Abstract
Bacterial biofilms are involved in various medical infections and food contamination episodes and, for this reason, it is of great importance to developing new strategies of its prevention and control. The subinhibitory concentration of nisin was determined, and its effect against Staphylococcus aureus and Staphylococcus epidermidis biofilms was evaluated. Results obtained by confocal laser microscopy demonstrated morphological changes in the architecture of the structure of biofilms. The main components (polysaccharides, proteins, and extracellular DNA (eDNA)) of the biofilm matrix were determined by spectrophotometry and showed that the formation of staphylococcal biofilms in the presence of nisin results in a less dense matrix structure with modification in its constituents. These results contribute to increase the knowledge of the composition and architecture of the extracellular matrix of biofilms of S. aureus, as well as evidence that the investigation of alternative products to assist in the control and combat of biofilms is a promising strategy.
Keywords: Staphylococcus aureus, Biofilm composition, Bacteriocin, Nisin subinhibitory concentrations
Introduction
Staphylococcus aureus is frequently involved in cases and outbreaks of food poisoning and is able to adhere and form biofilms on different surfaces causing serious problems of food contamination [1–4]. Besides, this pathogen is considered a common source of infections in medical implants in many host tissues [5–7]. Human diseases caused by S. aureus range from skin infections to endocarditis and hemolytic pneumonia, whereas in animals, this pathogen is frequently involved in mastitis [8, 9]. Therefore, it is essential to develop new strategies for prevention and control of the presence of pathogens in environment and surfaces, both in the food industry and in hospital areas.
Biofilm can be defined as a community of microorganisms that adhere to moist surfaces, multiply, and produce a viscous matrix comprising of extracellular polymeric substances (EPS) [10, 11]. These structures create a persistent source of contamination since microorganisms released from the biofilm are able to cause damage to the quality and safety of food through pathogens which may also be associated in the biofilms [12]. Biofilm formation on surfaces in contact with food can be controlled by cleaning using chemical and physical methods; however, bacteria living in biofilms are more resistant to antimicrobial agents and difficult to remove mechanically [11]. Biofilms in medical devices is also a public health problem [13], and the majority of device-associated infections are caused by staphylococci, especially Staphylococcus epidermidis. Actually recognized as an opportunistic pathogen, S. epidermidis adapts to form a biofilm, and the dissemination from biofilm-infected catheters represents the most frequent source of severe S. epidermidis infections such as sepsis [14, 15].
Biofilms are highly complex structures being their EPS matrix mainly comprised of polysaccharides, proteins, and eDNA [16–20]. Each component develops an important function within biofilms, like adhesion, aggregation of bacterial cells, cohesion of biofilms, retention of water, protective barrier, sorption of organic compounds, sorption of inorganic ions, enzymatic activity, nutrient source, exchange of genetic information, electron donor or acceptor, export of cellular components, reservoir of energy, and binding of enzymes [21]. Such structures facilitate the formation of highly resistant microcolonies, which are not desirable in food handling environments [22]. Thus, the destabilization of this complex matrix formed by these components, polysaccharides, proteins, and eDNA, may induce a biofilm disaggregation.
Alternative strategies for microbial control in both sessile and planktonic forms have been researched. Ribosomally synthesized peptides with antimicrobial properties are produced by many living organisms, from prokaryotes to higher eukaryotes [22]. These antimicrobials ribosomally synthesized peptides by bacteria are called bacteriocins [23] and have aroused interest as foodborne microbial inhibitors. Nisin, an antimicrobial peptide produced by Lactococcus lactis, is the best known and extensively studied class I (lantibiotic) bacteriocin [24]. Nisin is recognized since 1989 as a GRAS (Generally Recognized as Safe) substance and has been used in food preservation [25–27]. The antimicrobial activity of nisin is attributed to its interaction with anionic lipids on the bacterial cell membrane resulting in membrane disruption, by forming pores that lead to efflux of ions, dissipation of proton-motive force, ATP hydrolysis, and therefore, loss of viability and cell lysis [23]. Nisin has a rapid bactericidal activity against Gram-positive bacteria, in addition to being effective against planktonic cells [28], and has also demonstrated efficacy against biofilms established [28–31]. However, its effect on the components of biofilms has not been widely studied.
Understanding the effects of antimicrobials on biofilm formation and composition is important for improving the efficiency of disinfection strategies. Therefore, this work aimed to verify the interference of nisin on the composition and structure of biofilms formed by S. aureus and S. epidermidis.
Materials and methods
Microorganisms and growth conditions
Strains of Staphylococcus (Table 1) were pre-cultured in Luria Bertani (LB) broth (Sigma-Aldrich, Germany) aerobically under stirring at 150 rpm at 37 °C for 18 h. From these cultures, inocula were transferred to Erlenmeyer flasks containing fresh LB broth and adjusting the initial optical density at 600 nm (OD600nm) to 0.1. The flasks were incubated at 37 °C and the growth was monitored by a spectrophotometer model 1510 (Thermo Fisher Scientific, Finland) until cultures reached OD600nm of 0.2.
Table 1.
Bacteria used in this study
| Bacteria | Source | Reference |
|---|---|---|
| S. aureus COL | Humans, MRSA* | [32] |
| S. aureus FRI 722 | Food | [33] |
| S. aureus EMBRAPA 4018 | Bovine mastitis | [34] |
| S. epidermidis ATCC 35984 | Catheter sepsis | [35] |
*Methicillin-resistente S. aureus
The cultures were grown separately in LB broth. Cells were collected by centrifugation at 5000×g for 10 min at 4 °C and then, washed twice using 0.85% saline solution. For the study of biofilm, a mixed culture containing the three different strains of S. aureus (COL, FRI 722, and EMBRAPA 4018) was prepared, as described by Garcia-Hernandez et al. [36]. S. epidermidis ATCC 35984, previously characterized as a strong biofilm-forming strain [24], was used as a control.
Bacteriocin
A stock solution of the bacteriocin nisin (2.5% nisin, ≥ 1.000 UImg-1, Sigma-Aldrich, Germany) at 1 mM was prepared in phosphate-buffered saline (PBS, 10 mM, pH 7.2) and stored at 7 °C [20].
Minimal inhibitory concentration determination
To determine the minimum inhibitory concentration (MIC) of nisin, 200 μl of LB broth supplemented with different concentrations of the bacteriocin (ranging from 0.67 to 11.39 mg l−1) was transferred to 96-well polystyrene microtiter plates and inoculated with, approximately, 5 × 105 CFU ml−1 of the different cultures of Staphylococcus spp., previously propagated in LB medium without bacteriocin. The minimum concentration able to prevent the growth (OD600nm) after 18 h of incubation at 37 °C was considered as the minimal inventory concentration (MIC) value [20].
Biofilm observation by confocal laser scanning microscopy
Polystyrene coupons (1.0 × 1.0 × 0.1 cm) were first cleaned by washing with liquid neutral detergent and water, followed by rinsing with distilled water and then immersing in 70% ethyl alcohol for 1 h to remove fat. Subsequently, they were rinsed with distilled water and air-dried under UV light. Confirmation of the coupons sterility was performed by the incubation of some coupons in LB broth at 37 °C for 24 h following by turbidity verification.
For biofilm formation assays, polystyrene coupons were immersed in LB broth contained 24-well polystyrene microtiter plates and inoculated with the mixed culture of S. aureus in the presence and absence of nisin at a concentration of 2.01 mg l−1, which allow the growth of all strains. Then, after 46 h at 37 °C at static condition, the coupons were rinsed twice by immersion in PBS (0.2 M; pH 7.2) and incubated in the dark for 15 min with a mixture of 20 mg l−1 propidium iodide (PI) (Sigma-Aldrich, Germany) and 2 mg l−1 fluorescein isothiocyanate (FITC) (Sigma-Aldrich, Germany) in PBS (0.2 M, pH 7.2) prepared immediately before use. After incubation, the coupons were washed by immersion in PBS and analyzed by a confocal laser scanning microscope model LSM 510 META (Zeiss, Germany) using argon laser with a wavelength of 458 to 514 nm. Cells with green color were considered alive and those showing yellowish or reddish color were considered dead.
Characterization of the biofilm composition
The content of the three main components of the biofilm matrix of S. aureus (polysaccharides, proteins, and nucleic acids) was evaluated according to Fredheim et al. [37] using: (i) sodium meta-periodate (NaIO4) (Sigma-Aldrich, Germany) capable of degrading β-1,6-polysaccharide links; (ii) proteinase K (Promega, USA) to degrade proteins, and (iii) DNase I (Sigma-Aldrich, Germany) to degrade DNA molecules. The experiment was carried out in 96-well polystyrene microtiter plates. The mixed culture of S. aureus was inoculated in LB broth and biofilms were formed during 48 h of incubation at 37 °C. After this period, the plate contents were discarded remaining just the biofilm attached to the plastic surface. The wells were washed three times with PBS and treated for 24 h at 37 °C with (i) 0.04 mol l−1 NaIO4 in bidistilled water, (ii) 0.1 μg l−1 proteinase K in 0.02 mol l−1 Tris-HCl (pH 7.5) with 0.1 mol l−1 NaCl, or (iii) 0.5 μg l−1 DNase I in 0.5 mol l−1 MgCl2. The percentage of adherent cells was determined by spectrophotometry at OD590nm (Thermo Fisher Scientific, Finland) after the plates were washed and stained with 0.1% (w/v) crystal violet as described by Fredheim et al. [37].
Biofilm detachment assays were carried out in triplicate in three different occasions, and the percentages of polysaccharides, proteins, and nucleic acids were calculated based on the difference between the OD590nm of the average of treated and the control wells. The results of the mix S. aureus and S. epidermidis biofilms were separately analyzed and the data were submitted to one-way analysis of variance (ANOVA). The means were compared by the Tukey test at 5% significance. Analyses were performed using the R software environment (R Development Core Team, Auckland, New Zealand).
Results
Staphylococcus spp. inhibition by nisin
The differences in the MIC values among the isolates show variability in the sensitivity profile of S. aureus to the bacteriocin nisin. Among the evaluated cultures, S. aureus COL showed the greatest resistance to nisin (9.38 mg l−1), while S. aureus FRI 722, S. aureus Embrapa 4018, and S. epidermidis ATCC 35984 had the same sensitivity profile to the bacteriocin (4.02 mg l−1). Based on MIC values, the subinhibitory concentration of 2.01 mg l−1, which allows the growth of all strains, was chosen to conduct the subsequent assays.
Evaluation of the biofilm structure formed by Staphylococcus spp.
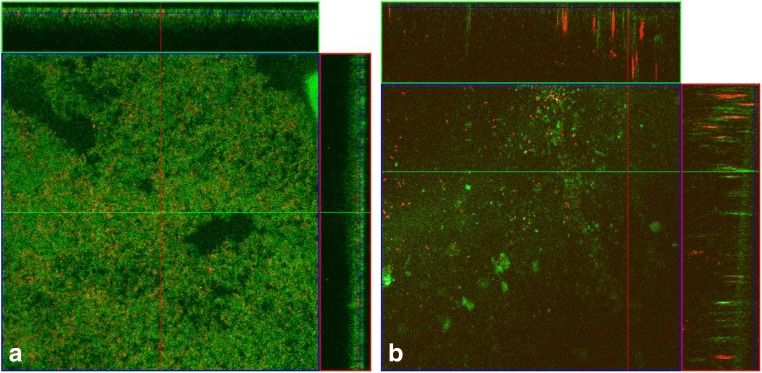
Images obtained by confocal laser scanning microscopy were used to observe the structure of biofilms in its hydrated state. In this assay, cell viability was verified by the fluorophores propidium iodide (PI), which stains injured and dead cells, and fluorescein isothiocyanate (FITC) that stains live cells. The image obtained in the control treatment without the addition of nisin (Fig. 1a) displays a balance in the proportion of live cells and dead cells in the biofilm. Moreover, it is possible to observe a decrease in biofilm formation (Fig. 1b), when they were formed in a culture medium containing nisin in subinhibitory concentrations. In the biofilm formed by the mixed culture of S. aureus (Fig. 1b) in the presence of 2.01 mg l−1 of nisin, more spaced cells in the array were observed together with higher numbers of dead cells.
Fig. 1.
Images of confocal laser microscopy. Biofilm produced during 46 h by the mixed culture of S. aureus COL, S. aureus FRI 722, and S. aureus Embrapa 4018 a without nisin and b in the presence of nisin (2.01 mg l−1). The green fluorescence represents live cells stained with FITC while red and yellow show dead cells stained with propidium iodide. Scale bar = 20 μm
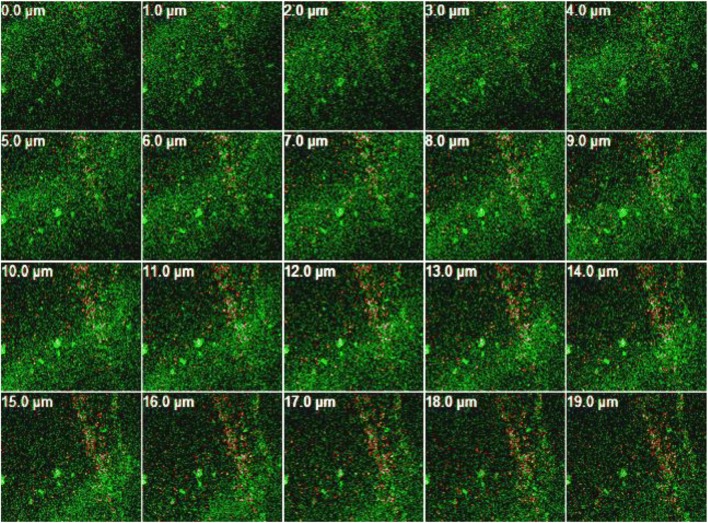
A sequence of cuts was made to observe interior parts of the biofilm structure from the bottom to the top (Figs. 2 and 3). To this end, the tool “z-stack” of the LSM META 510 software (Zeiss, Germany) was applied to the image generating biofilms slices with standardized cuts of 1.0 μm. Through these pictures, a reduction of biofilm formed in the presence of nisin can be clearly observed.
Fig. 2.
Images of confocal laser microscopy. Biofilm produced during 46 h by the mixed culture of S. aureus COL, S. aureus FRI 722, and S. aureus Embrapa 4018 on polystyrene surface in the absence of nisin. The green fluorescence represents live cells stained with FITC while red and yellow show dead cells stained with propidium iodide. Image generating biofilm slices with standardized cuts of 1.0 μm
Fig. 3.
Images of confocal laser microscopy. Biofilm produced during 46 h by the mixed culture of S. aureus COL, S. aureus FRI 722, and S. aureus Embrapa 4018, on polystyrene surface in the presence of nisin (2.01 mg l−1). The green fluorescence represents live cells stained with FITC while red and yellow show dead cells stained with propidium iodide. Image generating biofilm slices with standardized cuts of 1.0 μm
Composition of staphylococcal biofilm
Given the importance of the three main components of the bacterial biofilm matrix, the variation of polysaccharides, protein, and eDNA contents in the biofilms formed by the mixed culture of S. aureus and by S. epidermidis was evaluated in the presence and in the absence of nisin. The averages of the OD590nm values of the remained biofilms after treatment with the specific enzymes for each one of the main components of the biofilms are presented in Fig. 4. The values represent the cell detachment ratio based on the applied treatment.
Fig. 4.
Composition of the biofilm by the mixed culture of S. aureus and by S. epidermidis ATCC 35984. Optical density (590 nm) corresponding to the detached portion of each biofilm component in the absence (white bars) and presence (gray bars) of nisin (2.01 mg l−1) with respect to total biofilm, polysaccharides, proteins, and nucleic acid (eDNA) components. * indicates statistical significance by the Tukey test at 5% probability error
The presence of a subinhibitory concentration of nisin showed a significant reduction of the OD590nm corresponding to the detachment of polysaccharides (Fig. 4). Nisin was also effective in reducing the total biofilm and the polysaccharide content of the biofilm S. epidermidis (Fig. 4). However, no significant change in protein composition of the biofilm formed by the mixed culture of S. aureus or by S. epidermidis, in the presence of nisin, was observed (Fig. 4). Nisin also showed to reduce eDNA in S. aureus biofilm, but this effect was not observed in the S. epidermidis biofilm.
Discussion
This study aimed to verify the effect of bacteriocin nisin on biofilm formed by Staphylococcus spp. Biofilms are complex structures formed by microbial cells and EPS composed mainly of polysaccharides, proteins, and eDNA. In medicine and in the food sector, studies which aim to understand these structures have been performed in order to prevent biofilm development or to investigate methods for biofilm removal.
S. aureus and S. epidermidis are microorganisms able to adhere and form biofilm on different surfaces. Among different approaches that have been used for preventing and/or removing biofilm in food and medical environments, the use of disinfectants and more natural strategies has been highlighted. The use of nisin, an antimicrobial peptide that has a well-established mechanism of action with respect to the growth of S. aureus and other pathogens [23, 24], consists of an example of the least one.
To conduct this study, the MIC of nisin was determined for each strain of Staphylococcus spp., and similar values (4.02 mg l−1) were observed except for S. aureus COL (9.38 mg l−1), which showed increased resistance to this bacteriocin. Similar results were found by Pimentel-Filho et al. [20], who observed greater resistance of this strain to the bacteriocins nisin and bovicin HC5. According to Melchior et al. [38], different strains of S. aureus require a specific concentration for each antimicrobial evaluated. Vázquez-Sánchez et al. [39] showed the existence of a different profile of resistance and susceptibility to antimicrobial agents among S. aureus strains and suggested the need to use a large collection of strains for the evaluation of the bactericidal activity of antimicrobials. Based on MIC values obtained for each strain, the subinhibitory concentration of nisin for the following experiments with the mixed culture of S. aureus and with S. epidermidis ATCC 35984 was determined as the concentration equivalent to 50% of the MIC of the most sensitive strain to bacteriocin. Therefore, 2.01 mg l−1 of nisin was established as the subinhibitory concentration for the following experiments.
Images of the biofilm formed by mixed culture of S. aureus obtained by confocal laser scanning microscopy (Figs. 1, 2, and 3) showed that, in the presence of subinhibitory concentrations of the bacteriocin, there was a reduction in biofilm formation in the period of 46 h. Nisin has showed the highest bactericidal activity against both planktonic cells and biofilm cells [30, 40] and, even in the subinhibitory concentration used, the effect upon sessile cells was confirmed by microscopic images. This finding highlights the importance of subinhibitory nisin in biofilm formation, causing possible modifications in its constituents and consequently in its structure. One factor that can contribute to the reduction of biofilms in the presence of a subinhibitory concentration of nisin is the change of hydrophobicity of the polystyrene surface caused by the presence of the antimicrobial peptide. Pimentel-Filho et al. [20] attributed the reduction of S. aureus cell adhesion to polystyrene surface to the change in the surface hydrophobicity profile when exposed to the bacteriocins nisin and bovicin HC5. Another factor could be related to the alterations in the biofilm composition and this hypothesis was confirmed through the next result.
Biofilms have a complex matrix and, when mature, this matrix protects sessile cells against environmental stresses including antimicrobial agents [18]. The biofilm formation in the presence of nisin resulted in the reduction of polysaccharides, which can be associated with decreased biofilm formation or its disruption, thereby making the associated microorganisms more sensitive to antimicrobial agents. Both S. aureus and S. epidermidis produce of β-1,6-linked N-acetyl-d-glucosamines, a surface polysaccharide which is referred to as the polysaccharide intercellular adhesin (PIA) [41–43]. In S. aureus, the integrity of the biofilm architecture and its protection against the action of biocidal compounds is attributed the presence and expression of gene ica that encode the polysaccharide poly-N-acetylglucosamine (PNAG) [44]. However, the effect of nisin upon the polysaccharide structure of staphylococcal biofilm rest to be more studied.
Proteins are also among the main components of the biofilm, but nisin does not seem to affect their relative abundance in the biofilm (Fig. 4). In S. aureus, small peptides called phenol-soluble modulins can self-organize into amyloid fibers and participate in the assembly and integrity of the biofilm [18].
Besides interfering with the polysaccharide composition, nisin had an effect on reducing eDNA of the biofilm S. aureus (Fig. 4) but not of S. epidermidis. The eDNA seems to have an important role in the biofilm of S. aureus, and pre-formed biofilm treated with DNase becomes more sensitive to inactivation by antimicrobials [18]. There is evidence that eDNA is an important structural component of the S. aureus biofilm matrix and may function as an intercellular adhesion molecule [17]. The eDNA release likely interferes with the early stages of attachment and/or microcolony formation during biofilm development [16]. The mechanism of eDNA release in bacterial biofilm is not well known; one of the primary findings is that cell death and lysis are necessary and, apparently, a controlled process during the development of S. aureus biofilm [16–18].
The effect of nisin on S. aureus on formed biofilms has already been reported; in this way, our results contribute to a deeper knowledge of the composition and architecture of the extracellular matrix of biofilms. Information about the composition and architecture of the biofilms is quite useful for improving the efficiency of disinfection strategies. Indeed, biofilms are highly complex and the extraction and purification of extracellular matrix components are laborious so that it is still a challenge to provide a full biochemical profile of biofilms [16–19].
Funding information
Authors thank CNPq for the fellowship to Cleriane Andre and FAPEMIG (APQ 02600-14-1), for the financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shale K, Lues JFR, Venter P, Buys EM. The distribution of Staphylococcus sp. on bovine meat from abattoir deboning rooms. Food Microbiol. 2005;22:433–438. doi: 10.1016/j.fm.2004.09.007. [DOI] [Google Scholar]
- 2.Rode TM, Langsrud S, Holck A, Moretro T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int J Food Microbiol. 2007;116:372–383. doi: 10.1016/j.ijfoodmicro.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Di Ciccio P, Vergara A, Festino AR, Paludi D, Zanardi E, Ghidini S, Ianieri A. Biofilm formation by Staphylococcus aureus on food contact surfaces: relationship with temperature and cell surface hydrophobicity. Food Control. 2015;50:930–936. doi: 10.1016/j.foodcont.2014.10.048. [DOI] [Google Scholar]
- 4.Abdallah M, Khelissa O, Ibrahim A, Benoliel C, Heliot L, Dhulster P, Chihib NE. Impact of growth temperature and surface type on the resistance of Pseudomonas aeruginosa and Staphylococcus aureus biofilms to disinfectants. Int J Food Microbiol. 2015;214:38–47. doi: 10.1016/j.ijfoodmicro.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28:55–61. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 7.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay JA, Holden MTG. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 2004;12:378–385. doi: 10.1016/j.tim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald JR, Sturdevant DE, Mackie SM, Gill SR, Musser JM. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc Natl Acad Sci U S A. 2001;98:8821–8826. doi: 10.1073/pnas.161098098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpentier B, Cerf O. Biofilms and their consequences, with particular reference to hygiene in the food industry. Appl Bacteriol. 1993;75:499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 11.Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT Food Sci Technol. 2010;43:573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- 12.Marques SC, Rezende JGOS, Alves LAF, Silva BC, Alves E, Abreu LR, Piccoli RH. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Braz J Microbiol. 2007;38:538–543. doi: 10.1590/S1517-83822007000300029. [DOI] [Google Scholar]
- 13.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277–281. doi: 10.3201/eid0702.700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers KL, Fey PD, Rupp ME. Coagulase-negative staphylococcal infections. Infect Dis Clin N Am. 2009;23:73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Khan BA, Cheung GYC, Bach THL, Jameson-Lee M, Kong KF, Queck SY, Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allesen-Holm M, Barken B, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol. 2006;59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 17.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pimentel-Filho NDJ, Martins MCF, Nogueira GB, Mantovani HC, Vanetti MCD. Bovicin HC5 and nisin reduce Staphylococcus aureus adhesion to polystyrene and change the hydrophobicity profile and Gibbs free energy of adhesion. Int J Food Microbiol. 2014;190:1–8. doi: 10.1016/j.ijfoodmicro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Flemming H, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 22.Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv. 2003;21:465–499. doi: 10.1016/S0734-9750(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 23.Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 24.Deegan LH, Cotter PD, Hill C, Ross P. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J. 2006;16:1058–1071. doi: 10.1016/j.idairyj.2005.10.026. [DOI] [Google Scholar]
- 25.Breukink E, Heusden HE, Van Vollmerhaus PJ, Swiezewska E, Brunner L, Walker S, Heck AJR, De Kruijff B. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J Biol Chem. 2003;278:19898–19903. doi: 10.1074/jbc.M301463200. [DOI] [PubMed] [Google Scholar]
- 26.Ennahar S, Sashihara T, Sonomoto K, Ishizaki A. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev. 2000;24:85–106. doi: 10.1016/S0168-6445(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 27.Tong Z, Ni L, Ling J. Antibacterial peptide nisin: a potential role in the inhibition of oral pathogenic bacteria. Peptides. 2014;60:32–40. doi: 10.1016/j.peptides.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Dosler S, Dosler S, Gerceker AA. In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy. 2011;57:511–516. doi: 10.1159/000335598. [DOI] [PubMed] [Google Scholar]
- 29.Mataraci E, Dosler S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2012;56:6366–6371. doi: 10.1128/AAC.01180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field D, Gaudin N, Lyons F, O’Connor PM, Cotter PD, Hill C, Ross RP. A bioengineered nisin derivative to control biofilms of Staphylococcus pseudintermedius. PLoS One. 2015;10:e0119684. doi: 10.1371/journal.pone.0119684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davison WM, Pitts B, Stewart PS. Spatial and temporal patterns of biocide action against Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2010;54:2920–2927. doi: 10.1128/AAC.01734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer WM, Iandolo JJ. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979;25:902–911. doi: 10.1128/IAI.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braga LC, Shupp JW, Cummings C, Jett M, Takahashi JA, Carmo LS, Chartone-souza E, Nascimento AMA. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel-Filho NDJ, Mantovani HC, Diez-Gonzalez F, Vanetti MCD. Inhibition of Listeria and Staphylococcus aureus by Bovicin HC5 and Nisin combination in milk. J Agric Sci. 2013;5:188–196. doi: 10.5539/jas.v5n8p188. [DOI] [Google Scholar]
- 35.Simpson WA, Younger JJ, Larry M, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol Infect. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Hernandez R, McMullen L, Gänzle MG. Development and validation of a surrogate strain cocktail to evaluate bactericidal effects of pressure on verotoxigenic Escherichia coli. Int J Food Microbiol. 2015;205:16–22. doi: 10.1016/j.ijfoodmicro.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Fredheim EGA, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flaegstad T, Sollid JE. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47:1172–1180. doi: 10.1128/JCM.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melchior MB, Fink-Gremmels J, Gaastra W. Extended antimicrobial susceptibility assay for Staphylococcus aureus isolates from bovine mastitis growing in biofilms. Vet Microbiol. 2007;125:141–149. doi: 10.1016/j.vetmic.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Vázquez-Sánchez D, Cabo ML, Ibusquiza PS, Rodríguez-Herrera JJ. Biofilm-forming ability and resistance to industrial disinfectants of Staphylococcus aureus isolated from fishery products. Food Control. 2014;39:8–16. doi: 10.1016/j.foodcont.2013.09.029. [DOI] [Google Scholar]
- 40.Okuda KI, Zendo T, Sugimoto S, Iwase T, Tajima A, Yamada S, Sonomoto K, Mizunoe Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob Agents Chemother. 2013;57:5572–5579. doi: 10.1128/AAC.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/JB.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1111/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 43.Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Götz F, Goldmann DA, Pier GB. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]