand under what circumstances oxytocin induces sex-specific effects and to elucidate the impact sex steroids have on these processes. In the case of human studies, while more studies are incorporating women into their design, many studies fail to characterize or utilize less than optimal ways of determining menstrual cycle phase (e.g., relying on self-reported last menstrual cycle day [60]), do not examine responses according to specific menstrual cycle phase, do not appropriately account for hormonal contraceptive use, and very few obtain sex hormone concentrations to either confirm cycle phase or to examine the potential impact of circulating gonadal hormones. Addressing these limitations in future studies will help foster a better understanding of the interaction between oxytocin, stress, and the role of sex.
Acknowledgements
This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism 1K01AA024167-01. The author would also like to recognize this article’s anonymous reviewers for improving this manuscript.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Cross BA: Neurohormonal mechanisms in emotional inhibition of milk ejection. J Endocrinol 1955, 12:29–37. [DOI] [PubMed] [Google Scholar]
- 2.Bartz JA, Zaki J, Bolger N,Ochsner KN: Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 2011, 15:301–309. [DOI] [PubMed] [Google Scholar]
- 3.Lang RE, Heil JW, Ganten D, Hermann K, Linger T, Rascher W: Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983, 37:314–316. [DOI] [PubMed] [Google Scholar]
- 4.Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T: Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Res 2011, 1387:39–45. [DOI] [PubMed] [Google Scholar]
- 5.Neumann ID, Krömer SA, Toschi N, Ebner K: Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept 2000, 96:31–38. [DOI] [PubMed] [Google Scholar]
- 6.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM: Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 2005, 30:924–929. [DOI] [PubMed] [Google Scholar]
- 7.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD: Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci 2004, 24:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabrowska J, Hazra R, Ahem TH, Guo J-D, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG: Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology 2011, 36:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabrowska J, Hazra R, Guo J-D, Dewitt S, Rainnie DG: Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 2013, 7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamieson BB, Nair BB, lremonger KJ: Regulation of hypothalamic CRH neuron excitability by oxytocin. J Neuroendocrinol 2017. 10.1111/jne.12532. [DOI] [PubMed] [Google Scholar]
- 11. •.Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, Neumann ID, van den Burg EH: Oxytocin regulates stress-induced Crf gene transcription through creb-regulated transcription coactivator 3. J Neurosci 2015, 35:12248–12260.The authors describe a novel oxytocin-receptor mediated intracellular mechanism that alters the transcription of the gene encoding corticotropin releasing factor during acute stress exposure.
- 12.Torner L, Plotsky PM, Neumann ID, de Jong TR: Forced swimming-induced oxytocin release into blood and brain: effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 2017, 77:165–174. [DOI] [PubMed] [Google Scholar]
- 13.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA: Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013, 253:155–164. [DOI] [PubMed] [Google Scholar]
- 14.Lee S-Y, Park S-H, Chung C, Kim JJ, Choi S-Y, Han J-S: Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Sci Rep 2015, 5 srepl 8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID: Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology 2005, 30:223–230. [DOI] [PubMed] [Google Scholar]
- 16.Huber D, Veinante P, Stoop R: Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308:245–248. [DOI] [PubMed] [Google Scholar]
- 17.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R et al. : Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73:553–566. [DOI] [PubMed] [Google Scholar]
- 18.Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM: Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology 2015, 90:74–81. [DOI] [PubMed] [Google Scholar]
- 19.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R: Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 2011, 333:104–107. [DOI] [PubMed] [Google Scholar]
- 20. •.Dumais KM, Veenema AH: Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinoi 2016,40:1–23.The authors give a rich overview of the current state of knowledge in regards to sex differences in vasopressin and oxytocin systems in the brain in the context of social behavior. This work highlights how species-specific differences in both these systems may contribute to sex differences in social behavior observed among various animal models and humans.
- 21.Neumann ID: Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinoi 2008, 20:858–865. [DOI] [PubMed] [Google Scholar]
- 22.Neumann ID, Tomer L, Wigger A: Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 2000, 95:567–575. [DOI] [PubMed] [Google Scholar]
- 23.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S: Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res 2002,109:84–94. [DOI] [PubMed] [Google Scholar]
- 24.Acevedo-Rodriguez A, Mani SK, Handa RJ: Oxytocin and estrogen receptor β in the brain: an overview. Front Endocrinol 2015, 6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ: The androgen metabolite, 5α-androstane-3β,17β-diol (3β-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology 2013, 154:1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Bisschop PH, Eggels L, Foppen E, Fliers E, Zhou JN, Kalsbeek A: Intrahypothalamic estradiol modulates hypothalamus-pituitary-adrenal-axis activity in female rats.Endocrinology 2012, 153:3337–3344. [DOI] [PubMed] [Google Scholar]
- 27.Lund TD, Hinds LR, Handa RJ: The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 2006, 26:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudwa AE, McGivern RF, Handa RJ: Estrogen receptor β and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol Behav 2014, 129:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda MC, Yamaguchi N, Nakata M, Ogawa S: Modification of female and male social behaviors in estrogen receptor beta knockout mice by neonatal maternal separation. Front Neurosci 2014, 8:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ••.Li K, Nakajima M, Ibañez-Tallon I, Heintz N: A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors.Cell 2016, 167:60–72.e11.Authors describe how sexually dimorphic behavior may arise as a result of the actions of oxytocin and CRF within the medial prefrontal cortex. Specifically, Li and colleagues demonstrate that stimulation of oxytocinergic activity within the mPFC can alter stress and social behaviors in male and female animals, but in sex-specific ways.
- 31. •.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM et al. : Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry 2016, 80:406–414.This study examined the effects of social defeat on oxytocinergic system parameters in male and female mice and explored how intranasal oxytocin can impact social interaction and stress-related behaviors. This study described a unique enduring effect of social defeat on oxytocin systems and sex differences in the both the behavioral responses to stress and in the behavioral effects of intranasal oxytocin.
- 32.Wang D, Yan X, Li M, Ma Y: Neural substrates underlying the effects of oxytocin: a quantitative meta-analysis of pharmaco-imaging studies. Soc Cogn Affect Neurosci 2017,12:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leppanen J, Ng KW, Tchanturia K, Treasure J: Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci Biobehav Rev 2017, 78:125–144. [DOI] [PubMed] [Google Scholar]
- 34.Cardoso C, Kingdon D, Ellenbogen MA: A meta-analytic review of the impact of intranasal oxytocin administration on Cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology 2014, 49:161–170. [DOI] [PubMed] [Google Scholar]
- 35. •.Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB: Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry 2017. 10.1038/mp.2017.27.This recent study by Lee and colleagues was the first study to demonstrate that oxytocin administered by either intranasal or intravenous routes reaches the CSF, indicating peripherally administered oxytocin does penetrate the CNS.
- 36.Legros JJ, Chiodera P, Geenen V: Inhibitory action of exogenous oxytocin on plasma Cortisol in normal human subjects: evidence of action at the adrenal level. Neuroendocrinology 1988, 48:204–206. [DOI] [PubMed] [Google Scholar]
- 37.de Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, Jurek B, Bosch OJ, Hellhammer J, Neumann ID: Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 2015, 62:381–388. [DOI] [PubMed] [Google Scholar]
- 38.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U: Social support and oxytocin interact to suppress Cortisol and subjective responses to psychosocial stress. Biol Psychiatry 2003, 54:1389–1398. [DOI] [PubMed] [Google Scholar]
- 39.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH: Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab 2001, 86:4798–4804. [DOI] [PubMed] [Google Scholar]
- 40.McRae-Clark AL, Baker NL, Maria MM-S, Brady KT: Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology 2013, 228:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R: Oxytocin facilitates the sensation of social stress. Hum Brain Mapp 2014, 35:4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M: Intranasal oxytocin increases positive communication and reduces Cortisol levels during couple conflict. Biol Psychiatry 2009, 65:728–731. [DOI] [PubMed] [Google Scholar]
- 43.Page SR, Ang VT, Jackson R, White A, Nussey SS, Jenkins JS: The effect of oxytocin infusion on adenohypophyseal function in man. Clin Endocrinol 1990, 32:307–313. [DOI] [PubMed] [Google Scholar]
- 44.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A: Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 2005, 25:11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC: Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 2007, 62:1187–1190. [DOI] [PubMed] [Google Scholar]
- 46.Petrovic P, Kalisch R, Singer T, Dolan RJ: Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci 2008, 28:6607–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E: Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 2008, 58:639–650. [DOI] [PubMed] [Google Scholar]
- 48.Gamer M, Zurowski B, Büchel C: Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA 2010, 107:9400–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK: A heartfelt response: oxytocin effects on response to social stress in men and women. Biol Psychol 2012, 90:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardoso C, Linnen A-M, Joober R, Ellenbogen MA: Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol 2012, 20:84–91. [DOI] [PubMed] [Google Scholar]
- 51.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC: Effects of intranasal oxytocin on emotional face processing in women.Psychoneuroendocrinology 2010, 35:83–93. [DOI] [PubMed] [Google Scholar]
- 52.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR: Amygdala response to negative images in postpartum vs nulliparous women and intranasal oxytocin. Soc Cogn Affect Neurosci 2014, 9:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G: Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 2012, 37:1431–1438. [DOI] [PubMed] [Google Scholar]
- 54.Luo L, Becker B, Geng Y, Zhao Z, Gao S, Zhao W, Yao S, Zheng X, Ma X, Gao Z et al. : Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage 2017, 162:127–137. [DOI] [PubMed] [Google Scholar]
- 55. •.Bowen MT, Neumann ID: The multidimensional therapeutic potential of targeting the brain oxytocin system for the treatment of substance use disorders. Curr Top Behav Neurosci 2017. http://dx.d0i.0rg/10.1007/7854_2017_17.The authors provide an excellent overview of research relating to the interactions between oxytocin systems and the neurobiological systems associated with the pathophysiology of addiction.
- 56.Koob GF, Volkow ND: Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016, 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedersen CA: Oxytocin, tolerance, and the dark side of addiction. Int Rev Neurobiol 2017, 136:239–274. [DOI] [PubMed] [Google Scholar]
- 58.Pang RD, Zvolensky MJ, Schmidt NB, Leventhal AM: Gender differences in negative reinforcement smoking expectancies. Nicotine Tob Res 2015, 17:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker JB, McClellan ML, Reed BG: Sex differences, gender and addiction. J Neurosci Res 2017, 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wideman L, Montgomery MM, Levine BJ, Beynnon BD, Shultz SJ: Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sports Health 2013, 5:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]