Abstract
Control of brucellosis as a worldwide zoonotic disease is based on vaccination of animals and diagnosis of infected cases to be eradicated. Accurate and rapid detection of infected animals is of critical importance for preventing the spread of disease. Current detection of brucellosis is based on whole-cell antigens and investigating serum antibodies against Brucella lipopolysaccharide (LPS). The critical disadvantage is misdiagnosis of vaccinated animals as infected ones and also cross-reactions with other Gram-negative bacteria. Recombinant outer membrane protein 2b (Omp2b) of Brucella abortus was evaluated as a novel serodiagnostic target in comparison to conventional tests which are based on LPS. Recombinant Omp2b (rOmp2b) was expressed in Escherichia coli BL21 and purified by Ni2+-based chromatography. rOmp2b was evaluated in an indirect enzyme-linked immunosorbent assay (ELISA) system for diagnosis of brucellosis, with sera from Brucella-infected mice along with negative sera and sera from mice which were inoculated with other Gram-negative species for assurance of specificity. Thereafter, cattle sera collected from different regions were assessed along with known negative and known positive serum samples. We found that Omp2b can discriminate between Brucella-infected animals and non-infected ones. Results for assessment of two hundred and fifty cattle sera by Omp2b-based indirect ELISA which were compared to Rose Bengal plate agglutination test (RBPT) and serum tube agglutination test (SAT) showed that our proposed procedure has the sensitivity of 88.5%, specificity of 100%, and accuracy of 90.8%. We suggest that recombinant Omp2b could be used as a protein antigen for diagnosis of brucellosis in domestic animals and can be evaluated for detection of human brucellosis.
Keywords: Brucella; diagnosis, Omp2b, ELISA, Recombinant protein
Introduction
Brucellosis is a widespread zoonotic disease while its etiological agent, Brucella, is a Gram-negative, facultative intracellular bacterium. Brucellosis is a worldwide spread zoonosis infecting both animals and humans, especially in developing countries [1, 2]. Brucellosis is usually acquired after consumption of contaminated foods, especially dairy products, nonpasteurized or after occupational exposure to infected animals [3, 4]. The prevalence of the disease is very high in Iran [5], Turkey [6], and India [7, 8]. Vaccination of livestock by conventional attenuated strains, the B. melitensis Rev-1 and B. abortus S19, is the main strategy for controlling the disease in animals but could not protect humans against the disease [9]. Control of the disease is also depending upon efficient methods for screening and detection of brucellosis in livestock and humans [10–15].
The main target for serological diagnosis of Brucella infection is the smooth lipopolysaccharide (S-LPS) from the outer membrane of smooth strains [10, 14, 16–18]. Antibody titers against the O-polysaccharide portion of LPS last for a long time in the serum and there are no efficient method for discriminating antibodies elicited according to vaccination and infection [19]. Cross-reactions to other Gram-negative bacteria such as Yersinia enterocolitica O9, Vibrio, Escherichia, and Salmonella also occur that cause misdiagnosis of brucellosis in both humans and animals [20–23].
Brucellae are having the typical structure of Gram-negative bacteria with an outer membrane including a vast variety of proteins called outer membrane proteins (OMPs) [24]. Serological investigation of antibodies against Brucella outer membrane proteins significantly helped to overcome the false positive results due to cross-reacting antibodies which recognize O-polysaccharide epitopes and also differentiating vaccinated and infected animals; examples of which include Omp25 [25], BP26 [26], Omp28 [27, 28], and Omp31 [29]. Omp2b of Brucella is classified in the group II of OMPs which is known as major outer membrane proteins [24]. Omp2b is a well-known target for molecular detection of brucellosis which has been reported by some researchers [30–34] along with protective immunity assessment [35]. Here, we described the production and purification of B. abortus recombinant Omp2b protein in E. coli host and evaluating this protein as a serodiagnostic tool for detection of brucellosis in cattle sera.
Materials and methods
Bacterial strains and culture conditions
B. abortus S19, B. abortus 544, B. melitensis Rev-1, and B. melitensis (all from the culture collection in the Department of Bacteriology, Tarbiat Modares University) were routinely cultured on Brucella agar and incubated in 37 °C for 72 h. Escherichia coli O:157, Salmonella enterica serovar Enteritidis, and Yersinia enterocolitica O:9 were cultured on BHI agar and incubated in 37 °C for 18–24 h. E. coli BL21 (DE3) as prokaryotic hosts for expression of recombinant protein was cultured using either LB broth or LB agar.
Induction, expression, and purification of recombinant Omp2b
Recombinant pET-28a(+) which was inserted with omp2b gene (pET28-omp2b) between BamHI and HindIII sites was previously prepared in our laboratory. Expression and purification were accomplished as previously reported [36]. Briefly, E. coli BL21(DE3) transformed with pET28-omp2b plasmid was grown in an LB medium supplemented with kanamycin (30 μg/ml) while shaking (250 rpm) at 37 °C until the OD620nm reached 0.6. Protein expression was induced by adding IPTG to a final concentration of 1 mM; thereafter, the culture was incubated for another 2 h. Induced bacterial cells were harvested and purification of the recombinant Omp2b (rOmp2b) was performed by using Ni-NTA agarose through the hybrid method of denaturing-resolubilization procedure [36].
Mouse model for serum preparation
Female BALB/c mice, aged between 6 and 8 weeks were obtained from the Department of Laboratory Animal Production at Pasteur Institute of Iran. Mice were adopted for 1 week before the experiments. Bacterial suspensions with an OD620 nm of 0.08–0.1 which contained approximately 108 bacterial cells were prepared from fresh cultures of B. abortus S19, B. abortus 544, B. melitensis Rev-1, B. melitensis, Escherichia coli O:157, Salmonella enterica serovar Enteritidis, and Yersinia enterocolitica O:9, separately in phosphate-buffered saline (pH 7.2 ± 0.1). Bacteria were heat-inactivated in a 56 °C water bath for 30 min. Separate groups of 10 mice each were inoculated with 50 μL of heat-inactivated bacterial suspension subcutaneously at days 0, 10, 20, and 30 with no adjuvants added. Serum samples were collected from the mice by retroocular bleeding at day 35. A mock group of mice receiving no antigenic component also was considered the negative control. Animal experiments were carried out under Tarbiat Modares Institutional ethics guidelines on laboratory animals and National ethics guidelines for using laboratory animals [37].
Cattle serum samples
Serum samples from 200 cattle confirmed for brucellosis by both Rose Bengal plate agglutination test (RBPT) and serum tube agglutination test (SAT) were prepared as positive samples. Serum samples from 50 healthy cattle maintained under strict care which were negative for mentioned serological tests were used as negative sera.
Recombinant Omp2b ELISA
The immunoassay plates (Maxisorp, Nunc, Denmark) were coated with 100 μL of purified recombinant Omp2b protein (5 μg/mL) diluted in 0.1 M bicarbonate buffer (pH 9.0) and incubated at 4 °C overnight [36]. The wells were washed three times with phosphate-buffered saline-Tween20 (PBS-T) and then blocked with 250 μL of 5% BSA for 2 h at 37 °C. Two-fold dilutions of mouse sera started at 1:40 prepared in 0.5-mL microtubes (12 dilutions). Immunoassay plates were charged with 100 μL of sera dilutions and incubated at 37 °C for 1 h. After adequate washing with PBS-T, plates were incubated with 100 μL of HRP-conjugated anti-mouse IgG (1:2000) (Sigma) for 1 h at 37 °C. After washing with PBS-T, 100 μL of substrate solution containing TMB and H2O2 was added to each well and plates were incubated in the dark at room temperature. Color development was stopped by adding 50 μL of 2 M H2SO4 after 15 min. Absorbance recorded at 490 nm wavelength in an ELISA reader. Cattle sera were assessed at a single dilution of 1:100 and detected by HRP-conjugated anti-cow IgG (1:2000) (Sigma) through the same procedure. The relative sensitivity, specificity, and accuracy of recombinant Omp2b-based indirect ELISA were evaluated in comparison to RBPT and SAT as previously described [28].
Results
Production and purification of the recombinant Omp2b
Transformed E. coli BL21 with pET28-omp2b plasmid was grown in an LB medium and the cells induced with 1.0 mM IPTG. rOmp2b was expressed and purified using a previously described hybrid procedure of denaturing-renaturing procedure (Fig. 1). We obtained 3.1 mg of rOmp2b from 1 L of induced culture.
Fig. 1.
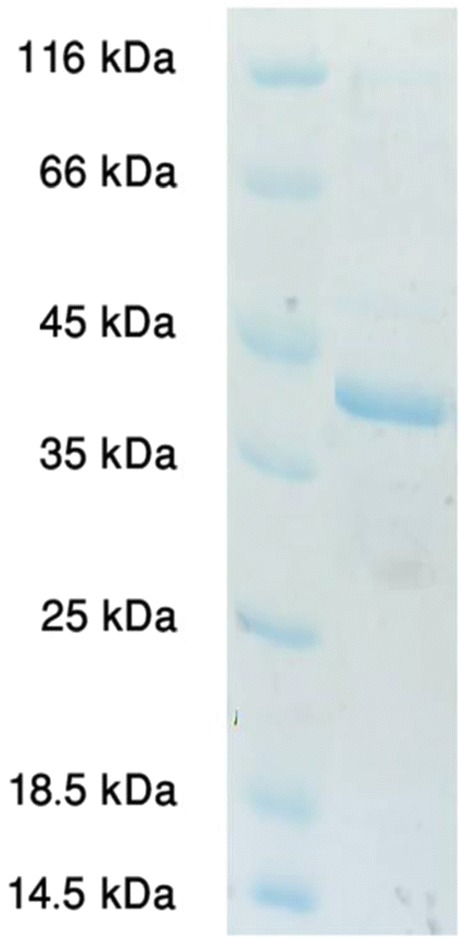
Purified recombinant Omp2b 44 kDa on 12.5% polyacrylamide gel which is stained with coomassie blue G-250
Serodiagnostic evaluation of rOmp2b by indirect ELISA
Indirect ELISA experiment with mouse sera immunized with Brucella and non-Brucella whole-cell antigen showed that rOmp2b can specifically detect Brucella-specific antibodies (Fig. 2). There was no significant cross-reaction between Escherichia coli, Salmonella enterica serovar Enteritidis, and Yersinia enterocolitica O:9 and Brucella (p < 0.001).
Fig. 2.
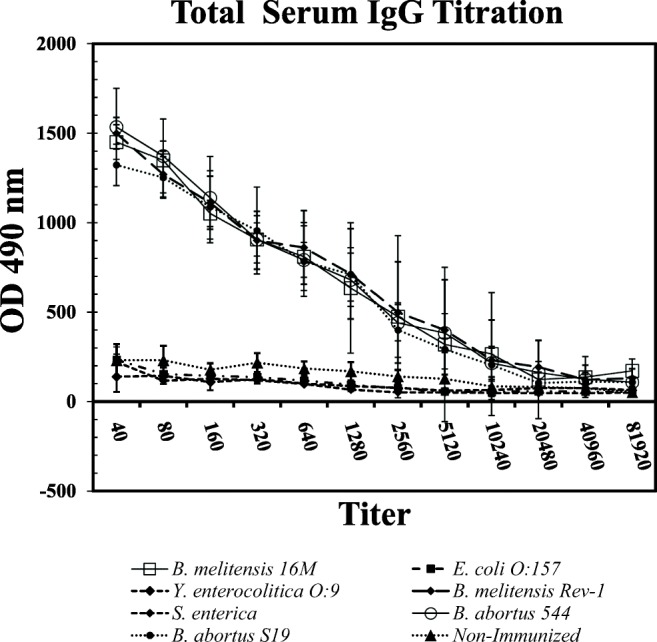
Titration of serum IgG of mice against rOmp2b antigen. Mice immunized with whole cell of B. melitensis 16M, B. melitensis Rev-1, B. abortus 544, and B. abortus S19 did not show any significant difference in reacting with rOmp2b (p > 0.05). There were no significant reactions between rOmp2b and sera from Escherichia coli O:157, Salmonella enterica serovar Enteritidis, and Yersinia enterocolitica O:9 (p > 0.05). There are significant differences between sera titers from mice which received Brucella antigens and those received non-Brucella antigens (p < 0.05) which confirms that there are no cross-reactions
An indirect ELISA experiment was performed with cattle sera at a fixed dilution of 1:100. Cut-off was determined by assessment of negative cattle serum results as mean OD490nm value +3 × SD (standard deviation) that was 0.162. Table 1 presents the result for cattle sera which were positive for RBPT and SAT and evaluated by indirect ELISA against the rOmp2b. The sensitivity, specificity, and accuracy of recombinant Omp2b-based ELISA relative to RBPT and SAT are 88.5%, 100%, and 90.8%, respectively.
Table 1.
Serological diagnostic values of rOmp2b-based indirect ELISA in comparison to Rose Bengal plate agglutination test (RBPT) and serum tube agglutination test (SAT) which are totally referred to as “Sero” here
| Sero (+) | Sero (−) | Total | |
|---|---|---|---|
| rOmp2b-ELISA (+) | 177 | 0 | 177 |
| rOmp2b-ELISA (−) | 23 | 50 | 73 |
| Total | 200 | 50 | 250 |
Sensitivity 88.5%, specificity 100%, accuracy 90.8%
Discussion
Brucellosis is a highly contagious zoonotic infection that affects people worldwide and cause significant economical loses [3, 38, 39]. Brucellae are highly infectious and there is currently no confirmed method of preventing human brucellosis; hence, attention should be directed toward effective control and eradicate of the disease in livestock [3, 40]. Brucellosis, in particular infections with B. abortus, B. melitensis, or B. suis, remains a significant human health threat [3, 38]. Although vaccination is the main control measure, administration of currently available vaccines alone is not sufficient for elimination of brucellosis in any host species [40, 41]. Rapid and accurate identification of infection in livestock is crucial for controlling the disease among both humans and animals. Especially in an endemic area, finding a reliable diagnostic method for brucellosis is still an important challenging problem [42]. The detection of antibodies by serological methods is very useful in the diagnosis of brucellosis in both humans and animals. Serological tests such as RBPT and SAT are routinely used in surveillance and control programs of animal brucellosis, but none of the available tests have been shown to be specific and accurate because of false positivity resulting from cross-reactions with other Gram-negative bacterial pathogens and inability to discriminate between vaccinated and infected animals [10].
In the present work, recombinant Omp2b was successfully produced using pET28a (+)/E. coli BL21 system. This recombinant outer membrane antigen was used to develop an indirect ELISA for detection of brucellosis. There are some reports in which protein antigens of Brucellae were used for the serological diagnosis of the disease and overcoming the cross-reaction problem of LPS-based serodiagnostic methods. Among previously investigated protein antigens for detection of brucellosis by ELISA method are Omp10 [43], Omp19 [43], Omp25 [44], Omp28 [28, 43–46], Omp31 [29, 43–45], BP26 [47–50], CP24 [50, 51], and lumazine synthase [50, 52]. These works confirmed that using protein antigens, especially outer membrane proteins of Brucella cell, is very useful in an efficient and accurate serological diagnosis of the disease.
Here, we used BALB/c mice for investigating the diagnostic ability of rOmp2b and its specificity, as described before [44]. Titration of sera from mice following injection of Brucella whole cell showed that specific antibodies against Omp2b are developed which can be detected by indirect ELISA with a significant affinity to the protein antigen. Although we did not detect a significant difference between sera from mice which were immunized with different strains of Brucella, results confirmed that the affinity of the antigen-antibody is high enough to suggest Omp2b as a serodiagnostic target. Sera from mice which received Escherichia coli O:157, Salmonella enterica serovar Enteritidis, and Yersinia enterocolitica O:9 did not react significantly with rOmp2b in ELISA; approving the specificity of the protein antigen against those bacterial species to which cross-reactions may occur in RBPT or SAT.
Ability of rOmp2b-based indirect ELISA to detect brucellosis in an endpoint method was assessed by a total of 250 cattle sera including 50 healthy and 200 infected samples. Twenty-three sera from those that were positive for RBPT and SAT were negative for rOmp2b-ELISA while none of healthy sera reacted with rOmp2b. These results suggest that rOmp2b is able to specifically detect antibodies elicited upon infection with Brucella, and sera which are falsely positive with LPS-based serological methods may be excluded.
Results showed that indirect ELISA, using recombinant Omp2b protein as the target antigen, yielded high sensitivity and specificity for serological diagnosis of brucellosis by detecting specific antibodies in the sera from infected cattle. The same results also may be achieved by evaluating serum samples from other animals like sheep, goat, and dog along with its possible potential for serological diagnosis of human brucellosis.
Compliance with ethical standards
Animal experiments were carried out under Tarbiat Modares Institutional ethics guidelines on laboratory animals and National ethics guidelines for using laboratory animals.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Melody Vatankhah and Nazanin Beheshti equally contributed to this work and both should be considered as first author.
Contributor Information
Shiva Mirkalantari, Email: mirkalantari.sh@iums.ac.ir.
Nima Khoramabadi, Email: nima.khoramabadi@gmail.com.
References
- 1.Boschiroli ML, Foulongne V, O’Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol. 2001;4(1):58–64. doi: 10.1016/s1369-5274(00)00165-x. [DOI] [PubMed] [Google Scholar]
- 2.Mirkalantari S, Zarnani AH, Nazari M, Irajian GR, Amirmozafari N. Brucella melitensis VirB12 recombinant protein is a potential marker for serodiagnosis of human brucellosis. Ann Clin Microbiol Antimicrob. 2017;16(1):8. Published 2017 Mar 3. doi:10.1186/s12941-017-0182-4 [DOI] [PMC free article] [PubMed]
- 3.Mirkalantari S, Amirmozafari N, Kazemi B, Irajian G. Molecular cloning of virB12 gene of Brucella melitensis 16M strain in pET28a vector. Asian Pac J Trop Med. 2012;5(7):511–513. doi: 10.1016/S1995-7645(12)60089-3. [DOI] [PubMed] [Google Scholar]
- 4.Ramin B, MacPherson P. Human brucellosis. Brucellose beim Menschen. 2011;100(5):305–307. doi: 10.1024/1661-8157/a000452. [DOI] [PubMed] [Google Scholar]
- 5.Leylabadlo HE, Bialvaei AZ, Samadi KH. Brucellosis in Iran: why not eradicated? Clin Infect Dis. 2015;61(10):1629–1630. doi: 10.1093/cid/civ646. [DOI] [PubMed] [Google Scholar]
- 6.Yumuk Z, O’Callaghan D. Brucellosis in Turkey -- an overview. Int J Infect Dis. 2012;16(4):e228–e235. doi: 10.1016/j.ijid.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Singh BB, Dhand NK, Gill JP. Economic losses occurring due to brucellosis in Indian livestock populations. Prev Vet Med. 2015;119(3–4):211–215. doi: 10.1016/j.prevetmed.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Mangalgi SS, Sajjan AG, Mohite ST, Kakade SV. Serological, clinical, and epidemiological profile of human brucellosis in rural India. Indian J Community Med : Off Publ Indian Assoc Prevent Soc Med. 2015;40(3):163–167. doi: 10.4103/0970-0218.158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev Sci Tech. 2013;32(1):249–261. doi: 10.20506/rst.32.1.2197. [DOI] [PubMed] [Google Scholar]
- 10.Ulu-Kilic A, Metan G, Alp E. Clinical presentations and diagnosis of brucellosis. Recent Patents Anti-Infect Drug Discov. 2013;8(1):34–41. [PubMed] [Google Scholar]
- 11.Al Dahouk S, Sprague LD, Neubauer H. New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev Sci Tech. 2013;32(1):177–188. doi: 10.20506/rst.32.1.2204. [DOI] [PubMed] [Google Scholar]
- 12.McGiven JA. New developments in the immunodiagnosis of brucellosis in livestock and wildlife. Rev Sci Tech. 2013;32(1):163–176. doi: 10.20506/rst.32.1.2205. [DOI] [PubMed] [Google Scholar]
- 13.Yu WL, Nielsen K. Review of detection of Brucella spp. by polymerase chain reaction. Croat Med J. 2010;51(4):306–313. doi: 10.3325/cmj.2010.51.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D. Laboratory-based diagnosis of brucellosis--a review of the literature. Part II: serological tests for brucellosis. Clin Lab. 2003;49(11–12):577–589. [PubMed] [Google Scholar]
- 15.Mohseni K, Mirnejad R, Piranfar V, Mirkalantari S. A comparative evaluation of ELISA, PCR, and serum agglutination tests for diagnosis of Brucella using Human [PMC free article] [PubMed]
- 16.Corrente M, Desario C, Parisi A, Grandolfo E, Scaltrito D, Vesco G, et al. Serological diagnosis of bovine brucellosis using B. melitensis strain B115. J Microbiol Methods. 2015;119:106–109. doi: 10.1016/j.mimet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Mathias Luis A., Meirelles Raphaella B., Buchala Fernando G. Estabilidade do antígeno de célula total de Brucella abortus para uso no diagnóstico sorológico da brucelose bovina pela reação de fixação de complemento. Pesquisa Veterinária Brasileira. 2007;27(1):18–22. [Google Scholar]
- 18.Gall D, Nielsen K. Serological diagnosis of bovine brucellosis: a review of test performance and cost comparison. Rev Sci Tech. 2004;23(3):989–1002. [PubMed] [Google Scholar]
- 19.Lord VR, Schurig GG, Cherwonogrodzky JW, Marcano MJ, Melendez GE. Field study of vaccination of cattle with Brucella abortus strains RB51 and 19 under high and low disease prevalence. Am J Vet Res. 1998;59(8):1016–1020. [PubMed] [Google Scholar]
- 20.Ko KY, Kim JW, Her M, Kang SI, Jung SC, Cho DH, Kim JY. Immunogenic proteins of Brucella abortus to minimize cross reactions in brucellosis diagnosis. Vet Microbiol. 2012;156(3–4):374–380. doi: 10.1016/j.vetmic.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Delpino MV, Fossati CA, Baldi PC. Occurrence and potential diagnostic applications of serological cross-reactivities between Brucella and other alpha-proteobacteria. Clin Diagn Lab Immunol. 2004;11(5):868–873. doi: 10.1128/CDLI.11.5.868-873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen K. Diagnosis of brucellosis by serology. Vet Microbiol. 2002;90(1–4):447–459. doi: 10.1016/s0378-1135(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen K, Smith P, Widdison J, Gall D, Kelly L, Kelly W, Nicoletti P. Serological relationship between cattle exposed to Brucella abortus, Yersinia enterocolitica O:9 and Escherichia coli O157:H7. Vet Microbiol. 2004;100(1–2):25–30. doi: 10.1016/j.vetmic.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Cloeckaert A, Vizcaino N, Paquet JY, Bowden RA, Elzer PH. Major outer membrane proteins of Brucella spp.: past, present and future. Vet Microbiol. 2002;90(1–4):229–247. doi: 10.1016/s0378-1135(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 25.Cloeckaert A, Zygmunt MS, Bezard G, Dubray G. Purification and antigenic analysis of the major 25-kilodalton outer membrane protein of Brucella abortus. Res Microbiol. 1996;147(4):225–235. doi: 10.1016/0923-2508(96)81383-0. [DOI] [PubMed] [Google Scholar]
- 26.Cloeckaert A, Baucheron S, Vizcaino N, Zygmunt MS. Use of recombinant BP26 protein in serological diagnosis of Brucella melitensis infection in sheep. Clin Diagn Lab Immunol. 2001;8(4):772–775. doi: 10.1128/CDLI.8.4.772-775.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salih-Alj Debbarh H, Cloeckaert A, Bezard G, Dubray G, Zygmunt MS. Enzyme-linked immunosorbent assay with partially purified cytosoluble 28-kilodalton protein for serological differentiation between Brucella melitensis-infected and B. melitensis Rev.1-vaccinated sheep. Clin Diagn Lab Immunol. 1996;3(3):305–308. doi: 10.1128/cdli.3.3.305-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhuri P, Prasad R, Kumar V, Gangaplara A. Recombinant OMP28 antigen-based indirect ELISA for serodiagnosis of bovine brucellosis. Mol Cell Probes. 2010;24(3):142–145. doi: 10.1016/j.mcp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Gupta VK, Verma DK, Singh SV, Vihan VS. Serological diagnostic potential of recombinant outer membrane protein (Omp31) from Brucella melitensis in goat and sheep brucellosis. Small Rumin Res. 2007;70(2–3):260–266. [Google Scholar]
- 30.Piranfar V, Sharif M, Hashemi M, Vahdati AR, Mirnejad R. Detection and discrimination of two Brucella species by multiplex real-time PCR and high-resolution melt analysis curve from human blood and comparison of results using RFLP. Iran J Basic Med Sci. 2015;18(9):909–914. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagalingam Mohandoss, Shome Rajeswari, Balamurugan Vinayagamurthy, Shome Bibek Ranjan, NarayanaRao Krishnamsetty, Vivekananda, Isloor Shrikrishna, Prabhudas Krishnamsetty. Molecular typing of Brucella species isolates from livestock and human. Tropical Animal Health and Production. 2011;44(1):5–9. doi: 10.1007/s11250-011-9886-1. [DOI] [PubMed] [Google Scholar]
- 32.Pishva E, Salehi R, Hoseini A, Kargar A, Taba FE, Hajiyan M, et al. Molecular typing of Brucella species isolates from human and livestock bloods in Isfahan province. Adv Biomed Res. 2015;4:104. doi: 10.4103/2277-9175.157798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmoock G, Ehricht R, Melzer F, Elschner M, Tomaso H, Neubauer H, al Dahouk S. Development of a diagnostic multiplex polymerase chain reaction microarray assay to detect and differentiate Brucella spp. Diagn Microbiol Infect Dis. 2011;71(4):341–353. doi: 10.1016/j.diagmicrobio.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Imaoka K, Kimura M, Suzuki M, Kamiyama T, Yamada A. Simultaneous detection of the genus Brucella by combinatorial PCR. Jpn J Infect Dis. 2007;60(2–3):137–139. [PubMed] [Google Scholar]
- 35.Sung KY, Jung M, Shin MK, Park HE, Lee JJ, Kim S, Yoo HS. Induction of immune responses by two recombinant proteins of brucella abortus, outer membrane proteins 2b porin and Cu/Zn superoxide dismutase, in mouse model. J Microbiol Biotechnol. 2014;24(6):854–861. doi: 10.4014/jmb.1312.12063. [DOI] [PubMed] [Google Scholar]
- 36.Aghababa H, Mohabati Mobarez A, Khoramabadi N, Behmanesh M, Mahdavi M, Tebianian M, Nejati M. A comparative approach to strategies for cloning, expression, and purification of mycobacterium tuberculosis mycolyl transferase 85B and evaluation of immune responses in BALB/c mice. Mol Biotechnol. 2014;56(6):487–497. doi: 10.1007/s12033-013-9696-y. [DOI] [PubMed] [Google Scholar]
- 37.National Research Council Committee for the Update of the Guide for the CUoL, Animals. (2011) The National Academies Collection: Reports funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health. 8th ed. Washington (DC): National Academies Press (US) National Academy of Sciences
- 38.Haque N, Bari MS, Hossain MA, Muhammad N, Ahmed S, Rahman A, Hoque SM, Islam A. An overview of brucellosis. Mymensingh Med J. 2011;20(4):742–747. [PubMed] [Google Scholar]
- 39.Ducrotoy MJ, Bertu WJ, Ocholi RA, Gusi AM, Bryssinckx W, Welburn S, Moriyón I. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl Trop Dis. 2014;8(7):e3008. doi: 10.1371/journal.pntd.0003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neubauer H. Brucellosis: new demands in a changing world. Prilozi. 2010;31(1):209–217. [PubMed] [Google Scholar]
- 41.Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. Progress in vaccine development. Front Biol. 2013;8(1):60–77. doi: 10.1007/s11515-012-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adone R, Pasquali P. Epidemiosurveillance of brucellosis. Rev Sci Tech. 2013;32(1):199–205. doi: 10.20506/rst.32.1.2202. [DOI] [PubMed] [Google Scholar]
- 43.Simborio HL, Lee JJ, Bernardo Reyes AW, Hop HT, Arayan LT, Min W, et al. Evaluation of the combined use of the recombinant Brucella abortus Omp10, Omp19 and Omp28 proteins for the clinical diagnosis of bovine brucellosis. Microb Pathog. 2015;83–84:41–46. doi: 10.1016/j.micpath.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed IM, Khairani-Bejo S, Hassan L, Bahaman AR, Omar AR. Serological diagnostic potential of recombinant outer membrane proteins (rOMPs) from Brucella melitensis in mouse model using indirect enzyme-linked immunosorbent assay. BMC Vet Res. 2015;11(1):275. doi: 10.1186/s12917-015-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari S, Kumar A, Thavaselvam D, Mangalgi S, Rathod V, Prakash A, Barua A, Arora S, Sathyaseelan K. Development and comparative evaluation of a plate enzyme-linked immunosorbent assay based on recombinant outer membrane antigens Omp28 and Omp31 for diagnosis of human brucellosis. Clin Vaccine Immunol. 2013;20(8):1217–1222. doi: 10.1128/CVI.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim JJ, Kim DH, Lee JJ, Kim DG, Min W, Lee HJ, et al. Evaluation of recombinant 28 kDa outer membrane protein of Brucella abortus for the clinical diagnosis of bovine brucellosis in Korea. J Vet Med Sci. 2012;74(6):687–691. doi: 10.1292/jvms.11-0512. [DOI] [PubMed] [Google Scholar]
- 47.Tiwari AK, Kumar S, Pal V, Bhardwaj B, Rai GP. Evaluation of the recombinant 10-kilodalton immunodominant region of the BP26 protein of Brucella abortus for specific diagnosis of bovine brucellosis. Clin Vaccine Immunol. 2011;18(10):1760–1764. doi: 10.1128/CVI.05159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu WX, Hu S, Qiao ZJ, Chen WY, Liu LT, Wang FK, Hua RH, Bu ZG, Li XR. Expression, purification, and improved antigenic specificity of a truncated recombinant bp26 protein of Brucella melitensis M5-90: a potential antigen for differential serodiagnosis of brucellosis in sheep and goats. Biotechnol Appl Biochem. 2011;58(1):32–38. doi: 10.1002/bab.11. [DOI] [PubMed] [Google Scholar]
- 49.Kumar S, Tuteja U, Kumar A, Batra HV. Expression and purification of the 26 kDa periplasmic protein of Brucella abortus: a reagent for the diagnosis of bovine brucellosis. Biotechnol Appl Biochem. 2008;49(Pt 3):213–218. doi: 10.1042/BA20070111. [DOI] [PubMed] [Google Scholar]
- 50.Thepsuriyanont P, Intarapuk A, Chanket P, Tunyong W, Kalambaheti T. ELISA for brucellosis detection based on three Brucella recombinant proteins. Southeast Asian J Trop Med Public Health. 2014;45(1):130–141. [PubMed] [Google Scholar]
- 51.Cassataro J, Delpino MV, Velikovsky CA, Bruno L, Fossati CA, Baldi PC. Diagnostic usefulness of antibodies against ribosome recycling factor from Brucella melitensis in human or canine brucellosis. Clin Diagn Lab Immunol. 2002;9(2):366–369. doi: 10.1128/CDLI.9.2.366-369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanke MM, Delpino MV, Baldi PC. Comparative performance of tests using cytosolic or outer membrane antigens of Brucella for the serodiagnosis of canine brucellosis. Vet Microbiol. 2002;88(4):367–375. doi: 10.1016/s0378-1135(02)00152-9. [DOI] [PubMed] [Google Scholar]


