Abstract
Activated monocytes/macrophages that produce a cytokine storm play an important role in the pathogenesis of dengue. Interleukin-18 (IL-18) is a proinflammatory cytokine produced by monocyte/macrophages that is increased during dengue. Ferritin is an acute-phase reactant and expressed by cells of the reticulo-endothelial system in response to infection by dengue virus. The aims of this study were to analyze the simultaneous expression of both IL-18 and ferritins in children infected by diverse serotypes of dengue virus (DENV) and determine their association with dengue severity. In this regard, children with dengue (n = 25) and healthy controls with similar age and sex (n = 20) were analyzed for circulating ferritin and cytokines. Monocytes were isolated by Hystopaque gradient and co-cultured with DENV-2. IL-18 and ferritin contents in blood, and IL-18 in culture supernatants were determined by ELISA. Increased levels of ferritin and IL-18 (p < 0.0001) were observed in dengue patients, not associated to NS1expression or type of infection (primary or secondary). Highest values of both molecules (p < 0.001) were observed in dengue with warning signs and severe dengue. Differential effect on IL-18/ferritin production was observed associated to viral serotype infection. There were no correlations between ferritin vs. IL-18 production, ferritin vs. NS1 status, and IL-18 vs. NS1 status. Viral-infected monocyte cultures showed increased production of IL-18 (p < 0.001). In conclusion, increased circulating ferritin and IL-18 are expressed in children infected by different serotypes of DENV associated with dengue severity.
Keywords: Dengue, Ferritin, IL-18, Children
Introduction
Dengue virus (DENV) is a flavivirus, which is transmitted by the bite of an Aedes mosquito. The symptoms of DENV infection are mild and self-limiting in the majority of cases, consisting of fever, headache, retro-orbital pain, myalgia, arthralgia, thrombocytopenia, minor mucosal bleeding, and skin manifestations. Some patients develop severe symptoms, such as shock, severe bleeding, or organ impairment. It has been hypothesized that severe dengue is caused by a cytokine storm inducing systemic inflammatory effects [19].
In addition to the current laboratory markers for dengue, ferritin levels were described to be associated with clinical disease severity [4, 6, 29, 39]. Ferritin is an acute-phase reactant and highly expressed by cells of the reticulo-endothelial system in response to infection and inflammation [36]. Hyperferritinemia is a hallmark of diseases, characterized by extensive immune activation, including hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS) [31] contributing to a cytokine storm [33].
Interleukin-18 (IL-18) is a member of the IL-1 family of cytokines. It is synthesized as an inactive precursor requiring processing by caspase-1 into an active cytokine. Dengue virus has been shown to activate the inflammasome and induces the production of IL-18 by human macrophages [37]. IL-18, together with IL-12, plays a major role in the production of gamma interferon (IFN-γ), considered central mediators in dengue host defense [5, 9]. Previous studies in patients with acute dengue have reported high serum IL-18 levels, which correlated with disease severity [21, 24, 34]. However, the activity of IL-18 is balanced by a naturally occurring IL-18 binding protein [20].The aim of this study was to investigate the association between hyperferritinemia, IL-18 production, viral serotype infection, and severity of disease in DENV-infected children.
Methods
Patients
Twenty five patients (1–14 years old) with a clinical diagnosis of dengue and classified as dengue without warning signs (DNWS), dengue with warning signs (DWWS), and severe dengue (SD) according to World Health Organization criteria (WHO, 2009) were studied. Patients were clinically diagnosed at University Hospital, Maracaibo, Venezuela. The laboratory diagnostic and the analysis of blood samples were performed at the Virology Section in the Instituto de Investigaciones Clínicas Dr. Américo Negrette, Maracaibo, Venezuela. In all patients, dengue virus infection was confirmed either by the presence of serum anti-dengue antibodies (DENV-1–4) or by virus isolation. Virus serotypes were determined using monoclonal antibodies specific to the different serotypes by indirect immunofluorescence. Experimental work was approved by the ethical committee of the Institution and University Hospital according to The Declaration of Helsinki, and informed consent was required from patients and controls. Blood samples were taken during the acute phase (1–6 days after the onset of the symptoms). The dengue immune response was considered as primary or secondary by serum immunoglobulin pattern. In addition to anti-dengue antibodies determination, the samples were subjected to virus isolation. The blood samples obtained from age- and sex-matched healthy individuals (n = 20) were used as controls. Samples from patients and controls were stored at − 70 °C until used. In addition, co-cultures of mononuclear leukocytes from healthy controls with DENV-2 were performed to determine the role of dengue virus in the production of IL-18 by monocytes.
Laboratory studies
IL-18 content was measured using a commercially available ELISA kit (R & D Systems, Inc. Camarillo, CA, USA) and the results expressed as pg/ml. Ferritin content was measured using a commercially available ELISA kit (DRG International. Springfield, NJ, USA) and the results expressed as μg/L. Qualitative presence of serum NS1 was determined using a Platelia dengue NS1 AG Elisa (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative determination of anti-dengue IgG or IgM was performed by corresponding capture ELISA (Diagnostic Automation, Inc. Calabasas, CA. USA). The samples were subjected to virus isolation through cell culturing with the C6/36 mosquito cell line obtained from Aedes albopictus (NIH; protocol C-524-1). Virus serotypes were determined by indirect immunofluorescence using monoclonal antibodies specific to the different serotypes (DENV-1–4).
Monocyte cultures
Mononuclear leukocytes were obtained from heparinized venous blood from five healthy donors. Cells were isolated through density gradient centrifugation in Hystopaque 1.077 (Sigma Chemical Co. St. Louis MO, USA). Cells were suspended at 2 × 106 cell/mL in RPMI 1640 supplemented with 100 U/ml penicillin, 10 μg/ml streptomycin, and 10% FBS and afterwards, incubated at 37 °C under humid atmosphere with 5% CO2. After 3-h-incubation, adherent cells were enriched by washing away unattached cells twice. After washing, a mean of 3 × 105 adherent cells/well was obtained. Monocyte cultures from each donor were infected (MOI 1) by referential strain dengue virus (DENV-2, New Guinea C). Monocytes cultured without virus were used as negative controls. After 24 h, supernatants were collected and IL-18 determinations were performed using ELISA. Results were expressed as pg/mg of cellular protein from triplicated cultures of five independent experiments. Monocyte purity was determined by an anti-human CD14 monoclonal antibody (Sigma Chemical Co. St. Louis, MO, USA). Monocyte protein content was determined by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Values were expressed as mean ± standard deviation. The differences between groups were tested by ANOVA and the Bonferroni post hoc test, or unpaired t test. Correlation analysis was performed using Pearson’s correlation. p values < 0.05 were considered statistically significant.
Results
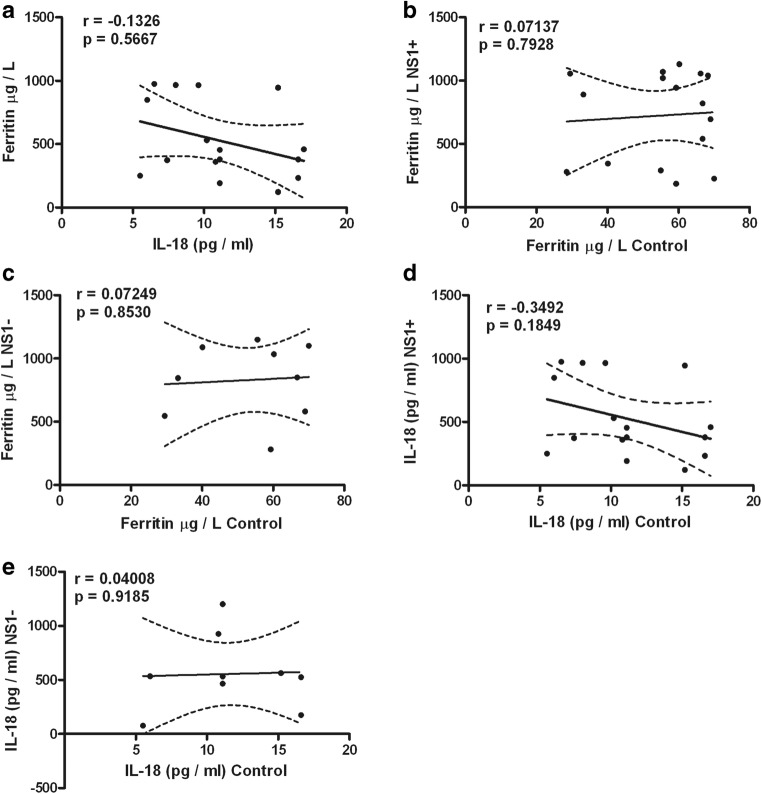
Table 1 shows the dengue patient distribution according to NS1 status, type of infection, clinical classification, and viral serotype infection. High percentage of NS1 positive secondary infected patients (64%) was observed. The highest percentage of patients was DWWN (44%). Blood samples were obtained at 3.53 ± 0.34 days (from 2 to 7 days) in NS1 positive patients and at 3.78 ± 0.43 days (from 1 to 6 days) in NS1 negative patients. Virus isolation was obtained in 79% of samples from patients during the acute period of disease. As shown in Table 2, lower values of hemoglobin in SD than DWWS were observed. However, hematocrit values were higher in SD and DWWS. Leukocyte values were higher in DWWS, and the lowest values of platelet number were observed in patients with severe dengue. Table 3 shows the number of patients according to the viral type infection related to dengue severity. Patients with DENV-2 infection were mainly related to SD degree of dengue disease. High levels of serum ferritin and IL-18 (Fig. 1) were observed in patients with dengue; however, association to type infection (Fig. 2) or NS1 status (Fig. 3) was not observed. Low values of ferritin accompanied with high values of IL-18 in patients infected by DENV-2 were observed. Conversely, high values of ferritin accompanied with low values of IL-18 in patients infected by DENV-1 or DENV-3 were also found (Fig. 4). Increased levels of ferritin and IL-18 were found in all patients with dengue compared to healthy controls; however, the highest levels were observed in DWWS and SD (Fig. 5). After 24 h of viral-monocyte interactions, DENV-2 was capable of inducing high amount of IL-18 (Fig. 6). There were no correlations between ferritin vs. IL-18 production, ferritin vs. NS1 status, and IL-18 vs. NS1 status (Fig. 7).
Table 1.
Distribution of dengue patients according to NS1 status, type of infection, clinical classification, and viral serotype infection
| Parameters | Patients (N) | Percentage |
|---|---|---|
| NS1 status | ||
| Positive | 16 | 64 |
| Negative | 9 | 36 |
| Type of infection | ||
| Primary | 9 | 36 |
| Secondary | 16 | 64 |
| Clinical classification | ||
| DNWS | 9 | 36 |
| DWWS | 11 | 44 |
| SD | 5 | 20 |
| Viral serotype | ||
| DENV-1 | 7 | 28 |
| DENV-2 | 6 | 24 |
| DENV-3 | 6 | 24 |
| DENV-4 | 6 | 24 |
DNWS, dengue without warning signs; DWWS, dengue with warning signs; SD, severe dengue
Table 2.
Hematological findings of dengue patients according to severity of disease
| Parameters | DNWS | DWWS | SD | P values |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 11.1 ± 0.42 | 12.21 ± 0.56 | 10.83 ± 0.48 | < 0.05a |
| Hematocrit (%) | 34.62 ± 0.99 | 38.52 ± 1.83 | 38.75 ± 2.36 | < 0.05b |
| Leukocytes/mm3 | 4916 ± 1078 | 5476 ± 1127 | 5225 ± 1618 | < 0.05c |
| Platelets × 103/mm3 | 142.5 ± 16.08 | 71.59 ± 11.27 | 48 ± 6.48 | < 0.01d |
DNWS, dengue without warning signs; DWWS, dengue with warning signs; SD, severe dengue. aSD vs. DWWS; bSD and DWWS vs. DNWS; cDWWS vs. DNWS; dSD and DWWS vs. DNWS
Table 3.
Stratification of viral type infection according to disease severity
| Dengue severity | DENV-1 | DENV-2 | DENV-3 | DENV-4 |
|---|---|---|---|---|
| DNWS | 3a | 1 | 2 | 3 |
| DWWS | 3 | 2 | 4 | 2 |
| SD | 1 | 3 | 1 |
DNWS, dengue without warning signs; DWWS, dengue with warning signs; SD, severe dengue. aNumber of patients
Fig. 1.
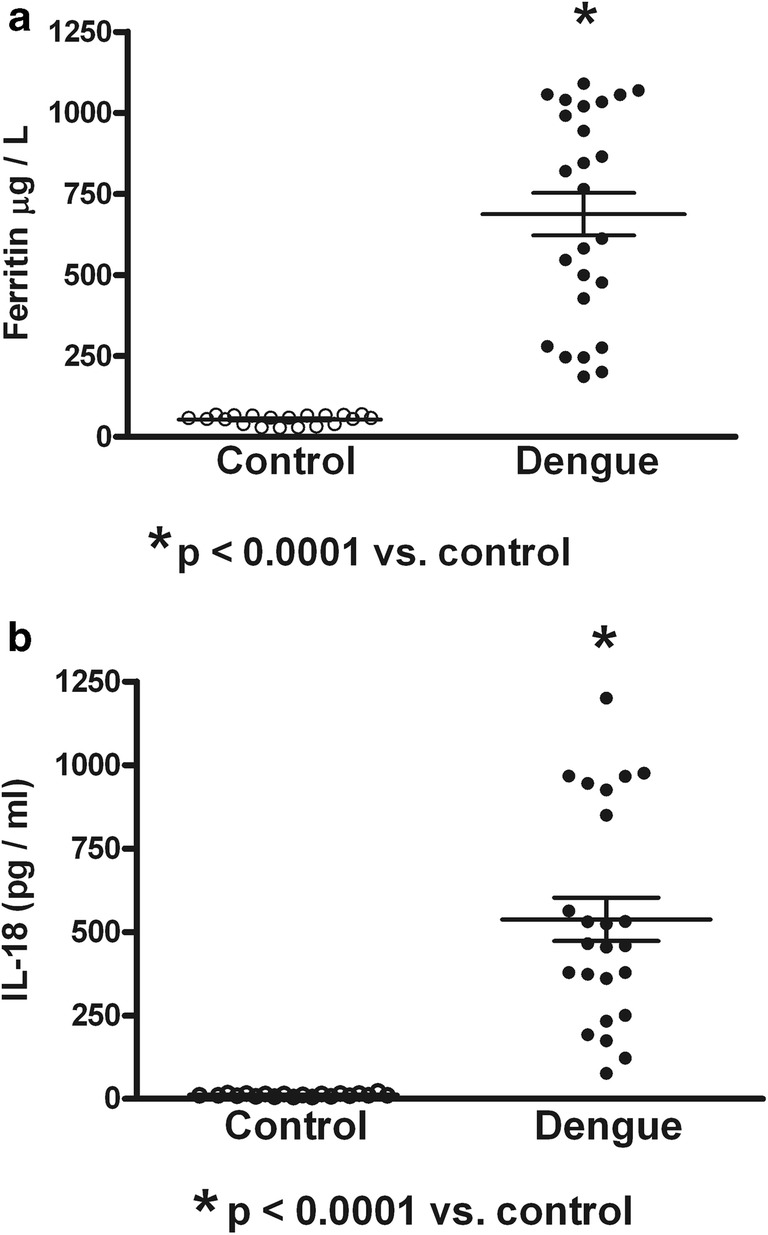
Serum contents of ferritin and interleukin-18 (IL-18) in healthy controls and children infected by dengue virus. a Values of ferritin were observed increased compared with controls. b High serum values of IL-18 were observed in dengue. Control (n = 20); dengue (n = 25). Unpaired T test was used
Fig. 2.
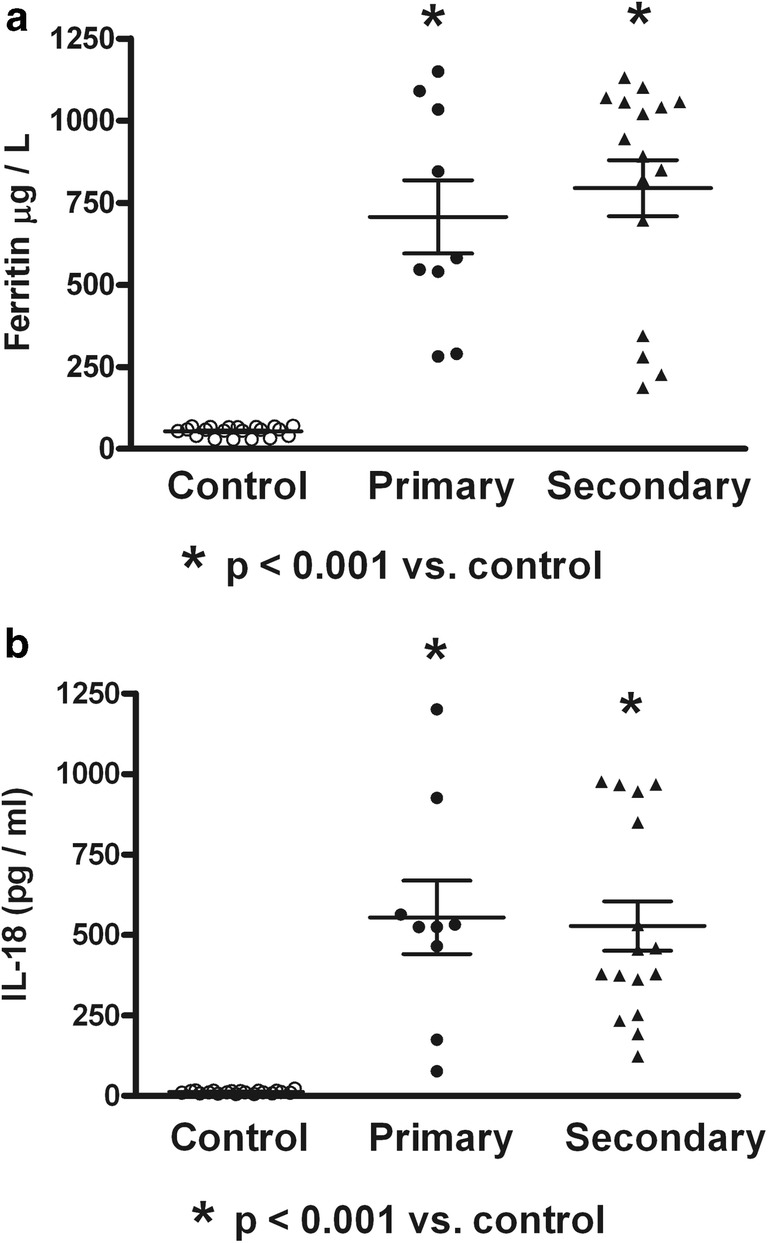
Serum contents of ferritin and interleukin-18 (IL-18) in primary or secondary dengue virus infection. Expression of circulating NS1 was not associated to increased serum content of ferritin (a) or IL-18 (b) in dengue patients. Control (n = 20); Primary (n = 9); Secondary (n = 16). ANOVA and the Bonferroni post hoc test were used
Fig. 3.
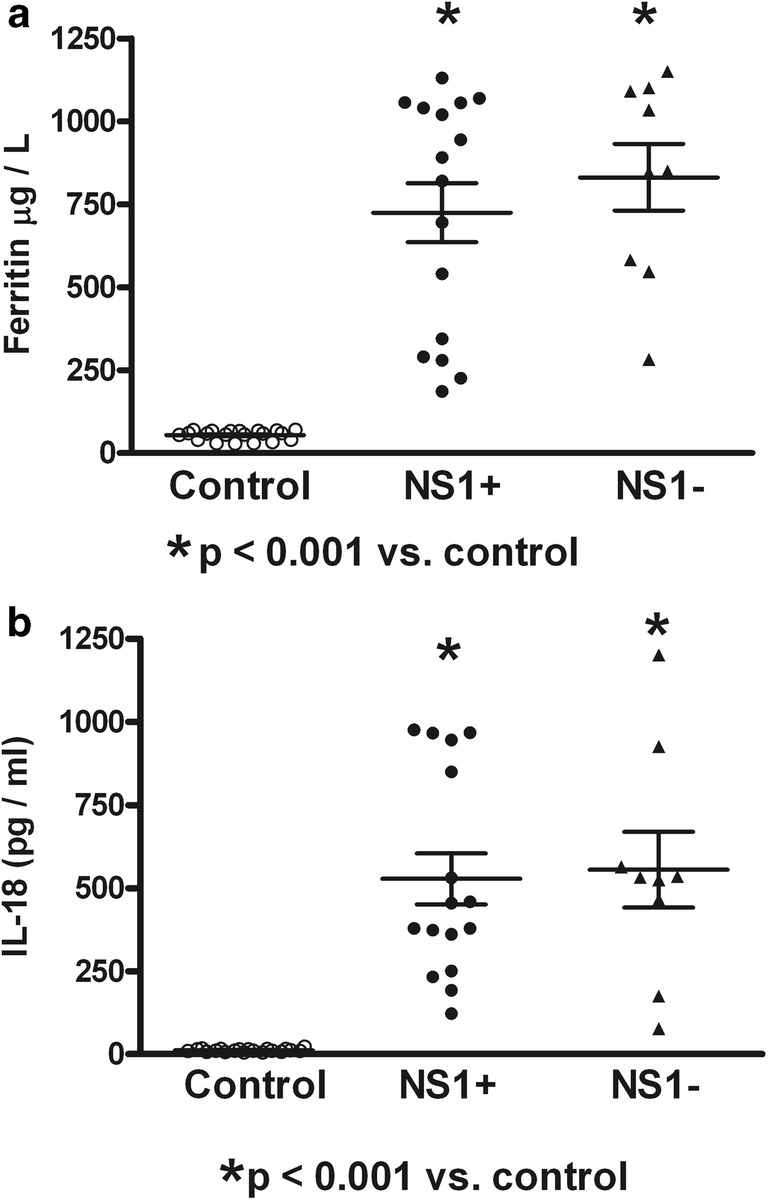
Serum contents of ferritin and interleukin-18 (IL-18) in dengue patients positive and negative to nonstructural protein-1 (NS1). Expression of circulating NS1 was not associated to increased serum content of ferritin (a) or IL-18 (b) in dengue patients. Control (n = 20); NS1+ (n = 16); NS1− (n = 9). ANOVA and the Bonferroni post hoc test were used
Fig. 4.

Serum contents of ferritin and interleukin-18 (IL-18) in children infected by different dengue virus serotypes. Differential induction of ferritin (a) and IL-18 (b) according to virus serotypes. Control (n = 20); DENV-1 (n = 7); DENV-2 (n = 6); DENV-3 (n = 6); DENV-4 (n = 6). ANOVA and the Bonferroni post hoc test were used
Fig. 5.
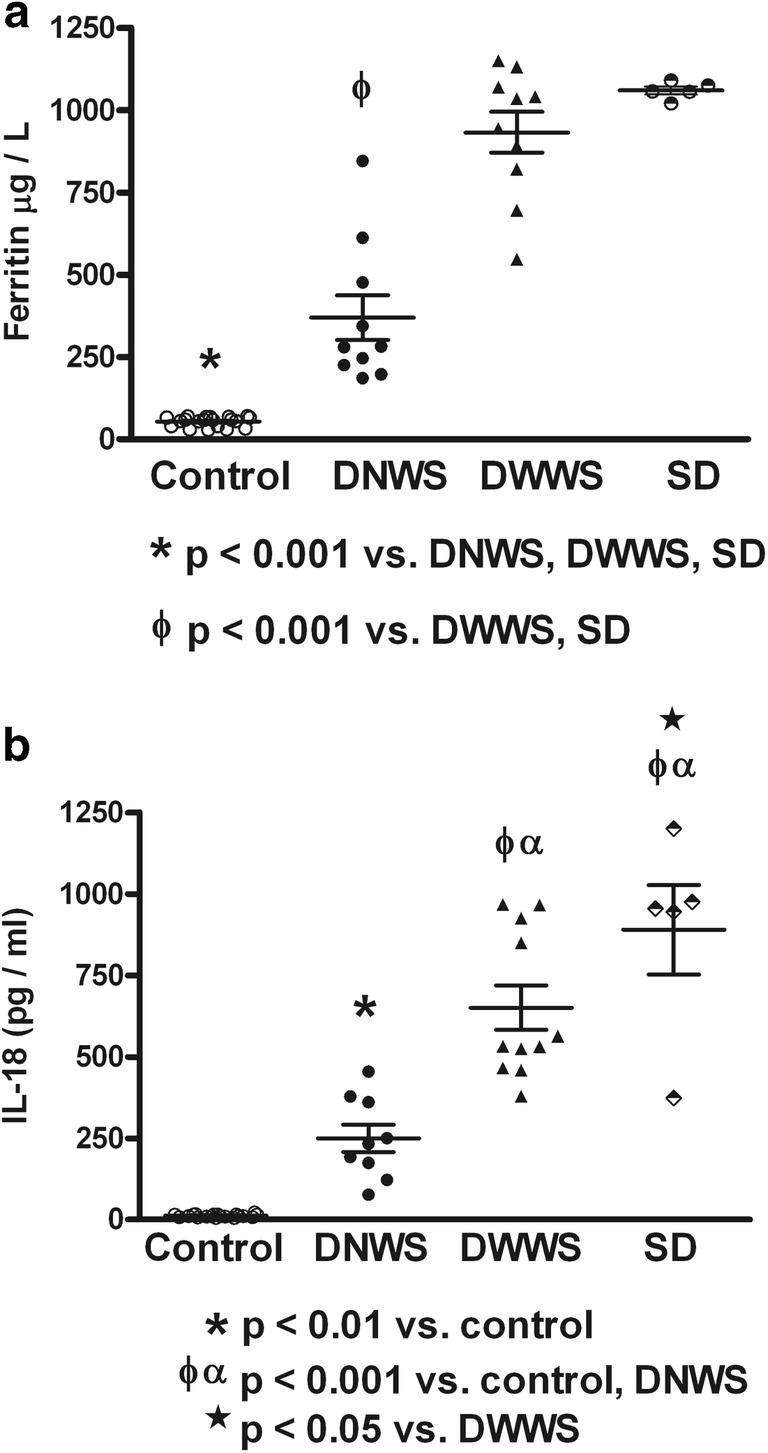
Serum contents of ferritin and interleukin-18 (IL-18) in different grades of dengue disease. Similar increment of serum ferritin (a) and IL-18 (b) according to dengue severity was observed. Control (n = 20); Dengue without warning signs (DNWS, n = 9); Dengue with warning signs (DWWS, n = 11); Severe dengue (SD, n = 5). ANOVA and the Bonferroni post hoc test were used
Fig. 6.
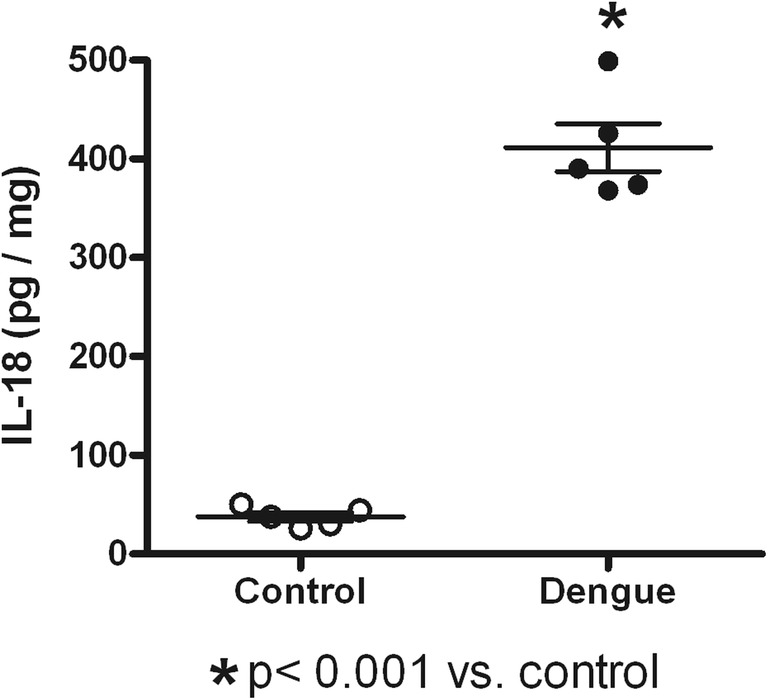
Interleukin-18 (IL-18) content on dengue virus type 2-infected human monocyte cultures. After 24 h of DENV-2 infection, increased amounts of IL-18 were found in supernatants from infected monocyte cultures. Values represent mean ± standard deviation from five independent experiments. Unpaired T test was used
Fig. 7.
Correlations between ferritin and interleukin-18 (IL-18) and nonstructural-1 (NS1) protein. There were no correlations when all of those variables were analyzed. a Ferritin vs. IL-18. b Ferritin in controls vs. ferritin in NS1 positive patients. c Ferritin in controls vs. ferritin in NS1 negative patients. d IL-18 in controls vs. IL-18 in NS1 positive patients. e IL-18 in controls vs. IL-18 in NS1 negative patients. Pearson correlation was used
Discussion
Ferritin is an acute-phase reactant, and a significant amount is produced by monocytes, macrophages, and hepatic cells. It has been shown that synthesis of ferritin can be induced by cytokines and iron [10, 17, 32]. Accordingly, increased levels of ferritin were associated with increased production of IL-18 in this study, suggesting a role of this cytokine in the induction of ferritin production and in immune activation in severe dengue. In this regard, the highest levels of ferritin and IL-18 in this study were found in severe dengue. The source of IL-18 in dengue probably involves the interaction of dengue virus with circulating monocytes, since increased production of this cytokine was found in human monocyte cultures infected by DENV-2.
The increased production of ferritin has been associated to severe manifestations of some diseases [1, 11, 14]. This common entity is termed “the hyperferritinemic syndrome.” In many cases, ferritin levels higher than 500 μg/L were defined as hyperferritinemia [12]. It has been proposed that ferritin may serve as a potential biomarker for an early prediction of dengue severity [29]. In this regard, recent reports have shown dengue patients with hyperferritinemia and hemophagocytic lymphohistiocytosis (HLH) [6, 39].The presence of hyperferritinemia with HLH is a potentially fatal condition [11, 14]; however, dengue infection with HLH is rare [39]. In this study, all dengue patients had elevated levels of serum ferritin, but, only patients with DWWS and SD with higher ferritin levels can be classified as hyperferritinemic [12], suggesting association of this syndrome with disease severity. As interesting finding, the highest levels of IL-18 were also found in patients with DWWS and SD, suggesting a role of IL-18 in the induction of ferritin production. However, correlation analysis showed no correlation between IL-18 and ferritin contents, descarting this possibility. Previous studies have shown that ferritin may serve as a significant marker for differentiating between dengue fever and other febrile illnesses of infective or inflammatory etiology, in the absence of a positive NS1antigen [27]. Our correlation analysis of ferritin and IL-18 vs. NS-1 status showed no correlation regarding those variables, suggesting that in children with dengue, that association failed to be a marker. A bone marrow evaluation to confirm the diagnosis of HLH was not performed in this study. Beside the deleterious effect of ferritin, associated to cytokine storm [33] and the presence of HLH [11, 14, 31, 39], potential beneficial effects have been reported. Ferritin can block the release of bradykinin, preventing vascular permeability and hypotension [2, 18, 23]. In addition, the capacity of ferritin to bind iron, can limit pathogenic microorganisms that need iron for their proliferation [36]. The association of deleterious or beneficial effects of ferritin regarding to mild or severe dengue has not been studied. However, similar to our findings, ferritin levels were described to be associated with clinical dengue severity in Asiatic children [4] suggesting an immunological response not based in ethnic background.
Differential effect regarding to ferritin and IL-18 productions in patients infected by different serotypes of DENV was found in this study. In general, the levels of ferritin and IL-18 were observed increased in dengue patients compared to healthy controls. However, DENV-2 infection induced high levels of IL-18 and low levels of ferritin (lesser than 500 μ/L), whereas, DENV-1 and DENV-3 induced high levels of ferritin (higher than 500 μ/L) and low levels of IL-18. It is assumed that a high level of ferritin associated to cytokine storm plays a pivotal role in the pathogenesis of dengue [29]. However, lower values of ferritin could diminish the effect of this molecule to block bradykinin release [23], with further inducing of vascular dilation, increased vascular permeability, and hypotension [2, 18]. On the other hand, increased level of IL-18 observed in DENV-2 infection could play a major role in the production of IFN-γ, involved in dengue host defense [5, 9]. Definition of mild or severe status in DENV-2 infection could depend of other factor. In this regard, DENV-2 has been associated to the severity of dengue during DENV epidemic in different countries, suggesting that the circulating DENV-2 might have become more virulent through passage in hosts during the epidemic [15, 16, 25, 26]. Under these concepts, high levels of ferritin and low levels of IL-18 from the others serotypes of viral infection (DENV-1 and DEVN-3) could be associated to mild dengue. However, it has also been shown that different geographical DENV strains or different serotypes may vary in their ability to infect different cell types or cause severe disease [7, 35]. According to the virus virulence hypothesis, certain DENV strains are responsible for more severe disease [19]. It is important to realize that virulence has traditionally been considered a microbial property, evaluated independently of the host or only in vitro or in often inbred animals. However, an increasing body of evidence incriminates the host immune response in the pathogenesis of many microbial infections [3].Therefore, in studying DENV virulence, both host and viral factors should be considered.
Macrophages, monocytes, and lymphocytes are the major target cells of DENV replication in vivo [8, 13]. Monocytes and macrophages are also important producers of ferritin, and therefore direct infection and subsequent viral replication in these cells may activate them and increase the ferritin production. Hepatocytes can also synthesize ferritin, and it has been demonstrated immunological liver alterations in patients infected by DENV [22]. DENV replicates very well in hepatic cell lines in vitro, but whether DENV replicates well in the liver in vivo is still a matter of debate [13]. However, it is likely that liver cells are also indirectly activated by cytokines and/or activated immune cells to produce high amounts of ferritin. It has been shown that DENV infection in mice resulted in NK and CD8+ T cell infiltration of the liver [30]. In this study, in vitro analysis showed increased production of IL-18 by cultured monocyte/macrophages infected by dengue virus, suggesting a viral cytokine induction and probably the source of this circulating cytokine in children with severe dengue. According to this, increased values of IL-18 found in patients during this study and reports [38] suggesting a pathogenic role for an aberrant inflammasome and monocyte activation in the high levels of IL-18 observed in adult severe form of dengue, suggest a viral IL-8 inducer effect on monocytes in both adults and children.
Previous reports suggest that it is important to assess the direct association between circulating concentrations of IL-18 and ferritin in viral infections, as current studies have only focused on either IL-18 or ferritin separately. It has been proposed a model in which the inflammatory response to viral (IL-18/ferritin) and bacterial (IL-6/CRP) infections presents with specific plasma patterns of immune biomarkers [28]. Accordingly, in this study, the increased IL-18 accompanied with increased values of ferritin suggests that IL-18/ferritin could be a biomarker for viral infection. In conclusion, this study report increased production of ferritin and IL-18 in children infected by various serotypes of dengue virus, in which both molecules were associated with the severity of disease.
Acknowledgments
This investigation was supported by Misión Ciencias, Fonacit, and Instituto de Investigaciones Clínicas “Dr. Américo Negrette” Facultad de Medicina, Universidad del Zulia, Maracaibo, Venezuela. Sponsors had no involvement in any aspect of manuscript preparation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Betancur JF, Navarro EP, Echeverry A, Moncada PA, Cañas CA, Tobó GJ. Hyperferritinemic syndrome: still’s disease and catastrophic antiphospholipid syndrome triggered by fulminant chikungunya infection: a case report of two patients. Clin Rheumatol. 2015;34:1989–1992. doi: 10.1007/s10067-015-3040-9. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch Intern Med. 1992;152:1381–1389. doi: 10.1001/archinte.1992.00400190023007. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaiyaratana W, Chuansumrit A, Atamasirikul K, Tangnararatchakit K. Serum ferritin levels in children with dengue infection. Southeast Asian J Trop Med Public Health. 2008;39:832–886. [PubMed] [Google Scholar]
- 5.Costa VV, Fagundes CT, Valadao DF, Cisalpino D, Dias AC, Silveira KD, Kangussu LM, Avila TV, Bonfim MR, Bonaventura D. A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-gamma in host resistance to infection. PLoS Negl Trop Dis. 2012;6:e1663. doi: 10.1371/journal.pntd.0001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Koninck AS, Dierick J, Steyaert S, Taelman P. Hemophagocytic lymphohistiocytosis and dengue infection: rare case report. Acta Clin Belg. 2014;69:210–213. doi: 10.1179/2295333714Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 7.Diamond MS, Edgil D, Roberts TG, Lu B, Harris E. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J Virol. 2000;74:7814–7823. doi: 10.1128/JVI.74.17.7814-7823.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008;376:429–435. doi: 10.1016/j.virol.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagundes CT, Costa VV, Cisalpino D, Amaral FA, Souza PR, Souza RS, Ryffel B, Vieira LQ, Silva TA, Atrasheuskaya A. IFN-gamma production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 2011;5:e1449. doi: 10.1371/journal.pntd.0001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahmy M, Young SP. Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: changes in transferrin uptake, iron handling and ferritin mRNA. Biochem J. 1993;296:175–181. doi: 10.1042/bj2960175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL study group of the histiocyte society. Semin Oncol. 1991;1:29–33. [PubMed] [Google Scholar]
- 12.Henter JI, Horne A, Arico M, Egeler RM, Filipovich S, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 13.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 14.Joshi R, Phatarpekar A, Currimbhoy Z, Desai M. Haemophagocytic lymphohistiocytosis: a case series from Mumbai. Ann Trop Paediatr. 2011;31:135–140. doi: 10.1179/1465328111Y.0000000009. [DOI] [PubMed] [Google Scholar]
- 15.Kouri GP, Guzman MG, Bravo JR. Why dengue haemorrhagic fever in Cuba? 2. An integral analysis. Trans R Soc Trop Med Hyg. 1987;81:821–823. doi: 10.1016/0035-9203(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 16.Kouri GP, Guzman MG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull W H O. 1989;67:375–380. [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H, Akaike T, Wu J, Noguchi Y, Sakata Y. Bradykinin and nitric oxide in infectious disease and cancer. Immunopharmacology. 1996;33:222–230. doi: 10.1016/0162-3109(96)00063-X. [DOI] [PubMed] [Google Scholar]
- 19.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhl H, Kampfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in non leukocytic cells. Biochem Biophys Res Commun. 2000;267:960–963. doi: 10.1006/bbrc.1999.2064. [DOI] [PubMed] [Google Scholar]
- 21.Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2001;30:229–233. doi: 10.1111/j.1574-695X.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 22.Pagliari C, Quaresma JA, Fernandes ER, Stegun FW, Brasil RA, de Andrade HF, Jr, Barros V, Vasconcelos PF, Duarte MI. Immunopathogenesis of dengue hemorrhagic fever: contribution to the study of human liver lesions. J Med Virol. 2014;86:1193–1197. doi: 10.1002/jmv.23758. [DOI] [PubMed] [Google Scholar]
- 23.Parthasarathy N, Torti SV, Torti FM. Ferritin binds to light chain of human H-kininogen and inhibits kallikrein-mediated bradykinin release. Biochem J. 2002;365:279–286. doi: 10.1042/bj20011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathakrishnan A, Wang SM, Hu Y, Khan AM, Ponnampalavanar S, Lum LC, Manikam R, Sekaran SD. Cytokine expression profile of dengue patients at different phases of illness. PLoS One. 2012;7:e52215. doi: 10.1371/journal.pone.0052215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 26.Rico-Hesse R, Harrison LM, Nisalak A, Vaughn DW, Kalayanarooj S, Green S, Rothman AL, Ennis FA. Molecular evolution of dengue type 2 virus in Thailand. Am J Trop Med Hyg. 1998;58:96–101. doi: 10.4269/ajtmh.1998.58.96. [DOI] [PubMed] [Google Scholar]
- 27.Roy Chaudhuri S, Bhattacharya S, Chakraborty M, Bhattacharjee K. Serum ferritin: a backstage weapon in diagnosis of dengue fever. Interdiscip Perspect Infect Dis. 2017;2017:1–6. doi: 10.1155/2017/7463489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slaats J, ten Oever J, van de Veerdonk FL, Netea MG. IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12:e1005973. doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soundravally R, Agieshkumar B, Daisy M, Sherin J, Cleetus CC. Ferritin levels predict severe dengue. Infection. 2015;43:13–19. doi: 10.1007/s15010-014-0683-4. [DOI] [PubMed] [Google Scholar]
- 30.Sung JM, Lee CK, Wu-Hsieh BA. Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS One. 2012;7:e46292. doi: 10.1371/journal.pone.0046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan LH, Lum LC, Omar SF, Kan FK. Hemophagocytosis in dengue: comprehensive report of six cases. J Clin Virol. 2012;55:79–82. doi: 10.1016/j.jcv.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90:4979–4986. [PubMed] [Google Scholar]
- 33.Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161:609–622. doi: 10.1111/bjh.12293. [DOI] [PubMed] [Google Scholar]
- 34.van de Weg CA, Huits RM, Pannuti CS, Brouns RM, van den Berg RW, van den Ham HJ, Martina BE, Osterhaus AD, Netea MG, Meijers JC. Hyperferritinaemia in dengue virus infected patients is associated with immune activation and coagulation disturbances. PLoS Negl Trop Dis. 2014;8:e3214. doi: 10.1371/journal.pntd.0003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 36.Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol. 2005;18:183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, Tsai IS, Li L, Hsieh SL. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood. 2013;121:95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]
- 38.Yong YK, Tan HY, Jen SH, Shankar EM, Natkunam SK, Sathar J, Manikam R, Sekaran SD. Aberrant monocyte responses predict and characterize dengue virus infection in individuals with severe disease. J Transl Med. 2017;15:121–131. doi: 10.1186/s12967-017-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshifuji K, Oshina T, Sonokawa S, Noguchi Y, Suzuki S, Tanaka K, Kumagai T. Domestic dengue infection with hemophagocytic lymphohistiocytosis successfully treated by early steroid therapy. Rinsho Ketsueki. 2016;57:864–868. doi: 10.11406/rinketsu.57.864. [DOI] [PubMed] [Google Scholar]