Abstract
Enterohemorrhagic (EHEC) and enteropathogenic Escherichia coli (EPEC) are human intestinal pathogens of clinical importance and their mechanism of pathogenicity is widely studied. However, both EHEC and EPEC poorly infect mice, whereas they do not develop important characteristics of the disease, hindering studies about mechanisms of virulence in vivo. Citrobacter rodentium exhibits high similarity of its genes with these human pathogens, including the island of pathogenicity Locus of Enterocyte Effacement (LEE). Therefore, C. rodentium becomes an alternative in vivo model for microorganisms that harbor LEE. The QseC directly regulates LEE as well as virulence mechanisms on these pathogens. Here, we report a novel surface motility in C. rodentium QseC-mediated in this non-flagellated bacterium. Moreover, we show norepinephrine and ethanolamine act as environmental signals in this movement. Hence, this study clarifies a novel role of the sensor QseC in completely unreported motility process of C. rodentium.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00123-0) contains supplementary material, which is available to authorized users.
Keywords: Citrobacter rodentium, Chemical signaling, Pathogenesis
Introduction
Citrobacter rodentium is a naturally occurring murine pathogen transmitted via fecal-oral route used as an in vivo model to study enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli pathogenesis for sharing 67% of its genes with these human gastrointestinal pathogens, including an important island of pathogenicity termed Locus of Enterocyte Effacement (LEE Island) [3, 22]. This island contains important virulence genes of these pathogens. The activation of the LEE genes leads to the production of factors such as the type III secretion system (T3SS), Tir, and Intimin to develop the Attaching and Effacement lesion (A/E lesion). The T3SS is a needle-like structure which injects effector proteins such as the receptor Tir that binds to the Intimin present in the bacterial cell membrane. This process induces the remodeling of the actin cytoskeleton of the host cell to form the characteristic pedestal-like structure (A/E lesion) which promotes the bacterial attachment to the host epithelium, leading to destruction of the microvilli [24].
Bacteria can establish cell-cell communication through the production and diffusion of small chemical molecules. These molecules are recognized by membrane receptors that detect changes in the environment, such as the increase in colony number, and modulate responses through gene expression [7]. Furthermore, bacteria and hosts establish inter-kingdom communication wherein bacterial cells modulate signal transduction in mammalian cells and host can, through cross-signaling, modulate gene expression in bacteria [10, 27, 28]. Chemical signaling gives bacteria the ability to control their genes through receptors and to adapt to changes in the environment. Pathogens such as EHEC and EPEC have in their membrane important receptors that are able to detect these changes [25].
The membrane receptor QseC is sensor histidine kinase which form a two-component system with the respective cognate regulator QseB [11, 26, 31]. The QseC sensor senses the adrenergic hormones norepinephrine (Nor) and epinephrine of mammals and autoinducer 3 (AI-3) secreted by bacteria from the microbiota [27]. Other molecules produced by the host, such as ethanolamine (EA), contribute as a signaling molecule to promote virulence expression in EHEC [14] as well as to colonize the gastrointestinal tract of cattle due to its metabolization by EHEC, which promote a growth advantage over the microbiota since EA is naturally present in the mammalian gut [1].
Bacteria can perform movement in surfaces as a way to access nutrients through the detection of environmental signals. Swarming is described as a flagellum-dependent surface movement and has been used to analyze the role of the flagellum in the expansion and surface growth of bacterial groups [8]. The movement on the surfaces plays an important role in the colonization of flagellated bacteria [8]. Furthermore, it is known that non-flagellated bacteria can perform surface movement through different mechanisms such as twitching, sliding, and gliding [17]. In sliding movement, the expansion of the colony occurs through the production of surfactants which reduce the surface tension [8, 9]. Bacteria employ mechanisms through pili retraction, such as type IV fimbria, called Twitching motility [8]. Another condition is the bacterial gliding, which is known as a smooth movement usually along the axis of the cell [17, 18].
C. rodentium is an important alternative infection model used to understand the molecular basis and mechanisms of virulence developed by EHEC and EPEC in vivo, since these human pathogens poorly infect animals [3, 22]. Is a non-flagellar bacterium classified as a non-motile organism, therefore, no descriptions about its surface motility have been done.
Based on that, here we studied if C. rodentium uses mechanisms to move along the surface, employing swarming like-assays, under similar conditions used for tests in flagellated bacteria. The assays were performed to analyze surface motility and the role of QseC sensor kinase during this movement. This is a novel surface motility mechanism of C. rodentium and the sensor QseC modulates this non-flagellated phenotype. Moreover, Nor and EA act as environmental signals via QseC during the movement in order to better understand the role of the QseC sensor kinases during this novel surface motility of this murine pathogen and to elucidate this feature in clinically important human pathogens such as EHEC.
Materials and methods
Bacterial strains
The C. rodentium strains used in this study are described in Table 1 [6]. All the strains were grown aerobically in Luria-Bertani (LB) broth supplemented with appropriate antibiotics at 37 °C, overnight in shaker (250 rpm) under aerobic conditions.
Table 1.
Strain and plasmids used in this study
Construction of isogenic mutants
The construction of isogenic single mutant ΔqseC of C. rodentium was achieved by using λ Red mutagenesis [5]. The ΔqseC single mutant was complemented with the qseC gene cloned into the vector pBADMycHisA, with an ampicillin resistance marker to generate the strain ΔqseC/qseC+.
Surface motility assays
The swarming-like assays were performed to measure the C. rodentium surface motility. For the assay, 5 μl of strains wild type (WT), ΔqseC, and qseC/qseC+ cultured overnight was inoculated on the surface of the plate with LB medium plus semi-solid agar (0.5%) with or without addition of EA (100 μM) or Nor (1 μM). The dimethyl sulfoxide (DMSO) used as solvent for Nor was added in control group in same concentration as Nor-treated group. The motility rings were measured in samples at 12, 24, and 48 h.
Results
The absence of QseC induces changes in the surface motility in C. rodentium
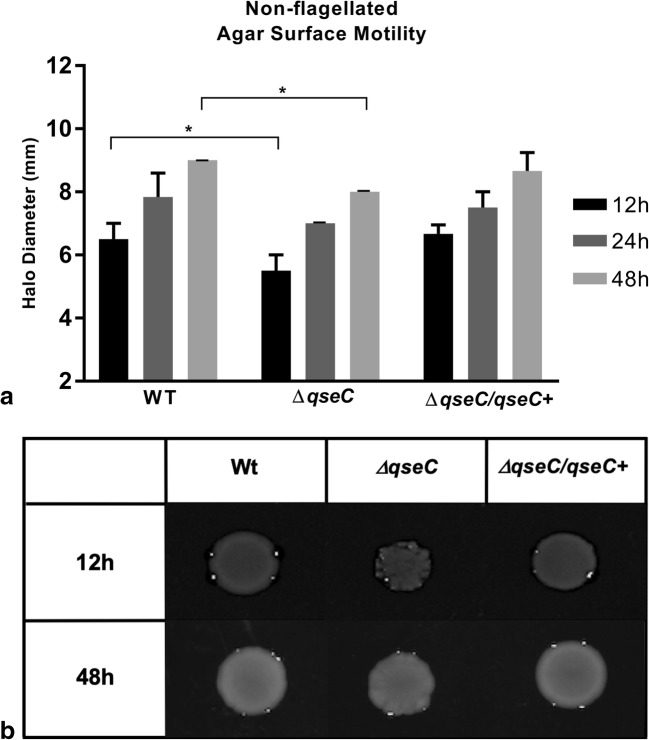
The motility swarming-like assays were performed in the same conditions used to swarming assay, in LB broth with 0.5% agar added. Herein, the main aim was to initially verify the C. rodentium ability to perform any sort of surface motility and investigate the QseC sensor role during the movement. The qseC mutant was employed and showed a significant decrease in the motility halo diameter when compared with WT levels in both 12 and 48 h period assayed (Fig. 1a). Thus, QseC complementation was also included to assure the restoration of the phenotype in comparison with WT and ΔqseC. There were no significant halo diameter differences between wild type (WT) and ΔqseC/ΔqseC+ during all time periods measured (Fig. 1a).
Fig. 1.
a Surface motility halo in C. rodentium. Halo sizes measured in semi-solid LB medium (0.5% agar) compared with wild type at 12, 24, and 48 h. Statistical significance P < 0.05 (*) (two-way ANOVA). All other not pointed had no statistic differences. b Differences in morphology after 48 h between WT, ΔqseC mutant, and its complemented strain
In addition to the motility halo size measurement, colony morphology differences were observed (Fig. 1b) and they were intensified in ΔqseC strain. The WT strain showed a uniform circular motility halo formation with regular edges at all times analyzed, in every condition assayed here. The ΔqseC strain presented visible motility halo differences with unregular edges in all conditions tested. During the first 12 h of the movement, in the absence of QseC sensor kinase, considerable differences in morphology with satellite colonies and deformed edges were present throughout the period tested. The motility halo consolidation was reached at the 48-h assay; however, the colony morphology still showed a different aspect than WT strain, which may indicate a possible LPS modification in this condition exacerbated during this motile phenotype. The genetic complementation with the qseC+ led to a similar morphology of WT relating the absence of the sensor to phenotype change observed in the ΔqseC strain.
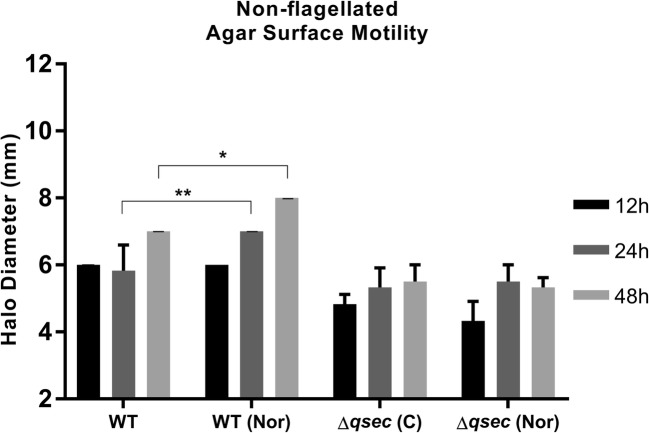
Norepinephrine acts as an adrenergic signal via QseC for this novel motility
Epinephrine and Nor adrenergic hormones induce LEE activation through QseC in C. rodentium [4, 20]; therefore, the assays were performed in presence of Nor (1 μM) to evaluate its influence in this non-flagellar motility phenotype (Fig. 2). In the ΔqseC mutant, significant changes were not observed in the motility halo at all measured times under Nor added conditions, when compared with its control group. Moreover, the observed increase in halo size was not related to Nor-induced growth, since these strains have not demonstrated advantages in Nor presence (Figure S1). The QseC-mediated motility increase in the WT strain started to show significant halo diameter when compared with its group without Nor at 24-h time point. These differences were more evident after 48 h, once the WT with Nor showed an increase of 1 mm in comparison with the control group, evidencing a clear phenotype QseC-mediated in WT strain since the mutant did not show this augment.
Fig. 2.
Surface motility by C. rodentium. Halo sizes measured in semi-solid LB medium (0.5% agar) with addition of norepinephrine (Nor) 1 μM, compared with its respective control group (C) with DMSO at 12, 24, and 48 h. Statistical significance P < 0.001 (**), P < 0.05 (*) (two-way ANOVA). All other not pointed had no statistic differences
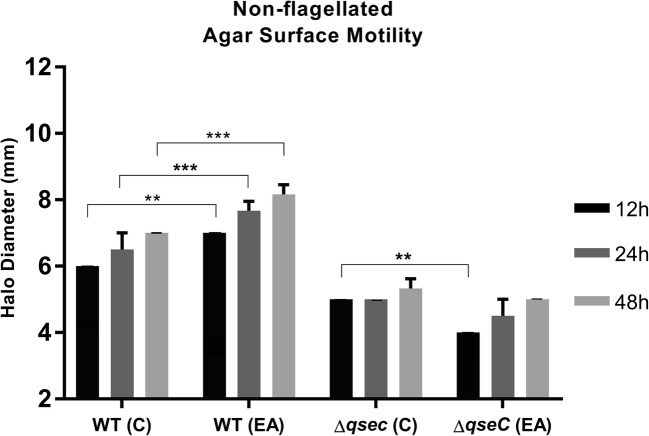
Ethanolamine contributes also as a signal to this bacterial motility
Chemical signals such as EA have been shown to affect bacterial pathogenesis [14]. The assay was performed in 100 μM of EA to evaluate how the different environment signals influence motility (Fig. 3). When comparing each strain with its respective control group (without EA) at 12 h, the WT had significant increase in the motility halo in EA. The ΔqseC strain showed lower motility halo in EA-treated group. After these 12 h, the mutant demonstrated no differences in the motility size. At 48 h, WT strain has clearly obtained advantages in EA presence, since the increase was ranged approximately 2 mm in the motility halo diameter if compared with its control group. Similar to the results observed in Nor presence, the increase in halo size was not correlated also with EA-induced growth, (Figure S2). These results indicate that EA contributes as signal to this surface motility and the absence of QseC impairs EA-mediated signaling.
Fig. 3.
Surface motility by C. rodentium. Halo sizes measured in semi-solid LB medium (0.5% agar) with addition of ethanolamine 100 μM (EA), compared with its respective control group (C) without ethanolamine at 12, 24, and 48 h. Statistical significance P < 0.001 (***), P < 0.01 (**) (two-way ANOVA). All other not pointed had no statistic differences
Discussion
Motility in bacteria is important for nutrient acquisition, immune system evasion, biofilm formation, and pathogenesis and supports bacterial survival upon environment hostile conditions [12]. The motility via rotational flagella is well studied and broadly distributed in several distinct bacteria [12, 13]. The motility associated with flagella, i.e., swimming and swarming, is directly linked to biosurfactants presence within semi-solid media and on the surfaces, respectively. Bacteria classified as non-motile or unflagellated are still able to move on surfaces through different types of mechanisms [8]. In the present study, we have verified clear evidences that C. rodentium performs a surface motility, totally independent of flagella, via QseC modulation, under Nor and EA influence during the mechanism.
In this study, the qseC mutant has presented differences in the motility halo in almost all assays performed, demonstrating the membrane sensor kinase QseC role in this mechanism. The QseBC two-component system regulates important virulence genes in EHEC and EPEC through external signals, such as AI-3 secreted by bacteria as a population signal [29]. In EHEC, a study demonstrated that qseC mutants showed less flagellin production and reduction in motility when compared with the wild type [26], evidences of QseC signaling role during bacterial flagella rotation.
Kendall and collaborators have previously shown that EA acts as a trigger molecule for cell-to-cell signaling to activate important virulence genes with increased in qseC expression in the presence of EA in EHEC. Although the EutR transcriptional factor is the key element for EA-mediated regulation, the study suggests a second sensor which responds at low EA concentrations. It was observed EA-dependent virulence gene induction in EHEC at micromolar concentrations and qseC had increase expression in eutR mutant when cells grown with 100 μM EA [14]. Here, C. rodentium WT strain had an increased in motility in the halo diameter in EA presence for all assayed hours, evidencing that the presence of EA contributes as favorable signaling during the progress of this surface motility. In contrast, the ΔqseC mutant exhibited significant disadvantage in EA presence at 12 h that suggests the QseC sensor kinase has important role sensing the ethanolamine-mediated signaling in C. rodentium, and for effective EA signaling, positive induction of the presence of the sensor is needed, since in the QseC sensor kinase absence, there was a significant decrease during motility at early stages of surface movement that clearly indicates QseC essential role during this initial stage of bacterial motility.
Our group has previously reported in vivo studies demonstrating significant increase C. rodentium colonization under Nor influence in mice infection and ΔqseC mutant attenuated in murine infection. Inasmuch, QseC adrenergic sensor is required for C. rodentium to sense neurotransmitters in vivo [20]. In this study, Nor showed modulation in the movement that seems to be even more advantageous in later time points tested for the surface movement. On the other hand, the absence of sensor QseC has shown to be detrimental in this process, which corroborates to illustrate the QseC importance to detect the Nor hormone in C. rodentium.
Important non-flagellated bacterial movements such twitching occur via retraction of fimbria type IV, particularly in aggregated cells that are highly aligned in close cell-cell contact to form a lattice-like structure, where the cells in the first movement go forward and towards other cells. They touch their own poles, then quickly fit into an aligned position again, which explains the featured spasmodic movement observed in this form of motility [8, 17]. This maneuver phenomenon is related to the way bacteria tend to translocate in low water environments and to colonize hydrated surfaces as opposed to free living in fluids [16]. It is well-known C. rodentium capacity to encode and fully assemble important fimbriae for its virulence such as gut colonization fimbria (Gfc) [2] and colonization factor Citrobacter (cfc) type IV fimbrial locus [21]; however, these studies did not demonstrate the participation of these fimbria on surface motility.
Previous studies have shown that the QseC sensor has a direct role in the regulation of fimbriae. The QseBC system regulates hemagglutination controlling the expression of fimbrial genes in Edwardsiella tarda [30]. The QseC presence increases the expression of fimbria type I leading to a more virulent phenotype in uropathogenic Escherichia coli [15].
Fimbriae are important in several processes, such as adhesion [23], survival, and phagocytosis in macrophages [19], as well as superficial movements like twitching [8]. Therefore, it is well known that bacteria may perform a sort of motility through fimbriae expression, based on the authors, we can speculate the role of these fimbriae of type IV in many aspects of C. rodentium virulence [3, 21]. Here, we hypothesize that these fimbriae may assist bacteria in order to position themselves in a lined up form to perform this sort of twitching motility and move along on the agar surface [8]. Speculative and more studies are still necessary to clarify it; however, the data presented here support this model. Thus, the absence or reduction of the fimbriae via QseC-mediated signaling in C. rodentium could lead bacteria to grow in different directions, which would explain the differentiated morphology presented by the strain ΔqseC observed in this study. In addition, chemical signaling such as Nor and EA may be important to regulate C. rodentium fimbrial assembly through QseC, and its lack led to decrease these proteic appendages production, once the pathogen does not have a proper assembled flagellum, becoming an interesting structure to be further investigate in future studies.
Non-flagellated bacteria are reported to demonstrate different types of surface movement; here, we describe for the first time a surface motility by C. rodentium. However, more studies are needed to better detail and understand this surface movement and to elucidate how that is performed and which are the triggers to the mechanism, as well as the full role of the QseC sensor kinase in this process. These data presented here converge to the novel important feature of the QseC sensor acting in a distinct flagella-independent surface motility mechanism in this important A/E lesion model pathogen.
Herein, we have substantiated C. rodentium non-flagellar surface motility mediated via QseC sensor. The C. rodentium chemical signaling also employs EA and Nor to redirect this novel surface motility response. Additional investigation is necessary to fully clarify the structures involved during surface translocation and to understand the signaling through sensor QseC that modulates this movement in this murine pathogen.
Electronic supplementary material
(PDF 96 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bertin Y, Girardeau JP, Chaucheyras-Durand F, Lyan B, Pujos-Guillot E, Harel J, Martin C. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ Microbiol. 2011;13(2):365–377. doi: 10.1111/j.1462-2920.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 2.Caballero-Flores GG, Croxen MA, Martínez-Santos VI, Finlay BB, Puente JL. Identification and regulation of a novel Citrobacter rodentium gut colonization fimbria (Gcf) J Bacteriol. 2015;197(8):1478–1491. doi: 10.1128/JB.02486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins JW, Keeney KM, Crepin VF, Rathinam VAK, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12(9):612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 4.Curtis MM, Russell R, Moreira CG, Adebesin AM, Wang C, Williams NS, Taussig R, Stewart D, Zimmern P, Lu B, Prasad RN, Zhu C, Rasko DA, Huntley JF, Falck JR, Sperandio V. QseC inhibitors as an antivirulence approach for Gram-negative pathogens. MBio. 2014;5(6):e02165. doi: 10.1128/mBio.02165-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, Ashman K, Lee S, Goode D, Pawson T, Finlay BB. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A. 2004;101(10):3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 8.Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 9.Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36(4):478–503. doi: 10.1128/MMBR.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6(2):111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. The QseC adrenergic signaling cascade in Enterohemorrhagic E. coli (EHEC) PLoS Pathog. 2009;5(8):e1000553. doi: 10.1371/journal.ppat.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam ST, Mignot T. The mysterious nature of bacterial surface (gliding) motility: a focal adhesion-based mechanism in Myxococcus xanthus. Semin Cell Dev Biol. 2015;46:143–154. doi: 10.1016/j.semcdb.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int J Med Microbiol. 2002;291(8):605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 14.Kendall MM, Gruber CC, Parker CT, Sperandio V (2012) Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. MBio 3(3) [DOI] [PMC free article] [PubMed]
- 15.Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ. QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol. 2009;73(6):1020–1031. doi: 10.1111/j.1365-2958.2009.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 17.Mattingly AE, et al (2018) Assessing travel conditions: environmental and host influences on bacterial surface motility [DOI] [PMC free article] [PubMed]
- 18.McBride MJ. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu Rev Microbiol. 2001;55:49–75. doi: 10.1146/annurev.micro.55.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Mokrievich AN, Kondakova AN, Valade E, Platonov ME, Vakhrameeva GM, Shaikhutdinova RZ, Mironova RI, Blaha D, Bakhteeva IV, Titareva GM, Kravchenko TB, Kombarova TI, Vidal D, Pavlov VM, Lindner B, Dyatlov IA, Knirel YA. Biological properties and structure of the lipopolysaccharide of a vaccine strain of Francisella tularensis generated by inactivation of a quorum sensing system gene qseC. Biochemistry (Mosc) 2010;75(4):443–451. doi: 10.1134/S0006297910040073. [DOI] [PubMed] [Google Scholar]
- 20.Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, Curtis MM, Winter SE, Weinshenker D, Sperandio V (2016) Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. MBio 7(3) [DOI] [PMC free article] [PubMed]
- 21.Mundy R, Pickard D, Wilson RK, Simmons CP, Dougan G, Frankel G. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol Microbiol. 2003;48(3):795–809. doi: 10.1046/j.1365-2958.2003.03470.x. [DOI] [PubMed] [Google Scholar]
- 22.Petty NK, Bulgin R, Crepin VF, Cerdeno-Tarraga AM, Schroeder GN, Quail MA, Lennard N, Corton C, Barron A, Clark L, Toribio AL, Parkhill J, Dougan G, Frankel G, Thomson NR. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J Bacteriol. 2010;192(2):525–538. doi: 10.1128/JB.01144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66(4):613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos AS, Finlay BB. Bringing down the host: enteropathogenic and enterohaemorrhagic Escherichia coli effector-mediated subversion of host innate immune pathways. Cell Microbiol. 2015;17(3):318–332. doi: 10.1111/cmi.12412. [DOI] [PubMed] [Google Scholar]
- 25.Sircili MP, et al (2004) Modulation of Enteropathogenic Escherichia coli virulence by quorum sensing [DOI] [PMC free article] [PubMed]
- 26.Sperandio V, Torres AG, Kaper JB. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol. 2002;43(3):809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 27.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A. 2003;100(15):8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GS, Bycroft BW, Pritchard DI. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66(1):36–42. doi: 10.1128/IAI.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters M, Sperandio V. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun. 2006;74(10):5445–5455. doi: 10.1128/IAI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Wang Q, Yang M, Xiao J, Liu Q, Wu H, Zhang Y. QseBC controls flagellar motility, fimbrial hemagglutination and intracellular virulence in fish pathogen Edwardsiella tarda. Fish Shellfish Immunol. 2011;30(3):944–953. doi: 10.1016/j.fsi.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K et al (2005) Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. 280:1448–56 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 96 kb)