Abstract
Salmonella enterica is an important animal and human pathogen that can cause enteritis and septicaemia in calves. Generally, antibiotics are prescribed for the treatment of salmonellosis in dairy calves. Here, we report the isolation of antibiotic resistant S. enterica serotypes from calves, including multidrug-resistant isolates. A total of 544 faecal samples from live healthy and diarrheic dairy calves from 29 commercial dairy farms and organ samples from 19 deceased calves that succumbed to salmonellosis in 12 commercial dairy farms in Uruguay were processed for selective S. enterica culture. In total, 41 isolates were serotyped, and susceptibility to 14 antibiotics, from 9 classes of compounds, was evaluated by disk-diffusion test. The minimum inhibitory concentration (MIC) was determined by microdilution. Salmonella Typhimurium was the most frequent serotype, followed by S. Dublin and S. Anatum. Whether determined by diffusion assay or microdilution, resistance to tetracycline, streptomycin and ampicillin were the most frequently pattern found. Based on MIC, 5 isolates were resistant to at least one antibiotic, 21 were resistant to 2 antibiotics, and 14 were multidrug-resistant (resistant to at least one antibiotic in 3 different categories of antibiotics). Eleven different resistance patterns were found. Multidrug resistance in S. enterica is a concern for animal and public health not only because of its zoonotic potential but also due to the possibility of transfer resistance determinants to other bacterial genera. This represents the first report of the antibiotic resistance in S. enterica in dairy farms in Uruguay.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00151-w) contains supplementary material, which is available to authorized users.
Keywords: Salmonella Typhimurium, Salmonella Dublin, Salmonella Anatum, Antibiotic resistance, Dairy calves
Salmonella enterica is an important pathogen that affects a wide range of animal species and humans. More than 2500 serotypes are documented within Salmonella spp. but only a few affect cattle [1]. In this species, Salmonella Dublin and Salmonella Typhimurium are by far the most frequent serotypes [2, 3], and can cause enteritis, diarrhoea and septicaemia [4, 5].
Salmonellosis in calves is often treated with antibiotics and ß-lactams, and sulphonamides are recommended in cases of septicaemia [6, 7]. When resistance to these antibiotics is suspected or confirmed, quinolones are the next therapeutic option, but emergence of resistance to this group of antibiotics has also been reported [8]. Moreover, antibiotics have been used as feed additives for decades [9, 10], which has promoted the occurrence and selection of resistant and multidrug-resistant (MDR) strains, affecting the therapeutic performance of antibiotics in both animals and humans [9]. The World Health Organization (WHO) recommendations point toward the preservation of antibiotics for human use, reducing their use in animals, and the promotion of sanitation and hygienic practices to avoid disease and, therefore, the use of antibiotics [10].
Multidrug resistance is an emerging issue worldwide, and the transference of resistance mechanisms and the potentially zoonotic pathogens presence bound not only the therapeutic options in animals, but also in humans [11]. The main objective of this work is to report the antibiotic resistance profiles of S. enterica isolated from diarrheic and non-diarrheic calves, and from deceased dairy calves diagnosed at postmortem with salmonellosis in commercial dairy farms in Uruguay.
Salmonella enterica isolation was attempted from two different sources. Firstly, individual faeces were taken directly from the rectal ampule of 544 live calves ≤ 30 days old, from 29 different dairy farms in Uruguay from March to November 2016. In this survey, 282 calves were non-diarrheic (healthy) and 262 were diarrheic based on the faecal score [12, 13]. Secondly, from March 2016 to October 2017, various organs from 19 autopsied calves ≤ 60 days of age, that had succumbed to salmonellosis in commercial dairy farms in Uruguay, were processed. These animals were from 12 different fatal outbreaks of spontaneous salmonellosis, submitted to the diagnostic service of the “Plataforma de Investigación en Salud Animal”, INIA- La Estanzuela, Colonia, Uruguay.
Organs and faecal samples were aerobically cultured in tetrathionate broth (37 °C, 24 to 48 h). After this selective enrichment, 100 μl of grown media were plated onto xylose lysine deoxycholate (XLD) agar, Salmonella-suspect colonies were selected, and identified to the species level (S. enterica) by routine biochemical tests [14]. To determine the S. enterica serotype for each isolate, the Kauffman-White-Le Minor serotyping scheme was performed at the bacteriology service of the “Instituto de Higiene, Facultad de Medicina, UdelaR”, in Montevideo, Uruguay, following a previously described procedure [1].
Antimicrobial susceptibility profiles were assessed using the disk diffusion assay following the procedures outlined by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) [15–17]. The agar disk diffusion is an accessible, low-cost and reproducible technique that provides rapid results. It is very widespread and easily available in most veterinary diagnostic laboratory. Commercial disks (Oxoid) containing fourteen antibiotics covering nine antimicrobial classes were employed, as follows: β-lactams -ampicillin (10 μg), amoxicillin-clavulanic acid (20 μg/10 μg), cefotaxime (30 μg); sulphonamide-diaminopyrimidine-sulfamethoxazole/trimethoprim (23.75/1.25 μg); quinolones -nalidixic acid (30 μg), ciprofloxacin (5 μg), enrofloxacin (5 μg); phenicols -chloramphenicol (30 μg); aminoglycosides -streptomycin (10 μg), gentamicin (10 μg); tetracyclines -tetracycline (30 μg); polypeptides -fosfomycin-trometamol (200 μg); nitrofurans -nitrofurantoin (300 μg); and macrolides -azithromycin (15 μg). Escherichia coli ATCC 25922 and E. coli ATCC 35218 were used for quality control. Salmonella enterica isolates were classified as susceptible: sensitive (S); or non-susceptible: intermediate (I) or resistant (R) to the antimicrobial compound tested according to EUCAST breakpoints [17], except for nalidixic acid, enrofloxacin, streptomycin, tetracycline and azithromycin, for which CLSI guides were followed, as there were no EUCAST breakpoints described. [15, 16].
Additionally, for every S. enterica isolate, the minimum inhibitory concentration (MIC) profiles were determined by the broth microdilution method, using a Gram-negative Sensititre plate (CMV3AGNF, Thermo Scientific, USA). The determination of MIC is the gold-standard methods for interpret the antimicrobial resistance. The panel of 14 antimicrobials tested included amoxicillin-clavulanic acid, ampicillin, azithromycin, cefoxitin, ceftiofur, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid, streptomycin, sulfisoxazole, trimethoprim-sulfamethoxazole and tetracycline. The MICs were determined, and breakpoints were interpreted based on EUCAST guidelines where possible [17]. For nalidixic acid, sulfisoxazole, tetracycline, azithromycin, ceftiofur and cefoxitin CLSI guidelines were followed [15, 16] and for streptomicyn, NARMS breakpoints were considered [18]. Escherichia coli ATCC 25922 was used as a quality control strain. According to the MIC, isolates were classified as multidrug-resistant (MDR) when they were resistant to at least one antibiotic in three or more antimicrobial classes [19].
To ordinate the antimicrobial susceptibility profiles for each isolate, and to infer similarities among them, a principal component analysis (PCA) was performed using the PAST software version 3.18 [20]. For this exploratory analysis, the diameters of the inhibition halos measured in mm were used as inputs.
Salmonella enterica was isolated from 22 faeces, from 16 diarrheic and 6 non-diarrheic calves (Table S1). These samples were from 9 of the 29 (31%) farms studied. In 8 of these farms, S. Typhimurium was the identified serotype, while S. Anatum was isolated in only one (3%) (Table S1). Also, 19 isolates were obtained, one from each calf from the 12 different mortality outbreaks. In this group, S. Typhimurium was isolated from 16 deceased calves in 10 outbreaks and S. Dublin from 3 calves in 2 outbreaks (Table S1). For further antimicrobial susceptibility assays, one isolate of each calf was selected (17 from mesenteric lymph node, one from the liver and one from intestine).
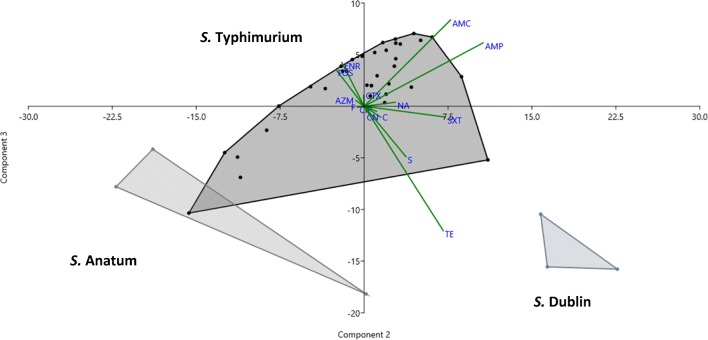
Based on the agar disk diffusion method, the most widespread resistance was against tetracycline (87.8%, 36/41 isolates), streptomycin (85.4%, 35/41 isolates) and ampicillin (22%, 9/41 isolates) (Table 1). The PCA analysis allowed to group the isolates according to their resistance-susceptibility profiles. Three different groups were formed corresponding to the three detected serotypes. In addition, the most resistant isolates were located in the left quadrants (Fig. 1). The three first principal components explained more than 70% of the variability observed.
Table 1.
Number of S. enterica isolates (total = 41) susceptible, resistant or with intermediate susceptibility to 14 antibiotics by serotype
| Antibiotics | Serotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Typhimurium | Dublin | Anatum | |||||||
| S | I | R | S | I | R | S | I | R | |
| AMP | 28 | 0 | 7 | 3 | 0 | 0 | 1 | 0 | 2 |
| AMC | 32 | 0 | 3 | 3 | 0 | 0 | 1 | 0 | 2 |
| CTX | 34 | 0 | 1 | 3 | 0 | 0 | 2 | 1 | 0 |
| C | 34 | 0 | 1 | 3 | 0 | 0 | 3 | 0 | 0 |
| NA | 15 | 2 | 5 | 3 | 0 | 0 | 3 | 0 | 0 |
| CIP | 12 | 22 | 1 | 2 | 1 | 0 | 0 | 3 | 0 |
| ENR | 14 | 2 | 0 | 2 | 0 | 1 | 3 | 0 | 0 |
| SXT | 35 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 2 |
| S | 1 | 1 | 33 | 2 | 1 | 0 | 0 | 1 | 2 |
| CN | 34 | 1 | 0 | 3 | 0 | 0 | 3 | 0 | 0 |
| TE | 1 | 0 | 34 | 3 | 0 | 0 | 1 | 0 | 2 |
| AZM | 35 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 |
| FOT | 35 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 |
| F | 26 | 8 | 1 | 3 | 0 | 0 | 2 | 1 | 0 |
AMP, ampicillin; AMC, amoxicillin-clavulanic acid; CTX, cefotaxime; C, chloramphenicol; NA, nalidixic acid; CIP, ciprofloxacin; ENR, enrofloxacin; SXT, trimethoprim-sulfamethoxazole; S, streptomycin; CN, gentamicin; TE tetracycline; AZM, azithromycin; FOT, fosfomycin-trometamol; F, nitrofurantoin; S, susceptible; I, intermediate; R, resistant according to the EUCAST and CLSI [15–17]
Fig. 1.
Principal components analysis (PCA) biplots. The graphic was constructed with the diameter of the inhibition halos in mm for 14 antimicrobials of the 41 tested Salmonella enterica isolates. Components 2 and 3 are graphed. The different isolates of a same serotype (S. Dublin, S. Anatum, S. Typhimurium) are contained in one convex hull. The contribution of each variable (diameter of the inhibition halo) to each component is graphed as a vector. Ampicillin, AMP; amoxicillin-clavulanic acid, AMC; cefotaxime, CTX; chloramphenicol, C; nalidixic acid, NA; ciprofloxacin, CIP; enrofloxacin, ENR; trimethoprim-sulfamethoxazole, SXT; streptomycin, S; gentamicin, CN; tetracycline, TE; azithromycin, AZM; fosfomycin FOS and nitrofurantoin, F
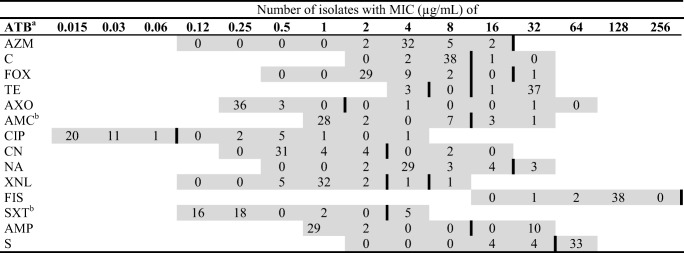
The determination of MIC showed that antimicrobial resistance was a common feature among the isolates; 92.7% (38/41 isolates) were resistant to tetracycline and 80.5% (33/41 isolates) to streptomycin (Table 2). In addition, resistance to ampicillin 24.4% (10/41) and ciprofloxacin 22% (9/41) was observed. Five isolates were resistant to at least one antibiotic and 21 were resistant to 2 antibiotics. Also, 14 isolates were classified as MDR [19], being resistant to at least three and up to six antimicrobial compound categories (Table 3). Eleven of these MDR isolates were S. Typhimurium and 3 were S. Anatum. No MDR was detected in S. Dublin isolates. The MDR isolates showed 11 different resistance pattern profiles being S/TE/CIP the most frequent combination, with six isolates (Table 3).
Table 2.
MIC distribution for S. enterica isolated from calves (n = 41)
Grey cells represent the concentration of each ATB in the Gram-negative Sensititre plate (CMV3AGNF, ThermoScientific, USA). Vertical black lines in each row represent the clinical breakpoint used [15–18]. Bold numbers are the concentration for each ATB. aATB antibiotic; AZM, azithromycin; C, chloramphenicol; FOX, cefoxitin; TE, tetracycline; AXO, ceftriaxona; AMC, amoxicillin /clavulanic acid (2:1); CIP, ciprofloxacin; CN, gentamicin; NA, nalidixic acid; XNL, ceftiofur; FIS, sulfisoxazole; SXT, trimethoprim/sulfamethoxazole; AMP, ampicillin; S, streptomycin. bFor AMC dilutions rank from 1/0.5 to 32/16 μg/mL; for SXT dilutions rank from 0.12/2.38 to 4/76 μg/mL
Table 3.
Antimicrobial resistance profiles of MDR-S. enterica isolates (n = 14)
| Number of classes of antibiotics | Classes of antibiotics | Antibiotics | n |
|---|---|---|---|
| 6 | AG-T-Q-BL-PH-S | S-TE-CIP-FOX-AXO-AMC-C-SXT | 1 |
| 4 | AG-T-Q-BL | S-TE-CIP-AXO | 1 |
| AG-T-BL-S | S-TE-AMP-SXT | 3 | |
| AG-T-Q-BL | S-CN-TE-NA-XNL | 1 | |
| AG-T-Q-BL | S-TE-NA-XNL | 1 | |
| AG-T-BL-S | S-TE-FOX-AMP-SXT | 1 | |
| AG-T-Q-BL | S-TE-CIP-AMP-AMC | 1 | |
| AG-T-Q-BL | S-TE-CIP-NA-AMP-XNL | 1 | |
| 3 | AG-T-BL | S-TE-AMC | 1 |
| AG-T-BL | S-TE-AMP-AMC | 1 | |
| AG-T-Q | S-TE-CIP | 2 |
Antibiotic resistance classified according compounds categories, AG aminoglycosides; T tetracyclines; Q, quinolones; BL, β-lactams; PH, phenicols; S, folate pathway inhibitor. Antibiotic resistance classified according to single antibiotics: AMP, ampicillin; AMC, amoxicillin-clavulanic acid; FOX, cefoxitin; AXO, ceftriaxona; XNL ceftiofur; SXT, trimethoprim-sulfamethoxazole; NA, nalidixic acid; CIP, ciprofloxacin; C, chloramphenicol; S, streptomycin; CN, gentamicin; TE, tetracycline
In this study, S. Typhimurium was isolated from calves with diarrhoea, septicaemia and mortality, as reported in previous works [7, 21]. The serotype S. Anatum was only isolated from faecal samples from only one dairy farm (Table S1). These samples were from two diarrhoeic and one non-diarrheic calves. Salmonella Anatum has been isolated from dairy and beef calves [22, 23] but the role of this serotype as a causative agent of diarrhoea in neonate calves still needs to be elucidated. Recently, a human salmonellosis outbreak was reported due to food contamination with this serotype [24]. Salmonella Dublin was only isolated from 3 autopsied calves and was associated to the cause of death based on the pathologic examination (data not shown). The affected calves, around 60 days old, were from 2 different dairy farms and were suffering mild diarrhoea and most of them died (data not shown). This serotype, adapted to cattle, generally causes septicaemia in calves and recently had been reported as a causative agent of urocystitis and ureteritis [25–27].
Although in Uruguay enrofloxacin is a very used quinolone in veterinary medicine, the Salmonella isolates from this work showed low frequency of resistance. Tetracycline and streptomycin are two antimicrobials with extended use worldwide in veterinary medicine [11]. Resistance to both antibiotics can be transferred concurrently in conjugative plasmids [28]. All MDR salmonellae were both tetracycline and streptomycin resistant (Table 3). Our results indicate that there is an association between resistances to these antibiotics, which might have a genetic basis. Additional molecular characterization of these strains is warranted.
As shown in Fig. 1, the three different S. enterica serotypes grouped separately, with S. Typhimurium isolates showing an ample diversity of antibiotic resistance profiles. This diversity is probably biased by the greater representation of this serotype in the collection of isolates analysed in this study. Despite of this bias, the antimicrobial resistance profiles seem to be influenced by the serotype. This has been observed in previous works [29, 30], where serotypes Typhimurium and Dublin were more resistant in cattle. In opposition, S. Dublin isolates in this study were the least resistant representatives of the collection, being susceptible to most of antibiotics assayed (Table 1, Fig. 1).
Several isolates, distributed in the left quadrants of the PCA graph (Fig. 1), were resistant to more than three classes of antibiotics. Resistance to ampicillin, amoxicillin-clavulanic acid, trimethoprim/sulfamethoxazole, tetracycline and streptomycin explains most of the grouping observed in Fig. 1.
As mentioned, MDR was common, and 31.4% of the S. Typhimurium isolates were included in this subset, while the three S. Anatum isolates were MDR, showing resistance to four antimicrobial classes. The occurrence of MDR bacteria has increased in the last years, [31] and worldwide public concern in this topic has been established [32]. Salmonella enterica infections have an important impact on animal and public health as a food-borne pathogen [33]. Misuse of antibiotics and subtherapeutic dosage had promoted pressure and selection on bacteria and transference of resistance determinants [34].
The three serotypes described in dairy calves in this study have also been reported in humans, highlighting their zoonotic potential. In South America, S. Typhimurium has been isolated from food-borne cases and resistance to β-lactams has been reported [35, 36]. Additionally, S. Dublin can cause invasive infection in humans and has been reported in the same geographic region of the isolates of the present study [37].
In this study, 34% of salmonellae isolated from diseased and healthy dairy calves showed MDR profiles, and all of them had the putative potential to infect humans. The diversity of antibiotic resistance profiles in this collection of isolates recovered in a relatively short period of time highlights the importance of this issue. Probably multiple diverse antibiotic resistance mechanisms coexist and represent a potential of transference to other bacteria. Efforts should be done to control MDR dissemination focusing in the implications for public health.
Electronic supplementary material
(DOCX 18 kb)
Acknowledgements
The authors thank Cecilia Monesiglio from INIA for technical assistance with microbiological routine, and Laura Betancor and Arací Martínez from the “Instituto de Higiene, Facultad de Medicina, UdelaR” for Salmonella serotyping.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
Funding information
This work was partially funded by INIA’s grant PL-15, and project FMV-104922 from the Uruguayan “Agencia Nacional de Investigación e Innovación” (ANII). M.L. Casaux received master’s scholarships from ANII (POS_NAC_2016_1_130296).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grimont PAD, Weill FX. Antigenic formulae of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella, 9th edition. Paris: Institut Pasteur; 2007. [Google Scholar]
- 2.Cho Y, Yoon KJ. An overview of calf diarrhea-infectious etiology, diagnosis, and intervention. J Vet Med Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzal FA, Plattner BL, Hostetter JM. Chapter 1 - Alimentary System. In: Grant Maxie M, editor. Jubb, Kennedy & Palmer’s pathology of domestic animals: Volume 2, 6th edn. St Louis: Elsevier; 2016. pp. 1–257. [Google Scholar]
- 4.Carrique-Mas JJ, Willmington JA, Papadopoulou C, Watson EN, Davies RH. Salmonella infection in cattle in Great Britain, 2003 to 2008. Vet Rec. 2008;67:560–565. doi: 10.1136/vr.c4943. [DOI] [PubMed] [Google Scholar]
- 5.Costa LF, Paixão TA, Tsolis RM, Bäumler AJ, Santos RL. Salmonellosis in cattle: advantages of being an experimental model. Res Vet Sci. 2012;93:1–6. doi: 10.1016/j.rvsc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Constable PD. Antimicrobial use in the treatment of calf diarrhea. J Vet Intern Med. 2004;18:8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohler VL, Izzo MM, House JK. Salmonella in calves. Vet Clin North Am Food Anim Pract. 2009;25:37–54. doi: 10.1016/j.cvfa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Pribul BR, Festivo ML, Rodrigues MS, Costa RG, Rodrigues EC, de Souza MM, Rodrigues DD. Characteristics of quinolone resistance in Salmonella spp. isolates from the food chain in Brazil. Front Microbiol. 2017;8:299. doi: 10.3389/fmicb.2017.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ, Ganshorn H, Sharma N, Kellner JD, Ghali WA. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (2017) WHO guidelines on use of medically important antimicrobials in food-producing animals: policy brief. https://www.who.int/foodsafety/publications/cia_guidelines/en/. Accessed 14 Jan 2019 [PubMed]
- 11.Eguale T, Engidawork E, Gebreyes WA, Asrat D, Alemayehu H, Medhin G, Johnson RP, Gunn JS. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC Microbiol. 2016;16:1–11. doi: 10.1186/s12866-016-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuirk SM. Disease management of dairy calves and heifers. Vet Clin North Am Food Anim Pract. 2008;24:139–153. doi: 10.1016/j.cvfa.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer MC, Stanton AL. Associations between health status and the probability of approaching a novel object or stationary human in preweaned group-housed dairy calves. J Dairy Sci. 2015;98:7298–7308. doi: 10.3168/jds.2015-9534. [DOI] [PubMed] [Google Scholar]
- 14.Octavia S, Lan R. The Family Enterobacteriaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes: Gammaproteobacteria. Berlin: Springer; 2014. pp. 225–286. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (2018) Performance standards for antimicrobial susceptibility testing; 25th informational supplement, M100-S28. Wayne, PA, USA
- 16.Clinical and Laboratory Standards Institute (2015) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. VET01S. Wayne, PA, USA
- 17.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. http://www.eucast.org
- 18.NARMS. The National Antimicrobial Resistance Monitoring System: enteric Bacteria. In: NARMS Integrated Report: 2012–2013: National Antimicrobial Resistance Monitoring System; 201
- 19.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Hammer Ø, Harper DA, Ryan DD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 178kb T Harper Geol Museum 4:5–7
- 21.Davidson KE, Byrne BA, Pires AFA, Magdesian KG, Pereira RV. Antimicrobial resistance trends in fecal Salmonella isolates from northern California dairy cattle admitted to a veterinary teaching hospital, 2002-2016. PLoS One. 2018;13:1–18. doi: 10.1371/journal.pone.0199928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilbao Gladys N., Malena Rosana, Passucci Juan A., Pinto de Almeida Castro Aldana M., Paolicchi Fernando, Soto Pedro, Cantón Juliana, Monteavaro Cristina E. Detección de serovares de Salmonella en terneros de crianza artificial de la región lechera Mar y Sierras, Argentina. Revista Argentina de Microbiología. 2019;51(3):241–246. doi: 10.1016/j.ram.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzo M, Mohler V, House JK. Antimicrobial susceptibility of Salmonella isolates recovered from calves with diarrhoea in Australia. Aust Vet J. 2011;89:402–408. doi: 10.1111/j.1751-0813.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (2017) Salmonella Anatum Infections Linked to Maradol Papayas. https://www.cdc.gov/salmonella/anatum-9-17/index.html. Accessed 14 Jan 2019
- 25.Nielsen LR. Review of pathogenesis and diagnostic methods of immediate relevance for epidemiology and control of Salmonella Dublin in cattle. Vet Microbiol. 2013;162:1–9. doi: 10.1016/j.vetmic.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Constable PD, Hinchcliff KW, Stanley H, Done SH, Grünberg W. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goat. 11th ed. St Louis: Elsevier; 2017. pp. 357–373. [Google Scholar]
- 27.Costa RA, Casaux ML, Caffarena RD, Macías-Rioseco M, Schild CO, Fraga M, Riet-Correa F, Giannitti F. Urocystitis and ureteritis in Holstein calves with septicaemia caused by Salmonella enterica serotype Dublin. J Comp Pathol. 2018;164:32–36. doi: 10.1016/j.jcpa.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzella C, Ricci A, DiGiannatale E, Luzzi I, Carattoli A. Tetracycline and streptomycin resistance genes, transposons, and plasmids in Salmonella enterica isolates from animals in Italy. Antimicrob Agents Chemother. 2004;48:903–908. doi: 10.1128/AAC.48.3.903-908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang SJ, Park KY, Kim SH, No KM, Besser TE, Yoo HS, Kim SH, Lee BK, Park YH. Antimicrobial resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from animals in Korea: comparison of phenotypic and genotypic resistance characterization. Vet Microbiol. 2002;86:295–301. doi: 10.1016/S0378-1135(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Rovira A, Davies P, Ahlstrom C, Muellner P, Rendahl A, Olsen K, Bender JB, Wells S, Perez A, Alvarez J. Serotypes and antimicrobial resistance in salmonella enterica recovered from clinical samples from cattle and swine in Minnesota, 2006 to 2015. PLoS One. 2016;11:1–20. doi: 10.1371/journal.pone.0168016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantón R, Ruiz-Garbajosa P. Co-resistance: an opportunity for the bacteria and resistance genes. Curr Opin Pharmacol. 2011;11:477–485. doi: 10.1016/j.coph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization; Food and agriculture organization of the United Nations; world organisation for animal health (2016) antimicrobial resistance a manual for developing national action plans (Ver.1). doi:10.17226/6121, 1998
- 33.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da Costa PM, Loureiro L, Matos AJF. Transfer of multidrug-resistant bacteria between intermingled ecological niches: the interface between humans, animals and the environment. Int J Environ Res Public Health. 2013;10:278–294. doi: 10.3390/ijerph10010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordeiro NF, Yim L, Betancor L, Cejas D, García-Fulgueiras V, Mota MI, Varela G, Anzalone L, Algorta G, Gutkind G, Ayala JA, Chabalgoity JA, Vignoli R. Identification of the first blaCMY-2 gene in Salmonella enterica serovar Typhimurium isolates obtained from cases of paediatric diarrhoea illness detected in South America. J Glob Antimicrob Resist. 2013;1:143–148. doi: 10.1016/j.jgar.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Cordeiro NF, Nabón A, García-Fulgueiras V, Álvez M, Sirok A, Camou T, Vignoli R. Analysis of plasmid-mediated quinolone and oxyimino-cephalosporin resistance mechanisms in Uruguayan Salmonella enterica isolates from 2011–2013. J Glob Antimicrob Resist. 2016;6:165–171. doi: 10.1016/j.jgar.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Sasías S, Martínez-Sanguiné A, Betancor L, Martínez A, D'Alessandro B, Iriarte A, Chabalgoity JA, Yim L. A naturally occurring deletion in FliE from Salmonella enterica serovar Dublin results in an aflagellate phenotype and defective proinflammatory properties. Infect Immun. 2018;86:1–20. doi: 10.1128/IAI.00517-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.