Abstract
An outbreak of infectious bronchitis caused by the IBVPR03 strain of the Massachusetts genotype affected H-120 vaccinated laying hens in South Brazil. We investigated the cross protection of the vaccine by assessing the traqueal ciliostasis, virus recovery, and histopathological changes typically observed in the respiratory tract. Although the IBVPR03 strain is S1-genotyped as Massachusetts with a high genomic similarity to the H-120 vaccine strains, surprisingly, we found no tropism or pathogenicity to the trachea in birds infected with this strain. On the other hand, we observed ovarian and testicle lesions. Here, we show that, despite belonging in the Massachusetts genotype, the IBVPR03 pathotype differs from the expected respiratory pattern, causing instead marked histopathological changes in the gonads, so far not associated with this group.
Keywords: Infectious bronchitis virus, Gonadal pathogenicity, Young chicks, Testis, Ovaries
Introduction
The Avian Infectious Bronchitis virus (IBV) causes a highly contagious disease and produces severe economic losses in the poultry industry worldwide. The etiologic agent, the infectious bronchitis virus, is a pleomorphic coronavirus with a single-stranded, positive-sense RNA genome that is 27.6 Kb long and encodes the following structural proteins: spike glycoprotein (S), membrane glycoprotein (M), the nucleocapsid protein (N), and the small membrane protein (E) [1]. The IBV replicates in the upper respiratory tract and also in some epithelial cells of the digestive, urinary, and reproductive systems [1, 2]. Literature has shown that in addition to its respiratory, digestive, and urinary signs, the IBV can cause declines in egg production and fertility of breeding flocks. Since it was first described in 1931 [3], several serotypes and strains of IBV have been isolated worldwide and there are currently no vaccines that stimulate a complete protection, demanding in many circumstances the adoption of multiple vaccines for the constantly arising antigenic variants [4]. Meanwhile, the emergence of new IBV variants requires constant characterization of these strains by genotyping, serotyping, and pathotyping for subsequent cross-protection studies with the available vaccines [5].
Here we investigated the tropism and pathogenicity among early age chicks of strain IBVPR03 isolated from field outbreaks in layer flocks from South Brazil, whose birds presented the renal form of the disease, despite vaccination with H-120. In a cross-protection study conducted previously with H-120 vaccinated birds challenged with IBVPR03, we found no tracheal lesions in unvaccinated birds and some birds whose gonads were examined presented lesions in the testis and ovary. Moreover, IBVPR03 was subsequently S1-genotyped as Massachusetts with a high genomic similarity between the IBVPR03 and H-120 vaccine strains [6]. Together, these facts led us to characterize the tropism and pathogenicity of this strain in specific-pathogen-free (SPF) early age chicks.
Materials and methods
Animals and experimental design
White Leghorn specific-pathogen-free (SPF) eggs, purchased from Biovet Laboratories S/A (São Paulo, Brazil), were incubated and hatched in the Microbiology Laboratory at the Departamento de Patologia Veterinária (FCAV/UNESP). All SPF birds were housed in P3-level isolators (Alesco®), under strict isolation and the same nutritional and environmental conditions. All animal procedures were approved by the Institutional Animal Care and Use Committee of Universidade de São Paulo (USP).
Experiment 1
For the cross-protection assay, we challenged the chicks, vaccinated or not, with M-41, as a reference strain, or IBVPR03. Thus, in Experiment 1 (Exp. 1), one-day-old chicks were separated into four groups isolated from each other: non-vaccinated and challenged with M-41 (NONVAC/M41, n = 12), non-vaccinated and challenged with IBVPR03 (NONVAC/PR03, n = 10), vaccinated and challenged with M-41 (VAC/M41, n = 12), and vaccinated and challenged with IBVPR03 (VAC/PR03, n = 11). Strict measures were taken to prevent cross contamination among groups. Vaccination was performed at 21 days of age with live commercial IBV vaccine, and challenge was made at 42 days of age. Most individuals in this experiment were euthanized 5 days after the challenge with the aim of collecting the trachea (for ciliary activity inhibition, histopathology, and virus recovery). However, because we observed macroscopic changes in the testis of one animal from the NONVAC/PR03 with 47 days old, we decided to keep 2 chickens from NONVAC/PR03 and VAC/PR03 groups until 49 days of age for the collection of gonads.
Vaccine
The commercial vaccine H-120 (Bio-Bronk-Vet H-120, Biovet®) used herein contained the live attenuated IBV (originated from Massachusetts serotype). The 21-day-old chicks were vaccinated with only one dose of 104 EID50/bird by oculo-nasal route.
Viruses
The virulent M-41 (Mass 41) strain of the Massachusetts type was used for the challenge test (Experiment 1), as a reference strain, in a dose of 104 EID50/bird. The strain IBVPR03 used in this study was isolated from field outbreaks in layer flocks presenting the renal form of the disease, despite vaccination. The IBVPR03 was previously analyzed for the nucleotide sequence of the 5′-terminal of the S1 gene (GenBank accession number—GQ169241), which, after phylogenetic analyses, was classified as Massachusetts genotype [6]. The dose of IBVPR03 used for challenge in Experiment 1 was 105 EID50/bird (NONVAC/PR03; VAC/PR03). In Experiment 2, the dose of IBVPR03 used for challenge was 104 EID50/bird. Challenges were made using the oculo-nasal route. All viruses were previously propagated and titrated in 10-day-old SPF chicken embryos. Infectivity titers were calculated according to Reed and Muench [7].
Clinical signs
The birds were observed daily for the onset of clinical signs.
Inhibition of ciliary activity
For evaluation of tracheal ciliostasis, three fragments of approximately 1.5 mm of each portion (total of nine rings per bird) were analyzed. The rings were placed in a Petri dish containing Eagle culture medium with 10% fetal bovine serum. They were then analyzed on an inverted microscope, observing the degree of integrity and preservation of the ciliary movement of the tracheal epithelial cells. The tracheal ciliary activity was classified in scores from 0 (100% ciliary activity—complete protection) to 4 (0 to 25% of ciliary activity—absence of protection). Since nine tracheal rings were assessed per bird, the maximum ciliostasis score reached 36, representing cases of complete destruction of tracheal epithelium [8–10].
Sample collection and histopathology
In chicks from Exp. 1, samples from different parts of the trachea (0.5 cm from the upper, middle, and lower portions) were collected and fixed in 10% buffered formaldehyde. Notwithstanding, as mentioned earlier, gonads were also collected from some individuals from groups NONVAC/PR03 and VAC/PR03. Tissue samples were processed using standard histological procedures, embedded in paraffin wax, and cut into 4-mm sections. Afterwards, sections were stained with hematoxylin and eosin for histological examination by light microscopy. Tracheal samples were assigned with scores ranging from 0 to 3. A score of zero identifies the absence of injury, while cases with mild, moderate, and severe injury were classified as 1, 2, and 3, respectively. The following morphological characteristics were recorded: loss of cilia (0 to 3), loss of epithelial cells (0 to 3), depletion of epithelial glands (0 to 3), inflammatory infiltration (0 to 3), edema (0 to 3), inflammatory infiltration in the adventitia (0 to 3), and epithelial hyperplasia (0 to 1). Final scores for the histopathology of the trachea (per bird) were calculated by summing the scores for each of the analyzed parameters. Since we observed macroscopic changes in the testis of one animal belonging to the NONVAC/PR03 unit at 47 days old, we kept 2 chicks from each group (NONVAC/PR03 and VAC/PR03) until 49 days for the collection of gonads, in addition to the trachea.
Virus recovery
Trachea sections of each bird were scraped for sample preparation and IBV isolation in 10-day-old SPF embryonated chicken eggs, according to the standard procedures [11, 12].
Statistical analyses
The Mann-Whitney test was applied for data set analyses of ciliary activity inhibition and histopathology for challenge protection state assessment. Results from virus recovery were compared by Fischer’s exact test. In all tests, confidence interval of 95% was applied. Description levels lower than 0.05 were considered significant.
Experiment 2
Subsequently, considering macro and microscopic findings in Exp. 1, a second set of SPF chicks was divided into two groups: non-vaccinated/non-challenged (CON, n = 9) and non-vaccinated/challenged with IBVPR03 (IBVPR03, n = 10). In this second experiment (Exp. 2), however, challenge and euthanasia were performed at 4 and 12 days of age, respectively. The IBVPR03 challenge dose was 104 EID50/bird (IBVPR03). The trachea, kidneys, and gonads from all individuals were collected for histopathological analysis. For kidney damage, injury was considered mild when there was slight tubular dilation with minimal infiltration of lymphocytic cells in the interstitial lumen. Moderate histological lesions were defined as the presence of small foci of necrosis and tubular dilation with moderate inflammatory infiltrate. Severe lesions are defined as the presence of large foci of acute necrosis with tubular dilation and severe inflammatory infiltrate around tubules. The testis was examined for the preservation of the seminiferous tubules, the presence or absence of inflammatory infiltrate, micro-bleeds, and testicular degeneration, whereas ovaries were investigated for the presence or absence of inflammatory infiltrate, follicular degeneration, and micro-bleeds. We performed the real-time RT-PCR to confirm the viral presence in the gonads.
Real-time RT-PCR and DNA sequencing
From Experiment 2, gonad samples from all IBVPR03 (ovaries n = 3 and testis n = 7) and 3 CON were submitted to real-time reverse transcriptase polymerase chain reaction (RT-qPCR) targeting the IBV 5′UTR sequence (143 bp) to verify the presence of IBV [13]. RNA was extracted from eight 9-μm formalin-fixed, paraffin-embedded (FFPE) tissue samples using the Recoverall™ Total Nucleic Acid Isolation Kit for FFPE (Thermo Fisher®) following the manufacturer’s instructions. RT-qPCR reaction was carried out in triplicate with GoTaq® Probe 1-Step RT-qPCR system (Promega®), 750 nM of each primer, 200 nM of probe, and 4 μL of RNA sample, with the manufacturer’s amplification conditions, using 7500 Real-Time PCR System (Applied Biosystems®). All samples were tested for chicken actin β as internal control [14] using the same reagents and thermocycler, but with 500 nM of each primer, 150 nM of probe, and 2 μL of RNA. After purification with ExoSAP-IT™ (Thermo Fisher®), the specificity of RT-qPCR reaction for IBV was checked by bidirectional sequencing using a fluorescent dye terminator kit (Applied Biosystems®), in a Mastercycler-Pro (Eppendorf®), followed by electrophoretic separation in a 3500XL sequencer (Applied Biosystems®). Sequencing data were edited using the DNAStar software and the nucleotide sequences were analyzed through GenBank searches.
Results
Experiment 1: clinical signs and gross pathology
In Experiment 1, mild respiratory symptoms characterized essentially by gasping were detected in the NONVAC/M41 group in all unvaccinated birds, over the 5 days after challenge with M-41, whereas animals from the VAC/M41 group exhibited no typical signs of IBV. At necropsy, we found slight amounts of tracheal mucus only in birds from the NONVAC/M41 group. In parallel, birds from both groups challenged with IBVPR03 had no clinical symptoms. Nevertheless, at macroscopic examination, one male from the NONVAC/PR03 group showed unilateral hypertrophy in the testis.
Inhibition of ciliary activity
Our findings indicated that vaccination offered protection against M-41, since ciliostasis score in the VAC/M41 group was on average four times lower than that seen in the NONVAC/M41 group (P < 0.001) (Fig. 1). On the other hand, ciliary activity was apparently preserved in both groups challenged with IBVPR03 as the difference between vaccinated and unvaccinated groups was at the limit of significance (median scores from VAC/PR03 and NONVAC/PR03 groups were 8 and 9, respectively) (P = 0.04).
Fig. 1.
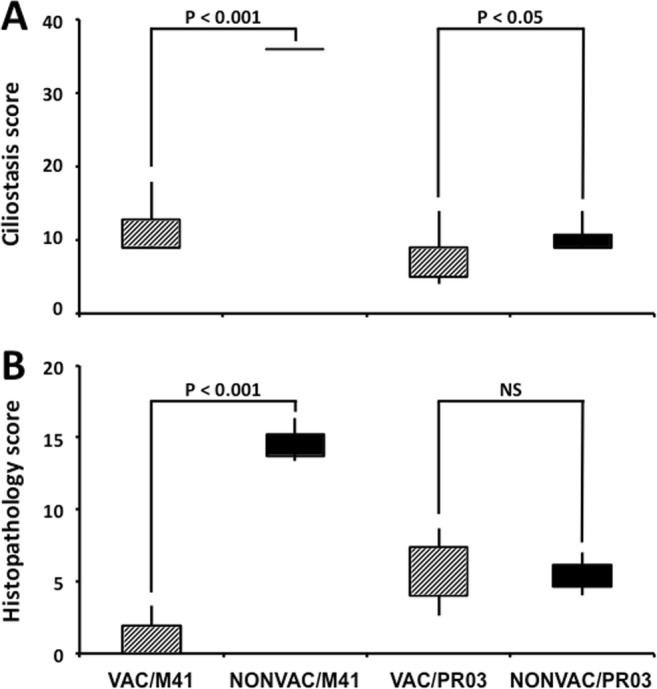
Ciliostasis (A) and histopathology (B) scores from the trachea of SPF chicks challenged and euthanized at 42 and 47 days of age, respectively (Exp. 1). NONVAC/M41 (non-vaccinated and challenged with M-41, n = 12), VAC/M41 (vaccinated and challenged with M-41, n = 12), NONVAC/PR03 (non-vaccinated and challenged with IBVPR03, n = 10), and VAC/PR03 (vaccinated and challenged with IBVPR03, n = 11). NS, non-significant (P > 0.05)
Histopathology
The histopathological assessment of the trachea also revealed great differences between VAC/M41 and NONVAC/M41 groups, which had average scores of 1.17 and 14.67, respectively (P < 0.001) (Fig. 1). All individuals from the NONVAC/M41 group presented intense inflammatory response with loss of cilia, epithelial cells, epithelial glands, and hyperplasia (Fig. 2). Conversely, tracheas from VAC/M41 birds were fully preserved (Fig. 2) as well as those from birds from the VAC/PR03 and NONVAC/PR03 groups that exhibited preservation of cilia, pseudostratified epithelia, and glands (median scores 6.67 and 5.67, respectively) (P > 0.05; Figs. 1 and 2). The 3 birds from NONVAC/PR03 group whose gonads were examined (1 male at 42 days old and 1 male and 1 female at 49 days old) showed inflammatory infiltrates (either heterophilic and/or mononuclear) in ovaries and testis. No injuries were found in gonads from the VAC/PR03 group (Fig. 3).
Fig. 2.
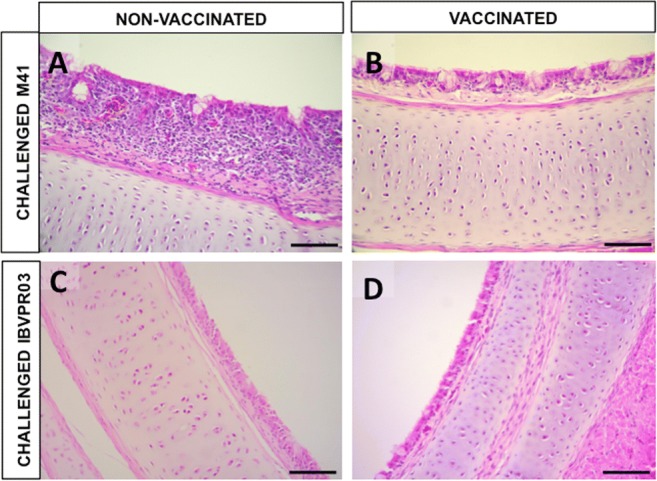
Histopathological sections of the trachea from SPF chicks challenged and euthanized at 42 and 47 days of age, respectively (Exp. 1). (A) Trachea from unvaccinated bird challenged with M-41 (NONVAC/M41), showing intense inflammatory infiltrate, vascular congestion, hyperplasia, loss of preservation of cilia, glands, and pseudostratified epithelial structure. (B) Trachea from vaccinated bird challenged with M-41 (VAC/M41) revealing complete preservation of cilia, glands, and epithelia. (C) and (D) Tracheas from unvaccinated (NONVAC/PR03) and vaccinated birds (VAC/PR03) challenged with IBVPR03, respectively, showing complete preservation of trachea structures with no presence of inflammatory infiltrate. H&E. Bar = 50 μm (A, B) and 100 μm (C, D)
Fig. 3.
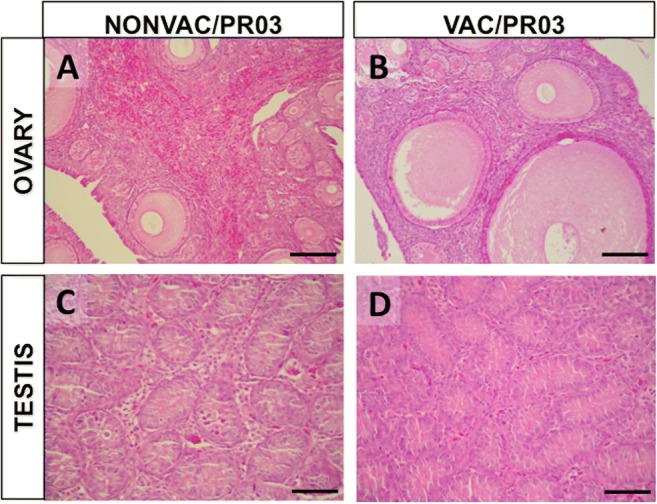
Histological sections of ovary and testis from SPF chicks euthanized 7 days after challenge with IBVPR03 (49 days of age—Exp. 1). (A) Intense heterophilic infiltration in the ovary of unvaccinated bird (NONVAC/PR03) and (B) preservation of gonadal structures in the ovary of a vaccinated bird (VAC/PR03). (C) Testicular degeneration, heterophillic, and mononuclear infiltrate in the interstitial space of unvaccinated male (NONVAC/PR03) and (D) preserved seminiferous tubules of a vaccinated male (VAC/PR03). H&E. Bar = 200 μm (A, B) and 100 μm (C, D)
Virus recovery
Analysis revealed that M-41 was recovered from the trachea of 11/12 individuals from the NONVAC/M41 group (first passage), whereas recovery was obtained from only 1/12 birds from the VAC/M41 group (P < 0.001, Fig. 4). In contrast, no statistical difference was observed between groups VAC/PR03 (18.3%; 2/11) and NONVAC/PR03 (40%; 4/10) (P > 0.05).
Fig. 4.
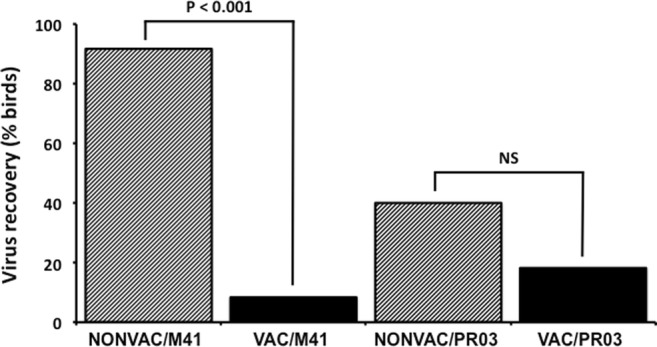
Virus recovery from tracheal scraping suspensions of SPF chicks from the following groups: non-vaccinated and challenged with M-41 (NONVAC/M41), vaccinated and challenged with M-41 (VAC/M41), non-vaccinated and challenged with IBVPR03 (NONVAC/PR03), and vaccinated and challenged with IBVPR03 (VAC/PR03). NS, non-significant (P > 0.05)
Experiment 2
Clinical signs and gross pathology
Neither CONPR03 nor IBVPR03 displayed clinical symptoms or macroscopic injuries in the trachea and gonads at necropsy.
Histopathology
Eight of the 10 chicks (8/10) from the IBVPR03 group had histological lesions in their gonads, while none of the birds (0/9) in the CONPR03 group displayed any gonadal injuries (Fig. 5). Histopathological findings in the testis included focal mononuclear cell infiltrate, tubular degeneration, diffuse heterophilic infiltrate, focal necrosis, and micro-bleeds (Fig. 5). In the ovary, we found infiltration of heterophils and mononuclear cells, destruction of follicles and, occasionally, micro-bleeds. Additionally, 7/10 of the individuals in the IBVPR03 group had kidneys with interstitial mononuclear infiltrates (compared to zero in the CONPR03 group) and all chicks from both groups exhibited no damage in the trachea (Fig. 5).
Fig. 5.
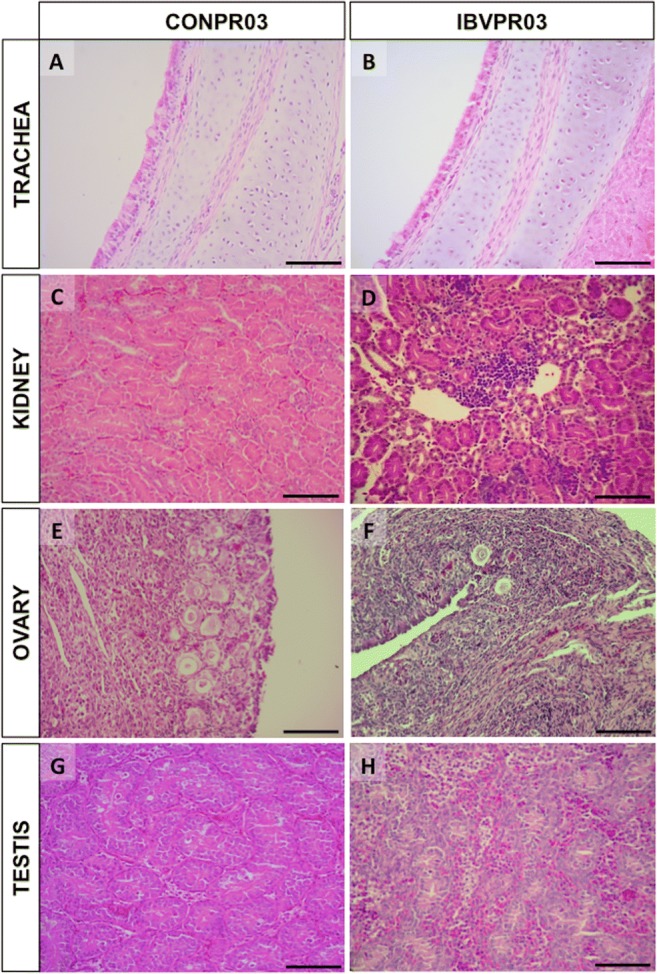
Histological sections of the trachea, kidney, ovary, and testis of 12-day-old SPF chicks from the non-challenged group (CONPR03—A, C, E, and G) and challenged with the IBVPR03 group (IBVPR03—B, D, F, and H), respectively. (A) and (B) Tracheas with preserved epithelia, cilia, and glands in the CONPR03 and IBVPR03 groups. (C) Kidney with structures preserved in CONPR03 and (D) interstitial mononuclear infiltrate (interstitial nephritis) in IBVPR03. (E) Ovary with follicles preserved with no inflammatory infiltrate in CONPR03 and (F) ovary in IBVPR03 displaying mononuclear infiltrate, destruction of follicles, and micro-bleeds. (G) Preserved seminiferous tubules with no inflammatory infiltrate in CONPR03 and (H) testis from IBVPR03 with diffuse heterophilic infiltrate with some mononuclear cells, tubular degeneration, and vascular congestion. H&E. Bar = 100 μm
IBV real-time RT-PCR and DNA sequencing
The viability of RNA was confirmed, as all sample showed amplification of internal control, but with high Ct values (29.36 to 32.69). Four of 10 (4/10) of the IBVPR03 samples showed amplification in the IBV 5′UTR reaction with Ct values from 35.84 to 37.42. Four of the 8 birds (4/8) with gonadal injuries showed amplification for IBV 5′UTR (3 ovaries and 1 testis). The specificity of RT-qPCR reaction was confirmed by sequencing of the 4 sample amplicons (143 bp) with identity of 100% with the IBV 5′UTRs (62 bp).
Discussion
The main finding of this work is that the IBVPR03 strain has tropism and pathogenicity for gonads but not by the trachea, although it belongs to the Massachusetts genotype. As expected, the reference strain M-41 caused injuries in the trachea in the unvaccinated group and the vaccine H-120 protected against the challenge. Unlike, the IBVPR03 presented neither tropism nor pathogenicity for the trachea since in both groups (NONVAC/IBVPR03 and VAC/IBVPR03) tracheas were fully preserved, as evaluated by virus recovery (tropism), ciliostasis, and histopathology (pathogenicity). On the other hand, although gonads from only 3 birds have been examined, the histopathological findings indicated that IBVPR03 was pathogenic for testis and ovaries (NONVAC/IBVPR03) but gonads from vaccinated birds (VAC/IBVPR03) were anatomically preserved, suggesting a protection by the H-120 vaccine. However, it is not possible to infer about the cross protection from the small number of birds whose gonads were evaluated.
In Experiment 2, the results from histopathology confirmed IBVPR03 displayed gonadal injuries corroborating with our first Experiment (1). The dose used to challenge the birds in Experiment 2 (104 EID50/bird) was 10 times lower than in Experiment 1 (105 EID50/bird) since we were evaluating birds at a younger age with an experimental design that also comprised the evaluation of nephropathogenicity of IBVPR03, which is held in early ages [15]. In fact, Experiment 2 showed that the Brazilian strain IBVPR03 is pathogenic to the kidneys (Fig. 5), corroborating clinical findings from outbreaks in South Brazil, and possibly, with the increase of the challenge dose, the injuries would be more severe, either in the kidneys or gonads. The most plausible explanation for the presence of IBV of the Mass genotype in birds vaccinated with the Massachusetts strain may be the insufficient immunity in the genital tract that was conferred by vaccination with the used Mass strain, despite the presence of elevated levels of systemic antibodies against this same strain that were induced by that same vaccine. Therefore, the anti-IBV antibodies that are present in the blood serum might exert a partial protective role preventing the development of lesions in some organs and/or tissues of the challenged birds, however not being able to prevent infection and the development of lesions in the genital tract of these birds. This could be due to other local immune mechanisms or ones that act more closely with these tissues, such as IgA antibodies or effector cytotoxic T cells that might not have been induced the vaccine [16, 17]. Also, it is important to emphasize that different IBV strains and other infectious agents usually co-infect commercial flocks enhancing the extent of pathogenic lesions and symptoms in the field.
In the ovary, we found infiltration of heterophils and mononuclear cells, destruction of follicles and, occasionally, micro-bleeds. To date, most research regarding IBV in the reproductive tract examined its pathogenicity in mature chickens primarily investigating the negative impact of the viral replication in the ciliated epithelium of the oviduct and epididymis [18–23]. Nevertheless, reproductive impairment caused by IBV is more severe and enduring whenever young chicks are affected and, accordingly, this study laid emphasis on immature birds [24, 25]. Despite the fact that declines in egg production and quality are largely associated with oviduct damage, four-day-old chicks challenged in this study with IBVPR03 often presented follicle degeneration at 12 days of age (Fig. 1). These data show that, in addition to possible changes in the oviduct, IBVPR03 may also reduce laying performance through its direct influence upon ovaries. Broadfoot et al. [ 26] reported that the earlier the age at which birds are infected with IBV, the greater the proportion of false layers (inoculation of 1- and 18-day-old chicks led to 26.6% and 9.3% of false layers, respectively). Afterwards, several other researchers focused on the IBV induction of false layers or abnormal oviducts [20, 21, 24, 25, 27, 28], but only a few studies investigated IBV effects on the gonads [29]. Recently, Hong et al. [ 30] noticed that infections with a Korean IBV strain (SNU8067) in unvaccinated 16-week-old SPF hens inhibited formation of hierarchal ovarian follicles (80% of individuals) and oviduct maturation (50% of individuals). This finding suggests that ovarian follicles may be more susceptible than the oviduct and, in turn, drops in egg production may be a consequence of irregular ovarian follicle development. Furthermore, the same authors observed that 30% of vaccinated chickens exhibited moderate to marked aplasia of the ovarian follicles and moderate atrophy of oviducts, possibly from the use of inactivated oil-emulsion vaccines in SPF chickens without priming [30]. According to the latest molecular epidemiological surveys, 16.80 to 33.71% of the Brazilian poultry flocks positive for IBV were comprised of breeders. Among them, 21.68 to 81.35% had gonad samples positive for IBV or exhibited some sort of reproductive disorder [31, 32]. Together, these evidences strengthen the need of further studies to assess not only the significance of IBVPR03 or other Brazilian field isolates on egg output of laying hens and breeders, but also the efficacy of the existing vaccination protocols to the challenges posed by the emerging IBV variants.
Histopathological findings in testis included focal mononuclear cell infiltrate, tubular degeneration, diffuse heterophilic infiltrate, focal necrosis, and micro-bleeds (Fig. 1). In roosters, some authors suggest that IBV is able to replicate in the epithelial lining of the efferent duct increasing the incidence of epididymal stones, which, in turn, decreases daily sperm production and fertility [18, 19, 33, 34], but an unequivocal conclusion has not yet been established as a result of contradictory data [18, 19, 34, 35]. Besides, to our knowledge, very few studies include experimental IBV infections of SPF chicks in order to monitor their impact on male physiology and fertility. Most of the available information comes from correlations between vaccinations (with both attenuated and killed viruses) or epidemiological surveys and the occurrence of lithiasis, orchitis, or decreased sperm production [18, 19, 34]. It cannot be excluded that we may be unaware of some relevant, missed articles, but it seems that our study is one of the few providing experimental data describing the microscopic lesions of IBV in testis. In other words, there is a lack of knowledge regarding the pathology of IBV infection in the male reproductive tract. Although IBV venereal transmission has been previously documented, inoculation of IBV (Arkansas and Massachusetts strains) in 10-week-old roosters apparently failed to induce histopathological changes in testes [36]. Thus, it has yet to be determined whether, as observed in females, there is any negative correlation between the age of exposure to IBVPR03 (and other strains) and the degree of testicular alterations.
One limitation of this study is that in Experiment 2, no material was collected for viral re-isolation. In an attempt to demonstrate the presence of viral RNA in the gonads, we extracted the RNA from formalin fixed paraffin-embedded (FFPE) samples. However, multiple factors influence the content and integrity of nucleic acids such as tissue size, fixation temperature and duration, and the amount of time that elapses before the sample is fixed [37], by the age of the tissue block [38]. In this context, we performed the RT-qPCR in order to detect the IBV RNA. From the 8 birds with gonadal injuries, 4 were positive to IBV RT-qPCR and although the high Ct values (35.84 to 37.42), probably by low viral load and RNA degradation in FFPE samples, the specificity of RT-qPCR reaction was also confirmed by bidirectional sequencing of the 4 sample amplicons.
Additionally, chicks from both groups (Exp. 2) exhibited no damage in the trachea (Fig. 5). These data suggest that IBVPR03 may not use the respiratory tract as the primary site for replication. Earlier work, where the authors sequenced the 5′-terminal of the S1 gene, indicates a high genomic similarity between the IBVPR03 and H-120 vaccine strains. Consequently, this field isolate was categorized as a Massachusetts genotype [6]. Subunit S1 is a primary target for host immune responses (via neutralizing antibodies) and it is determinant for the tissue tropism of IBV strains. These changes in tissue tropism could be a consequence of escaping mechanisms of the virus associated with the immune pressures driven by vaccination protocols for IBV [39, 40]. Furthermore, it has been demonstrated that immunity is not the only selective pressure on IBV [41] and the genetic diversity in viral isolates is attributed to shifts in population equilibrium of the replicating viral genomes even in the absence of immune selection pressure [42]. Such divergence between genotype and predominant pathotype or tissue tropism has also been reported in an IBV isolate from Egypt (Egypt/F/03), which, despite being classified as Massachusetts, showed a marked nephropathogenicity in experimentally infected SPF chickens [43].
Overall, our results demonstrate that, despite belonging to the Massachusetts genotype, IBVPR03 presents tropism and pathogenicity for gonads showing a different pathotype from the expected respiratory pattern. We do not exclude that other Massachusetts strains do not cause lesions in gonads and we consider that the other strains classified in this group should be investigated in this respect. This change in tissue tropism may be due to microenvironment selective pressures (once gonads are considered an immune-privileged site, i.e., an ideal tissue for the virus to avoid or attenuate the immune response) [44, 45]. This information, coupled with the discovery of venereal transmission of IBV [36], highlights the relevance of studying [1] how and to what extent this disease impairs reproduction, as well as [2] the role of males in IBV epidemiology.
Acknowledgments
We thank Adriana da Costa Neves (Laboratório de Genética, Instituto Butantan, São Paulo, Brazil) for pathology suggestions of the manuscript and Magna Aparecida Maltauro Soares by the assistance in histological sections.
Funding information
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) under grant 04/11040-4.
Compliance with ethical standards
All animal procedures were approved by the Institutional Animal Care and Use Committee of Universidade de São Paulo (USP).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Roekel H. Chronic respiratory disease of chickens. Am J Vet Res. 1952;13:252–259. [PubMed] [Google Scholar]
- 3.Schalk AF, Hanw MC. An apparently new respiratory disease in baby chicks. J Am Vet Med Assoc. 1931;78:413–422. [Google Scholar]
- 4.Ignjatović J, Sapats S (2000) Avian infectious bronchitis virus. In: Revue scientifique et technique (International Office of Epizootics), vol 19. p 493–508 [DOI] [PubMed]
- 5.Cavanagh D, Gelb J., Jr . Infectious bronchitis. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of poultry. 12. Iowa State: University Press; 2008. pp. 117–135. [Google Scholar]
- 6.Montassier MFS, Brentano L, Richtzenhain L, Montassier HJ, Lierz M, Heffels-Redmann U, Kaleta E, Heckmann J. VII. International symposium on avian corona- and pneumoviruses and complicating pathogens. Germany: Rauischholzhausen; 2012. Molecular analysis and evolution study of infectious bronchitis viruses isolated in Brazil over a twenty-one-year period; pp. 19–30. [Google Scholar]
- 7.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 8.Cavanagh D, Ellis MM, Cook JKA. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- 9.Cook JK, Darbyshire J, Peters R. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch Virol. 1976;50:109–118. doi: 10.1007/BF01318005. [DOI] [PubMed] [Google Scholar]
- 10.Andrade LF, Villegas P, Fletcher O, Laudencia R. Evaluation of ciliary movement in tracheal rings to assess immunity against infectious bronchitis virus. Avian Dis. 1982;26:805–815. doi: 10.2307/1589867. [DOI] [PubMed] [Google Scholar]
- 11.Gelb J, Jr, Rosenberger JK, Fries PA, Cloud SS, Odor EM, Dohms JE, Jaeger JS. Protection afforded infectious bronchitis virus-vaccinated sentinel chickens raised in a commercial environment. Avian Dis. 1989;33:764–769. doi: 10.2307/1591158. [DOI] [PubMed] [Google Scholar]
- 12.Owen RL, Cowen BS, Hattel AL, Naqi SA, Wilson RA. Detection of viral-antigen following exposure of one-day-old chickens to the Holland-52 strain of infectious-bronchitis virus. Avian Pathol. 1991;20:663–673. doi: 10.1080/03079459108418805. [DOI] [PubMed] [Google Scholar]
- 13.Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, Jackwood MW. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staines K, Batra A, Mwangi W, et al. A versatile panel of reference gene assays for the measurement of chicken mRNA by quantitative PCR. PLoS One. 2016;11:e0160173. doi: 10.1371/journal.pone.0160173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winterfield RW, Albassam MA. Nephropathogenicity of infectious bronchitis virus. Poult Sci. 1984;63:2358–2363. doi: 10.3382/ps.0632358. [DOI] [PubMed] [Google Scholar]
- 16.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 17.Chhabra R, Kuchipudi SV, Chantrey J, Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–241. doi: 10.1016/j.virol.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boltz DA, Nakai M, Bahr JM. Avian infectious bronchitis virus: a possible cause of reduced fertility in the rooster. Avian Dis. 2004;48:909–915. doi: 10.1637/7192-040808R1. [DOI] [PubMed] [Google Scholar]
- 19.Boltz DA, Zimmerman CR, Nakai M, Bunick D, Scherba G, Bahr JM. Epididymal stone formation and decreased sperm production in roosters vaccinated with a killed strain of avian infectious bronchitis virus. Avian Dis. 2006;50:594–598. doi: 10.1637/7654-052506R.1. [DOI] [PubMed] [Google Scholar]
- 20.Crinion RA, Ball RA, Hofstad MS. Pathogenesis of oviduct lesions in immature chickens following exposure to infectious bronchitis virus at one day old. Avian Dis. 1971;15:32–41. doi: 10.2307/1588385. [DOI] [PubMed] [Google Scholar]
- 21.Crinion RA, Hofstad MS. Pathogenicity of four serotypes of avian infectious bronchitis virus for the oviduct of young chickens of various ages. Avian Dis. 1972;16:351–363. doi: 10.2307/1588800. [DOI] [PubMed] [Google Scholar]
- 22.Raj GD, Jones R. Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet Immunol Immunopathol. 1996;53:147–161. doi: 10.1016/0165-2427(95)05545-2. [DOI] [PubMed] [Google Scholar]
- 23.Raj GD, Jones R. Growth of infectious bronchitis virus vaccines in oviducts derived from oestrogen-treated chicks and embryos. Vaccine. 1997;15:163–168. doi: 10.1016/S0264-410X(96)00157-0. [DOI] [PubMed] [Google Scholar]
- 24.Crinion RA, Ball RA, Hofstad MS. Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old. Avian Dis. 1971;15:42–48. doi: 10.2307/1588386. [DOI] [PubMed] [Google Scholar]
- 25.Jones RC, Jordan FT. Persistence of virus in the tissues and development of the oviduct in the fowl following infection at day old with infectious bronchitis virus. Res Vet Sci. 1972;13:52–60. doi: 10.1016/S0034-5288(18)34088-8. [DOI] [PubMed] [Google Scholar]
- 26.Broadfoot DL, Pomeroy BS, Smith WM. Effects of infectious bronchitis in baby chicks. Poult Sci. 1956;35:757–762. doi: 10.3382/ps.0350757. [DOI] [Google Scholar]
- 27.Benyeda Z, Mato T, Süveges T, et al. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathol. 2009;38:449–456. doi: 10.1080/03079450903349196. [DOI] [PubMed] [Google Scholar]
- 28.Crinion RA. Egg quality and production following infectious bronchitis virus exposure at one day old. Poult Sci. 1972;51:582–585. doi: 10.3382/ps.0510582. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Q, Hu Y-x, Jin J-h, Zhao Y, Zhao J, Zhang G-z. Pathogenicity of virulent infectious bronchitis virus isolate YN on hen ovary and oviduct. Vet Mic. 2016;193:100–105. doi: 10.1016/j.vetmic.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Hong SM, Kwon HJ, Kim IH, Mo ML, Kim JH. Comparative genomics of Korean infectious bronchitis viruses (IBVs) and an animal model to evaluate pathogenicity of IBVs to the reproductive organs. Viruses. 2012;4:2670–2683. doi: 10.3390/v4112670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balestrin E, Fraga AP, Ikuta N, Canal CW, Fonseca AS, Lunge VR. Infectious bronchitis virus in different avian physiological systems—a field study in Brazilian poultry flocks. Poult Sci. 2014;93:1922–1929. doi: 10.3382/ps.2014-03875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacón JL, Rodrigues JN, Assayag MS, Jr, Peloso C, Pedroso AC, Ferreira AJP. Epidemiological survey and molecular characterization of avian infectious bronchitis virus in Brazil between 2003 and 2009. Avian Pathol. 2011;40:153–162. doi: 10.1080/03079457.2010.544641. [DOI] [PubMed] [Google Scholar]
- 33.Janssen SJ, Kirby JD, Hess RA, Rhoads M, Bunick D, Bailey KL, Parsons CM, Wang H, Bahr JM. Identification of epididymal stones in diverse rooster populations. Poult Sci. 2000;79:568–574. doi: 10.1093/ps/79.4.568. [DOI] [PubMed] [Google Scholar]
- 34.Villarreal LYB, Brandao PE, Chacón JL, Assayag MS, Jr, Maiorka PC, Raffi P, Saidenberg ABS, Jones RC, Ferreira AJP. Orchitis in roosters with reduced fertility associated with avian infectious bronchitis virus and avian metapneumovirus infections. Avian Dis. 2007;51:900–904. doi: 10.1637/7815-121306-REGR4.1. [DOI] [PubMed] [Google Scholar]
- 35.Mahecha G, Oliveira C, Balzuweit K, Hess R. Epididymal lithiasis in roosters and efferent ductule and testicular damage. Reproduction. 2002;124:821–834. doi: 10.1530/rep.0.1240821. [DOI] [PubMed] [Google Scholar]
- 36.Gallardo RA, Hoerr FJ, Berry WD, van Santen VL, Toro H. Infectious bronchitis virus in testicles and venereal transmission. Avian Dis. 2011;55:255–258. doi: 10.1637/9592-102910-Reg.1. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore H, Compton C, Alper J, Vaught T. Biospecimen research network symposium: advancing cancer research through biospecimen science. Cancer Res. 2009;69:6770–6772. doi: 10.1158/0008-5472.CAN-09-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casais R, Dove B, Cavanagh D, Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toro H, Van Santen VL, Jackwood MW. Genetic diversity and selection regulates evolution of infectious bronchitis virus. Avian Dis. 2012;56:449–455. doi: 10.1637/10072-020212-Review.1. [DOI] [PubMed] [Google Scholar]
- 41.Gallardo RA, van Santen VL, Toro H. Effects of chicken anaemia virus and infectious bursal disease virus-induced immunodeficiency on infectious bronchitis virus replication and genotypic drift. Avian Pathol. 2012;41:451–458. doi: 10.1080/03079457.2012.702889. [DOI] [PubMed] [Google Scholar]
- 42.Rocha E, Cox NJ, Black RA, Harmon MW, Harrison CJ, Kendal AP. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J Virol. 1991;65:2340–2350. doi: 10.1128/jvi.65.5.2340-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Moneim AS, El-Kady MF, Ladman BS, Gelb J., Jr S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol J. 2006;3:78. doi: 10.1186/1743-422X-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 45.Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]


