Abstract
Nine bacterial strains were previously isolated in association with pinewood nematode (PWN) from wilted pine trees. They proved to be nematicidal in vitro, and one of the highest activities, with potential to control PWN, was showed by Serratia sp. M24T3. Its ecology in association with plants remains unclear. This study aimed to evaluate the ability of strain M24T3 to colonize the internal tissues of the model plant Arabidopsis thaliana using confocal microscopy. Plant growth–promoting bacteria (PGPB) functional traits were tested and retrieved in the genome of strain M24T3. In greenhouse conditions, the bacterial effects of all nematicidal strains were also evaluated, co-inoculated or not with Bradyrhizobium sp. 3267, on Vigna unguiculata fitness. Inoculation of strain M24T3 increased the number of A. thaliana lateral roots and the confocal analysis confirmed effective bacterial colonization in the plant. Strain M24T3 showed cellulolytic activity, siderophores production, phosphate and zinc solubilization ability, and indole acetic acid production independent of supplementation with l-tryptophan. In the genome of strain M24T3, genes involved in the interaction with the plants such as 1-aminocyclopropane-1-carboxylate (ACC) deaminase, chitinolytic activity, and quorum sensing were also detected. The genomic organization showed ACC deaminase and its leucine-responsive transcriptional regulator, and the activity of ACC deaminase was 594.6 nmol α-ketobutyrate μg protein−1 μl−1. Strain M24T3 in co-inoculation with Bradyrhizobium sp. 3267 promoted the growth of V. unguiculata. In conclusion, this study demonstrated the ability of strain M24T3 to colonize other plants besides pine trees as an endophyte and displays PGPB traits that probably increased plant tolerance to stresses.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00098-y) contains supplementary material, which is available to authorized users.
Keywords: Serratia, ACC deaminase, Genome, Endophytic colonization, PGPB, Nematode, Arabidopsis thaliana, Vigna unguiculata
Introduction
Agrochemicals are pesticides, when used to protect plants against invasive pests, or fertilizers, when used for plant improvement in yield. The massive use of chemicals has impacted farmers’ economy and has also decreased environmental quality. Besides chemical compounds, the use of plant growth–promoting bacteria (PGPB) has been suggested as an alternative to classical strategies [8].
PGPB constitute a diverse group of microorganisms found in soil, rhizosphere, or inside plant tissues (i.e., endophytes). PGPB provide direct beneficial functions for the host plant such as those involved in (i) the production of phytohormones that support plant growth—indole acetic acid (IAA), ethylene, gibberellins, cytokinins, or abscisic acid; (ii) the production of enzymes, including bacterial 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which has been reported to reduce plant ethylene levels, a substance that proved to function as an attractant for nematodes [53]; (iii) phosphate and zinc solubilization, iron chelation by siderophores, nitrogen fixation, and nitric oxide production; and (iv) plant protection against pathogenic organisms by producing antagonistic compounds or through induced systemic resistance (ISR) as, for example, biosynthesizing the quorum sensing-molecule acyl-homoserine lactone [14, 15, 19, 39]. On the other hand, PGPB that exert their function as endophytes need to cope with the inner plant environment. Reactive oxygen species (ROS) and also nitric oxide (NO) are known to be part of the resistance system of plants [3]. Therefore, as a prerequisite for plant colonization, microbes need to have antioxidant enzymes, for example, superoxide dismutase (SOD) and catalase [1]. A variety of bacterial species of the genera Azoarcus, Azospirillum, Burkholderia, Gluconacetobacter, Herbaspirillum [39], Bradyrhizobium [40], Bacillus [12], or Serratia [27] have been recognized to play a role as PGPB. Bradyrhizobium strains were able to promote nodulation in Vigna unguiculata (cowpea) and showed the presence of genes involved in nitrogen fixation [40]. Cowpea is an important food legume and part of traditional cropping systems in the semi-arid regions of the tropics but is largely consumed around the world [23]. Especially, strains from genera Serratia, Pseudomonas, and Bacillus have been used as biological controllers of plant pathogens by triggering induced systemic resistance (ISR) in the host plant [52] and/or synthesizing antibiotic volatile organic compounds (VOCs) in plants [9].
Serratia sp. M24T3, previously isolated in association with pinewood nematode Bursaphelenchus xylophilus (PWN), the causal agent of pine wilt disease [34], was part of a group of strains with in vitro nematicidal activity [28]. The strain was identified as belonging to the species Serratia plymuthica based on the similarity of the 16S rRNA gene [35]. Other strains belonging to the genus Serratia were identified as tolerant to oxidative stress [51] and have capabilities as opportunistic endophytes [26, 50]. However, the role of bacterial strains that are isolated with the nematodes present inside pine trees with pine wilt disease remains unclear. A positive role (nematotoxic activity against B. xylophilus) or a negative role (mutualistic relation with the nematode) has been suggested [28, 34, 35, 37]. Some strains of the genera Curtobacterium, Pantoea, Rhanella, Pseudomonas, and Serratia proved to be nematicidal in vitro [28]. Serratia sp. M24T3 showed 100% nematicidal activity towards PWN [28, 35], revealing its potential as antagonist against phytopathogenic nematodes. Since nematodes enter the tree through the aerial parts [11], an efficient function of these bacterial strains as antagonists can only be performed if they have the potential to express enzymes which lower the impact of a range of different stresses that affect plant growth and development. In addition, as an efficient biocontrol strain, it should be able to colonize plant tissues endophytically. In fact, the 16S rRNA gene sequence of this species was also found as part of the natural endophytic community of Pinus pinaster [33, 36].
The main goal of this study was to evaluate the ability of the Serratia sp. M24T3 strain to colonize the internal tissues of the model plant Arabidopsis thaliana and evaluate its role on plant fitness. In order to support these characteristics, in silico analysis was performed on the published genome of strain M24T3 [35] to detect genes encoding for proteins important for plant colonization processes, as well as for plant growth promotion.
Material and methods
Bacterial strain manipulation and growth conditions
Strains tested in this study were previously isolated from PWN [34], showed 100% nematicidal activity towards PWN [28], and are presented in Table 1. For strain M24T3 harboring plasmid pHRGFPGUS (gfp::gusA, AmpRKanR) [38], kanamycin (50 μg ml−1) was added to the growth medium.
Table 1.
Differential characteristics for plant growth promotion of bacterial strains with nematicidal activity
| Treatment | Strain | 16S rRNA gene Accession number (GenBank/EMBL) |
Siderophores | Proteases | Phosphate solubilization | Zinc solubilization | IAA μg(IAA).(μg(protein)/ml)−1 | Nematicidal activity (100%)a |
|---|---|---|---|---|---|---|---|---|
| 1 | Serratia sp. M24T3 | HQ538811 | + | – | + | + | 0.036 | + |
| 2 | Serratia sp. M24tronco5 | HQ538813 | + | – | + | – | 0.030 | + |
| 3 | Serratia sp. A88copa7 | HQ538800 | + | + | + | + | 0 | + |
| 4 | Serratia sp. A88copa13 | HQ538801 | + | + | + | + | 0 | + |
| 5 | Pantoea sp. A37tronco3 | HQ538809 | + | – | + | – | 0 | + |
| 6 | Rhanella sp. M72troncoD | HQ538817 | + | – | + | – | 0 | + |
| 7 | Pseudomonas sp. M47T1 | HQ538790 | + | – | – | – | 0 | + |
| 8 | Pseudomonas sp. M67copa9 | HQ538784 | + | – | + | – | 0 | + |
| 9 | Curtobacterium sp. M24copaD2 | HQ538819 | + | – | – | – | 0 | + |
+, positive; −, negative. Families of Enterobacteriaceae (1–6), Pseudomonadaceae (7–8), and Microbacteriaceae (9)
aData obtained from Paiva et al. (2013)
Electroporation of Serratia sp. M24T3 with plasmid pHRGFPGUS was performed using a Gene Pulser Xcell™ (Bio-Rad Laboratories, Richmond, CA), according to the manufacturer’s instructions for Escherichia coli (2.5 kV, 25 μF, 200 Ω, 5.2 ms). Fresh culture cells from strain M24T3 (OD600 = 0.6 ≈ 106 cells ml−1), grown in LB medium at 30 °C, were used to prepare electrocompetent cells [4]. All centrifugation steps were performed at 7000g for 25 min at 4 °C. Two microliters of plasmid pHRGFPGUS was used for transformation. The cells were harvested and then resuspended in 1 ml of Super Optimal broth with Catabolite repression (SOC) medium and incubated for 1 h at 30 °C. The cell suspensions were plated onto the LB agar medium with kanamycin.
Plant growth conditions for A. thaliana and bacterial inoculation
A. thaliana seeds, of the genotype Columbia or its derivative DR5::GUS, were disinfected in sterile microtubes by adding 500 μl of a mixture of ethanol (70%) and Tween (0.05%) and incubating for 5 min. The supernatant was discarded; 500 μl of 100% ethanol was added and incubated by 5 min. The ethanol was discarded and evaporated. The disinfected seeds were seeded on half strength MS medium, supplemented with 1 ml l−1 of vitamins [24], 1.5% glucose as carbon source and 0.8% agar. The plates were incubated at 4 °C for 3 days to enhance germination. No contamination was observed in the whole incubation period at 24 °C.
The bacterial inoculation was performed with exponential growth phase culture of OD600 = 0.6. The bacterial suspension for plant inoculation consisted in 1 ml of bacterial growth washed with MS medium half strength with vitamins and glucose but without agar (see above), pelleted (1 min, 16,000g) and resuspended in 1 ml of half strength MS medium. In order to test promotion of seed germination, five seeds of A. thaliana were inoculated after disinfection, together with 500 μl suspension of M24T3. Controls were performed using non-inoculated medium and E. coli S17 suspension. All the tests were performed in triplicate.
For root elongation assays, seeds were spread in a single line on MS agar plates (see above). Fifty-microliter suspensions of strain M24T3 transformed with plasmid pHRGFPGUS and wild type or bacterial supernatants were added separately in the center of different plates, on a sterile filter paper (1-cm diameter) at a 2-cm distance from the seed line. Plates were incubated vertically and plants were grown at 24 °C under a 16/8-h light-dark cycle.
Colonization assays were performed after germination of plantlets at 24 °C for 7 days. Plantlets were transferred under sterile conditions, after germination on MS agar plates, into wells of 12-well plates. Each well contained 500 μl of bacterial suspensions (M24T3, transformed with plasmid pHRGFPGUS, wild type, or E. coli S17 transformed with plasmid pHRGFPGUS) and 5 plantlets. MS medium without bacteria was used as a negative control. Plantlets were grown at 24 °C under a 16/8-h light-dark cycle. All the tests were performed in triplicate. After 7 days of inoculation, A. thaliana plantlets were collected for microscopy analysis.
Confocal laser scanning microscopy
A. thaliana plantlets non-inoculated and inoculated with bacteria were removed from the 12-multiwell plate, after 7-day inoculation in liquid MS medium, washed with sterile water and mounted on bridge slide with 10% (v/v) glycerol. In order to observe colonization of A. thaliana by bacteria, roots were observed directly in a LEICA TCS-SP5 AOBS confocal system with 40× lens and Leica Application Suite—Advanced Fluorescence Lite software (release 2.4.1). GFP-tagged bacterial cells were excited with the 488-nm Argon laser line (25% power). Chlorophylls from A. thaliana cells were excited with the 405-nm Diode laser line (20% power). All images were collected in a z-series with 15 optical sections, step size 2 μm and 52 μm of average thickness.
Evaluation of the beneficial effects on Vigna unguiculata
Seeds of V. unguiculata were sterilized by ethanol (70%) for 1 min, H2O2 for 5 min, and followed by eight washes with sterile water. The disinfected seeds were planted in sterile sand with vermiculite (2:1 ratio) in Leonard jars [49]. One milliliter containing approximately 106 of the bacterial cells from each strain present in Table 2 was added to the seeds at the time of planting. The co-inoculation assay consisted of 1 ml of each bacterial strain (106 of the bacterial cells). A control with nitrogen supplementation was established as well as a control without mineral nitrogen supplementation, both controls without bacterial inoculations. The experiment was conducted under greenhouse conditions for 35 days. Plant size, dry weight of aerial part and roots, ethylene levels for measuring nitrogenase activity, and the number of nodules were evaluated in triplicate for all samples.
Table 2.
Principal effects on V. unguiculata promoted by bacterial inoculums
| Treatment | Size of shoot (cm plant−1) | Dry root weight (g plant−1) | Dry shoot weight (g plant−1) | Ethylene (ARA) (nmol plant−1) | Number of nodules | Dry nodules weight (g plant−1) |
|---|---|---|---|---|---|---|
| 1 | 18.7ª | 0.2a | 0.5ª | 0.2ª | 2.7ª | 0NC |
| 2 | 25.7afg | 0.5a | 0.9ª | 2ª | 21ª | 0.3ª |
| 3 | 22.0a | 0.3a | 0.5ª | 0.1ª | 3.7ª | 0NC |
| 4 | 21.3ª | 0.3a | 0.3ª | 0NC | 2.3ª | 0NC |
| 5 | 21.8ª | 0.4a | 0.5ª | 0.8ª | 10ª | 0.01b |
| 6 | 21.8a | 0.5a | 0.9ª | 3.2ª | 25.3ª | 0.1ac |
| 7 | 26.5afg | 0.5ac | 1.1ª | 1.7ª | 119.7acde | 0.2ac |
| 8 | 22.0a | 0.3a | 0.4ª | 0.5ª | 12ª | 0.1ª |
| 9 | 25.2af | 0.3a | 1.0ª | 3.8ª | 35.0ac | 0.2ac |
| A | 25.3af | 0.5ac | 1.4ac | 4.8ª | 40.7ac | 0.2acd |
| Br | 41.5bcdf | 0.9ac | 3.4bcd | 4.3ª | 221.3bc | 0.5acd |
| Br+1 | 44.3bcdg | 1.3bc | 4.2bcd | 5.3ª | 342.3b | 0.6acd |
| Br+7 | 51.4bcdh | 0.9ac | 4.0bcd | 6.8ª | 248bd | 0.6acd |
| Br+1+7 | 36.5afgh | 0.5ac | 2.7acd | 3.8ª | 234.7be | 0.4acd |
| N | 37.0afgh | 3.9b | 6.1bd | 0NC | 0NC | 0NC |
| C | 19.3a | 0.5a | 0.6a | 0NC | 1.0ª | 0NC |
Treatment numbers 1 to 9 correspond to inoculums performed with bacterial strains included in Table 1. Values are means of three replicates. Values followed by the presence of the same letter means no statistical differences between the treatments (p > 0.05). NC, not considered for statistical analysis. A, co-inoculum containing all the first-nine strains in the table. Br, Bradyrhizobium 3267. Br+1, Br+7, Br+1+7, co-inoculum of Br with treatment 1, 7, or 1 and 7 (Table 1), respectively. N, nitrogen supplied control; C, absolute control
Assay for the production of IAA, siderophores, and proteases
The ability of the wild type strain M24T3 and transformed strain to produce IAA was measured based on the colorimetric method [16, 29] with modifications. The strains were grown in LB broth, either in the absence or presence of 500 μg ml−1l-tryptophan at 30 °C up to a final OD600 = 0.6. One milliliter of each culture was centrifuged, and 100 μl supernatant was mixed with 150 μl Salkowski’s reagent [17] and incubated for 30 min at room temperature, and absorbance was measured at 540 nm. A standard plot was performed from 5 to 50 μg ml−1 pure IAA (Sigma) in order to calculate the concentration of each sample. Assays were performed in triplicate. Protein quantification was performed in order to normalize the concentration of the production of IAA. The cell pellet was resuspended in 1 ml 0.8% NaCl, 100 μl was removed, and 100 μl 1 M NaOH was added, mixed, and incubated for 5 min at 100 °C. Twenty microliters was added to 180 μl of Bradford reagent (Bio-Rad) and incubated 10 min at room temperature in the dark, and absorbance was measured at 595 nm. Assays were performed in triplicate.
The capability to produce siderophores and proteases was previously evaluated [34] and revisited in this study. Briefly, the bacterial production of siderophores was evaluated when cultivated on CAS agar plates at 25 °C for 48 h [43]. Strains developing an orange halo were considered as positive. Skim Milk Agar (R2A:skim milk, 1:1, w/w) was used to detect proteolytic activity. Strains showing a transparent halo around the zone of growth were considered positive.
Inorganic phosphate and zinc solubilization
The ability for inorganic phosphate and zinc solubilization of strain was determined on Pikovskaya’s agar plates supplemented with 0.5% Ca3(PO4)2 and 0.12% ZnO as inorganic P and Zn sources, respectively [31]. The plates were incubated at 30 °C up to 7 days, and the presence of transparent halos around the zone of growth was considered positive for both tests.
Cellulolytic, chitinolytic, catalase, and ACC deaminase activities
Detection of cellulase activity for strain M24T3 was performed on R2A agar plates containing 1% (w/v) carboxymethyl cellulose (CMC), and an evaluation test was performed according to [6]. The ability to hydrolyze chitin of strain M24T3 at a concentration of 1.0% (w/v) on R2A agar after incubation at 30 °C up to 5 days was determined according to [47]. The catalase of strain M24T3 was determined after 24 h of incubation on R2A agar, as previously described [45]. Other enzyme activities of strain M24T3 were determined using the API ZYM test strips (bioMérieux) at 37 °C according to the manufacturer’s instructions.
ACC deaminase activity was determined on agar plates supplemented with ACC as a sole nitrogen source [30]. The quantification of the activity of the ACC deaminase was performed according to Penrose and Glick [34] with modification of the liquid media to M9.
Genomic analysis for plant growth–promoting properties
The genome of Serratia sp. M24T3 was previously published [35] and it is available in DDBJ/EMBL/GenBank under accession no. AJHJ00000000. Some genes encoding for proteins that are recognized as important for plant colonization processes as well as for plant growth promotion were identified in the genome using the RAST server tools [5] as well as BLAST [2]. These included ACC deaminase, cellulase, pectinase, SOD, catalase, chitinase, nod genes, nitrogen fixation genes, and genes involved in ammonia, IAA, acetoin, 2,3-butanediol biosynthesis, and quorum sensing.
Phylogenetic analysis of ACC deaminase sequences
The sequence of ACC deaminase from the genome of Serratia sp. M24T3 was compared with sequences available in the EMBL/GenBank database using BLAST network services in order to reveal its phylogenetic relationship with the other ACC from the genus Serratia [2]. Sequences were aligned with MEGA 7 software [22] and were verified by the presence of the amino acids Glu295 and Leu322 at the active site confirming them as ACC deaminase [48]. Phylogenetic dendrograms were constructed by maximum likelihood and neighbor-joining methods and Poisson correction as substitution model included in MEGA 7 software. Bootstrap analysis with 1000 replicates was used to evaluate the robustness of the phylogeny.
Statistical analysis
In the plant growth test, the differences between test treatments for assays with A. thaliana (control vs. Serratia sp. M24T3 wild type vs. transformed) and for assays with V. unguiculata (bacterial strains separately vs. co-inoculation) were evaluated through one-way analysis of variance (one-way ANOVA), followed by Tukey’s pairwise. Post hoc tests were run to confirm significant differences occurring between treatments. The characteristics considered for assays with A. thaliana were (1) length of plantlets (from seed to the first shoot), (2) length of primary roots, and (3) number of lateral roots. The characteristics considered for assays with V. unguiculata were (1) size of shoot, (2) dry root weight, (3) dry shoot weight, (4) quantity of ethylene in nmol plant−1, (5) number of nodules, and (6) dry nodules’ weight. The statistical analysis was performed with PAST 2.08 software [18]. The level of statistical significance for all analysis was α = 0.05.
Results
Effects of strain M24T3 on plant development and confocal analysis
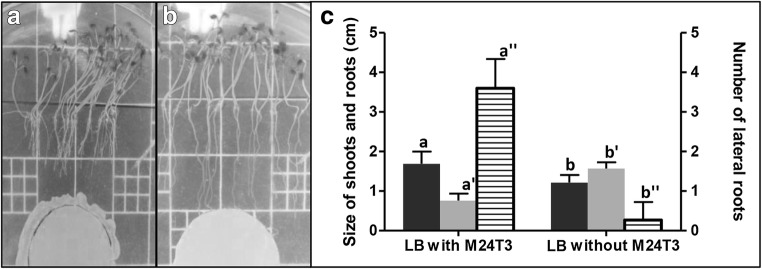
The presence of a cell suspension of the strain M24T3 partially affected the germination of A. thaliana. When added to germinated-plantlet strain, M24T3 significantly promoted (one-way ANOVA F(1,28) = 25.46, p < 0.001) the length of plantlets but the primary root elongation was significantly inhibited (one-way ANOVA F(1,28) = 172.4, p < 0.001) (Fig. 1). Moreover, the number of lateral roots was significantly increased (one-way ANOVA F(1,28) = 221.5, p < 0.001) (Fig. 1). The roots obtained from of A. thaliana were evaluated under a microscope to determine the position and number of lateral roots at top, intermediary location, and at the bottom level. Roots from the uninoculated plants did not show lateral roots. Roots from plantlets grown in the presence of strain M24T3 wild type or gfp/gusA-tagged M24T3 and supernatant from the growth of strain gfp/gusA-tagged M24T3 showed lateral root formation (Fig. 1).
Fig. 1.
Alteration of root elongation in Arabidopsis thaliana seedlings by Serratia M24T3. Seeds were germinated in a line on half strength MS agar and 50 μl of bacterial suspensions added on sterile paper. a Primary root elongation was inhibited by suspensions of the strain M24T3 wild type. b The root elongation in control with LB broth without strain M24T3 was not inhibited. c The length of shoots (black bar) and roots (gray bar) and the number of lateral roots (stripes bar) were compared between plants with or without M24T3. Bars within the different symbols (a,b; a′,b′; a″,b″) mean statistically different between each other (p < 0.05)
The effective endophytic colonization of gfp/gusA-tagged Serratia sp. M24T3 in A. thaliana intercellular spaces (Fig. 2a), in the cortex region as well as inner zones (Fig. 2b), was confirmed using the confocal laser scanning microscopy (CLSM). The localization of the M24T3 (gfp/gusA) cells was also supported by the fact that the chlorophylls were located in the same optical section. The gfp/gusA-tagged E. coli negative control showed no endophytic colonization (Fig. 2c).
Fig. 2.
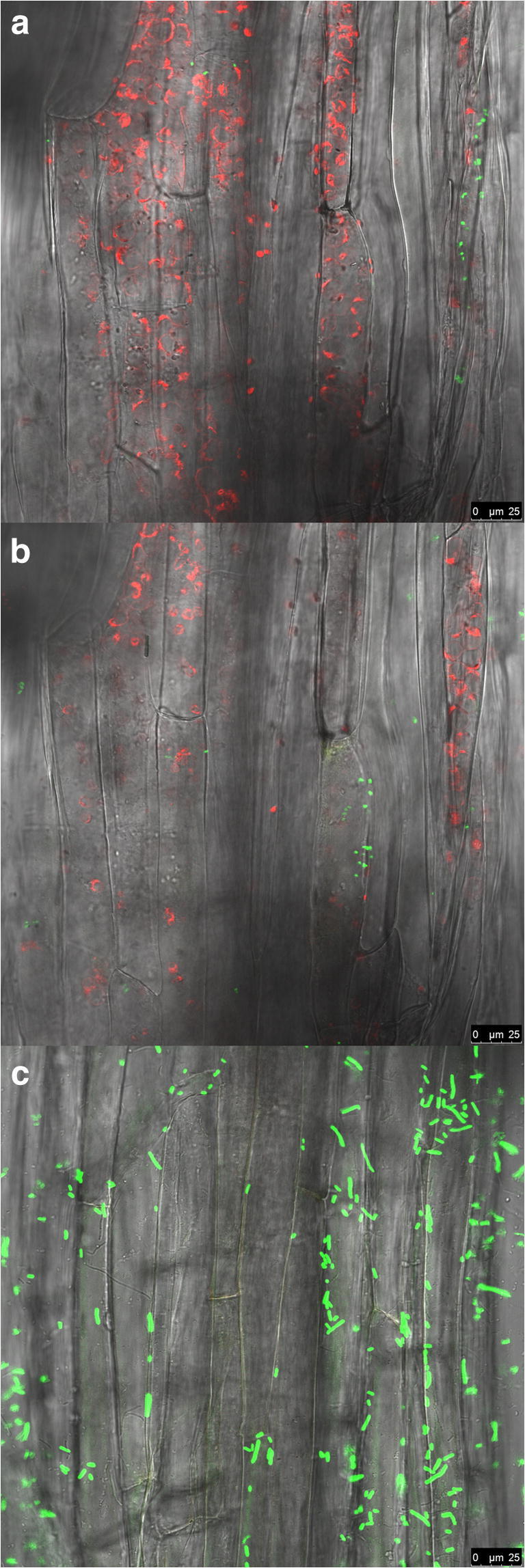
Confocal laser scanning microscopy micrographs of gfp/gusA-tagged Serratia sp. M24T3 cells colonizing A. thaliana roots. Longitudinal section of A. thaliana root inoculated with strain M24T3 and grown for 7 days in a 12 multiwell plate. The images a–b show different optical sections through one sample after M24T3 (green) colonizing a inner tissues, b intercellular spaces as well as in the cortex region. Chloroplasts are visible in red fluorescence. c Negative control with gfp/gusA-tagged E. coli (green) outside the tissue (top layer) (bar 25 μm)
Growth promotion effects in V. unguiculata by all nine strains were registered after 35 days as summarized in Table 2. Serratia M24T3 co-inoculated with Bradyrhizobium sp. 3267 showed the best results for all the characteristics, only surpassed by the co-inoculation of Bradyrhizobium sp. 3267-Pseudomonas sp. M47T1 with respect to the size of shoot and ethylene production, but no statistical differences were detected between these two co-inoculums.
In vitro assessment of PGPB properties
The strain Serratia sp. M24T3, that was selected based on its biochemical properties and on the availability of its genome sequence, showed positive results for inorganic phosphate and zinc solubilization, cellulolytic activity, siderophores, but did not exhibit proteolytic activity. It also showed chitinolytic activity, as per the chitin degradation test as well as catalase.
The production of IAA was similar in both Serratia sp. M24T3 wild type and transformed strain with plasmid pHRGFPGUS. The strain M24T3 produced IAA identically when grown in LB and LB supplemented with l-tryptophan (0.035 μg(IAA).(μg(protein)/ml)−1 and 0.036 μg(IAA).(μg(protein)/ml)−1, respectively).
The strain M24T3 was able to grow on agar plates supplemented with ACC as the only nitrogen source. Therefore, the strain was evaluated in terms of the activity of ACC deaminase in M9 liquid media. The activity of ACC deaminase was 594.6 nmol α-ketobutyrate μg protein−1 μl−1.
Presence of conserved PGPB genes in strain M24T3
In the genome of Serratia M24T3, genes likely encoding for ACC deaminase (WP_009638971) and its AsnC family leucine-responsive transcriptional regulator (WP_009638972); cellulase—β-glucosidase (WP_009635056; WP_009636821) and periplasmic β-glucosidase (WP_009638496); catalase (WP_009638086) and catalase/peroxidase (WP_009636268); pectinase—pectinesterase B (WP_009638872); superoxide dismutase (WP_009635546); chitinase (WP_009638226), polysaccharide deacetylase (nodB) (WP_009639040, WP_009638391, WP_009639041), and several genes involved in IAA, acetoin, and 2,3-butanodiol biosynthesis were identified.
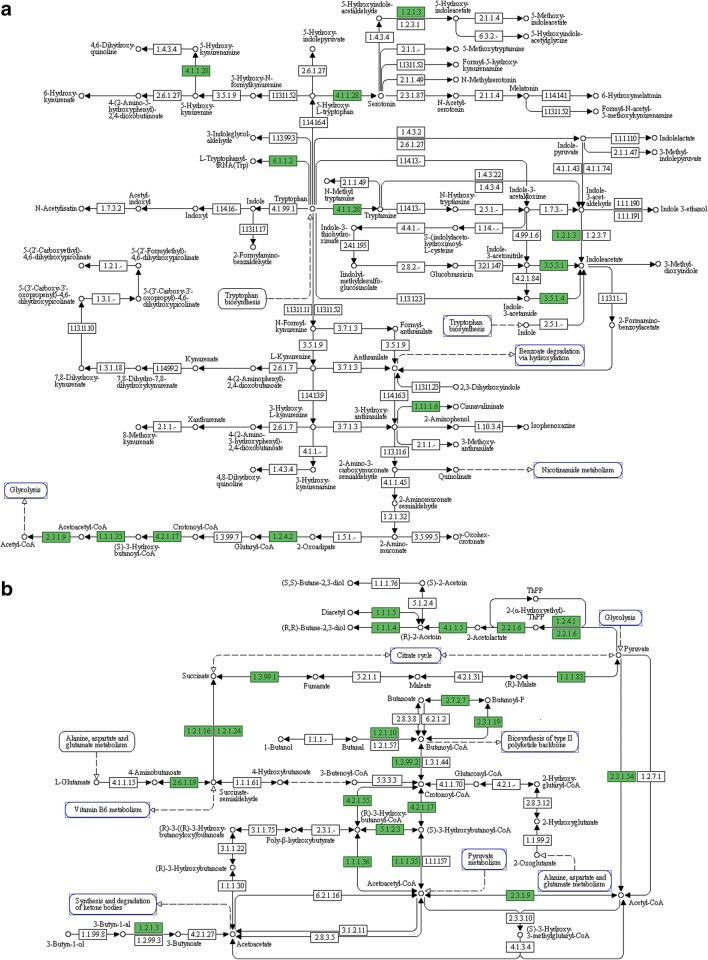
Ten genes involved in IAA biosynthesis were identified in the genome of M24T3, indicating the possibility of three IAA biosynthesis pathways. In the indole-3-acetamide (IAM) pathway, tryptophan is converted to tryptamine by aromatic-l-amino-acid decarboxylase (WP_009635138), and indole-3-acetamide is converted to IAA by aliphatic amidase AmiE (WP_009634947, WP_009639043). In the indole-3-acetonitrile (IAN) pathway, IAN is converted directly to IAA by nitrilase (WP_009634944). Indole-3-acetoaldehyde is converted to IAA by aldehyde dehydrogenase (WP_009635369, WP_009635627, WP_009636235, WP_009637232, WP_009639021, and WP_009639329) (Fig. 3a).
Fig. 3.
KEGG pathways of tryptophan metabolism (a) and butanoate metabolism (b) of Serratia sp. M24T3. KEGG pathway diagrams showing the genes of strain M24T3 (in green) encoding for enzymes involved in these two metabolisms
For butanoate metabolism, eight genes were found in Serratia sp. M24T3 and might be involved in the production of acetoin and 2,3-butanediol. Pyruvate is converted to 2-(α-hydroxyethyl)-ThPP by pyruvate dehydrogenase (WP_009634667); ThPP (thiamine pyrophosphate) is converted to 2-(α-hydroxyethyl)-ThPP followed by conversion to 2-acetolactate by acetolactate synthase (WP_009635236; small subunit—WP_009634704 and large subunit—WP_009634705; small subunit—WP_009637883 and large subunit—WP_009637884). Moreover, acetoin is produced by two pathways, one from the conversion of 2-acetolactate by alpha-acetolactate decarboxylase (WP_009635235), and another from the conversion of diacetyl by acetoin reductase/2,3-butanediol dehydrogenase (WP_009635231). Acetoin is converted to 2,3-butanediol by acetoin reductase/2,3-butanediol dehydrogenase (Fig. 3b).
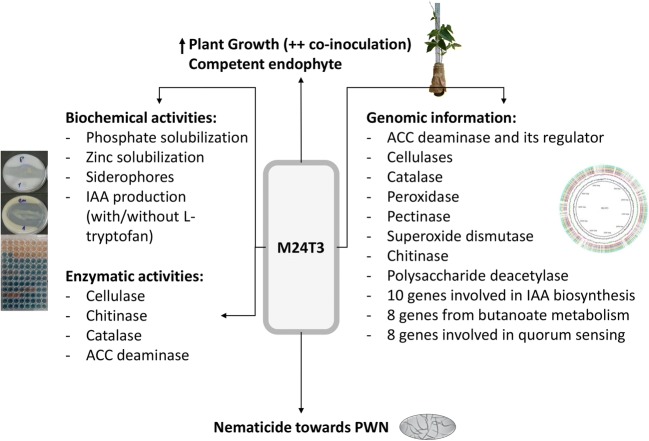
Eight genes involved in quorum sensing were found in strain M24T3 encoding for acyl-homoserine-lactone synthase (WP_009638744, WP_009635293) and LuxR family transcriptional regulator (WP_009638745, WP_009635292, WP_009637809, WP_009638756, WP_009635139, WP_037377293). All the biochemical, enzymatic, genomic, PGPB, and nematicidal characteristics of strain M24T3 were summarized in Fig. 4.
Fig. 4.
Summary of multiple traits of Serratia sp. M24T3 in the plant-nematode-bacteria interaction. The strain M24T3 showed multiple genes involved in plant growth promotion; some showed its biochemical/enzymatic activities. Serratia sp. M24T3 showed beneficial effects on plant growth and has nematicidal activity towards PWN
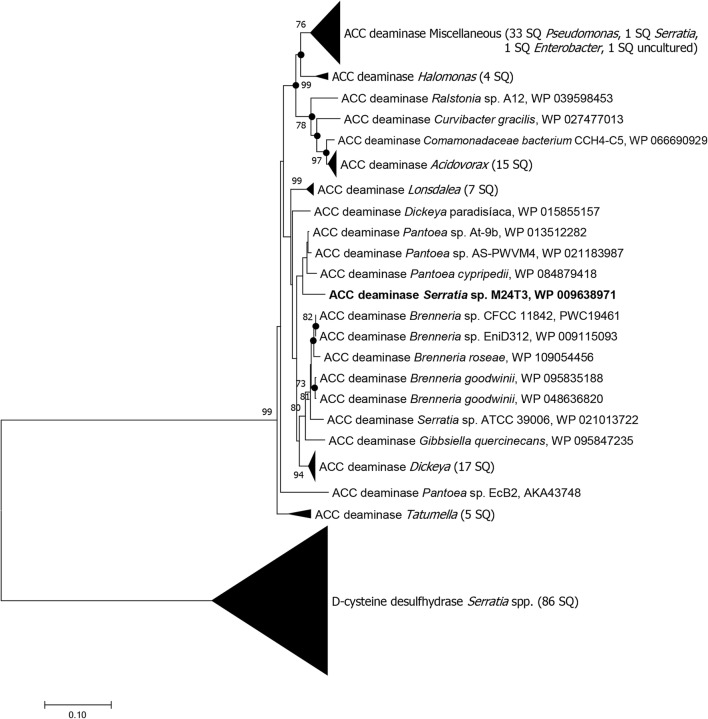
The ACC deaminase sequence from strain M24T3 and all the closest sequences considered for the phylogenetic tree showed the presence of the amino acids Glu295 and Leu322 at the active site, differentiating themselves from d-cysteine desulfhydrase (Fig. S1). The phylogenetic tree indicated five main branches: one branch with sequences belonging to the class Gammaproteobacteria—ACC deaminase miscellaneous sequences of the genera Pseudomonas (33 sequences), Serratia (S. rubidaea, 1 sequence), Enterobacter (1 sequence) and one uncultured bacteria, and Halomonas (4 sequences); one branch with sequences belonging to the class Betaproteobacteria—sequences of the genera Ralstonia, Curvibacter, and Acidovorax (15 sequences); one branch that includes Serratia sp. M24T3 and with sequences belonging to the class Gammaproteobacteria, family Enterobacteriaceae—sequences of the genera Lonsdalea (7 sequences), Dickeya (18 sequences), Pantoea (3 sequences), Brenneria (5 sequences), Serratia (1 sequence), and Gibbsiella (1 sequence); one branch that includes sequences belonging to the class Gammaproteobacteria, family Enterobacteriaceae—5 sequences of the genus Tatumella; and the last branch composed by sequences of the genus Serratia with homology with ACC deaminase of Serratia sp. M24T3 but all of them are d-cysteine desulfhydrases (Fig. 5, Fig. S1).
Fig. 5.
Dendrogram of 1-aminocyclopropane-1-carboxylate (ACC) deaminase. The tree topology was obtained by neighbor-joining analysis of protein sequences in MEGA 7, previously aligned by CLUSTALW. Reference protein sequences were obtained from NCBI database (cutoff of 86%) and sequence from Serratia sp. M24T3 is indicated in bold. The numbers indicate the percentages of bootstrap sampling, derived from 1000 replications; values below 70% are not shown. Symbol (●) indicates node branches conserved when the tree was reconstructed using the maximum likelihood algorithm. Scale bar 10 inferred nucleotide substitutions per 100 nucleotides. SQ, sequence(s)
ACC deaminase from Serratia sp. M24T3 showed more similarity to Pantoea cypripedii (WP_084879418) (95.5%), Pantoea At-9b (WP_013512282) (94.9%), and Pantoea AS-PWVM4 (WP_021183987) (94.2%). These are known as plant growth–promoting bacteria, and Pantoea At-9b is known to be associated with Eukaryotic organisms as the leaf cutter ant symbiont [32]. The other two members from the genus Serratia with similarities in databases were Serratia ATCC 39006 (WP_021013722) (91.8%) and Serratia rubidaea AcdSPB1 (AEQ29824) (85.5%) (Fig. 5). Moreover, the closest gene upstream of the acdS gene (codding for ACC deaminase) that was found in the genome was acdR gene that encodes the AsnC family leucine-responsive regulatory protein (WP_009638972), shown to regulate expression of acdS gene [15]. Both genes are oriented in the same direction that was in agreement with Serratia ATCC39006 genome sequence. The G+C content of acdS gene of strain M24T3 was 54.18%, different from the G+C content of the total genome of 49.03% [35]. The gene encoding for d-cysteine desulfhydrase (WP_009636121) was also found.
Discussion
New biological strategies for sustainability and pest control are among the concerns of today for the near future. Lately, bacteria have been seen as potential new tools for natural and sustainable agriculture, since they are able to colonize plants, inside and outside, and proved to have a role in plants. Several studies reported mechanisms of plant growth promotion by PGPB and their direct or indirect beneficial role for plants [3, 6].
The group of nine strains included in this study proved to be nematicidal in vitro [14, 15]. This characteristic makes these strains potentially antagonist to phytopathogenic nematodes such as B. xylophilus, if they are able to colonize plants as endophytes.
In our study, Serratia sp. M24T3 carrying the pHRGFPGUS plasmid showed green fluorescence inside A. thaliana intercellular spaces, in the cortex region as well as inner zones. These results suggest efficient plant colonization obtained with gfp/gusA-tagged Serratia sp. M24T3. The presence of the bacterial strain M24T3 in the plant inhibited the primary root elongation but increased the number of lateral roots. In previous work, Serratia marcescens strain 90–166, a plant growth–promoting rhizobacterium (PGPR), produced the same effect in A. thaliana, which may have had resulted from IAA-production by the bacterium [44]. Such alterations might be beneficial for plants to increase water absorption and nutrients uptake [29]. The morphological effects induced in the plant by strain M24T3 might be related to the ability of the strain to produce IAA and is supported by the presence of 10 genes in the genome related to IAA biosynthesis pathways. When querying the KEGG database, genes encoding for aldehyde dehydrogenase were present in all genomes available for Serratia spp. Aliphatic amidase amiE genes were present only in S. proteamaculans and S. liquefaciens but nitrilase genes were not previously found in any Serratia genome sequences and this nitrilase (WP_009634944) from Serratia sp. M24T3 constitutes the first report in the genus Serratia. Moreover, the concentration of IAA produced by Serratia sp. M24T3 was basically the same when grown in LB broth supplemented with and without l-tryptophan. This suggests and supports the genomic information that the IAA synthesis pathway is independent of l-tryptophan.
The properties determined for Serratia strain M24T3 in this study seem to indicate that this strain can act as PGPB. Firstly, strain M24T3 solubilized inorganic phosphate and zinc oxide, and these findings demonstrated that this strain might support plant growth, since P and Zn are essential macro and micronutrients, respectively, for plant growth [14]. Secondly, zinc solubilization by Gluconacetobacter diazotrophicus PAL5 promoted the nematicidal activity against root-knot nematode Meloidogyne incognita [42]. Therefore, Serratia sp. M24T3 ability to solubilize Zn may be part of the mechanism of toxicity against the PWN demonstrated by the strain in vitro [14, 15]. In contrast, other Serratia strains, previously isolated from a different laboratory, did not have nematicidal activity towards PWN [26, 50, 51].
In addition, Serratia sp. M24T3 showed cellulolytic activity, the ability for CMC degradation, which was also supported by genomic information with genes encoding for cellulase—β-glucosidase (WP_009635056; WP_009636821) and periplasmic β-glucosidase (WP_009638496) and pectinase—pectinesterase B (WP_009638872) present. The high phosphate and zinc solubilization activity and the cellulolytic activity support the idea of strain M24T3 being a PGPB since cellulases produced by the bacteria seem to potentiate the endophytic colonization [39] without phytopathogenic relevance.
According to sequence analysis of 16S rRNA gene, Serratia sp. M24T3 is closely related to S. plymuthica, considered non-pathogenic [35]. Several studies on the species S. plymuthica have shown its PGPB role as well as its ability to act against pathogenic Agrobacterium [9, 27]. Strains from the species S. plymuthica are ubiquitous and have been preferentially recovered from rhizospheres, both as free-living and endophytic organisms. Strains from this species are also described as producers of homoserine lactones which were proven to induce ISR-like system protection as a biocontrol against Botrytis cinnera [19]. Serratia sp. M24T3 produces siderophores and lipases but was not able to degrade casein. As with some strains of S. plymuthica, strain M24T3 has the gene coding for AHL synthase and its regulator (LuxR) which indicates its potential to induce ISR protection against pathogens.
The strain M24T3 showed a gene coding for nitrilase, an enzyme involved in the hydrolysis of nitrile compounds (such as indole-3-acetonitrile), resulting in the production of ammonia as well as involved in several plant-microbe interactions [21].
Ethylene is a stress-induced plant hormone involved in inhibition of plant growth; at low levels, it is required for normal plant development [15]. It was shown that Pseudomonas putida UW4 containing ACC deaminase was able to reduce the PWD symptoms and the presence of PWN in pine seedlings [25]. The gene encoding for ACC deaminase is present in Serratia sp. M24T3 and might be involved in lowering ethylene levels in the plant and therefore contribute to protecting the plant from nematodes infection. The ACC deaminase present in Serratia sp. M24T3 was only found in two other Serratia strains based on NCBI/KEEG databases, revealing that this function is not shared by most Serratia species [20]. Therefore, based on the G+C content of this gene (54.18%) that is much higher than the total genomic G+C content (49.03%), we believe that this gene was acquired by horizontal gene transfer proposed also by Hontzeas et al. [20]. The closest relatives, Pantoea At-9b and Serratia rubidae, were associated with fungus development [7, 32], and the closest Serratia strain ATCC 39006 was associated with plant growing under salt stress [13]. ACC deaminase-producing organisms have been found to be more abundant in the rhizosphere of plants growing in a stressed environment contributing to lower the stress in the plant [46].
Additional characteristics of Serratia sp. M24T3 support its potential as PGPB. Strain M24T3 is catalase positive and harbors genes encoding for catalase and SOD that can be part of the mechanism of bacterial protection against ROS released by plants as a defense strategy against microbial colonization. The presence of ROS-degrading enzymes promotes an effective endophytic bacterial colonization of the plant as shown, for example, for G. diazotrophicus [1]. Moreover, Serratia sp. M24T3 was able to degrade chitin and harbored a gene encoding for a chitinase. Its genome also harbored eight genes that might be related to butanoate metabolism and involved in the production of acetoin and 2,3-butanediol. These volatiles showed an important role in plant growth promotion of Arabidopsis [41]. The acetoin reductase/2,3-butanediol dehydrogenase was found only in half of the Serratia genome sequences closely related to S. plymuthica included in KEGG database.
Inoculation of the nematicidal group strains includes Serratia sp. M24T3, in co-inoculation with Bradyrhizobium sp. 3267, in the V. unguiculata enhanced, at different levels, various physiological plant activities. Moreover, all the plant’s physiological characteristics were enhanced to a higher level when in co-inoculation including Bradyrhizobium sp. 3267 and the Serratia sp. M24T3. However, the nematicidal strains without co-inoculation were not able to produce an impact on plant growth. Effects on V. unguiculata promoted by strain M24T3 alone showed no statistical differences compared to absolute control. The use of bacterial co-inoculum to promote plant growth has been reported with better results than the use of a single strain in plants under stress conditions [10].
Conclusion
In conclusion, the results with the model plant Arabidopsis showed that Serratia sp. M24T3 was a competent potential endophyte. When inoculated in the plant, strain M24T3 increased the number of plant lateral roots, showing potential as a direct PGPB which was supported by the biochemical characteristics determined in vitro and on genomic analysis. These results were supported by the tests in Leonard jars where the strain M24T3 co-inoculated with Bradyrhizobium sp. 3267 promoted the growth of V. unguiculata by increasing all growth characteristics. Indirectly, strain M24T3 could act as PGPB in pine trees, by decreasing plant attractiveness to nematodes, since it has genes that encode for ACC deaminase with a demonstrated activity that in turn is recognized to decrease ethylene levels in plants. These data together with previous results concerning nematicidal activity make the strain M24T3 a very promising candidate as an alternative for sustainable agriculture using less agrochemicals.
Electronic supplementary material
Alignment of sequences showed differences between ACC deaminases and D-cysteine desulfhydrase. The sequences were aligned with CLUSTALW and it was verified the presence of the amino acids Glu295 and Leu322 at the active site confirming them as ACC deaminase or Thr at the both active sites confirming them as D-cysteine desulfhydrase. Serratia sp. M24T3 showed sequence of ACC deaminase whereas the most common sequences of the strains in the genus Serratia are D-cysteine desulfhydrases. (PNG 42557 kb)
Acknowledgments
The authors thank Grasiella Ventura Matioszek, Unidade Multiusuária de Microscopia Confocal—ICB/UFRJ for support on CSLM. We thank Jakson Leite for his support on cowpea assays. We thank Tiago Natal da Luz for his help with statistical analysis.
Funding statement
This work was financed by FEDER funds through the Programa Operacional Factores de Competitividade—COMPETE and by national funds through the Fundação para a Ciência e a Tecnologia (FCT), Portugal, under the projects UID/EMS/00285/2013 and PTDC/AGR-CFL/115373/2009, and Programa CNPq/Universidade de Coimbra/Associação Grupo de Coimbra de Dirigentes de Universidades Brasileiras—2010, process no. 590041/2010-0.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Diogo Neves Proença, Phone: +351 239240700, Email: diogo.proenca@uc.pt.
Paula V. Morais, Phone: +351 239240700, Email: pvmorais@ci.uc.pt
References
- 1.Alquéres S, Meneses C, Rouws L, Rothballer M, Baldani I, Schmid M, Hartmann A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol Plant-Microbe Interact. 2013;26(8):937–945. doi: 10.1094/MPMI-12-12-0286-R. [DOI] [PubMed] [Google Scholar]
- 2.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 5.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carder JH. Detection and quantitation of cellulase by Congo red staining of substrates in a cup-plate diffusion assay. Anal Biochem. 1986;153(1):75–79. doi: 10.1016/0003-2697(86)90063-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Qiu C, Huang T, Zhou W, Qi Y, Gao Y, Shen J, Qiu L. Effect of 1-aminocyclopropane-1-carboxylic acid deaminase producing bacteria on the hyphal growth and primordium initiation of Agaricus bisporus. Fungal Ecol. 2013;6(1):110–118. doi: 10.1016/j.funeco.2012.08.003. [DOI] [Google Scholar]
- 8.Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem. 2010;42(5):669–678. doi: 10.1016/j.soilbio.2009.11.024. [DOI] [Google Scholar]
- 9.Dandurishvili N, Toklikishvili N, Ovadis M, Eliashvili P, Giorgobiani N, Keshelava R, Tediashvili M, Vainstein A, Khmel I, Szegedi E, Chernin L. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J Appl Microbiol. 2011;110(1):341–352. doi: 10.1111/j.1365-2672.2010.04891.x. [DOI] [PubMed] [Google Scholar]
- 10.Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater. 2010;177(1–3):323–330. doi: 10.1016/j.jhazmat.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Evans HF, McNamara DG, Braasch H, Chadoeuf J, Magnusson C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull. 1996;26(2):199–249. doi: 10.1111/j.1365-2338.1996.tb00594.x. [DOI] [Google Scholar]
- 12.Fan B, Chen XH, Budiharjo A, Bleiss W, Vater J, Borriss R. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J Biotechnol. 2011;151(4):303–311. doi: 10.1016/j.jbiotec.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Fineran PC, Cans CI, Ramsay JP, Wilf NM, Cossyleon D, Mcneil MB, Williamson NR, Monson RE, Becher SA, Stanton JL, Brügger K, Brown SD, Salmond PC. Draft genome sequence of Serratia sp. strain ATCC 39006, a model bacterium for analysis of the biosynthesis and regulation of prodigiosin, a carbapenem, and gas vesicles. Genome Announc. 2013;1(6):e01039–e01013. doi: 10.1128/genomeA.01039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169(1):30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer Ø, Harper DAT, Ryan PD. Past: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):1–9. [Google Scholar]
- 19.Hartmann A, Rothballer M, Hense BA, Schröder P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front Plant Sci. 2014;5:131. doi: 10.3389/fpls.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hontzeas N, Richardson AO, Belimov A, Safronova V, Abu-Omar MM, Glick BR. Evidence for horizontal transfer of 1-aminocyclopropane-1-carboxylate deaminase genes. Appl Environ Microbiol. 2005;71(11):7556–7558. doi: 10.1128/AEM.71.11.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howden AJM, Preston GM. Nitrilase enzymes and their role in plant-microbe interactions. Microb Biotechnol. 2009;2(4):441–451. doi: 10.1111/j.1751-7915.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leite J, Seido SL, Passos SR, Xavier GR, Rumjanek NG, Martins LMV. Biodiversity of rhizobia associated with cowpea cultivars in soils of the lower half of the São Francisco River Valley. Rev Bras Ciência Do Solo. 2009;33(5):1215–1226. doi: 10.1590/S0100-06832009000500015. [DOI] [Google Scholar]
- 24.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 25.Nascimento FX, Vicente CSL, Barbosa P, Espada M, Glick BR, Mota M, Oliveira S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl. 2012;58(3):427–433. doi: 10.1007/s10526-012-9500-0. [DOI] [Google Scholar]
- 26.Nascimento F, Vicente C, Cock P, Tavares M, Rossi M, Hasegawa K, Mota M (2018) From plants to nematodes: Serratia grimesii BXF1 genome reveals an adaptation to the modulation of multi-species interactions. Microb Genomics 4(7). doi 10.1099/mgen.0.000178 [DOI] [PMC free article] [PubMed]
- 27.Neupane S. Genomics and transcriptomics of plant beneficial Serratia spp. Sweden: Swedish University of Agricultural Sciences; 2013. [Google Scholar]
- 28.Paiva G, Proença DN, Francisco R, Verissimo P, Santos SS, Fonseca L, Abrantes IMO, Morais PV. Nematicidal bacteria associated to pinewood nematode produce extracellular proteases. PLoS One. 2013;8(11):e79705. doi: 10.1371/journal.pone.0079705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68(8):3795–3801. doi: 10.1128/AEM.68.8.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118(1):10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 31.Pikovskaya RI. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 32.Pinto-Tomás AA, Anderson MA, Suen G, Stevenson DM, Chu FST, Cleland WW, Weimer PJ, Currie CR. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science. 2009;326(5956):1120–1123. doi: 10.1126/science.1173036. [DOI] [PubMed] [Google Scholar]
- 33.Proença DN. Role of endophytic microbial community in pine wilt disease. Portugal: University of Coimbra; 2014. [Google Scholar]
- 34.Proença DN, Francisco R, Santos CV, Lopes A, Fonseca L, Abrantes IMO, Morais PV. Diversity of bacteria associated with Bursaphelenchus xylophilus and other nematodes isolated from Pinus pinaster trees with pine wilt disease. PLoS One. 2010;5(12):e15191. doi: 10.1371/journal.pone.0015191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proença DN, Espírito Santo C, Grass G, Morais PV. Draft genome sequence of Serratia sp. strain M24T3, isolated from pinewood disease nematode Bursaphelenchus xylophilus. J Bacteriol. 2012;194(14):3764. doi: 10.1128/JB.00670-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proença DN, Francisco R, Kublik S, Scholer A, Vestergaard G, Schloter M, Morais PV. The microbiome of endophytic, wood colonizing bacteria from pine trees as affected by Pine Wilt Disease. Sci Rep. 2017;7(1):4205. doi: 10.1038/s41598-017-04141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proença DN, Grass G, Morais PV. Understanding pine wilt disease: roles of the pine endophytic bacteria and of the bacteria carried by the disease-causing pinewood nematode. Microbiologyopen. 2017;6(2):e00415. doi: 10.1002/mbo3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos HJO, Roncato-Maccari LDB, Souza EM, Soares-Ramos JRL, Hungria M, Pedrosa FO. Monitoring Azospirillum-wheat interactions using the gfp and gusA genes constitutively expressed from a new broad-host range vector. J Biotechnol. 2002;97(3):243–252. doi: 10.1016/S0168-1656(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 39.Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011;14(4):435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Rouws LFM, Leite J, de Matos GF, Zilli JE, Coelho MRR, Xavier GR, Fischer D, Hartmann A, Reis VM, Baldani JI. Endophytic Bradyrhizobium spp. isolates from sugarcane obtained through different culture strategies. Environ Microbiol Rep. 2013;6(4):354–363. doi: 10.1111/1758-2229.12122. [DOI] [PubMed] [Google Scholar]
- 41.Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci. 2003;100(8):4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangaraju M. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett Appl Microbiol. 2007;44(3):235–241. doi: 10.1111/j.1472-765X.2006.02079.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 44.Shi C-L, Park H-B, Lee JS, Ryu S, Ryu C-M. Inhibition of primary roots and stimulation of lateral root development in Arabidopsis thaliana by the rhizobacterium Serratia marcescens 90-166 is through both auxin-dependent and -independent signaling pathways. Mol Cells. 2010;29(3):251–258. doi: 10.1007/s10059-010-0032-0. [DOI] [PubMed] [Google Scholar]
- 45.Smibert RM, Krieg NR. Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for general and molecular bacteriology. Washington, DC: American Society for Microbiology; 1994. pp. 607–654. [Google Scholar]
- 46.Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E. Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS One. 2011;6(3):e17968. doi: 10.1371/journal.pone.0017968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tindall JB, Sikorski J, Smibert AR, Krieg RN. Phenotypic characterization and the principles of comparative systematics. In: Reddy CA, Beveridge JT, Breznak JA, Marzluf GA, Schmidt TM, Snyder LR, editors. Methods for general and molecular microbiology. 3. Washington, DC: American Society for Microbiology; 2007. pp. 330–393. [Google Scholar]
- 48.Todorovic B, Glick BR. The interconversion of ACC deaminase and D-cysteine desulfhydrase by directed mutagenesis. Planta. 2008;229(1):193–205. doi: 10.1007/s00425-008-0820-3. [DOI] [PubMed] [Google Scholar]
- 49.Vicente GM. Manual of the practical study of root nodule bacteria. International biology program, 15. Oxford: Blackwell; 1970. p. 163. [Google Scholar]
- 50.Vicente CSL, Nascimento FX, Barbosa P, Ke H-M, Tsai IJ, Hirao T, Cock PJA, Kikuchi T, Hasegawa K, Mota M. Evidence for an opportunistic and endophytic lifestyle of the Bursaphelenchus xylophilus-associated bacteria Serratia marcescens PWN146 isolated from wilting Pinus pinaster. Microb Ecol. 2016;72(3):669–681. doi: 10.1007/s00248-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 51.Vicente CSL, Nascimento FX, Ikuyo Y, Cock PJA, Mota M, Hasegawa K. The genome and genetics of a high oxidative stress tolerant Serratia sp. LCN16 isolated from the plant parasitic nematode Bursaphelenchus xylophilus. BMC Genomics. 2016;17(1):1–15. doi: 10.1186/s12864-016-2626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei G, Kloepper JW, Tuzum S. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology. 1996;86(2):221. doi: 10.1094/Phyto-86-221. [DOI] [Google Scholar]
- 53.Wubben MJ, Su H, Rodermel SR, Baum TJ. Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Mol Plant-Microbe Interact. 2001;14(10):1206–1212. doi: 10.1094/MPMI.2001.14.10.1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of sequences showed differences between ACC deaminases and D-cysteine desulfhydrase. The sequences were aligned with CLUSTALW and it was verified the presence of the amino acids Glu295 and Leu322 at the active site confirming them as ACC deaminase or Thr at the both active sites confirming them as D-cysteine desulfhydrase. Serratia sp. M24T3 showed sequence of ACC deaminase whereas the most common sequences of the strains in the genus Serratia are D-cysteine desulfhydrases. (PNG 42557 kb)