Abstract
The effect of gene dosage on the production of Candida antarctica lipase B (CalB) in the methylotrophic yeast Komagataella phaffii, at high densities in a simple medium containing crude glycerin as the sole carbon source, is described. The use of crude glycerin, the main by-product of biodiesel production from vegetable oils, will reduce the production cost of the bioprocess. Two K. phaffii strains were constructed with one or three copies of LipB, an optimized version of the gene encoding CalB under the control of the constitutive PPGK1 promoter. These two constructs were tested and compared on batches using minimal-salts medium with crude glycerin. The strain with three copies achieved a higher enzyme yield (48,760 U/L, 2.3-fold higher than the one-copy strain), with 42 g/L biomass, with no effects on growth.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00056-8) contains supplementary material, which is available to authorized users.
Keywords: Candida antarctica, lipase B; Komagataella phaffii; Constitutive expression; Multicopy vector integration; Gene dosage
Introduction
The yeast Candida antarctica is capable of producing two lipases, lipase A (CalA) and lipase B (CalB). The latter is one of the lipases most used for biocatalysis thanks to its high enantioselectivity with a broad range of substrates and good thermal stability [1–3]. From an industrial angle, CalB tested has high efficiency in transesterification [4] and also catalyze alcoholysis to produce biodiesel [5, 6]. CalB is produced commercially by Novozymes as a recombinant enzyme from Aspergillus niger, which is widely used in organic synthesis for resolution of racemic mixtures of alcohols, amines, and acids or in the preparation of optically active compounds [2, 7]. The 33 kDa CalB has the following characteristics: isoelectric point of 6.0, 15% retained activity after 20 min of incubation at 70 °C pH 7, stable in basic pH [7–10] and regiospecificity of hydrolysis of triacylglycerol Sn-3 [7].
The methylotrophic yeast Komagataella phaffii (formerly known as Pichia pastoris) has several advantages over other eukaryotic or prokaryotic systems as an expression platform for CalB production [1]: high productivity in simple medium containing glycerol as the sole carbon source [2]; growth at high cell densities [3]; well-established fermentation protocols [4]; GRAS status [5]; ability to perform various post-translational modifications, including glycosylation, methylation, acylation, and proteolysis; and [6] extracellular soluble-protein purification [8, 9].
Most vectors developed for K. phaffii are based on the methanol-induced PAOX1 promoter [10]. However, the use of methanol may be undesirable in large-scale fermentation processes because of its flammability and toxicity. Variants of PAOX1 are being developed, in order to regulate the production without the need for methanol, but the production rates are not as well studied as the classic PAOX1 [11]. Constitutive expression systems represent an attractive alternative, with the advantage that different carbon sources can be employed, including crude glycerol, a by-product of the biodiesel industry [12]. The most commonly used promoter is PGAP, which is strongly regulated and capable of hyperexpression [13]. Other promoters of the glycolytic pathway can be explored, such as a reduced-version PPGK1 used in this study, which has potential for use in the development of new engineered pathways [14].
In order to improve the protein production of K. phaffii, several studies have focused on increasing the copy number of the desired gene, using different approaches such as in vitro multimerization, PVTA (post-transformational vector amplification), integration targeted to the rDNA locus, and the use of defective markers [15]. The drug-resistance genes Sh ble and kan (zeocin and G418 resistance, respectively) are typically used to select yeast multicopy clones. Although the use of these markers is laborious and requires expensive antibiotics, they can be used in wild-type yeast strains and provide the opportunity to obtain a multicopy strain. In most cases, a higher gene dosage may greatly enhance the expression of recombinant protein. However, an excessively high gene dosage will lead to a plateau in expression, and may even be detrimental [16, 17]. A high level of overexpression of heterologous proteins in K. phaffii saturates or overloads the secretory pathway, or even triggers the protein to unfold [18].
Defective markers are typically auxotrophic genes, which are transcriptionally impaired. In this case, prototrophy is normally obtained when several copies of the transforming vector are present to compensate for the weak transcription from the defective marker [19]. This approach has been used successfully to obtain multicopy transformants in many types of yeast, such as Saccharomyces cerevisiae [20–22], Yarrowia lipolytica [23, 24], and Hansenula polymorpha [25]. Recently, Betancur et al. [26] developed a transformation system for K. phaffii based on the leucine-defective marker leu2-d, which proved to be an easy and efficient way to obtain multicopy integration of the desired gene after a single transformation event.
In this study, we sought the development of K. phaffii strains that express a synthetic gene coding for CalB under the control of a constitutive promoter, PPGK1, and evaluated the effect of gene dosage in order to improve lipase production. In addition, we evaluated several fermentation batches based on crude glycerol as the sole carbon source, in order to compare the productivity of each strain in a controlled environment.
Material and methods
Material
The crude glycerol used in this study was derived from biodiesel synthesis from soybeans and was kindly provided by Petrobras (Rio de Janeiro, Brazil). The main features of this crude glycerol were: density 1.25 g/L, NaCl content about 7 g/L, and less than 1000 ppm methanol. The purity of the glycerol was 84%.
Strains and media
Chemically competent Escherichia coli Stellar™ (Clontech, USA) and XL10-Gold® (Stratagene, USA) were used for the DNA cloning procedures. Bacterial cells were cultured in LB modified medium (in w/v, 1% peptone, 0.5% yeast extract, 1% NaCl, pH 7.2), with 100 μg/mL ampicillin or 50 μg/mL kanamycin added when necessary, at 37 °C and 200 rpm. Low-salt LB (0.5% w/v NaCl) was used for selection with zeocin (25 μg/mL). Solid medium was prepared by adding 1.5% agar.
K. phaffii X-33 (Invitrogen) and M12 (leu2) [26] were used for lipase expression. K. phaffii was routinely cultured in YPD medium (in w/v, 1% yeast extract, 2% peptone, 2% glucose) at 28 °C and 200 rpm. For transformation, K. phaffii X-33 cells were plated on YPDS (YPD with 1 M sorbitol) with 100 μg/mL zeocin added, while M12 cells were selected on MD medium (1.34% yeast nitrogen base without amino acids—YNB, 2% glucose, 4 × 10−5% biotin). Lipase activity was detected on MDT (MD plus 100 mM phosphate buffer and 1% tributyrin) or YPDT (YPD plus 1% tributyrin) medium plates. For solid medium, 2% agar was added.
Plasmid construction
A 954-bp version of the C. antarctica CalB gene corresponding to the mature protein was codon-optimized for expression in K. phaffii and synthesized de novo by Epoch Biosciences (USA). The synthetic gene, LipB, contained in its 5′-end a XhoI site followed by 18 nt required to reestablish the Kex2 and Ste13 proteolytic sites present in the S. cerevisiae α-factor signal sequence; while the 3′-end had a NotI site after the stop codon. LipB was cloned into pBluescript II SK, resulting in pBSK_LIPB. LipB was removed after double digestion of this vector with XhoI/NotI following subcloning in pPGKΔ3AMY [14] digested with the same enzymes, resulting in pPGKΔ3LIPB. To further improve CalB production, the original α-factor secretion sequences were replaced with codon-optimized sequences, resulting in pPGKΔ3PRO_LIPB. This vector has the Sh ble dominant marker, and LipB is under the control of the constitutive pPGK1 promoter.
For multicopy integration of LipB mediated by a defective marker, the pK-ld vector [26] was used. This vector contains pPGK1 and the leu2-d selection marker. A fragment corresponding to LipB and the optimized α-factor sequences was amplified by PCR from pPGKΔ3PRO_LIPB with the primers EGFif-F (GATTACGAAAGGATCACGATGAGATTCCCATCTATCTTCACTG) and Liprec-R (ATGGTCGACGCGGCCGCTTATGGAGTAACGATACCAGAG) using CloneAmp HiFi PCR Premix (Clontech). The ~ 1.2 kb amplicon was cloned into pK-ld previously double-digested with BamHI/NotI by in vitro recombination (In-Fusion® HD Cloning Kit, Clontech). The resulting vectors pPGKΔ3PRO_LIPB and pKLip-ld were linearized with SacI and PvuI, respectively, for direct integration into the K. phaffii PGK1 locus. Schemes of the constructed plasmids are shown as supplemental figures.
Yeast transformation
K. phaffii cells were transformed by electroporation following the protocol described in the Pichia Expression Kit (Invitrogen). Transformed cells were plated on YPDS containing zeocin (pPGKΔ3PRO_LIPB) or MD (pKLip-ld). Transformants were screened for lipase activity on YPDT (pPGKΔ3PRO_LIPB) or MDT (pKLip-ld) plates.
DNA extraction
Genomic DNA (gDNA) was isolated using a DNA purification kit (Wizard® Genomic DNA Purification Kit, Promega). DNA concentration and quality were verified with Nanodrop™ (Thermo Scientific™).
Primer design
For DNA amplification, a set of primers for the gene of interest (LipB) was used, and the reference β-actin gene (ACT1) was used as an endogenous control to normalize the data. Primers for ddPCR were designed according to the Bio-Rad guidelines. Primer sequences are listed in Table 1.
Table 1.
Construction design of primers for LipB and ACT1
| Oligonucleotide | Sequence | Tm | %GC | Amplicon size (nt) |
|---|---|---|---|---|
| LipB Fw | 5′-TGGCTTTCGCTCCAGACTAC-3′ | 63 | 55 | 137 |
| LipB Rev | 5′-AGTCAAACCACCAGCGTTTC-3′ | 63 | 50 | |
| ACT1 Fw | 5′-CACCACACCTTCTACAAC-3′ | 56 | 50 | 139 |
| ACT1 Rev | 5′-AGAAGGCTGGAACGTTG-3′ | 58 | 52 |
ddPCR methodology
To ensure accurate quantification and avoid a negative impact on the droplet volume, genomic DNA was digested with EcoRI (Thermo Scientific). As a result, different gDNA fragments were obtained, containing individual expression cassettes. EcoRI was selected on the following basis: (i) the LipB expression cassette was not cut by this enzyme and (ii) LipB was cloned into a standard plasmid backbone such as pPICZα, i.e., containing a single restriction site for EcoRI in its multicloning site. ddPCR reactions contained 10 μL of QX200™ ddPCR™ EvaGreen® Supermix, 200 nM of forward primer, 100 nM of reverse primer, 0.4 ng of digested gDNA, and the required amount of DNase/RNase-free water up to 20 μL of final volume. Reactions were incubated at 95 °C for 10 min, followed by a denaturation (94 °C, 30 s), and an annealing/extension step (58 °C, 1 min for LipB primers; 56.5 °C, 1 min for ACT1 primers) during 40 cycles. Droplets were detected by using the QX100™ Droplet Digital™ PCR System and the software QuantaSoft™ v. 1.7.4.0917. All the reagents used in the ddPCR were purchased from Bio-Rad. This methodology was adapted based on [16].
Storage and inoculum
Transformed K. phaffii cells were stored in 20% glycerol and maintained at − 80 °C for storage. A Petri dish with YPD (in w/v, 1% yeast extract, 2% peptone, and 2% glucose) and 2% w/v agar-agar was maintained in a refrigerator to be used as the inoculum. A single colony from the Petri dish was propagated in shaker flasks at 200 rpm and 30 °C in YPD medium.
Culture medium
The culture medium used for fermentation in bioreactors was adapted from [27] containing (per liter): 2 g citric acid, 12.4 g (NH4)2HPO4, 0.022 g CaCl2·2H2O, 0.9 g KCl, 0.5 g MgSO4·7H2O, 100 g crude glycerol, and 4.6 mL of PTM1 trace salts. The pH was maintained at 7.0 using 5% H2SO4 and 20% NH4OH.
The composition of PTM1 trace salts used contains (per liter): 2 g CuSO4·5H2O, 0.08 g KI, 3 g MnSO4·H2O, 0.2 g MnSO4·H2O, 0.02 g H3BO3, 0.5 g CoCl2 ·6H2O, 6.7 g ZnCl2, 21.7 g FeSO4·7H2O, 0.2 g biotin, and 1.7 mL H2SO4.
Fermentation process
The bioreactor used was a bench-top Multifors from Infors with three vessels of 500 mL working volume. The bioreactor was maintained at 30 °C. Aeration was controlled in order to try to maintain the dissolved-oxygen saturation at 30%. This control was effected through a cascade with stirring between 250 and 700 rpm and gas flow between 0 and 1 vvm. The inoculum was produced as mentioned in the “Storage and inoculum” section and used to inoculate the bioreactor at the desired concentration, typically between 0.7 and 1 g/L. The fermentations were performed in duplicate.
Titrimetric activity
Titrimetric activity was performed with 56 mM tributyrin for hydrolysis at 40 °C and pH 7.0, based on the method described by [28] using a pHstat and 0.06 M NaOH as titrating reagent. One unit of lipase activity (U) is defined as the quantity of enzyme needed to catalyze the production of 1 μmol butyric acid (volumetric analysis) per minute under the assay conditions. This analysis was conducted at the peak of production activity, to obtain the parameters associated with the fermentation. The results were determined as the mean of triplicates.
Biomass measurement
Cell growth was quantified based on a standard curve of dry weight, related to the measurement of optical density at 600 nm in a spectrophotometer (Hach Lange DR 3900™): DCW (g/L) = 0.4382 abs.
Glycerol concentration
Glycerol was determined using HPLC (HPLC Agilent Technologies) equipped with an HPX-87H column (Bio-Rad 300 mm × 7.8 mm). The temperature was maintained at 65 °C, using as the mobile phase a 0.005 M sulfuric acid solution at a flow rate of 0.6 mL/min. The sample volume injected was 20 μL.
N-NH4+ concentration
This was measured using the method adapted from [29, 30]. Ammonium sulfate was used as a standard. The nitrogen concentration was determined as the mean of triplicates.
Results
Copy number determination
In order to produce CalB in K. phaffii, a synthetic gene (LipB) was designed with codon optimization for this yeast. LipB was cloned under the transcriptional control of the K. phaffii constitutive promoter PPGK1, resulting in two vectors: pPGKΔ3PRO_LIPB and pKLip-ld. K. phaffii was transformed with linearized pPGKΔ3PRO_LIPB and pKLip-ld in order to target integration to the PGK1 locus. Over 85% of transformants from both systems showed lipolytic activity on plates containing tributyrin (data not shown). A clone showing the largest halo from each system was selected for further study. Clones derived from pPGKΔ3PRO_LIPB and pKLip-ld were denominated 04-MR and 34-MR, respectively.
DNA quality and concentration
Because of the low quality and quantity of DNA samples, genomic extractions were repeated. The results of the Nanodrop quantification are shown in Table 2.
Table 2.
Results obtained from Nanodrop quantification for both strains tested
| Strain | Concentration (ng/μg) | 260/280 ratio | 260/230 ratio |
|---|---|---|---|
| 04-MR | 1018 | 2.09 | 1.56 |
| 34-MR | 523 | 1.95 | 1.2 |
Gene dosage quantification
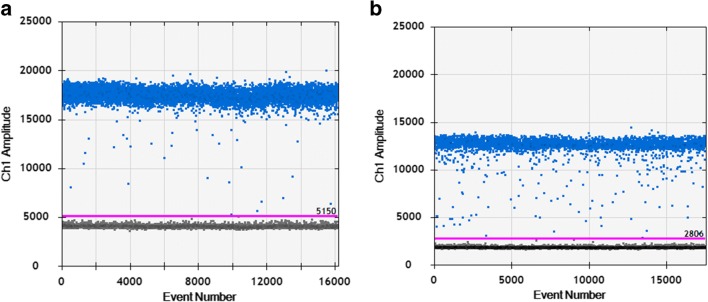
For each sample, five replicates were performed. The number of copies of LipB was determined by calculating the ratio between positive droplets of LipB and ACT1 amplification (Fig. 1).
Fig. 1.
In blue, positive droplets (PCR amplification detected). Pink line indicates threshold between positive and negative droplets. a Amplification with CalB primers. b Amplification with Act1 primers
These results established that strain 04-MR has one gene copy and strain 34-MR has three copies.
Comparison of CalB production between K. phaffii strains
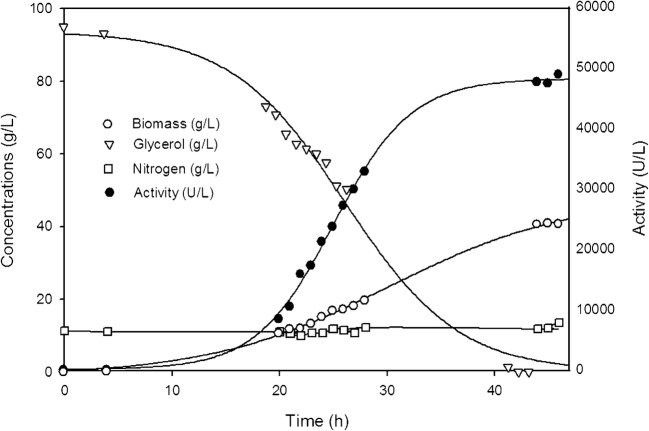
In a previous study, the best conditions for Clone 04-MR in terms of production were evaluated, and the use of 100 g/L of crude glycerin as initial in a batch culture was the best condition obtained [3]. In order to conduct a comparative analysis of the new strain, the same conditions were used in this study. Figure 2 shows the culture progress of CalB production using Clone 04-MR.
Fig. 2.
Kinetics of cell growth, enzymatic activity ,and consumption of glycerol and nitrogen with Clone 04-MR on minimal medium with 100 g/L crude glycerin, pH 7, with a cascade control to maintain oxygen saturation at 30%
The fermentation attained a biomass/substrate yield (Yx/s) of 0.47 gX/gS. The specific growth rate was 0.10 h−1 and the volumetric productivity was 453 U/L·h, with the crude glycerin being completely consumed after 46 h of growth. No nitrogen limitation occurred; as can be seen, the NH4OH added to maintain the pH assured a nearly constant nitrogen concentration in the culture.
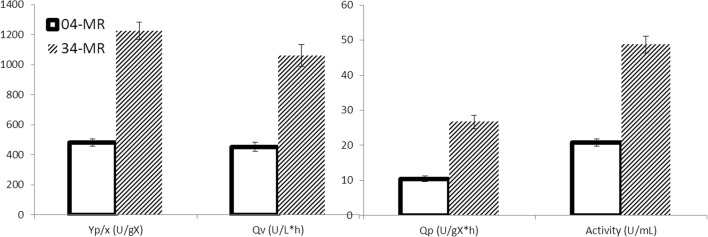
Figure 3 shows the fermentation process using Clone 34-MR. Biomass/substrate yield (Yx/s) was 0.44 gX/gS, the specific growth rate was 0.11 h−1 and the volumetric productivity and activity were 2.3-fold higher than in the previous case. Figure 4 shows the comparison of the main parameters obtained with the two strains.
Fig. 3.
Kinetics of cell growth, enzymatic activity, and consumption of glycerol and nitrogen of Clone 34-MR on minimal medium with 100 g/L crude glycerin, pH 7, with a cascade control to maintain oxygen saturation at 30%
Fig. 4.
Comparison of biomass/product yield, enzymatic activity, and volumetric and product productivity for both strains on minimal medium with 100 g/L crude glycerin, pH 7, with a cascade control to maintain oxygen saturation at 30%
Discussion
Two integrative vectors for lipase production were constructed in this work, pPGKΔ3PRO_LIPB and pKLip-ld. The main difference between these vectors is that the latter allows the direct isolation of transformants with multiple copies of the expression cassettes [26]. This vector contains the defective auxotrophic marker leu2-d, which is an allele bearing only 29 bp of the 5′ flanking region of the S. cerevisiae LEU2 gene [31]. In S. cerevisiae, this marker is typically used to maintain multiple copies of episomal plasmids [31] but since this class of vectors is not common in K. phaffii, prototrophy is necessarily achieved after multiple plasmid integration events in the host’s chromosome as previously shown [21].
Over 85% of transformants from both systems showed lipolytic activity on plates containing tributyrin (data not shown), thus showing that CalB was efficiently secreted by the S. cerevisiae α-factor signal sequence.
The recommended 260/230 ratio (related to some of the extraction contaminants, e.g., phenol and guanidine) to the ddPCR methodology is around 2 [32, 33], but the samples were of sufficient quality to continue with the procedure, although it achieved only 1.6 and 1.2, respectively.
For both cultivations, the growths were coupled to the production of CalB as expected, since a constitutive promoter was used. In general, both clones had the same behavior of production. Nevertheless, the biomass/substrate yield (Yx/s) for strain 34-MR was slightly smaller (0.44 gX/gS); this is explainable since the strain used for the modification is auxotrophic. Volumetric productivity and activity were 2.3-fold higher in this case, which shows that this multicopy construction generates higher lipase production, as has previously been reported for other multicopy integration processes. The production of tumor necrosis factor (TNF) increased 200-fold with more than 20 integrated copies [34]; hepatitis B surface antigen showed a linear correlation between copy number, mRNA and protein [35]; and proinsulin secretion increased 13-fold with 11 copies of the expression cassette [36]. Marx et al. (2009) [37] found a linear correlation between copy number and hSOD production. Specifically, for K. phaffii, some studies with lipase expression found an increase in protein production with multicopy integration [38–41].
For all parameters, the 34-MR strain showed better performance, around 2.4-fold. This behavior was expected because of the larger number of copies integrated; the increase was nearly linearly related to the number of integrations, as there are 3 times more copies of the lipase B gene from C. antarctica. It is also possible that this number of copies is close to saturation, due to limits on secretion or transcription, and the integration of more copies would reduce the correlation until the productivity becomes stable, independently of the number of copies, as for the HSA (human serum albumin) productivity reported by Marx et al. [37]. Notably, the maximum specific growth rate achieved was half the rate normally obtained with glycerol (0.2 h–1 3), with both copy numbers, also a reflection of the auxotrophic characteristic.
The effect of gene dosage on the production of different microbial recombinant lipases in K. phaffii has also been reported. Under PAOX1, the maximum gene dosage was around five copies for C. rugosa Lip1 [18] and Rhizopus chinensis CCTCC M201021 lipase [39]. For the constitutive promoter PGAP, the gene dosage was lower, three copies; for C. rugosa Lip2 [42], the same optimal copy number found in this study.
Although this strain produced much less than the highest cell density (which would be about 120 g/L as previously achieved in K. phaffii culturing [43]), because of the decrease in Yx/s, the fed-batch strategy can circumvent this problem and further increase the parameters obtained. Attempts to find a strong constitutive promoter and construction to match or even exceed the activities obtained with the PAOX1 promoter are important, in order to eliminate the use of methanol. This system produces the highest standards reported in the literature, as obtained by Vadhana et al. [44]: 300 U mL−1 at 6 days of induction. In some cases, such as the hepatitis B surface antigen, constitutive expression is better than induction because no induction is necessary and the specific growth rate is generally higher using glucose or glycerol than using methanol, which leads to faster processes [45]. Obviously, one must be careful when comparing activity numbers, because different substrates and methods used generate divergent results. Vadhana and colleagues used the same method to measure the activity as in this study, and they achieved a production, per hour, very near that obtained with methanol induction (1.87 U/ml·h with methanol induction, while the present study achieved 1.5 U/ml·h). These results demonstrate the excellent potential of K. phaffii for CalB production, and different culture strategies should be studied in order to explore its full potential. The enzyme produced on this work was already used to produce myo-inositol derivates that are intermediates from pharmacological pathways involved in the synthesis of building blocks for drugs that inhibit the etiological agent of Chagas disease, Trypanosoma cruzi [46].
Conclusion
Evaluating the newly constructed K. phaffii strain with a multicopy gene of C. antarctica lipase B, the increase in the number of gene copies directly impacted the lipase productivity. Clone 34-MR, with three copies, showed around 2.4-fold better performance in terms of volumetric and product productivity, although the final biomass decreased by 10%.
The optimal gene dosage to maximize CalB production under control of the PPGK1 promoter was three copies, similar to the number obtained with the well-known constitutive promoter PGAP.
Therefore, the novel constitutive promoter PPGK1 showed high potential for production of this lipase when coupled with multicopy integration, close to the productivities achieved when using methanol induction, but without the downsides of toxicity and hazards involved in using methanol. The process described here can easily be scaled up (pilot and industrial), as it is simple and low in cost.
Electronic supplementary material
(DOCX 405 kb)
Funding information
This work was financially supported by PETROBRAS/ANP and CAPES/DGPU from Brazil. Also by the Spanish project CTQ2016-74959-R (MINECO/FEDER, UE). The Spanish group is a member of 2014-SGR-452 and the Reference Network in Biotechnology (XRB) (Generalitat de Catalunya).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julia de Macedo Robert and Maritza Ocampo Betancur contributed equally to this work.
Contributor Information
Francisco Valero, Email: francisco.valero@uab.cat.
Denise Maria Guimarães Freire, Email: freire@iq.ufrj.br.
References
- 1.Gotor-Fernández V, Busto E, Gotor V. Candida antarctica Lipase B: an ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv Synth Catal. 2006;348(7–8):797–812. doi: 10.1002/adsc.200606057. [DOI] [Google Scholar]
- 2.Anderson EM, Larsson KM, Kirk O. One biocatalyst–many applications: the use of Candida Antarctica B-lipase in organic synthesis. Biocatal Biotransformation. 1998;16(3):181–204. doi: 10.3109/10242429809003198. [DOI] [Google Scholar]
- 3.Robert JM, Lattari FS, Machado AC, de Castro AM, Almeida RV, Torres FAG, Valero F, Freire DMG. Production of recombinant lipase B from Candida antarctica in Pichia pastoris under control of the promoter PGK using crude glycerol from biodiesel production as carbon source. Biochem Eng J. 2016;118:123–131. doi: 10.1016/j.bej.2016.11.018. [DOI] [Google Scholar]
- 4.Wu Q, Soni P, Reetz MT. Laboratory evolution of enantiocomplementary Candida antarctica lipase B mutants with broad substrate scope. J Am Chem Soc. 2013;135(5):1872–1881. doi: 10.1021/ja310455t. [DOI] [PubMed] [Google Scholar]
- 5.Ghaly AE, Dave D, Brooks MS, Budge S. Production of biodiesel by enzymatic transesterification: review. Am J Biochem Biotechnol. 2010;6(2):54–76. doi: 10.3844/ajbbsp.2010.54.76. [DOI] [Google Scholar]
- 6.Aguieiras ECG, Cavalcanti-Oliveira ED, De Castro AM, Langone M a P, Freire DMG. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel. 2014;135:315–321. doi: 10.1016/j.fuel.2014.06.069. [DOI] [Google Scholar]
- 7.Kirk O, Christensen MW. Lipases from Candida antarctica: unique biocatalysts from a unique origin. Org Process Res Dev. 2002;6(4):446–451. doi: 10.1021/op0200165. [DOI] [Google Scholar]
- 8.Cregg JM, Vedvick TS, Raschke WC (1993) Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology (N Y);11(8):905–910. http://www.ncbi.nlm.nih.gov/pubmed/7763913. Accessed 10 Jan 2016 [DOI] [PubMed]
- 9.Li P, Anumanthan A, Gao X-G, Ilangovan K, Suzara VV, Düzgüneş N (2007) Renugopalakrishnan V Expression of recombinant proteins in Pichia pastoris. Appl Biochem Biotechnol;142(2):105–124. http://www.ncbi.nlm.nih.gov/pubmed/18025573. Accessed 20 Jan 2016 [DOI] [PubMed]
- 10.Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24(1):45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 11.Looser V, Lüthy D, Straumann M, Hecht K, Melzoch K, Kovar K Effects of glycerol supply and specific growth rate on methanol-free production of CALB by P. pastoris: functional characterisation of a novel promoter. Applied Microbiol and Biotech 101(8):3163–317610.1007/s00253-017-8123-x [DOI] [PMC free article] [PubMed]
- 12.Anastácio GS, Santos KO, Suarez PAZ, Torres FAG, De Marco JL, Parachin NS. Utilization of glycerin byproduct derived from soybean oil biodiesel as a carbon source for heterologous protein production in Pichia pastoris. Bioresour Technol. 2014;152:505–510. doi: 10.1016/j.biortech.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Zhang AL, Luo JX, Zhang TY, Pan YW, Tan YH, Fu CY, Tu FZ. Recent advances on the GAP promoter derived expression system of Pichia pastoris. Mol Biol Rep. 2009;36(6):1611–1619. doi: 10.1007/s11033-008-9359-4. [DOI] [PubMed] [Google Scholar]
- 14.Arruda A, Reis VCB, Batista VDF, Daher BS, Piva LC, de Marco JL, de Moraes LMP, Torres FAG. A constitutive expression system for Pichia pastoris based on the PGK1 promoter. Biotechnol Lett. 2016;38(3):509–517. doi: 10.1007/s10529-015-2002-2. [DOI] [PubMed] [Google Scholar]
- 15.Piva LC, Bentacur MO, Reis VCB, De Marco JL, de Moraes LMP, Torres FAG. Molecular strategies to increase the levels of heterologous transcripts in Komagataella phaffii for protein production. Bioengineered. 2017;8:1–5. doi: 10.1080/21655979.2017.1296613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cámara E, Albiol J, Ferrer P. Droplet digital PCR-aided screening and characterization of Pichia pastoris multiple gene copy strains. Biotechnol Bioeng. 2016;113(7):1542–1551. doi: 10.1002/bit.25916. [DOI] [PubMed] [Google Scholar]
- 17.Cos O, Serrano A, Montesinos JL, Ferrer P, Cregg JM, Valero F. Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures. J Biotechnol. 2005;116(4):321–335. doi: 10.1016/j.jbiotec.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Liu Z, Wang G, Pan D, Jiao L, Yan Y. Overexpression of Candida rugosa lipase Lip1 via combined strategies in Pichia pastoris. Enzym Microb Technol. 2016;82:115–124. doi: 10.1016/j.enzmictec.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kazemi Seresht A, Nørgaard P, Palmqvist EA, Andersen AS, Olsson L. Modulating heterologous protein production in yeast: the applicability of truncated auxotrophic markers. Appl Microbiol Biotechnol. 2013;97(9):3939–3948. doi: 10.1007/s00253-012-4263-1. [DOI] [PubMed] [Google Scholar]
- 20.Servienë E, Melvydas V. Effect of the defective leucine gene leu2-d, on the properties of recombinant plasmid in yeast Saccharomyces cerevisiae. Biologia (Bratisl) 2001;4:30–33. [Google Scholar]
- 21.OKKELS JS. A URA3-promoter deletion in a pYES vector increases the expression level of a fungal lipase in Saccharomyces cerevisiae. Ann N Y Acad Sci. 1996;782(1):202–207. doi: 10.1111/j.1749-6632.1996.tb40561.x. [DOI] [PubMed] [Google Scholar]
- 22.Moon HY, Lee DW, Sim GH, Kim HJ, Hwang JY, Kwon MG, Kang BK, Kim JM, Kang HA. A new set of rDNA-NTS-based multiple integrative cassettes for the development of antibiotic-marker-free recombinant yeasts. J Biotechnol. 2016;233:190–199. doi: 10.1016/j.jbiotec.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Pignede G, Wang H-J, Fudalej F, Seman M, Gaillardin C, Nicaud J-M. Autocloning and amplification of LIP2 in Yarrowia lipolytica. Appl Environ Microbiol. 2000;66(8):3283–3289. doi: 10.1128/AEM.66.8.3283-3289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicaud J-M, Madzak C, Broek P, Gysler C, Duboc P, Niederberger P, Gaillardin C. Protein expression and secretion in the yeast Yarrowia lipolytica. FEMS Yeast Res. 2002;2(3):371–379. doi: 10.1111/j.1567-1364.2002.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 25.Krasovska OS, Stasyk OV, Sibirny AA. Stable overproducer of hepatitis B surface antigen in the methylotrophic yeast Hansenula polymorpha due to multiple integration of heterologous auxotrophic selective markers and defect in peroxisome biogenesis. Appl Microbiol Biotechnol. 2013;97(23):9969–9979. doi: 10.1007/s00253-013-5223-0. [DOI] [PubMed] [Google Scholar]
- 26.Betancur MO, Reis VCB, Nicola AM, De Marco JL, de Moraes LMP, Torres FAG. Multicopy plasmid integration in Komagataella phaffii mediated by a defective auxotrophic marker. Microb Cell Factories. 2017;16(1):99. doi: 10.1186/s12934-017-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer M, Kühleitner M, Gasser B, Mattanovich D. Versatile modeling and optimization of fed batch processes for the production of secreted heterologous proteins with Pichia pastoris. Microb Cell Factories. 2006;5(37):37. doi: 10.1186/1475-2859-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freire DM, Teles EM, Bon EP, Sant’ Anna GL. Lipase production by Penicillium restrictum in a bench-scale fermenter: effect of carbon and nitrogen nutrition, agitation, and aeration. Appl Biochem Biotechnol. 1997;63-65:409–421. doi: 10.1007/BF02920442. [DOI] [PubMed] [Google Scholar]
- 29.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabacco A, Meiattini F, Moda E, Tarli P. Simplified enzymic/colorimetrlc serum urea nitrogen determination thymol is a suitable preservative for uric acid standards in the Uricase technique CHEMISTRY, in the CK-BB least-squares evaluation of linearity more on the detection of serum CK-BB Acti. Clin Chem. 1979;25(2):4–5. [Google Scholar]
- 31.Erhart E, Hollenberg CP. The presence of a defective LEU2 gene on 2μ DNA recombinant plasmids of Saccharomyces cerevisiae is responsible for curing and high copy number. J Bacteriol. 1983;156(2):625–635. doi: 10.1128/jb.156.2.625-635.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robin JD, Ludlow AT, La Ranger R, Wright WE, Shay JW. Comparison of DNA quantification methods for next generation sequencing. Sci Rep. 2016;6:1–10. doi: 10.1038/srep24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endrullat C, Glökler J, Franke P, Frohme M. Standardization and quality management in next-generation sequencing. Appl Transl Genomics. 2016;10:2–9. doi: 10.1016/j.atg.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sreekrishna K, Nelles L, Potenz R, Cruze J, Mazzaferro P, Fish W, Fuke M, Holden K, Phelps D. High-level expression, purification, and characterization of recombinant human tumor necrosis factor synthesized in the methylotrophic yeast Pichia pastoris. Biochemistry. 1989;28(9):4117–4125. doi: 10.1021/bi00435a074. [DOI] [PubMed] [Google Scholar]
- 35.Vassileva A, Arora Chugh D, Swaminathan S, Khanna N. Effect of copy number on the expression levels of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Protein Expr Purif. 2001;21(1):71–80. doi: 10.1006/prep.2000.1335. [DOI] [PubMed] [Google Scholar]
- 36.Mansur M, Cabello C, Hernández L, et al. Multiple gene copy number enhances insulin precursor secretion in the yeast Pichia pastoris. Biotechnol Lett. 2005;27(5):339–345. doi: 10.1007/s10529-005-1007-7. [DOI] [PubMed] [Google Scholar]
- 37.Marx H, Mecklenbräuker A, Gasser B, Sauer M, Mattanovich D. Directed gene copy number amplification in Pichia pastoris by vector integration into the ribosomal DNA locus. FEMS Yeast Res. 2009;9(8):1260–1270. doi: 10.1111/j.1567-1364.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 38.Brunel L (2004) High-level expression of Candida parapsilosis lipase/acyltransferase in Pichia pastoris. J Biotechnol. 10.1016/S0168-1656(04)00162-2 [DOI] [PubMed]
- 39.Sha C, Yu X-W, Li F, Xu Y. Impact of gene dosage on the production of lipase from Rhizopus chinensis CCTCC M201021 in Pichia pastoris. Appl Biochem Biotechnol. 2013;169(4):1160–1172. doi: 10.1007/s12010-012-0050-9. [DOI] [PubMed] [Google Scholar]
- 40.Sha C, Yu X-W, Lin N-X, Zhang M, Xu Y. Enhancement of lipase r27RCL production in Pichia pastoris by regulating gene dosage and co-expression with chaperone protein disulfide isomerase. Enzym Microb Technol. 2013;53(6–7):438–443. doi: 10.1016/j.enzmictec.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 41.He D, Luo W, Wang Z, Lv P, Yuan Z. Combined use of GAP and AOX1 promoters and optimization of culture conditions to enhance expression of Rhizomucor miehei lipase. J Ind Microbiol Biotechnol. 2015;42(8):1175–1182. doi: 10.1007/s10295-015-1633-6. [DOI] [PubMed] [Google Scholar]
- 42.Kuo T-C, Shaw J-F, Lee G-C. Improvement in the secretory expression of recombinant Candida rugosa lipase in Pichia pastoris. Process Biochem. 2015;50(12):2137–2143. doi: 10.1016/j.procbio.2015.09.013. [DOI] [Google Scholar]
- 43.Charoenrat T, Ketudat-Cairns M, Stendahl-Andersen H, Jahic M, Enfors SO. Oxygen-limited fed-batch process: an alternative control for Pichia pastoris recombinant protein processes. Bioprocess Biosyst Eng. 2005;27(6):399–406. doi: 10.1007/s00449-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 44.Vadhana AKP, Samuel P, Berin RM, Krishna J, Kamatchi K, Meenakshisundaram S. Improved secretion of Candida antarctica lipase B with its native signal peptide in Pichia pastoris. Enzym Microb Technol. 2013;52(3):177–183. doi: 10.1016/j.enzmictec.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Vassileva A, Chugh DA, Swaminathan S, Khanna N. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter. J Biotechnol. 2001;88(1):21–35. doi: 10.1016/S0168-1656(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 46.Manoel EA, Robert JM, Pinto MCC, Machado ACO, Besteti MD, Coelho MAZ, Simas ABC, Fernandez-Lafuente R, Pinto JC, Freire DMG. Evaluation of the performance of differently immobilized recombinant lipase B from Candida antarctica preparations for the synthesis of pharmacological derivatives in organic media. RSC Adv. 2016;6:4043–4052. doi: 10.1039/c5ra22508f. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 405 kb)