Abstract
Brazilian data for maternal GBS colonization shows different prevalence rates. This conflicting data may be related to the absence of an official recommendation from the Federal Brazilian Health Authorities describing guidelines and protocols to perform GBS screening in pregnant women, in both public and private clinics. In the present review, we evaluated published reports addressing the prevalence of GBS in different regions of the country, methods used, and, when available, information regarding antibiotic resistance and serological typing of clinical isolates. According to this review, GBS prevalence in pregnant women in Brazil ranged from 4.2 to 28.4%, in the last 10 years. Serotype Ia was the most prevalent. The highest antibiotic resistance rates were found for tetarcycline, although its use to treat GBS infections is not common. Our results also show high resistance rates to clindamycin and erythromycin, which are commonly used as an alternative to penicillin in GBS infecctions. The increased antibiotic resistance, variations in serotype distribution, and high GBS prevalences need to be further investigated. Based on the present situation, recommendations regarding GBS surveillance in the country were raised and may improve our strategies for preventing neonatal infections.
Keywords: Streptococcus agalactiae, Sepsis, Antibiotic resistance, Pregnancy
Introduction
Group B Streptococcus (GBS) encompasses different Streptococcus agalactiae strains, frequently found as a commensal bacterium in vaginal and intestinal microbiota, but also represents a leading cause of newborn sepsis [1, 2]. Ascension of GBS into the uterus can potentially infect the fetus through aspiration of the amniotic fluid and could cause infection in the neonate in the case of a vaginal delivery [3]. According to the World Health Organization (WHO), GBS causes 150,000 stillbirths and infant deaths globally, despite the effectiveness of intrapartum antibiotic prophylaxis (IAP) [4]. The Centers for Disease Control and Prevention (CDC) released a set of guidelines in 2010, which included recommendations for GBS screening and intrapartum prophylaxis [5].
Different rates of maternal colonization with GBS are observed worldwide, depending on the geographic region and national income. In 2017, a Cochrane Library Report was published discussing the effectiveness of intrapartum antibiotic prophylaxis for GBS. In this review, the global rates of maternal colonization reported ranged from 6 to 36% in Europe, from 19 to 22% in Africa, and 14% in the Americas [6]. The colonization rate in non-pregnant women also varies around the world.
GBS screening, if carried out between 35 and 37 weeks of gestation along with intrapartum prophylaxis, is essential for reducing negative outcomes in pregnancy [7]. An important step in sepsis prevention is the identification of the microorganism, with specimen recovery, and the use of an enrichment broth for better culture yield [5]. The mother-to-neonate GBS transmission rates also vary around the world, depending on the prophylaxis protocol adopted [6], and the reports describe rates of around 0.53 per 1000 live births [1]. The CDC recommendation for a positive GBS test is IAP. Samples are collected using swabs from both the lower vagina and the rectum. Penicillin G is administered intravenously every 4 h until the fetus is completely expelled, which is the end of labor [5]. Intrapartum prophylaxis is also recommended for patients with the following risk factors: gestational age below 37 weeks, intrapartum temperature above 38 °C, membrane rupture lasting for 18 h or more, intra-amniotic infection, and previous delivery of an infant with GBS infection [5].
There are two main approaches to detect a potential Group B Streptococcus infection: a risk-based approach and a culture-based one [8]. Both strategies have advantages and flaws [9, 10]. However, the rate of GBS infections has decreased significantly since universal screening was adopted, while it has increased in the United Kingdom (UK), where universal screening was denied in favor of a risk-based approach [10]. In this risk-based approach adopted in the UK, the mother receives intrapartum antibiotics if there are a few risk factors involved, such as intrapartum fever or premature membranes rupture [8, 10, 11].
The culture-based approach consists in universal screening during prenatal care [5] and is adopted in the USA [10]. According to the Brazilian Health Regulatory Agency (Anvisa), the first step for GBS identification is to differentiate it from Staphylococcus spp. using the catalase test. If catalase is negative, more specific tests are conducted [12]. However; the most common methods for GBS identification are latex agglutination and the CAMP test, due to their specificity [13].
In Brazil, there are recommendations for the prevention of GBS infection and newborn sepsis [14]. We also found a few local protocols containing recommendations for GBS screening [15–18], although there is not a national consensus. According to the Brazilian Health Authorities, there are no sufficient elements that would justify constant screening and intrapartum prophylaxis [19]. This review aimed at searching the Brazilian literature from the past 10 years (2008–2018) in order to provide information that could help understanding GBS profile in Brazil and guide improved guidelines for GBS screening.
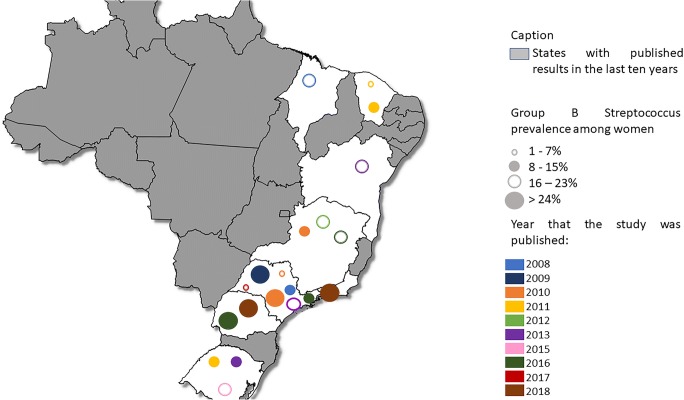
A total of twenty-one studies on GBS prevalence in Brazil were used in this review. One of these studies obtained data on newborns, and the other twenty articles researched prevalence in pregnant women, and their results are summarized in Table 1 and Fig. 1. Only eight states were represented, showing that although a lot of information on GBS from the past 10 years was gathered, all data is concentrated in a few regions and might underrepresent the real prevalence in the Brazilian population. Most of the Brazilian studies on GBS prevalence among women are from the state of São Paulo, followed by Minas Gerais, and Rio Grande do Sul (Table 1). There are no studies reporting GBS prevalence in the North and Midwest regions in Brazil.
Table 1.
Overview of GBS prevalence among pregnant women in Brazil from 2008 to 2018
| Year of publication | Location | Number of women | Prevalence n(%) | Specimen* (E, V, R) |
Analysis** (B, S, M) |
Reference |
|---|---|---|---|---|---|---|
| Northeast Region | ||||||
| 2008 | Maranhão | 201 | 41(20.4) | V + Ra | B | 21 |
| 2011 | Ceará | 112 | 10(8.9) | V + Ra | B | 23 |
| 2011 | Ceará | 213 | 9(4.2) | V + Rb | B;S | 22 |
| 2013 | Bahia | 23 | 4(17.4) | V + Rb | B;S | 24 |
| Southwest Region | ||||||
| 2008 | São Paulo | 212 | 20(9.4) | E | B | 30 |
| 2009 | São Paulo | 203 | 56(27.6) | V + Ra | B | 25 |
| 2010 | São Paulo | 405 | 103(25.4) | V + Rc | B | 28,29 |
| 2010 | São Paulo | 129 | 3(2.33) | V + Ra | B | 26 |
| 2010 | Minas Gerais | 221 | 21(9.5) | V + Ra | B; M | 34 |
| 2012 | Minas Gerais | 911 | 152(16.7) | V + Re | B | 36 |
| 2013 | São Paulo | 208 | 4(17,4) | V | B | 32 |
| 2016 | Minas Gerais | 108 | 19(17.5) | V + Ra | B; S | 37 |
| 2016 | São Paulo | 1.717 | 193(11.24) | V + Ra | B | 31 |
| 2017 | São Paulo | 560 | 24(4.3) | V | B | 27 |
| 2018 | Rio de Janeiro | 689 | 956(26.2) | V + Rb | B; S | 33 |
| South Region | ||||||
| 2011 | Rio Grande do Sul | 36 | 4(11.11) | V + Ra | B | 42 |
| 2013 | Rio Grande do Sul | 1.146 | 83(7.2) | V + Re | B | 40 |
| 2015 | Rio Grande do Sul | 80 | 18(22.5) | V + Ra | B; S | 41 |
| 2016 | Paraná | 544 | 136(25) | V + Ra | B; S | 39 |
| 2018 | Paraná | 496 | 141(28.4) | V + Rd | B; S | 38 |
*Specimen – E: endocervix. V: vaginal. R: rectal
**Analysis – B: bacteriological methods. S: serology. M: molecular methods
a: Two swabs inoculated separately; b: One swab used for both vaginal and rectal region. c: Two vaginal swabs and one rectal swab. d: Three vaginal swabs and three rectal swabs. e: Information regarding swabs was not clear in the article.
Fig. 1.
Geographic distribution of GBS prevalence in pregnant women
The Northeast region
Only three states from the Northeast region were represented in GBS screening of the last 10 years in Brazil: Maranhão, Ceará, and Salvador. One study was conducted in Maranhão, in the year of 2005–2006, and the prevalence found was 20.4% [20]. Two studies from Ceará were published in 2011, and the prevalence rates of GBS colonization in pregnant women were 9.8% [21] and 8.9% [22]. Despite the difference in prevalence rates, each of these three studies enrolled around 200–210 pregnant women and all of them used standard microbiology methods for GBS identification. In 2012, a study with 23 pregnant women in Vitoria da Conquista, Salvador, Bahia [23], identified GBS in 17.4% of the enrolled participants. No recent articles for the Northeast region were found.
The Southeast region
Most of the available data from GBS screening in Brazil in the last 10 years is from studies conducted in the Southeast region, mainly in the states of São Paulo and Rio de Janeiro. A lot of fluctuation is seen in São Paulo, with a large gap between the highest (27.6%) [24] and the lowest (2.33%) [25] prevalence. Among the studies conducted in the state of São Paulo, only two were conducted in the city of São Paulo.
The colonization rate in Votuporanga was the lowest one, 2.33% in 123 pregnant women [25]. In Campinas, the prevalence was 27.6% in 203 pregnant women, including those with premature labor, premature membranes rupture, or both, between the 22nd and 36th gestational weeks, which could be associated with the higher prevalence rate in the state of São Paulo [24], as these conditions are considered risk factors for GBS infection [5, 24]. In the city of Bauru, the prevalence rate was 4.3%, based on a retrospective study collecting documents from a hospital [26].
In Botucatu, 405 specimens were obtained from pregnant women and evaluated in two studies [27, 28], showing a colonization rate of 25.4%. One study evaluated the bacterioscopic exams of vaginal specimens with a positive correlation between candidiasis and cytolytic vaginosis and a higher rate of GBS colonization [28]. The other study evaluated the differences in collection swabs from the rectal and vaginal sites, or both. Vaginal specimens had high positivity rates, but there were more GBS-positive swabs obtained from the rectum [27]. Both studies lead to the conclusion that candidiasis and cytolytic vaginosis could be considered risk factors for GBS infection, and also that an effective protocol needs to include swabs from both the rectal and vaginal regions in order to obtain higher sensitivity [27, 28].
A study performed in the city of Sumaré, São Paulo, investigated GBS colonization in pregnant women with premature labor or premature membranes rupture. In this study, the authors collected specimens from the endocervix, and 14.2% of the specimens were colonized by GBS, although other pathogens were also found, such as Candida sp. GBS was isolated in 9.4% of the specimens [29]. The authors pointed out the correlation between GBS in the endocervix and infectious morbidity in mothers and neonates, highlighting the pathogenicity of the microorganism, which is capable of ascending from the lower urinary tract, as well as crossing the placenta and reaching the amniotic fluid [29].
In the city of São Paulo, both prevalence studies were retrospective studies conducted in a hospital, in which the records were analyzed looking for GBS positivity in swabs from both the rectal and vaginal regions of pregnant women. The prevalence rates were 11.24% [30] and 17.4% [31]. The authors highlight an issue with the lack of screening protocols: in one study, 22.3% of women were not screened at proper gestational age, which could influence not only the prevalence results but also antibiotic prophylaxis, which is essential to prevent newborn GBS infection [30]. In the other study, only 76.7% of women were screened for GBS [31].
Recently, Botelho et al. published an observational study for an 8-year period, from 2008 to 2015, collecting specimens from 3647 pregnant women with a gestational age of 35 to 37 weeks in Rio de Janeiro [32]. The prevalence rate was 26.32%, which is the second highest prevalence in Brazil in the past 10 years. There was no significant fluctuation in prevalence throughout the years covered by this study [32], which means that colonization rates have been high ever since the study started, reinforcing the need for screening and prophylaxis strategies. A few clinical aspects were collected, such as preterm birth, urinary tract infection, history of GBS, use of antibiotics, and vaginal discharge. Only the latter was significantly associated with GBS colonization. In this study, 14% of women who had a positive culture did not show positive results in a risk-based approach, which considered their clinical conditions [32]. Certainly, clinical aspects must be taken into account; however, they are not reliable as the only condition to determine the administration of IAP [10].
Among all the studies in the last 10 years in Brazil, only one used molecular methods (polymerase chain reaction (PCR)) in order to confirm GBS-positive specimens. The study was conducted in Juiz de Fora, Minas Gerais. The prevalence obtained by PCR was 32.6%, and the one obtained using classic methods was 9.5% [33]. The classic microbiological method is currently considered the gold standard for GBS isolation. However, the molecular method showed a much higher prevalence rate, which suggests that the cultured-based approach might generate false-negative results. In this research paper, the authors highlight the need for systematization of the protocols [33]. Nevertheless, a faster, more sensitive, and reliable method such as PCR could be employed alongside the culture in order to diminish false-negative results obtained using PCR alone, thereby allowing more women to receive treatment, especially in cases when there is no proper prenatal care [34].
GBS prevalence in Minas Gerais from 2007 to 2009 suffered a lot of fluctuations: in 2007, it was 2.8%; in 2008, it was 14%; and in 2009, it was 27.5%. The average colonization rate was 16.7% [35]. In 2011, the prevalence was 17.5%, with specimens from 108 pregnant women, using the microbiological approach [36]. The authors proposed a constant epidemiological surveillance system and the strengthening of the current public health system in order to prevent and treat GBS infections and reduce the risk for newborn sepsis [36].
The South region
Two studies were conducted in the State of Paraná from 2011 to 2014, with a sample size of 496 [37] and 544 [38] women. These studies showed prevalence rates of 28.4% [37] and 25% [38], which are the highest prevalence rates among all studies analyzed in this review. In addition to classical microbiological methods, they used latex agglutination [37, 38].
There was an increase in GBS prevalence in the state of Rio Grande do Sul, considering the results over the last 10 years [39–41]. A study with 36 specimens from pregnant women during the year 2006 using culture and latex agglutination for GBS identification found a prevalence of 11.11%. In addition, there was one case of meningitis caused by GBS in a newborn whose mother was colonized by GBS [41]. In 2011 and 2012, 1041 non-pregnant and 105 pregnant women were enrolled in a study where the prevalence was 15.2% among pregnant women and 6.4% in non-pregnant women [39]. In 2013, another study evaluated specimens from 80 pregnant women and the prevalence rate was 22.5% [40]. The authors compared two identification methods: when the classical method was used alone, 33.75% of women were considered positive, while when it was used along with latex agglutination, the prevalence obtained was 22.5% [40]. The difference shows that the serology must be used with culture-based methods in order to estimate prevalence more accurately [8, 40].
Despite the lack of references to newborn infection in other studies over the last 10 years, the increase in colonization seen in these three studies and the risk for newborn infection reinforce the need for GBS screening and prophylaxis [39–41].
Serotypes
The serological classification of GBS is based on the specific capsular polysaccharide [42]. Currently, 10 different capsular serotypes (Ia, Ib, and II–IX) have been described [8, 43, 44]. All serotypes are capable of causing invasive diseases; however, serotypes Ia, Ib, II, III, and V are responsible for most diseases in neonates and adults worldwide [45, 46]. There is a correlation between serotype and pathogenicity, for example, serotype III is responsible for 90% of all late sepsis cases [47], and it has a high efficiency in crossing the blood-brain barrier [3]. Furthermore, an increase in serotype V prevalence among the population could have an impact on the rate of diseases, since it is commonly associated with invasive diseases in adults [48], newborn sepsis, and infections in pregnant women [8].
The prevalence and distribution of serotypes differ between geographic regions, ethnic populations, and clinical presentations [49]. Data collected about serotype distribution in distinct geographic areas should be used as a basis for the development of vaccine proposals [47, 50].
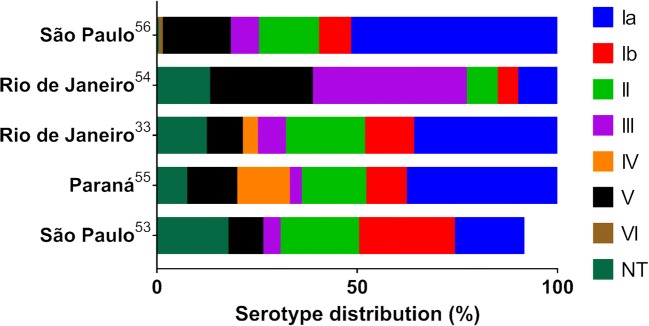
There is a divergence in serotype distribution among pregnant women from different locations in Brazil [32, 51–54]. Few studies evaluated GBS serotypes, and from 2008 to 2017, most of them were performed in the Southeast Region (Fig. 2). In 2002, serotype III was the most abundant in pregnant women, followed by serotypes V and Ia [52]. Some years later, serotype Ia was the most prevalent, followed by serotypes II and Ib. [32] Both studies were conducted in Rio de Janeiro. In São Paulo, serotype Ia was the most abundant followed by V and II in a study conducted in 2015–2016 [54], suggesting that the increased prevalence in serotype Ia may be related with a period of time. In Paraná, serotype Ia was also the most prevalent; however, there was a surprisingly high number of serotype IV strains [53]. The authors suggest that the microorganism was acquired from the environment [53], and although GBS could survive the hospital environment [55, 56], it is not possible to determine the source precisely [53].
Fig. 2.
GBS serotype prevalence in pregnant women
In human specimens, including non-pregnant women, serotype Ia has been the most frequent in recent years [57, 58]. The global data from serotyping prevalence also reports Ia, Ib, II, III, and V as the most prevalent serotypes in pregnant women in all continents in reports published between 1997 and 2015 [60] (Fig. 2).
GBS neonatal sepsis
Newborn sepsis is a major health issue, and one of the reasons is that the symptoms are not specific, such as hyperglycemia, respiratory insufficiency, apnea, and bleeding. When symptoms appear between the first 24 h and 6 days of life, the condition is called early-onset sepsis (EOS). Late-onset sepsis (LOS) is when symptoms appear after this period [8, 33]. There is insufficient information on newborn sepsis in the Brazilian literature, which might be the reason why this subject receives so little attention, but there should be public policies structured and shaped by scientific knowledge.
Barbosa et al. highlight the lack of an official program for GBS prophylaxis in Brazil [60]. Their group gathered data on EOS in a Brazilian hospital (Hospital de Clínicas de Uberlandia, HC-U). The incidence of newborn sepsis caused by GBS was 0.90 cases per 1000 live births, the fatality rate was 50%, and one of the surviving patients had neurological damage as a consequence of the GBS infection. The mothers either were not screened for GBS or, despite a positive GBS culture, the result was not delivered in time for antibiotic prophylaxis. According to Barbosa et al., these results not only reinforce the need for GBS screening but also demand the development of a faster diagnosis method [60].
Cases of GBS infection without any diagnosis from the mother are not rare. In a study in a hospital in Brasilia, for all cases of GBS infection in newborns, none of the mothers had been screened for S. agalactiae, even when the patients presented risk factors. The fatality for GBS infection in neonates for this report was 62.5%, five deaths in eight infected neotnates. Five newborns were delivered by vaginal labor (three deceases), and the other three were delivered by cesarean surgery (two deceases). All of those cases might be preventable if universal screening and antibiotic prophylaxis had been implemented [61, 62].
An analysis of medical records from both pregnant women and their newborns in Rio de Janeiro showed that over 48% of the colonized mothers did not receive appropriate therapy. Although prophylaxis has been prescribed, the prescription, dose, and intervals in between antibiotic administration were incorrect. Also, women went through labor less than 4 h after antibiotic administration. Many of the swabs were not collected in the recommended time frame during the prenatal period, which could underestimate the incidence of positive cultures. In these conditions, the incidence of sepsis was higher in newborns from mothers in which intrapartum antibiotic prophylaxis was not done properly [63].
Prophylaxis in Brazil
Besides local government recommendations [15–18], a group from the Federal University of Pernambuco proposed a protocol based on the CDC guidelines, and submitted it to obstetricians and pediatricians, who evaluated the protocol as judges. The sepsis prevention protocol was considered adequate, and the group recommends the implementation of universal screening at other institutions [64]. Despite this validation, no other research or institution was using this protocol at the time of this publication.
Universal screening is strongly recommended, and there are a few local Brazilian protocols. However, low adherence in clinical routine impairs prophylaxis. One effort to improve adherence to universal GBS screening is simply to provide information to doctors. When detailed information is provided to obstetricians, there is an improvement in the number of women screened for GBS, although more long-term research is needed [65].
In regard to prenatal care, both healthcare professionals and mothers need to be aware of the risk factors, the importance of screening for this microorganism, and antibiotic prophylaxis. A study by the University of São Paulo applied a questionnaire to women in a maternity ward, and the results showed that these patients did not have enough information about GBS screening, and the authors concluded that this could happen in other regions and hospitals [66].
A joint effort to inform both healthcare professionals and pregnant women of the importance of GBS is critical to improve adherence to universal screening guidelines and antibiotic prophylaxis, and may decrease the incidence of newborn sepsis among Brazilian neonates.
An alternative to IAP is a vaccine given to pregnant women. Currently there are vaccines on the clinical trial phase, but they are still not available to the general population [46]. Vaccines could be a more powerful tool to prevent diseases caused by GBS, protecting mothers and children [46, 67].
Antibiotic resistance
GBS was initially associated with bovine mastitis; however, they can infect and cause diseases in humans [8]. According to Multilocus Sequence Typing (MLST), most of human isolated GBS belong to six clonal complexes (CCs) [68]. Sequencing the genome of 229 strains isolated from humans all over the globe showed that the increase of GBS diseases is associated with the dominance of tetracycline-resistant clones [69].
Due to certain advantages, such as its broad spectrum of action, low toxicity, and low cost, tetracycline has been widely used, and resulted in a high rate of GBS resistance, as detected in many studies [38]. The acquisition of additional resistance markers was an important step for its evolution as a human pathogen. Resistance to erythromycin emerged not only among pregnant women but also among non-pregnant adults [70], and resistance to clindamycin was found in 28% of the isolates from severe GBS disease in the USA [71].
The strategy recommended by the Centers for Disease Control to reduce neonatal GBS infection is the IAP [71]. Although IAP leads to an 80% reduction in the incidence of GBS in the first days of life, significantly reducing the rates of disease caused by the microorganism, the frequent use of antibiotics has contributed to the selection of resistant strains [72], which may appear during the perinatal period.
Although GBS strains isolated from humans in other countries have shown an antibiotic resistance profile [73], there is poor quality of information regarding changes in the profile of antibiotic-resistant strains in Brazil, where we count on fragmented data based mainly on local studies [57].
A study performed in Rio de Janeiro, Brazil, found results indicating that there is an increase in erythromycin resistance, affecting 13.2% of the clinical isolates tested, more than 85% of which had the cMLSB phenotype. Resistance to tetracycline was 81.7% [74]. Another study conducted in Rio de Janeiro reported 14% erythromycin resistance and 5% clindamycin resistance. For tetracycline, resistance was found in 83% of the isolates [75].
A group from Paraná, Brazil, evaluated 544 pregnant women, and among these, 136 (25%) were positive for GBS. Resistance levels were identified for erythromycin (8.1%) and clindamycin (2.2%). In addition, the authors showed a high rate of GBS resistance to tetracycline (82.3%) [38].
Botelho et al. (2018), in Rio de Janeiro, showed that 592 GBS strains were resistant to different antibiotics. The resistance percentages observed among isolates were 5% for chloramphenicol, 2% for clindamycin, 14% for erythromycin, 5% for levofloxacin, and 86% for tetracycline [32].
To date, based on the studies conducted in Brazil, all isolates presented sensitivity to ceftriaxone, penicillin, and vancomycin [74, 75]. In relation to this, recent studies have described strains with reduced susceptibility to penicillin in other countries, an alert situation for the main antibiotic used in IAP [76].
High levels of resistance to tetracycline have been found, reaching 86% [32, 74]. In a study conducted by Dutra et al. (2014), the level of resistance to tetracycline reached 97%. Resistance to erythromycin was found to be 4.1% and 3% to clindamycin [57]. Clindamycin or erythromycin are recommended as alternatives in IAP for penicillin-allergic women [71], and its resistance showed that in a short period of time, these antibiotics will no longer be a reliable alternative empiric therapy. This data can be evaluated in Table 2.
Table 2.
Overview of antibiotic resistance between isolates of GBS in Brazil from 1980 to 2015
| Period | Location | Antibiotic resistance rates (%) | Reference | ||
|---|---|---|---|---|---|
| Clindamycin | Erythromycin | Tetracycline | |||
| 1980–2006 |
Rio de Janeiro São Paulo Santa Catarina |
1.9 | 4 | 8.2 | 59 |
| 2008 | Rio de Janeiro | 16.7 | 13.2 | 81.7 | 74 |
| 2008–2009 | Rio de Janeiro | 5 | 14 | 83 | 75 |
| 2010 | Paraná | 2.2 | 8.1 | 82.3 | 39 |
| 2008–2015 | Rio de Janeiro | 2 | 14 | 86 | 33 |
Conclusion
Brazilian data for maternal GBS colonization shows different prevalence rates, as seen elsewhere, and might be explained by regional differences, as well as serotyping distribution and individual immunity [6]. The absence of an official recommendation from the Brazilian Health Authorities describing guidelines and protocols for performing GBS screening in pregnant women, both in public and private clinics, may contribute to the conflicting data described in this review. Universal screening could provide an adequate prophylaxis in colonized pregnant women, contributing to a reduction in the incidence of illness and the occurrence of sequelae in newborns, as indicated by other studies [23, 32, 33, 35].
The gold standard method for GBS identification is still the microbiological approach, with cultures from vaginal and anal swabs. Molecular biology identification may have a higher specificity and sensitivity than cultures; however, the higher cost and the need for skilled labor are major limitations for the public health service. Therefore, a higher rate of GBS identification could be obtained using cultures from both vaginal and anal swabs.
Unnecessary use of intrapartum antibiotic prophylaxis is also harmful to both mother and newborn. In order to avoid this, the recommendation of GBS screening earlier than 36 weeks of gestation, with subsequent delivery of the result in time for antibiotic prophylaxis, needs to be evaluated.
Recommendations regarding the present situation of GBS in Brazil and priorities to be followed in order to improve the present surveillance methods and/or approaches to combat antimicrobial resistance may be relevant for our Health Authorities.
As such, it would be premature to establish the withdrawal of GBS screening during the monitoring of pregnancies, as proposed for some states in Brazil [17].
Twenty studies were published in the last 10 years in Brazil, focusing in GBS screening and prevalence, but not all regions and states were studied in this period of time. Therefore, more studies are necessary around the country in order to reveal the actual prevalence of GBS, and even more relevant, the real consequences for newborn’s health.
Acknowledgments
We would like to thank Luis Carlos Ferreira for his helpful revision and comments.
Authors’ contribution
CSN conducted the literature research and wrote the manuscript. NFBS help to collected data and helped with manuscript writing. RCCF helped with the idea design and critically reviewed the manuscript. CRT designed the idea, followed the literature research and wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AKM, Cousens S, Heath PT. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379(9815):547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosen GH, Randis TM, Desai PV, Sapra KJ, Ma B, Gajer P, Humphrys MS, Ravel J, Gelber SE, Ratner AJ. Group B Streptococcus and the vaginal microbiota. J Infect Dis. 2017;216(6):744–751. doi: 10.1093/infdis/jix395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doran KS, Nizet V. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol Microbiol. 2004;54(1):23–31. doi: 10.1111/j.1365-2958.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- 4.WHO (2017) Group B Streptococcus infection causes an estimated 150,000 preventable stillbirths and infant deaths every year. Immunization, Vaccines Biol
- 5.Centers for Disease Control and Prevention. Verani JR, McGee L, Schrag SJ. Prevention of perinatal Group B Streptococcal disease. Revised Guidelines from CDC, 2010. Morb Mortal Wkly. 2010;59(RR-10):1–32. [PubMed] [Google Scholar]
- 6.Ohlsson A, Shah VS (2016) Intrapartum antibiotics for known maternal Group B streptococcal colonization (Review) Intrapartum antibiotics for known maternal Group B streptococcal colonization. (6):2014–2016. 10.1002/14651858.CD007467.pub4.Copyright
- 7.Taminato M, Fram D, Torloni MR, Belasco AGS, Saconato H, Barbosa DA. Screening for group b streptococcus in pregnant women: a systematic review and meta-analysis. Rev Lat Am Enfermagem. 2011;19(6):1470–1478. doi: 10.1590/S0104-11692011000600026. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MS, Nizet V, Baker CJ. Infectious diseases of the fetus and newborn. 6. Philadelphia, PA: Elsevier Saunders; 2011. Group B streptococcal infections; pp. 419–469. [Google Scholar]
- 9.Vornhagen J, Adams KMA, Rajagopal L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol. 2017;25(11):919–931. doi: 10.1016/j.tim.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns G, Plumb J. GBS public awareness, advocacy, and prevention-what’s working, what’s not and why we need a maternal GBS vaccine. Vaccine. 2013;31(S4):D58–D65. doi: 10.1016/j.vaccine.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Royal College of Obstetricians and Gynaecologists Prevention of early-onset neonatal Group B Streptococcal disease: Green-top Guideline No. 36. BJOG An Int J Obstet Gynaecol. 2017;124(12):e280–e305. doi: 10.1111/1471-0528.14821. [DOI] [PubMed] [Google Scholar]
- 12.Agência Nacional de Vigilância Sanitária Microbiologia clínica para o controle de infecção relacionada à assistência à saúde. Agência Nac Vigilância Sanitária - Anvisa. 2013;6:1–154. [Google Scholar]
- 13.Lang S, Palmer M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem. 2003;278(40):38167–38173. doi: 10.1074/jbc.M303544200. [DOI] [PubMed] [Google Scholar]
- 14.Costa HdPF (2011) Prevenção Da Doença Perinatal Pelo Estreptococo Do Grupo B 1–18
- 15.Júnior AdOF (2014) Protocolo de Assistência Materno Infantil Do Estado Do Rio Grande Do Norte
- 16.Kahhale S (2012) Protocolos de Obstetrícia : Descrição, Diagnóstico, Tratamento 1–4. 10.15713/ins.mmj.3
- 17.Secretaria de Estado da Saúde (2017) Protocolo de Atenção Ao Pré-Natal: Risco Habitual:1–4. 10.15713/ins.mmj.3
- 18.Secretaria Municipal de Saúde do Rio de Janeiro (SMS-Rio) (2016) Atenção ao Pré-Natal Rotinas para gestantes de baixo risco. Coleção Guia Ref Rápida. http://www.rio.rj.gov.br/dlstatic/10112/6552790/4176323/GuiaPrenatal_reunido.pdf
- 19.Brasil. Ministério Da Saúde (2012) Atenção Ao Pré-Natal de Baixo Risco. Brasilia: Editora do Ministério da Saúde. http://bvsms.saude.gov.br/bvs/publicacoes/cadernos_atencao_basica_32_prenatal.pdf
- 20.Costa AL d R, Lamy Filho F, Chein MB d C, Brito LMO, Lamy ZC, Andrade KL. Prevalência de colonização por estreptococos do grupo B em gestantes atendidas em maternidade pública da região Nordeste do Brasil. Rev Bras Ginecol Obs. 2008;30(6):274–280. doi: 10.1590/S0100-72032008000600002. [DOI] [PubMed] [Google Scholar]
- 21.Linhares JJ, Neto PGC, Vasconcelos JLM, et al. Prevalência de colonização por Streptococcus agalactiae em gestantes atendidas em maternidade do Ceará, no Brasil, correlacionando com os resultados perinatais. Rev Bras Ginecol e Obs. 2011;33(12):395–400. doi: 10.1590/S0100-72032011001200004. [DOI] [PubMed] [Google Scholar]
- 22.Ventura MSM, Rodrigues JLN, Feitosa FE d L, Junior CA d A, Almeida PC. Colonização por Streptococcus do Grupo B em Gestantes com Trabalho de Parto Prematuro e/ou Ruptura Prematura das Membranas. Arq Med. 2011;25(2):61–66. [Google Scholar]
- 23.Oliveira MV, Teles MF, Viana TA (2013) PREVALÊNCIA E FATORES DE RISCO ASSOCIADOS À COLONIZAÇÃO POR Streptococcusagalactiae EM GESTANTES ATENDIDAS NO HOSPITAL MUNICIPAL ESAÚ MATOS EM VITÓRIA DA CONQUISTA – BA. C&D-Revista Eletrônica da Fainor,. 6(1):172–184. http://srv02.fainor.com.br/revista/index.php/memorias/article/view/197%5Cnsrv02.fainor.com.br/revista237/index.php/memorias/article/.../197/146?
- 24.Nomura ML, Passini Júnior R, Oliveira UM, Calil R. Colonização materna e neonatal por estreptococo do grupo B em situações de ruptura pré-termo de membranas e no trabalho de parto prematuro. Rev Bras Ginecol e Obs. 2009;31(8):397–403. doi: 10.1590/S0100-72032009000800005. [DOI] [PubMed] [Google Scholar]
- 25.Rezende C, Azeredo A, Silveira DG, Malta RCG, de Castro V d CO, Miziara RC. PESQUISA DE Streptococcus agalactiae NA SECREÇÃO VAGINAL E ANAL DE GESTANTES DE UM MUNICÍPIO DO NOROESTE PAULISTA. Rev Uniara. 2010;13(2):194–201. [Google Scholar]
- 26.Martins BL, Jacob T, De Oliveira C. Prevalência de Streptococcus agalactiae em secreção vaginal de gestantes atendidas em um laboratório de análises clínicas do interior do estado de são Paulo. SALUSVITA. 2017;36(3):695–707. [Google Scholar]
- 27.Marconi C, Rocchetti TT, Rall VLM, De Carvalho LR, Borges VTM, Da Silva MG. Detection of Streptococcus agalactiae colonization in pregnant women by using combined swab cultures: cross-sectional prevalence study. Sao Paulo Med J. 2010;128(2):60–62. doi: 10.1590/S1516-31802010000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocchetti TT, Marconi C, Rall VLM, Borges VTM, Corrente JE, Da Silva MG. Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet. 2010;283(4):717–721. doi: 10.1007/s00404-010-1439-8. [DOI] [PubMed] [Google Scholar]
- 29.Lajos GJ, Passini Junior R, Nomura ML, Amaral E, Pereira BG, Milanez H, Parpinelli MÂ. Colonização bacteriana do canal cervical em gestantes com trabalho de parto prematuro ou ruptura prematura de membranas. Rev Bras Ginecol e Obs. 2008;30(8):393–399. doi: 10.1590/S0100-72032008000800004. [DOI] [PubMed] [Google Scholar]
- 30.Higashi AB, da Silva IR, Goldman RE. Prevalence of Streptococcus of Group B in pregnant women and the relation with neonatal infection. Rev Enferm e Atenção à Saúde. 2016;5(1):24–36. [Google Scholar]
- 31.Função JM, Narchi NZ. PESQUISA DO ESTREPTOCOCO DO GRUPO B EM GESTANTES DA ZONA LESTE DE SÃO PAULO. Rev da Esc Enferm da USP. 2013;47(1):22–29. doi: 10.1590/S0080-62342013000100003. [DOI] [PubMed] [Google Scholar]
- 32.Botelho ACN, Oliveira JG, Damasco AP, Santos KTB, Ferreira AFM, Rocha GT, Marinho PS, Bornia RBG, Pinto TCA, Américo MA, Fracalanzza SEL, Teixeira LM. Streptococcus agalactiae carriage among pregnant women living in Rio de Janeiro, Brazil, over a period of eight years. PLoS One. 2018;13(5):e0196925. doi: 10.1371/journal.pone.0196925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellano-Filho DS, da Silva VL, Nascimento TC, Vieira M d T, Diniz CG. Detection of group B Streptococcus in Brazilian pregnant women and antimicrobial susceptibility patterns. Braz J Microbiol. 2010;41(4):1047–1055. doi: 10.1590/S1517-83822010000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkins KL, Atkinson RM, Shanks A, Parvin CA, Dunne WM, Gross G. Evaluation of polymerase chain reaction for group B streptococcus detection using an improved culture method. Obstet Gynecol. 2006;108(3 Pt 1):488–491. doi: 10.1097/01.AOG.0000228961.42272.31. [DOI] [PubMed] [Google Scholar]
- 35.Bastos AN, Bastos RV, Dias VC, Bastos LQ d A, Souza RC d, Bastos VQ d A. Streptococcus agalactiae em gestantes : incidência em laboratório clínico de Juiz de Fora ( MG ) - 2007 a 2009. HU Rev. 2012;38(2):45–50. [Google Scholar]
- 36.Barbosa NG, de Brito DVD, dos Reis H, et al. Colonização Materna Por Estreptococos Do Grupo B : Prevalência E Suscetibilidade Aos Antimicrobianos. Rev Pesqui em Saúde. 2016;17(1):13–16. [Google Scholar]
- 37.de Melo SCCS, Balandis Costa A, Teixeira F, et al. Prevalence of Streptococcus agalactiae colonization in pregnant women from the 18th Health Region of Paraná State. Rev Inst Med Trop Sao Paulo. 2018;60(2):2–7. doi: 10.1590/S1678-9946201860002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Melo SCCS, Santos NC d S, Oliveira M d, et al. Antimicrobial susceptibility of Streptococcus agalactiae isolated from pregnant women. Rev Inst Med Trop Sao Paulo. 2016;58(1):83. doi: 10.1590/S1678-9946201658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiss FS, Rossato J d S, Graudenz MS, Gutierrez LLP. Prevalência da colonização por Streptococcus agalactiae em uma amostra de mulheres grávidas e não grávidas de Porto Alegre, estado do Rio Grande do Sul. Sci Med (Porto Alegre) 2013;23(3):169–174. [Google Scholar]
- 40.Senger FR, Alves IA, Pellegrini D d CP, Prestes DC, Souza EF d, Corte ED. Prevalência da colonização por Streptococcus agalactiae em gestantes atendidas na rede pública de saúde de Santo Ângelo/RS. Rev Epidemiol e Control Infecção. 2015;6(1):1–5. doi: 10.17058/reci.v6i1.6272. [DOI] [Google Scholar]
- 41.Veit AR, Roehrs MCSM, Mayer LE, Santos SO, Martini R, Tizotti MK, Kempfer CB, Martins VR, Fronza L, Cunha MDC, Colpo PR, Konopka CK, Horner R. Colonization prevalence and susceptibility of Streptococcus agalactiae in pregnant women at HUSM. Saúde (Santa Maria) 2011;36(1):9–14. doi: 10.5902/223658342391. [DOI] [Google Scholar]
- 42.Kapatai G, Patel D, Efstratiou A, Chalker VJ. Comparison of molecular serotyping approaches of Streptococcus agalactiae from genomic sequences. BMC Genomics. 2017;18(1):1–11. doi: 10.1186/s12864-017-3820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods. 2010;80(2):212–214. doi: 10.1016/j.mimet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol. 2007;45(9):2929–2936. doi: 10.1128/JCM.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis. 2009;49(1):85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 46.Nuccitelli A, Rinaudo CD, Maione D. Group B Streptococcus vaccine: state of the art. Ther Adv Vaccines. 2015;3(3):76–90. doi: 10.1177/2051013615579869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;2008(17):2056–2065. doi: 10.1016/S0084-3873(08)79076-X. [DOI] [PubMed] [Google Scholar]
- 48.Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, Wessels MR, Paulsen IT, Nelson KE, Margarit I, Read TD, Madoff LC, Wolf AM, Beanan MJ, Brinkac LM, Daugherty SC, DeBoy RT, Durkin AS, Kolonay JF, Madupu R, Lewis MR, Radune D, Fedorova NB, Scanlan D, Khouri H, Mulligan S, Carty HA, Cline RT, van Aken SE, Gill J, Scarselli M, Mora M, Iacobini ET, Brettoni C, Galli G, Mariani M, Vegni F, Maione D, Rinaudo D, Rappuoli R, Telford JL, Kasper DL, Grandi G, Fraser CM. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A. 2002;99(19):12391–12396. doi: 10.1073/pnas.182380799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11(3):497–513. doi: 10.1128/CMR.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Doare K, Faal A, Jaiteh M, et al. Association between functional antibody against Group B Streptococcus and maternal and infant colonization in a Gambian cohort. Vaccine. 2017;35(22):2970–2978. doi: 10.1016/j.vaccine.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simoes JA, Alves VMN, Fracalanzza SEL, Camargo RPS, Mathias L, Milanez HMBP, Brolazo EM. Phenotypical characteristics of group B streptococcus in parturients. Braz J Infect Dis. 2007;11(2):261–266. doi: 10.1590/S1413-86702007000200019. [DOI] [PubMed] [Google Scholar]
- 52.Soares GCT, Alviano DS, da Silva Santos G, Alviano CS, Mattos-Guaraldi AL, Nagao PE. Prevalence of Group B Streptococcus serotypes III and V in pregnant women of Rio de Janeiro, Brazil. Braz J Microbiol. 2013;44(3):869–872. doi: 10.1590/S1517-83822013000300032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmeiro JK, Dalla-Costa LM, Fracalanzza SEL, Botelho ACN, da Silva Nogueira K, Scheffer MC, de Almeida Torres RSL, de Carvalho NS, Cogo LL, Madeira HMF. Phenotypic and genotypic characterization of group B streptococcal isolates in southern Brazil. J Clin Microbiol. 2010;48(12):4397–4403. doi: 10.1128/JCM.00419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade PD, Russo J d S, Gouveia JB, et al. Molecular characterization of group B Streptococcus serotypes by multiplex polymerase chain reaction. Med Express. 2017;4(4):6–8. doi: 10.5935/MedicalExpress.2017.04.06. [DOI] [Google Scholar]
- 55.Nagano N, Nagano Y, Toyama M, Kimura K, Tamura T, Shibayama K, Arakawa Y. Nosocomial spread of multidrug-resistant group B streptococci with reduced penicillin susceptibility belonging to clonal complex 1. J Antimicrob Chemother. 2012;67(4):849–856. doi: 10.1093/jac/dkr546. [DOI] [PubMed] [Google Scholar]
- 56.Easmon CSF, Hastings MJG, Clare AJ, et al. Nosocomial transmission of group B streptococci. Br Med J (Clin Res Ed) 1981;283(6289):459–461. doi: 10.1136/bmj.283.6289.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutra VG, Alves VMN, Olendzki AN, Dias CAG, de Bastos AFA, Santos GO, de Amorin ELT, Sousa MÂB, Santos R, Ribeiro PCS, Fontes CF, Andrey M, Magalhães K, Araujo AA, Paffadore LF, Marconi C, Murta EFC, Fernandes Jr PC, Raddi MSG, Marinho PS, Bornia RBG, Palmeiro JK, Dalla-Costa LM, Pinto TCA, Botelho ACN, Teixeira LM, Fracalanzza SEL. Streptococcus agalactie in Brazil: serotype distribution, virulence determinants and antimicrobiol suscetibility. BMC Infect Dis. 2014;14(1):323–330. doi: 10.1186/1471-2334-14-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinto TCA, Costa NS, Vianna Souza AR, da Silva LG, Corrêa ABA, Fernandes FG, Oliveira ICM, de Mattos MC, Rosado AS, Benchetrit LC. Distribution of serotypes and evaluation of antimicrobial susceptibility among human and bovine Streptococcus agalactiae strains isolated in Brazil between 1980 and 2006. Braz J Infect Dis. 2013;17(2):131–136. doi: 10.1016/j.bjid.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwatra Gaurav, Cunnington Marianne C, Merrall Elizabeth, Adrian Peter V, Ip Margaret, Klugman Keith P, Tam Wing Hung, Madhi Shabir A. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2016;16(9):1076–1084. doi: 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 60.Barbosa NG, dos Reis H, Mantese OC, Mussi-Pinhata MM, Abdallah VOS, Gontijo Filho PP. Early-onset neonatal sepsis by Group B Streptococcus in a Brazilian public hospital. Braz J Infect Dis. 2016;20(6):647–648. doi: 10.1016/j.bjid.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evangelista MLB, de Mello Freitas FT. Group B streptococcus neonatal infection in an intensive care unit in Brazil: high fatality and missed opportunities for antibiotic prophylaxis. Braz J Infect Dis. 2015;19(1):98–99. doi: 10.1016/j.bjid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (2010) Prevention of perinatal Group B Streptococcal disease. Morb Mortal Wkly. 10.1097/01.EDE.0000032431.83648.8D
- 63.Costa NDVL, De Carvalho M, Pone SM, Saint CG. Gestantes colonizadas pelo Streptococcus do grupo B e seus recémnascidos: Análise crítica da conduta adotada no Instituto Fernandes Figueira, Fundação Oswaldo Cruz. Rev Paul Pediatr. 2010;28(2):155–161. doi: 10.1590/S0103-05822010000200005. [DOI] [Google Scholar]
- 64.da Silva FA, Vidal CF d L, Araújo EC d. Validation of the content of the prevention protocol for early sepsis caused by Streptococcus agalactiaein newborns. Rev Lat Am Enfermagem. 2015;23(4):635–641. doi: 10.1590/0104-1169.0179.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva JM, Stein AT, Schünemann HJ, Bordin R, Kuchenbecker R, de Lourdes Drachler M. Academic detailing and adherence to guidelines for Group B streptococci prenatal screening: a randomized controlled trial. BMC Pregnancy Childbirth. 2013;13(68):1–5. doi: 10.1186/1471-2393-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Mello DS, Tsunechiro MA, Mendelski CA, Pierre SA, Silva AR, Padoveze MC. Group B Streptococcus: compliance with the information in prenatal card records and knowledge of pregnant women. Am J Infect Control. 2015;43(4):400–401. doi: 10.1016/j.ajic.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 67.Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-ter Meulen A, Dull PM. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31(S4):D52–D57. doi: 10.1016/j.vaccine.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 68.Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N. Serotype distribution, population structure, and antimicrobial resistance of Group B Streptococcus strains recovered from colonized pregnant women. Burnham C-AD, ed. J Clin Microbiol. 2017;55(2):412–422. doi: 10.1128/JCM.01615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Da Cunha V, Davies MR, Douarre PE, et al. Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amundson NR, Flores AE, Hillier SL, Baker CJ, Ferrieri P. DNA macrorestriction analysis of nontypeable group b streptococcal isolates: clonal evolution of nontypeable and type v isolates. J Clin Microbiol. 2005;43(2):572–576. doi: 10.1128/JCM.43.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States. Atlanta, Georgia; 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html
- 72.Glezen WP, Alpers M. Maternal immunization. Clin Infect Dis. 1999;28(2):219–224. doi: 10.1086/515122. [DOI] [PubMed] [Google Scholar]
- 73.Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MRB, Mavenyengwa RT, Lebelo SL, Monokoane ST, Tshepuwane C, Moyo SR. Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes. 2015;8(1):6–12. doi: 10.1186/s13104-015-1328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corrrêa AB d A, da Silva LG, Pinto T d CA, et al. The genetic diversity and phenotypic characterization of Streptococcus agalactiae isolates from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2011;106(8):1002–1006. doi: 10.1590/S0074-02762011000800017. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura PAM, Schuab RBB, Neves FPG, Pereira CFA, de Paula GR, Barros RR. Antimicrobial resistance profiles and genetic characterisation of macrolide resistant isolates of Streptococcus agalactiae. Mem Inst Oswaldo Cruz. 2011;106(2):119–122. doi: 10.1590/S0074-02762011000200001. [DOI] [PubMed] [Google Scholar]
- 76.Dahesh S, Hensler ME, Van Sorge NM, et al. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob Agents Chemother. 2008;52(8):2915–2918. doi: 10.1128/AAC.00461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]