Abstract
The use of lactic bacteria in the development of functional foods has increased in recent years. In addition to their probiotic characteristics, they can ferment a variety of substrates, such as cereals, roots, and tubers. Phytase producer lactic acid bacteria strains and their behavior during the fermentation process of yam-based food were studied. Leuconostoc lactis CCMA 0415, Lactobacillus plantarum CCMA 0744, and Lactobacillus fermentum CCMA 0745 were selected due to phytase production, pH reduction, and growth during 24 h of fermentation. Oxalate activity was not detected in all assays, suggesting its concentration was reduced due to the bleaching process. Among the selected strains, L. lactis CCMA 0415 appeared to be a promising strain in yam-based fermentations because it maintained a cell viability above 8 log CFU/mL and did not reduce diosgenin concentrations (around 8.0 μg/mL) after fermentation for 24 h, thereby, generating a potentially functional yam food. Furthermore, this strain promoted the decrease of pH value from 6.1 to 3.8 and produced 8.1 g/L lactic acid, at 6 h of fermentation. The L. lactis CCMA 0415 was reported as a starter culture in fermented products based on cereals, roots, and tubers.
Keywords: Leuconostoc lactis, Fermentation, Phytase producer, Yam, Starter culture
Introduction
Consumer demand for functional foods has increased and stimulated food companies to invest in their research and development sector. Thus, fermented products have gained market space due to the functional properties conferred to the final product [1]. Yams belong to the Dioscorea genus and are mainly produced in Africa (96% of the world’s production), followed by America (2.5%), Oceania (0.6%), and Asia (0.3%) [2]. In some West African countries, yam has been used to produce several processed foods, such as amala, elubo, and gbodo [3].
Yam is a valuable source of nutritional components, such as carbohydrates, essential amino acids, minerals (Ca, K, Na, Mg, Fe, Zn, Fe, Co, Cu, and Mn), vitamins (thiamine, riboflavin, niacin, vitamin A, and ascorbic acid), and sapogenin steroids [4]. Sapogenin steroids have been extensively studied for their pharmacological properties [5]. The main steroids are found in monocotyledons, predominantly in the species belonging to the Dioscoreaceae family [5]. Among them, diosgenin can act as a hypoglycemic, hypocholesterolemic, anti-inflammatory, prebiotic, and antioxidant agent [6, 7].
Conversely, a significant amount of anti-nutritional compounds, such as oxalate and phytate, can be found in yam. Oxalates are salts that are not metabolized by humans, being excreted in the urine. However, its high concentration in urine increases the risk of calcium oxalate kidney stones, also known as nephrolithiasis, due to the formation of insoluble compounds when complexed with calcium ions. In foods, the cooking process promotes a reduction in oxalate concentration as it promotes tissue rupture, leading to the release of soluble oxalate [8, 9].
The phytates are phytic acid salts that form insoluble compounds when complexed with minerals, such as Cu2+, Zn2+, Fe3+, and Ca2+, reducing the mineral bioavailability [10, 11]. Phytase enzymes are produced by a wide range of plants, bacteria, filamentous fungi, and yeast, which catalyze the hydrolysis of phytate to phosphate and inositol [10]. Microbial phytase has shown effectiveness in improving the bioavailability of minerals [12]. Some bacteria such as Lactobacillus plantarum, Lactobacillus acidophilus, and Leuconostoc mesenteroides subsp. mesenteroides have shown phytase activity [13].
Lactobacillus and other lactic acid bacteria (LAB) are commonly used in dairy and also nondairy fermentations because of their ability to metabolize different substrates [14, 15]. LAB produce metabolites, such as volatile and non-volatile compounds, during the fermentation process, which contribute to their use in the food industry. The ability of LAB to ferment cereal, root, and tuber foods is due to their ability to produce amylases, an enzyme capable of converting the substrate starch into fermentable carbohydrates. The rapid acidification of the food matrix occurs through conversion of fermentable carbohydrates, mainly into lactic acid. Reduced pH and the production of antimicrobial agents (such as bacteriocins) decrease the growth and development of pathogenic microorganisms and food spoilers, extending the shelf life and ensuring food safety of the final product [16]. The nutritional quality is maintained by reducing anti-nutritional compounds (e.g., phytate) present in the various substrates, such as cereals, vegetables, nuts, seeds, and tubers. Also, the sensorial characteristics of each product is a result of the diverse routes used by microorganisms to metabolize carbohydrates, amino acids, fatty acids, and organic acids, releasing flavor compounds [16, 17].
Selected wild LAB strains with the capability to reduce the concentration of anti-nutritional compounds present in fermented yam and their behavior during the fermentation process were evaluated through the volatile compounds profile. Bioactive compounds present in yam and fermented yam were also evaluated.
Material and methods
Lactic bacteria strains
The bacteria strains, Leuconostoc lactis CCMA 0415, Lactobacillus plantarum (CCMA 0743, CCMA 0744, and CCMA 0746), Pediococcus acidilactici (CCMA 0748 and CCMA 0749), and Lactobacillus fermentum (CCMA 0201, CCMA 0208, CCMA 0211, CCMA 0212, CCMA 0745, CCMA 0751, and CCMA 0752), were provided by the Agricultural Microbiology Culture Collection (CCMA) of the Federal University of Lavras (Minas Gerais, Brazil). These bacteria were selected for this study, based on their ability to produce α-amylase as evaluated by Freire et al. [14]. It is an important characteristic for growth in starch-rich substrates including yam.
Phytase activity
Phytase production was assessed according to Raghavendra and Halam [17]. Lactic bacteria were grown in the de Man, Rogosa, and Sharpe (MRS) broth (Merck, Darmstadt, Germany) for approximately 24 h, until to reach 108 CFU/mL. Then, they were transferred to modified MRS agar, in which the inorganic phosphate KH2PO4, was replaced with 0.65 g/L sodium phytate and the concentrations of glucose, yeast extract, and meat extract were decreased to 10, 2, and 4 g/L, respectively, to reduce the final phosphate content and promote enzymatic synthesis. Positive strains produced a halo after incubation at 35 °C for 24 h, following the addition of 2% w/v cobalt chloride aqueous solution for 5 min and equal volumes of ammonium molybdate (6.25% w/v) and ammonium metavanadate (0.42% w/v), for 5 min. The positive strains were selected for fermentation assay.
Fermentation
Approximately 2 kg of yams (purchased from a local market in Lavras, Brazil) was used in each experiment. The yams were washed and peeled and the pieces (3 mm thick) pre-cooked at 60 °C for 10 min [8]. Each fermentation contained a mixture of 40% (w/v) yam, 60% water, and 1% (w/v) aqueous carboxymethylcellulose, which was crushed, filtrated, and pasteurized at 65 °C for 30 min. The selected LAB strains were previously grown in MRS broth at 35 °C for 24 h, recovered by centrifugation (10,000×g), washed twice in sterile water, and inoculated into the yam medium at 107 CFU/mL. The fermentation was performed for 24 h. The LAB viability was evaluated by plating in MRS agar and incubation at 35 °C for 24 h. The LAB viability and pH were evaluated during the fermentation process. The samples were withdrawn at 0, 6, 12, and 24 h of fermentation and stored at − 20 °C, for chemical analysis. The fermentations were done in triplicate. The fermentation media of the selected lactic bacteria were analyzed for phytate, organic acids, volatile compounds, and bioactive compounds.
Phytate determination
Phytate was extracted from fermented and non-fermented samples as described by Latta and Eskin [18], with minor modifications. Twenty milliliters of HCl (0.66 N) was added in 1 g of samples, adjusted the pH to 0.6 and incubated for 2 h under stirring (magnetic stir bar). Then, the extract was centrifuged at 200×g for 60 min and 3750×g for 5 min. The supernatant was filtered and diluted into 24 mL of distilled water (pH 6). Samples of 10 mL were passed through the AGL-X8 (Supelco Co., Bellefonte, PA, USA) ion exchange column and eluted in 15 mL of NaCl (0.7 M) and the pH adjusted to 3. One milliliter of Wage reagent (0.03% FeC13-6H20 and 0.3% sulfosalicylic acid in distilled water) was added to 3 mL of obtained samples and homogenized for 5 s. The absorbance at 500 nm was determined by a spectrophotometer. The quantification was performed from the calibration curve constructed from the different concentrations of phytic acid.
Lactic acid and oxalic acid analysis
Lactic acid and oxalic acid were analyzed throughout the fermentation process, by high-performance liquid chromatography (HPLC), according to Duarte et al. [19] and Ross et al. [20], respectively. The high-performance liquid chromatography (Shimadzu LC-10AI, Shimadzu Corp, Japan) was equipped with a Shimadzu SP-10Ai UV-Vis detector, set at 210 nm for the detection of the organic acids and a Shimadzu ion exclusion column (Shim-pack SCR-101H, 7.9 mm × 30 cm), operated at 50 °C. Perchloric acid (16 mM) was eluted at a rate of 0.6 mL/min. Each compound was identified and quantified based on its retention time and a standard curve [21]. Analyses were done in triplicate.
Analysis of volatile compounds
Aliquots (2 mL) of the food samples were previously removed from non-fermented yam medium and also at the end of the fermentation process. The volatile compounds were extracted by headspace solid-phase microextraction (HS-SPME), as described by Gaujac et al. [22], with minor modifications. A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 50/30-mm SPME fiber (Supelco Co., Bellefonte, PA, USA) was used to extract the volatile constituents from the yam food headspace. The fiber was equilibrated at 60 °C for 15 min and then exposed to the samples (yam food) for 30 min, at the same temperature.
The volatile compounds were evaluated by gas chromatography-mass spectrometry (GC-MS), using a Shimadzu GC-MS-QP2010 SE (Tokyo, Japan), equipped with a Carbowax column (30 m × 0.25 mm i.d. × 0.25-μm film thickness). The initial oven temperature was 40 °C, which was held for 5 min, then increased to 200 °C at 10 °C/min, and held at this temperature for 30 min. The carrier gas was helium, at a flow rate of 0.7 mL/min. A splitless injector was used. The mass detector was a quadrupole type, with an electron impact ionization system, operated at 70 eV and 260 °C. Compound identification was performed using GC-MS solution software (version 2.6) and comparison of the mass spectra of the peaks with those available in the NIST11 mass spectral library.
Determination of total allantoin and diosgenin content
Diosgenin and allantoin were extracted according to Avula et al. [23] and evaluated before fermentation and at the end of fermentation. Samples (1 mL) were combined with 9 mL methanol and then homogenized. The solutions were vortexed for 30 s, sonicated for 30 min, vortexed for 30 s, and, finally, centrifuged at 959×g for 10 min. The supernatant was filtered through a 0.22-μm membrane (Millipore), before HPLC analysis.
Diosgenin and allantoin were identified by HPLC (Shimadzu LC-10AI) with UV detection at 214 nm, using a Shimadzu C18 column, operated at 35 °C. The mobile phase consisted of acetonitrile/water (90:10 v/v) at a flow rate of 1 mL/min [24]. For quantification, an external calibration curve was constructed, by injecting various concentrations of the standards under the same conditions as the samples.
Statistical analysis
Analysis of variance (ANOVA) and the Scott-Knott test (p < 0.05) were performed using SISVAR software version 5.3 [25].
Results
Selection of LAB strains producing phytase and evaluation of their ability to reduce the pH of the yam medium
Thirteen amylase-producing LAB strains were evaluated for phytase production in MRS agar medium, pH reduction, and growth in yam. Among them, L. lactis CCMA 0415, L. plantarum CCMA 0744, L. fermentum CCMA 0745, L. fermentum CCMA 0212, P. acidilactici CCMA 0748, and P. acidilactici CCMA 0749 were distinguished by their ability to hydrolyze phytate (Table 1) in the modified MRS agar medium, as confirmed by halo presence.
Table 1.
Conditions evaluated during the selection of lactic acid bacteria strains
| Isolate | Phytase activity | pH at 24 h | Growth at 24 h (log CFU/mL) |
|---|---|---|---|
| Lactobacillus fermentum CCMA 0201 | – | 4.5 ± 0.04c | 8.6 ± 0.05a |
| Lactobacillus fermentum CCMA 0208 | – | 4.4 ± 0.00c | 8.5 ± 0.00a |
| Lactobacillus fermentum CCMA 0211 | – | 4.8 ± 0.07d | 8.4 ± 0.16a |
| Lactobacillus fermentum CCMA 0212 | + | 4.2 ± 0.06b | 8.7 ± 0.04a |
| Lactobacillus fermentum CCMA 0745 | + | 3.8 ± 0,01a | 7.8 ± 0.04b |
| Lactobacillus fermentum CCMA 0751 | – | 5.1 ± 0,06e | 8.5 ± 0.4a |
| Lactobacillus fermentum CCMA 0752 | – | 4.9 ± 0,13d | 8.5 ± 0.14a |
| Lactobacillus plantarum CCMA 0743 | – | 5.5 ± 0.02f | 8.3 ± 0.03a |
| Lactobacillus plantarum CCMA 0744 | + | 3.7 ± 0,02a | 8.5 ± 0.06a |
| Lactobacillus plantarum CCMA 0746 | – | 4.3 ± 0.04b | 8.5 ± 0.11a |
| Leuconostoc lactis CCMA 0415 | + | 3.7 ± 0.14a | 8.6 ± 0.00a |
| Pediococcus acidilactici CCMA 0748 | + | 4.8 ± 0.02d | 7.5 ± 0.08b |
| Pediococcus acidilactici CCMA 0749 | + | 4.8 ± 0.04d | 7.7 ± 0.17b |
Mean values followed by a different letter differ significantly (p < 0.05) by the Scott-Knott test
*Values are means ± SD of determinations
Among these, L. lactis CCMA 0415, L. plantarum CCMA 0744, and L. fermentum CCMA 0745 (Table 1) differed significantly (p < 0.05) in their pH (3.8, 3.7, and 3.8) and remained viable (around 8 log CFU/mL) during yam fermentation (Table 1). Therefore, they were selected for further analyses.
Evaluation of the reduction of anti-nutritional compounds by LAB selected
Anti-nutritional compounds such as oxalate and phytate were evaluated in this work. No significant oxalate concentrations were detected in the fermented yam. The phytate concentration was 54.92 mg/100 g in the substrate and remained constant in fermentation with L. plantarum CCMA 0744 and L. fermentum CCMA 0745. The fermentation with L. lactis CCMA 0415 showed a concentration of 9.79 mg/100 g.
Bioactive compounds in yams fermented with LAB selected
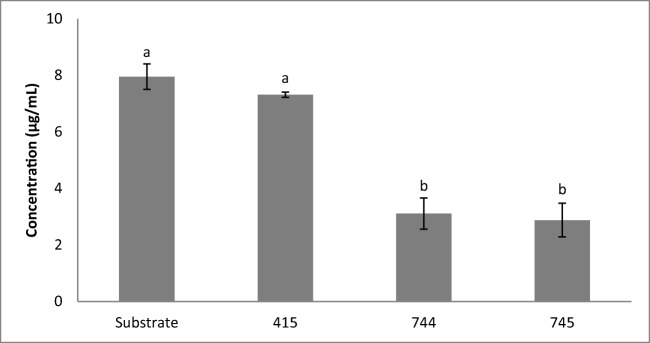
The bioactive compounds evaluated were allantoin and diosgenin. The allantoin concentration remained constant at around 0.5 mg/mL in all fermentations. The diosgenin concentration in the non-fermented yam was 8.0 μg/mL, while fermentations inoculated with L. plantarum CCMA 0744 and L. fermentum CCMA 0745 were around 3.0 μg/mL and L. lactis CCMA 0415 was 7.3 μg/mL (Fig. 1).
Fig. 1.
Diosgenin concentrations in the non-fermented and at 24 h of fermentation with Leuconostoc lactis CCMA 0415 (415), Lactobacillus plantarum CCMA 0744 (744), and Lactobacillus fermentum CCMA 0745 (745). The mean values followed by a different letter differ significantly (p < 0.05) by the Scott-Knott test
Volatile compounds produced by selected lactic acid bacteria
A total of 26 volatile compounds were identified by GC-MS at 24 h of fermentation (Table 2). The volatile compounds detected were grouped into acids (2), aldehydes (1), alkanes (6), alcohols (6), ketones (3), esters (4), lactones (1), and others (3). Differences were observed regarding the profile of the volatile compounds of each culture starter.
Table 2.
Volatile compounds detected in yam non-fermented and after yam fermentation by Leuconostoc lactis CCMA 0415 (CCMA 415), Lactobacillus plantarum CCMA 0744 (CCMA 744), and Lactobacillus fermentum CCMA 0745 (CCMA 745) for 24 h
| Compound | Sample | |||
|---|---|---|---|---|
| Non-fermented | CCMA0415 | CCMA0744 | CCMA0745 | |
| Acid | ||||
| Acetic acid | nd | + | + | + |
| Pentadecanoic acid | + | nd | nd | nd |
| Ester | ||||
| Ethyl palmitate | + | nd | nd | nd |
| Methyl palmitate | + | + | + | + |
| Phthalic acid, diisobutyl ester | + | + | + | + |
| Salicylic acid, 2-ethylhexyl ester | + | nd | nd | nd |
| Aldehyde | ||||
| Octadecanal | + | nd | nd | nd |
| Alkane | ||||
| Isopentacosane | + | nd | nd | nd |
| Heptadecane | + | + | + | + |
| Hexadecane | nd | + | + | + |
| Nonadecane | + | nd | nd | + |
| Octadecane | + | nd | + | nd |
| Tetradecane | + | + | nd | nd |
| Alcohol | ||||
| 1-Dodecanol | + | + | + | + |
| 1-Hexadecanol | + | nd | + | nd |
| 1-Tetradecanol | + | + | + | + |
| 2-Hexyldodecanol | nd | + | nd | nd |
| Heptadecanol | + | nd | nd | nd |
| Tridecanol | + | nd | + | nd |
| Ketone | ||||
| 2-Tridecanone | nd | + | + | + |
| 2-Undecanone | nd | nd | nd | + |
| Acetoin | nd | + | + | + |
| Lactone | ||||
| γ-Nonalactone | nd | nd | nd | + |
| Other | ||||
| 2-Ethylhexyl salicylate | + | nd | + | + |
| Homosalate | + | + | + | + |
| Isopropyl myristate | nd | nd | + | nd |
nd not detected
Discussion
LAB strains isolated from the natural fermentations have shown peculiar characteristics regarding their metabolic properties, such as amylase and organic acid production [14], which are essential for starch-based fermented foods. L. lactis CCMA 0415, L. plantarum CCMA 0744, L. fermentum CCMA 0745, L. fermentum CCMA 0212, P. acidilactici CCMA 0748, and P. acidilactici CCMA 0749 were distinguished by their ability to hydrolyze phytate. Phytate reduction occurred via the enzymatic action of phytase, which can be produced by plants, animal tissues, and microorganisms [26]. Phytate (hexaphosphoric acid myo-inositol) is the primary phosphorus source stored by plant tissues and, when consumed, the phosphates from phytic acid hydrolysis can react with divalent ions, such as Zn2+, Fe2+/3+, Ca2+, Mg2+, Mn2+, and Cu2+, to form insoluble complexes that compromise ionic intestinal absorption [10]. Phytate can also interact with proteins, affecting their solubility and hydrolysis and inhibiting the activity of some digestive enzymes, such as trypsin, pepsin, α-amylase, and β-galactosidase, reducing their bioavailability [27].
LAB, such as Pediococcus sp. and Lactobacillus sp., isolated from cereal-based foods and beverages, have shown to be potentially effective in reducing phytates present in foods [17]. Thirteen LAB, including L. lactis CCMA 0415, L. plantarum CCMA 0744, and L. fermentum CCMA 0745, were highlighted in relation to the phytase production, reduction of pH, and growth during yam fermentation.
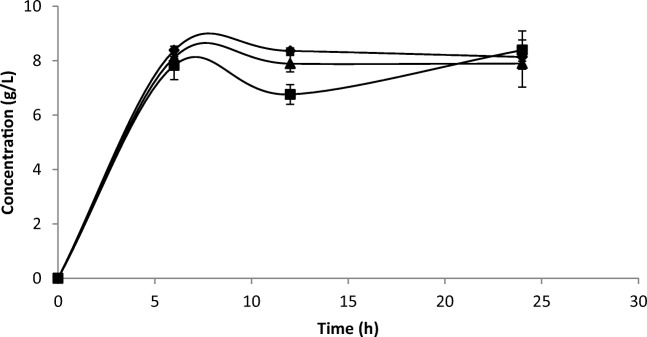
The pH is an important intrinsic factor in fermented food because it determines the microbiological stability against pathogenic microorganisms and food deterioration, besides being associated with the product flavor. The evaluated LAB in yam fermentation can produce α-amylase, resulting in rapid degradation of the starch into fermentable carbohydrates and consequent production of organic acids (mainly lactic acid). The pH value decrease detected in the fermentations probably occurred due to the lactic acid generated that reached a concentration of around 8 g/L at 6 h and remained almost constant for 24 h, for all fermentations (Fig. 2). Similar results (7.8 g/L) were found by Freire et al. [28] during spontaneous cassava fermentation, and low pH values (pH 3–4) have been documented in previous studies for fermented cereal-based beverages [14, 28]. Moreover, lactic acid contributes to the desirable refreshing attribute associated with thirst satiation of acidic dairy-based foods [29].
Fig. 2.
Lactic acid production (g/L) by Leuconostoc lactis CCMA 0415 (diamond), Lactobacillus plantarum CCMA 0744 (box), and Lactobacillus fermentum CCMA 0745 (triangle) during yam fermentation for 24 h
Plant foods contain anti-nutritional components, such as trypsin inhibitors, phytate, oxalate, cyanogenic glycosides, and nitrates. Oxalate is one of the constituents of yam, and the amount absorbed in vivo depends on its complexed form, the calcium and magnesium present in the food, and the presence of oxalate-degrading bacteria in the gastrointestinal tract [30]. In the present study, no significant oxalate concentrations were detected in the fermented yam, due to the pre-cooking process, given that cooking can reduce the soluble oxalate by 30 to 87% [31]. Regarding phytate concentration, the fermentation with L. lactis CCMA 0415 showed a reduction of 82% (9.79 mg/100 g) while the other strains maintained the concentration found in the substrate (54.92 mg/100 g).
Post-harvest processing, such as the fermentation process, also assists in the development of volatile compounds, contributing to the unique flavor of fermented foods [21]. The 26 volatile compounds were grouped into acids (2), aldehydes (1), alkanes (6), alcohols (6), ketones (3), esters (4), lactones (1), and others (3).
In all fermentations, acetoin, acetic acid, hexadecane, and 2-tridecanone were probably produced by LAB metabolism because they were not detected in the non-fermented samples. LAB may utilize citric acid and accumulate acetic acid and acetoin, which are correlated to butter and cream flavors [32]. Hexadecane, detected in all fermentations, has been shown to exhibit anti-inflammatory, antibacterial, antioxidant, and thermogenic functions [33].
Most of the volatile compounds identified belonged to esters, alcohols, and alkanes. The alcohols 1-dodecanol and 1-tetradecanol remained throughout the fermentation process. 1-Hexadecanol and tridecanol were produced by L. plantarum CCMA 0744, and 2-hexyldodecanol was produced by L. lactis CCMA 0415. The production of the various alcohols may have been influenced by differences in amino acid degradation reactions, resulting in the formation of corresponding alcohols or aldehydes [34]. The alkane tetradecane was present in the non-fermented and remained during the fermentation with L. lactis CCMA 0415. Some bacterial species can degrade tetradecane under anaerobic conditions through pathways other than β-oxidation, stimulating the lipases and proteases production that may negatively interfere with the food technological properties [35].
It is well established that microbial activity during fermentation affects the volatile compound profiles of plant-based foods and beverages. However, the environmental conditions influence the metabolites produced by plant tissues. According to Karlsson et al. [36], the presence of decanal, nonanal, isopropyl myristate, phenylacetaldehyde, benzothiazole, heptadecane, octadecane, myristicin, E-α-farnesene, and verbenone indicates that the tuber has undergone stress. In this sense, heptadecane, isopropyl myristate, and octadecane, detected in the present study, may infer that the tubers experienced stress during post-harvest treatment.
Conclusion
L. lactis CCMA 0415, L. plantarum CCMA 0744, and L. fermentum CCMA 0745 were selected and used as starter cultures for yam fermentation. Among them, L. lactis CCMA 0415 showed to be a better starter culture for yam-based fermentations. This phytase-producing microorganism maintained cell viability and did not reduce diosgenin concentrations, affording yam food potentially functional. Furthermore, this strain was able to decrease the pH value from 6.1 to 3.8 and to produce 8.1 g/L of lactic acid at 6 h of fermentation. Therefore, this strain might be useful as a starter culture, to produce fermented foods based on cereals, roots, and tubers.
Acknowledgements
The authors thank the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors gratefully acknowledge the anonymous referees for their comments and constructive suggestions for improving the quality of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nádia Nara Batista, Email: nadia.nb@hotmail.com.
Cíntia Lacerda Ramos, Email: cintia.ramos@ufvjm.edu.br.
Leonardo de Figueiredo Vilela, Email: leo.biomed@gmail.com.
Disney Ribeiro Dias, Email: diasdr@dca.ufla.br.
Rosane Freitas Schwan, Phone: +55 38291614, Email: rschwan@dbi.ufla.br.
References
- 1.Carrillo E, Prado-Gascó V, Fiszman S, Varela P. Why buying functional foods? Understanding spending behaviour through structural equation modelling. Food Res Int. 2013;50(1):361–368. doi: 10.1016/j.foodres.2012.10.045. [DOI] [Google Scholar]
- 2.FAOSTAT. FAOSTAT. Crop, food and agriculture organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Published 2014. Accessed 1 July 2017
- 3.Babajide JM, Oyewole OB, Henshaw FO, Babajide SO, Olasantan FO. Effect of local preservatives on quality of traditional dry yam slices “Gbodo” and its products. World J Agric Sci. 2006;2(3):267–273. [Google Scholar]
- 4.Wanasundera JPD, Ravindran G. Nutritional assessment of yam (Dioscorea alata) tubers. Plant Foods Hum Nutr. 1994;46:33–39. doi: 10.1007/BF01088459. [DOI] [PubMed] [Google Scholar]
- 5.Hostettmann K, Marston A. Occurrence and distribution. Cambridge: Cambridge University Press; 1995. Saponins; pp. 18–121. [Google Scholar]
- 6.Huang CH, Cheng JY, Deng MC, Chou CH, Jan TR. Prebiotic effect of diosgenin, an immunoactive steroidal sapogenin of the Chinese yam. Food Chem. 2012;132(1):428–432. doi: 10.1016/j.foodchem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Patel K, Gadewar M, Tahilyani V, Patel DK. A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect. 2012;2(2):46–52. doi: 10.1007/s13659-012-0014-3. [DOI] [Google Scholar]
- 8.Bhandari MR, Kawabata J. Cooking effects on oxalate, phytate, trypsin and α-amylase inhibitors of wild yam tubers of Nepal. J Food Compos Anal. 2006;19(6–7):524–530. doi: 10.1016/j.jfca.2004.09.010. [DOI] [Google Scholar]
- 9.Finch AM, Kasidas GP, Rose GA. Urine composition in normal subjects after oral ingestion of oxalate-rich foods. Clin Sci. 1981;60:411–418. doi: 10.1042/cs0600411. [DOI] [PubMed] [Google Scholar]
- 10.Greiner R, Konietzny U. Phytase for food application. Food Technol Biotechnol. 2006;44(2):125–140. [Google Scholar]
- 11.Lopez HW, Leenhardt F, Coudray C, Remesy C. Minerals and phytic acid interactions: is it a real problem for human nutrition? Int J Food Sci Technol. 2002;37:727–739. doi: 10.1046/j.1365-2621.2002.00618.x. [DOI] [Google Scholar]
- 12.Qian H, Kornegay ET, Denbow DM. Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium: total phosphorus ratio in broiler diets. Poult Sci. 1997;76(1):37–46. doi: 10.1093/ps/76.1.37. [DOI] [PubMed] [Google Scholar]
- 13.Lopez HW, Ouvry A, Bervas E, Guy C, Messager A, Demigne C, Remesy C. Strains of lactic acid bacteria isolated from sour doughs degrade phytic acid and improve calcium and magnesium solubility from whole wheat flour. J Agric Food Chem. 2000;48(6):2281–2285. doi: 10.1021/jf000061g. [DOI] [PubMed] [Google Scholar]
- 14.Freire AL, Ramos CL, Souza PNC, Cardoso MGB, Schwan RF. Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int J Food Microbiol. 2017;248:39–46. doi: 10.1016/j.ijfoodmicro.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Luana N, Rossana C, Curiel JA, Kaisa P, Marco G, Rizzello CG. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int J Food Microbiol. 2014;185:17–26. doi: 10.1016/j.ijfoodmicro.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Smid EJ, Kleerebezem M. Production of aroma compounds in lactic fermentations. Annu Rev Food Sci Technol. 2014;5(3):313–326. doi: 10.1146/annurev-food-030713-092339. [DOI] [PubMed] [Google Scholar]
- 17.Raghavendra P, Halami PM. Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int J Food Microbiol. 2009;133(1–2):129–134. doi: 10.1016/j.ijfoodmicro.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Latta M, Eskin M. A simple and rapid colorimetric method for phytate determination. J Agric Food Chem. 1980;28(6):1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- 19.Duarte WF, Dias DR, Oliveira JM, Teixeira JA, de Almeida e Silva JB, Schwan RF. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci Technol. 2010;43(10):1564–1572. doi: 10.1016/j.lwt.2010.03.010. [DOI] [Google Scholar]
- 20.Ross AB, Savage GP, Martin RJ, Vanhanen L. Oxalates in oca (New Zealand yam) (Oxalis tuberosa Mol.) J Agric Food Chem. 1999;47(12):5019–5022. doi: 10.1021/jf990332r. [DOI] [PubMed] [Google Scholar]
- 21.Ramos CL, Dias DR, Miguel MG d CP, Schwan RF. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res Int. 2014;64:908–918. doi: 10.1016/j.foodres.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 22.Gaujac A, Dempster N, Navickiene S, Brandt SD, de Andrade JB. Determination of N,N-dimethyltryptamine in beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta. 2013;106:394–398. doi: 10.1016/j.talanta.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Avula B, Wang YH, Ali Z, Smillie TJ, Khan IA. Chemical fingerprint analysis and quantitative determination of steroidal compounds from Dioscorea villosa, Dioscorea species and dietary supplements using UHPLC-ELSD. Biomed Chromatogr. 2014;28(2):281–294. doi: 10.1002/bmc.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu YC, Ferng LHA, Huang PY. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chem. 2006;94(4):541–549. doi: 10.1016/j.foodchem.2004.12.006. [DOI] [Google Scholar]
- 25.Ferreira DF. SISVAR: a program for statistical analysis and teaching. Rev Científica Symp. 2008;6(2):36–41. [Google Scholar]
- 26.Lopez Y, Gordon DT, Fields ML. Release of phosphorus from phytate by natural lactic acid fermentation. J Food Sci. 1983;48:953–954. doi: 10.1111/j.1365-2621.1983.tb14938.x. [DOI] [Google Scholar]
- 27.Reddy NR, Pierson MD. Reduction in antinutritional and toxic components in plant foods by fermentation. Food Res Int. 1994;27:281–290. doi: 10.1016/0963-9969(94)90096-5. [DOI] [Google Scholar]
- 28.Freire AL, Ramos CL, de Almeida EG, Duarte WF, Schwan RF. Study of the physicochemical parameters and spontaneous fermentation during the traditional production of yakupa, an indigenous beverage produced by Brazilian Amerindians. World J Microbiol Biotechnol. 2014;30(2):567–577. doi: 10.1007/s11274-013-1476-0. [DOI] [PubMed] [Google Scholar]
- 29.Ishimori Y, Koizumi T (2017) Acid lactic beverage and method for producing same: Pat. US 2017/0006889 A1, 8p
- 30.Massey LK. Food oxalate: factors affecting measurement, biological variation, and bioavailability. J Am Diet Assoc. 2007;107(7):1191–1194. doi: 10.1016/j.jada.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Chai W, Liebman M. Effect of different cooking methods on vegetable oxalate content. J Agric Food Chem. 2005;53(8):3027–3030. doi: 10.1021/jf048128d. [DOI] [PubMed] [Google Scholar]
- 32.Shimazu Y, Uehara M, Watanabe M. Transformation of citric acid to acetic acid, acetoin and diacetyl by wine making lactic acid bacteria. Agric Biol Chem. 1985;49(7):2147–2157. doi: 10.1271/bbb1961.49.2147. [DOI] [Google Scholar]
- 33.Musharraf SG, Iqbal A, Ansari SH, Parveen S, Khan IA, Siddiqui AJ. β-Thalassemia patients revealed a significant change of untargeted metabolites in comparison to healthy individuals. Sci Rep. 2017;7:42249. doi: 10.1038/srep42249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schieberle P. Intense aroma compounds: useful tools to monitor the influence of processing and storage on bread aroma. Adv Food Sci. 1996;18(5–6):237–244. [Google Scholar]
- 35.Gaur R, Khare SK. Cellular response mechanisms in Pseudomonas aeruginosa PseA during growth in organic solvents. Lett Appl Microbiol. 2009;49(3):372–377. doi: 10.1111/j.1472-765X.2009.02671.x. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson MF, Birgersson G, Witzgall P, Lekfeldt JDS, Nimal Punyasiri PA, Bengtsson M. Guatemalan potato moth Tecia solanivora distinguish odour profiles from qualitatively different potatoes Solanum tuberosum L. Phytochemistry. 2013;85:72–81. doi: 10.1016/j.phytochem.2012.09.015. [DOI] [PubMed] [Google Scholar]