Abstract
Tuberculosis is becoming a global issue with raising occurrences; particularly in developing countries, the situation is alarming. Besides environmental factors, host genetic factors are vital in disease development. A demographical and genotypic analysis in relation to tuberculosis commencement is conducted in a Pakistani population, and genotypic frequency of EBI3 (rs4740) was analyzed. Allelic frequencies of EBI3 (rs4740) were significantly associated with disease susceptibility in the reviewed population. Analysis for EBI3 (rs4740) genotyping showed a significant association of “GG” with reduced risk for disease. Moreover, females and older age found to be more perilous to develop TB while smoking and a family history of TB are additional risk factors for disease development. Further work with a larger population is necessary to identify the true causative variants of tuberculosis.
Keywords: Tuberculosis, Polymorphism, EBI3 (rs4740) , Smoking
Introduction
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (M. tuberculosis) and is one of the most devastating chronic infectious diseases, which remains the leading cause of death in developing countries [1]. Worldwide, there is a heavy burden of TB with 9.6 million new cases besides the 1.5 million deaths reported in the year 2014 [2]. It is estimated that nearly one-third of the world’s population is infected with M. tuberculosis while a large number of the population are left with no clinical symptoms of this infectious disease. Since merely 5–15% of individuals will develop the active disease [3, 4], it is assumed that susceptibility and progression to active TB are partly regulated by the host genetic factors [5]. In this regard, the identification of host genes and genetic variations would lead to a better understanding of the pathogenesis of TB and undoubtedly lead to novel strategies of treatment or prophylaxis.
It has already been acknowledged that innate and adaptive immune responses are imperative in the control of TB infection [6]. At some point during the infectious cycle, immune competent–infected humans will show the presence of Mycobacterium and start to generate an immune response, destroying macrophages containing bacilli. This leads to the presentation of Mycobacterium antigens to the host immune system resulting in the generation of a specific immune response against M. tuberculosis7.
Numerous immune regulatory genes involved in host immune responses have been proven to contain multiple polymorphisms thus contributing to TB susceptibility among different populations [8]. Among them, EBI3 has been found to be an important regulator in inflammation and infection. It has been shown to modulate differentiation of hematopoietic progenitor cells and regulate activation of immune cells as well as chemokines and cytokine production [9]. It was identified as a susceptible gene for pulmonary TB (PTB) and its deficiency protected mice against mycobacterial infection [10]. The G allele at EBI3 (rs4740) reduced the risk of developing tuberculosis in the Chinese population [10]. EBI3 is a member of the IL-12 heterodimeric cytokine family [11, 12]. Deficiency of EBI3 caused a reduction in bacterial burden and histopathological injury in lungs infected with Mycobacterium bovis. EBI3 was also found in higher abundance in the granuloma of PTB patients and in lung tissues infected with BCG. Mycobacterial infection extraordinarily induced the expression of EBI3 at both mRNA and protein levels. Consequently, polymorphism in the EBI3 gene rs4740 is closely linked with PTB susceptibility [10]. The expression of EBI3 is significantly upregulated in a variety of cancers such as breast cancer [13], gastric cancer [14], and pancreatic ductal adenocarcinoma [15]. Moreover, EBI3 polymorphisms were also reported to be closely associated with susceptibility to other diseases such as allergic rhinitis [16] and chronic rhino sinusitis [17].
In order to investigate the role of EBI3 in accordance with the reported findings, we performed a study to evaluate the association of EBI3 (rs4740) with PTB susceptibility in a Pakistani population through genotyping.
Methods
Study population
A total of 292 PTB patients and 199 healthy controls were analyzed in this study. Patients were consecutively recruited from Nishtar Hospital, Multan, after complete microbiological (smear positive and/or culture positive) and radiographical (x-ray) examination. Subjects showing either only a positive smear culture or only positive radio-graphical reports were excluded. Moreover, subjects under anti-TB medication were also omitted from the study. The control subjects were unrelated adults selected through the population without recent sign, symptom, or history of TB, and they were living in the same region as the patients with PTB. Inclusion criteria for controls were no history of previous TB or anti-mycobacterial treatments, no evidence of TB-related infiltrates in chest x-rays and no microbiological finding of Mycobacterium in their sputum. All cases and controls were HIV negative. Written informed consents were obtained from all the participants of the present study, and they donated a blood sample for genotyping analysis. The Ethical Committee of Bahauddin Zakriya University, Multan, approved the study.
Genomic DNA preparation and genotyping
Genomic DNA was isolated from peripheral blood mononuclear cells and granulocytes obtained from the blood of patients and controls using salting out procedure. EBI3 A/G (rs4740) polymorphism was studied using PCR-based restriction fragment length polymorphism (RFLP) methods. The sequences of primers, restriction digestion enzyme used, and restriction digestion pattern (Fig. 1) for different alleles are given in Table 1. Some randomly selected samples were sequenced (TSINGKE Biological Technology) for the conformation of PCR-RFLP, which showed complete matching of results.
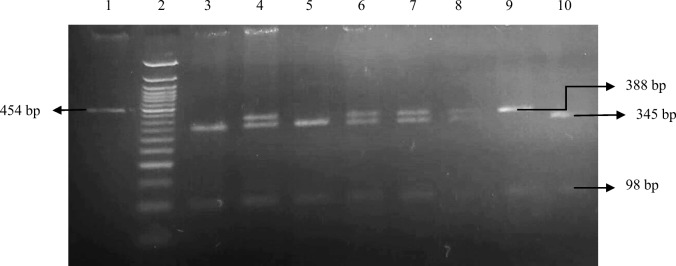
Fig. 1.
Electrophoresis of PCR and restriction digestion products for EBI3 rs4740. Ethidium bromide–stained electrophoresed representative RFLP products samples: 50-bp ladder (lane 2); EBI3 rs4740; PCR product (lane 1) 454 bp; homozygous wild A/A (lane 9) 388 bp, 98 bp; homozygous mutant G/G (lanes 3, 5, 10) 98 bp and 345 bp; heterozygous A/G (lanes 4, 6, 7, 8) 98 bp, 345 bp, and 388 bp
Table 1.
Enzymes and primers used in the study
| SNPs studied | Sequence of primers | Product size | Restriction enzyme used | Restriction digestion patterns for different alleles and their band sizes |
|---|---|---|---|---|
| EBI3 A/G (rs4740) |
5′-GCTCCGTTGTGTGGTTCTGT-3′ 5′-AGTGACAGTTCAGTCAGCCC-3′ |
486 bp | HpyCH4IV |
A allele: 98 + 388 bp G allele: 98 + 43+ 345 bp |
Statistical analysis
SPSS 17.0 was used to carry out statistical analysis. The association between phenotype (TB) and various demographic and genotypic parameters was determined using cross-tabulation in complex samples. Analysis with χ2 was used to test the statistical significance of the association. Stratified analyses were used to explore the correlation between phenotype and genotypes. The odds ratios (OR) and 95% confidence limits (CL) were calculated as an estimate of the relative risk and strength of association using logistic regression analysis. The result was considered significant when its associated P values were less than 0.05.
Results
Overall, this study consists of 292 cases (41.4% males, 58.6% females) and 199 controls (70.8% male, 29.1% female). The age (mean ± SD) was 36.36 and ± 17.17 for cases and 30.35 ± 10.87 years for controls. Despite the difference in number, significant differences in the distribution of gender and age between cases and controls were observed (Table 2) as a result of frequency matching. Despite the different ratios of males:females in the selected population, females tend to be more prevalent in the case group. Furthermore, patients aging 51–70 years had a higher prevalence of the disease (21.6%) compared with the same age group from the controls (11.5%). In addition to this, smoking and a family history of TB were also found to be significantly associated with the disease (P value < 0.0001) as the frequency distribution showed both of these factors are more prevalent in cases (Table 2).
Table 2.
Influence of various demographic factors on phenotype
| Factors | Categories | Cases (n = 292) |
Control (n = 199) |
Significance | OR (95% CI) |
|---|---|---|---|---|---|
| Gender | Male | 121 (41.4%) | 141 (70.8%) |
χ2 = 41.148, df = 1, P value = 0.000 |
3.44 (2.34–5.05) |
| Female | 171 (58.6%) | 58 (29.1%) | |||
| Age | 11–30 | 132 (45.2%) | 90 (45.2%) | χ2 = 9.954, df = 2, P value = 0.007 | 0.69 (0.53 0.90) |
| 31–50 | 97 (33.2%) | 86 (43.2%) | |||
| 51–70 | 63 (21.6%) | 23 (11.5%) | |||
| Smoking | Smokers | 57 (19.5%) | 15 (7.5%) |
χ2 = 13.580, df = 1, P value = 0.000 |
0.34 (0.18 0.61) |
| Non smokers | 235 (80.4%) | 184 (92.5%) | |||
| Family history of TB | TB present in family history | 137 (46.9%) | 10 (5%) |
χ2 = 99.019, df = 1, P value = 0.000 |
0.06 (0.03–012) |
| No TB history in family | 155 (53.1%) | 189 (95%) |
Genotypic and allelic frequency of various genotypes in controls or cases
On exploring EBI3 (rs4740 A/G) polymorphism in controls and PTB subjects, significant difference was observed among case and control groups (Table 3). Frequency of “AA” and “AG” genotypes is higher in cases (53.1% and 36.6% respectively) than in controls (48.2% and 29.6% respectively) while the “GG” genotype is higher in controls (22.1%) than in cases (10.2%). This showed a significant association of “GG” with reduced risk for developing tuberculosis (P < .0001; Table 3). Similarly, allelic frequency of the “G” allele is also higher in the control group. These results display that EBI3 genetic polymorphism is associated with susceptibility to PTB and allele “G” denotes protection against infection.
Table 3.
Distribution of genotypic and allelic frequencies among cases and controls and their possible association with tuberculosis
| Genes | Genotypes/allele | Cases (n = 292) | Control (n = 199) | P value | OR (95%CI) |
|---|---|---|---|---|---|
|
EBI3 (rs4740 A/G) |
AA | 155 (53.1%) | 96 (48.2%) | 0.292 | 1.21 (0.85–1.74) |
| AG | 107 (36.6%) | 59 (29.6%) | 0.108 | 1.17 (0.97–1.42) | |
| GG | 30 (10.2%) | 44 (22.1%) | 0.0005 | 0.74 (0.62–0.87) | |
| A | 417 (0.71) | 251 (0.63) | 0.0066 | 1.4624 (1.1148–1.9184) | |
| G | 167 (0.29) | 147 (0.37) | 0.6838 (0.5213–0.897) |
Effect of stratification by gender and age on the incidence of tuberculosis
To explore studied genes to environment interactions, we examined the association between genotype and TB; the data was stratified by selected characteristics such as sex and age (Tables 4 and 5). While stratifying rs4740 with gender, although we found a higher frequency of “GG” genotype and “G” allele in controls of male and female subjects, we only found a significant association in females only (χ2 = 6.324, df = 2, P = 0.042; Table 4). It depicts the protective role of the “G” allele against TB, while a higher frequency of “AA” genotype (52.6%) and “A” allele (0.72) in female cases illustrates that the “A” allele is involved in increasing the risk of TB in females.
Table 4.
Association of genotypes and tuberculosis stratified by gender
| Genes | Genotypes | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| Cases (121) | Control (141) | Significance | Cases (171) |
Control (58) | Significance | ||
| EBI3 (rs4740 A/G) | AA | 65 (53.7%) | 72 (51%) | χ2 = 5.215, df = 2, P value = 0.074 | 90 (52.6%) | 24 (41.3%) | χ2 = 6.324, df = 2, P value = 0.042 |
| AG | 41 (33.8%) | 37 (26.2%) | 66 (38.5%) | 22 (37.9%) | |||
| GG | 15 (12.3%) | 32 (22.6%) | 15 (8.7%) | 12 (20.6%) | |||
| A | 0.71 | 0.64 | 0.72 | 0.60 | |||
| G | 0.29 | 0.36 | 0.28 | 0.40 | |||
Table 5.
Association of genotypes and tuberculosis stratified by age
| Genes | Genotypes | 11–30 years | 31–50 years | 51–70 years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (132) | Cont. (90) | Significance | Cases (97) | Cont. (86) | Significance | Cases (63) | Cont. (23) | Significance | ||
| EBI3 (rs4740 A/G) | AA | 72 (54.5%) | 48 (53.3%) | χ2 = 7.262, df = 2, P value = 0.026 | 49 (50.5%) | 37 (43%) | χ2 = 2.478, df = 2, P value = 0.290 | 34 (54%) | 11 (47.8%) | χ2 = 6.124, df = 2, P value = 0.047 |
| AG | 50 (37.8%) | 25 (27.7%) | 34 (35.05%) | 29 (33.7%) | 23 (36.5%) | 5 (21.7%) | ||||
| GG | 10 (7.5%) | 17 (18.8%) | 14 (14.4%) | 20 (23.3%) | 6 (9.5%) | 7 (30.4%) | ||||
On stratification of patients with different age groups, it was observed that subjects 11–30 years and 51–70 years of age had a significant interaction with EBI3 (χ2 = 7.262, df = 2, P = 0.026 and χ2 = 6.124, df = 2, P = 0.047, respectively; Table 5), while the interaction of genotypes with age group of 31–50 years was not significant.
Discussion
The magnitude and complexity of the human immune response to Mycobacterium have historically been underestimated [18]. It is vital to determine whether those who remain healthy have a genetically endowed high level of resistance to tuberculosis or whether the resistance is affected by environmental or other exogenous factors [19].
The genome-wide association studies (GWA) identified several susceptibility loci for tuberculosis in sub-Saharan African, Russian, and Moroccan populations [20–22]. However, follow-up studies reported conflicting results [23]. In the present study, we explored the genetic polymorphism of EBI3 (rs4740) in association with pulmonary tuberculosis in a Pakistani population. EBI3 is a soluble glycosylated protein initially identified as a transcriptionally activated gene in Epsteine-Barr virus (EBV)-infected human B lymphocytes [24]. Our results were in agreement with a previous finding [10] that the “G/G” genotype was significantly associated with a reduced risk of TB where the allele “G” located in rs4740 protects against the disease. In addition to this, “AA” genotypes increase the risk of TB in our female population. This type of association in females was not found in previous studies. To the best of our knowledge, no data of this SNP have been published yet in association with TB except in the Chinese population [10] making us the pioneer to explore the role of genetic polymorphisms in this region. Variant rs4740 is a non-synonymous SNP (ns SNPs) and “G” allele replaced by “A” allele leads to substitution of valine with isoleucine at position 201 which is located in fibronectin type III domains of EBI3. This missense mutation of rs4740 affects the stability, structure, or biological function of the EBI3 gene disturbing the bacterial processing during infection. Although, in the present study, we only focused on rs4740, other SNPs of EBI3 are also involved in various other conditions and we cannot rule out their role. For instance, rs428253 is associated with the risk of coronary heart disease [25] while has protective effects against allergic rhinitis [16]. Similarly, rs568408 and not rs2243115 is associated with asthma [26].
Furthermore, a significant association of the incidence of TB was observed with demographic factors such as gender, age, smoking pattern, and the presence of TB in a family history. Like a previous study from Pakistan [27], we observed that females are at higher risk of TB development, divergent to the Taiwanese population [28], since females in our society have worsened conditions concerning TB diagnosis, treatment, and cure. The interaction of genders with the EBI3 (rs4740 A/G) revealed that the females carrying “AA” genotypes were at significantly higher risk of disease development than males while the “GG” genotype plays a protective role in males only. Old age was recorded as the risk factor for the disease development, as in aged people, the immune system is compromised. Age-related factors enhance TB susceptibility as well as increase the possibility of TB reactivation [29]. The incidence of TB among older people is almost three times higher than that of young adults worldwide [30].
We additionally observe smoking as a risk factor for TB susceptibility as described by other studies [31, 32]. Smoking results in malfunctioning of alveolar macrophages and tuberculosis leads to apoptosis of these cells [33–35]. Antigen presentation of alveolar macrophages is impaired by nicotine present in cigarette smoke; thus, prolonged acquaintance to smoking diminishes the expression of surface proteins involved in antigen presentation [36–39] consequently resulting in disease development. Lastly, a noteworthy association of TB sensitivity was observed in relation to the presence of TB in a family history strengthening the perception that host genetic factors are equally contributive.
In summary, our data suggest that allelic frequencies of EBI3 (rs4740) are associated with the risk of TB in a Pakistani population. In the present study, heterogeneity was found, which is possibly due to the ethnic origin of the included TB patients as ethnicity-specific genetic variations may influence the host immunity to bacterial infection. Further studies of SNPs in high linkage disequilibrium covering a larger cohort are under process in our institutes for further investigation.
Acknowledgements
We are thankful to the study participants for their active cooperation and support. We are also appreciative to all officers and officials of Nishtar Hospital, Multan, for their provision and consideration. This work was supported by the Institute of Molecular Biology and Biotechnology, Bahauddin Zakariya University Multan, Pakistan, through usage of facilities available at the institute.
Compliance with Ethical Standards
Written informed consents were obtained from all the participants of the present study and they donated a blood sample for genotyping analysis. The Ethical Committee of Bahauddin Zakriya University, Multan, approved the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abrar Ul Haq Khan, Email: abrarulhaqkhan@gmail.com.
Muhammad Ali, Email: alisam007@hotmail.com.
References
- 1.Hu Q, et al. Association of genetic polymorphisms with pulmonary tuberculosis in a Chinese Tibetan population: a case-control study. Int J Clin Exp Pathol. 2016;9:267–274. [Google Scholar]
- 2.WHO. (2017) Tuberculosis fact sheet
- 3.Schluger NW. Recent advances in our understanding of human host responses to tuberculosis. Respir Res. 2001;2:157–163. doi: 10.1186/rr53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A, George A, Sharma V, Herbert N. WHO’s 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382:1765–1767. doi: 10.1016/S0140-6736(13)62078-4. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira MMd, et al. Single Nucleotide Polymorphisms (SNPs) of the TNF-alpha (-238/-308) gene among TB and nom TB patients: susceptibility markers of TB occurrence? J Bras Pneumol. 2004;30:371–377. doi: 10.1590/S1806-37132004000400012. [DOI] [Google Scholar]
- 6.Leandro A, Rocha M, Cardoso C, Bonecini-Almeida M. Genetic polymorphisms in vitamin D receptor, vitamin D-binding protein, Toll-like receptor 2, nitric oxide synthase 2, and interferon-γ genes and its association with susceptibility to tuberculosis. Braz J Med Biol Res. 2009;42:312–322. doi: 10.1590/S0100-879X2009000400002. [DOI] [PubMed] [Google Scholar]
- 7.Ducati RG, Ruffino-Netto A, Basso LA, Santos DS. The resumption of consumption: a review on tuberculosis. Mem Inst Oswaldo Cruz. 2006;101:697–714. doi: 10.1590/S0074-02762006000700001. [DOI] [PubMed] [Google Scholar]
- 8.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80(10):3343–3359. doi: 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berod L, Stüve P, Swallow M, Arnold-Schrauf C, Kruse F, Gentilini MV, Freitag J, Holzmann B, Sparwasser T. MyD88 signalling in myeloid cells is sufficient to prevent chronic mycobacterial infection. Eur J Immunol. 2014;44:1399–1409. doi: 10.1002/eji.201344039. [DOI] [PubMed] [Google Scholar]
- 10.Zheng R, Liu H, Song P, Feng Y, Qin L, Huang X, Chen J, Yang H, Liu Z, Cui Z, Hu Z, Ge B. Epstein–Barr virus-induced gene 3 (EBI3) polymorphisms and expression are associated with susceptibility to pulmonary tuberculosis. Tuberculosis. 2015;95:497–504. doi: 10.1016/j.tube.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 12.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DAA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 13.Hamidinia M, Ghafourian Boroujerdnia M, Talaiezadeh A, Solgi G, Roshani R, Iranprast S, Khodadadi A. Increased P-35, EBI3 transcripts and other treg markers in peripheral blood mononuclear cells of breast cancer patients with different clinical stages. Adv Pharm Bull. 2015;5:261–267. doi: 10.15171/apb.2015.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y-G, Zhai JM, Wang W, Feng B, Yao GL, An YH, Zeng C. IL-35 over-expression is associated with genesis of gastric cancer. Asian Pac J Cancer Prev: APJCP. 2015;16:2845–2849. doi: 10.7314/APJCP.2015.16.7.2845. [DOI] [PubMed] [Google Scholar]
- 15.Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol. 2014;75:29–33. doi: 10.1016/j.humimm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Duan S, Wei X, Zhao Y, Zhao L, Zhang L. Association between polymorphisms in FOXP3 and EBI3 genes and the risk for development of allergic rhinitis in Chinese subjects. Hum Immunol. 2012;73:939–945. doi: 10.1016/j.humimm.2012.07.319. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang C, Zhao Y, Zhang L. Some polymorphisms in Epstein-Barr virus-induced gene 3 modify the risk for chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27:91–97. doi: 10.2500/ajra.2013.27.3851. [DOI] [PubMed] [Google Scholar]
- 18.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17-and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P, Grange J. Factors affecting susceptibility and resistance to tuberculosis. Thorax. 2001;56:ii23–ii29. doi: 10.1136/thorax.56.11.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thye T, et al. Corrigendum: genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q112. Nat Genet. 2011;43:1040. doi: 10.1038/ng1011-1040a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis J, Luo Y, Zenner HL, Cuchet-Lourenço D, Wu C, Lo K, Maes M, Alisaac A, Stebbings E, Liu JZ, Kopanitsa L, Ignatyeva O, Balabanova Y, Nikolayevskyy V, Baessmann I, Thye T, Meyer CG, Nürnberg P, Horstmann RD, Drobniewski F, Plagnol V, Barrett JC, Nejentsev S. Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat Genet. 2015;47:523–527. doi: 10.1038/ng.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant A, et al. A genome-wide association study of pulmonary tuberculosis in Morocco. Hum Genet. 2016;135:299–307. doi: 10.1007/s00439-016-1633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji L-D, Chai PF, Zhou BB, Tang NLS, Xing WH, Yuan F, Fei LJ, Zhang LN, Xu J. Lack of association between polymorphisms from genome-wide association studies and tuberculosis in the Chinese population. Scand J Infect Dis. 2013;45:310–314. doi: 10.3109/00365548.2012.726739. [DOI] [PubMed] [Google Scholar]
- 24.Devergne O, Hummel M, Koeppen H, le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Xue Y, Huang X, Lu J, Yang Z, Ye J, Zhang S, Liu L, Liu Y, Shi Y. Association between interleukin-35 polymorphisms and coronary heart disease in the Chinese Zhuang population: a case–control study. Coron Artery Dis. 2018;29:423–428. doi: 10.1097/MCA.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 26.Shen T-C, Tsai CW, Chang WS, Wang S, Chao CY, Hsiao CL, Chen WC, Hsia TC, Bau DT. Association of interleukin-12A rs568408 with susceptibility to asthma in Taiwan. Sci Rep. 2017;7:3199. doi: 10.1038/s41598-017-03523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AuH, Aslam MA, Hussain I, Naz AG, Rana IA, Ahmad MM, Ali M, Ahmad S. Role of Toll-like receptor 2 (–196 to–174) polymorphism in susceptibility to pulmonary tuberculosis in Pakistani population. Int J Immunogen. 2014;41:105–111. doi: 10.1111/iji.12086. [DOI] [PubMed] [Google Scholar]
- 28.Lee S-W, Chuang TY, Huang HH, Lee KF, Chen TTW, Kao YH, Wu LSH. Interferon gamma polymorphisms associated with susceptibility to tuberculosis in a Han Taiwanese population. J Microbiol Immunol Infect. 2015;48:376–380. doi: 10.1016/j.jmii.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Dutt AK, Stead WW. Tuberculosis in the elderly. Med Clin North Am. 1993;77:1353–1368. doi: 10.1016/S0025-7125(16)30198-5. [DOI] [PubMed] [Google Scholar]
- 30.Gavazzi G, Herrmann F, Krause K-H. Aging and infectious diseases in the developing world. Clin Infect Dis. 2004;39:83–91. doi: 10.1086/421559. [DOI] [PubMed] [Google Scholar]
- 31.Yu G-p, Hsieht C-c, Peng J. Risk factors associated with the prevalence of pulmonary tuberculosis among sanitary workers in Shanghai. Tubercle. 1988;69:105–112. doi: 10.1016/0041-3879(88)90072-4. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemian R, Najafi N, Yadegarinia D, Alian S. Association between cigarette smoking and pulmonary tuberculosis in men: a case-control study in Mazandaran, Iran. Arch Clin Infect Dis. 2009;4:135–141. [Google Scholar]
- 33.Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Phys Lung Cell Mol Phys. 2001;281:L1392–L1401. doi: 10.1152/ajplung.2001.281.6.L1392. [DOI] [PubMed] [Google Scholar]
- 35.Elssner A, Carter JE, Yunger TM, Wewers MD. HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. CHEST J. 2004;125:1071–1076. doi: 10.1378/chest.125.3.1071. [DOI] [PubMed] [Google Scholar]
- 36.Pankow W, Neumann K, Rüschoff J, Schröder R, von Wichert P. Reduction in HLA-DR antigen density on alveolar macrophages of smokers. Lung. 1991;169:255–262. doi: 10.1007/BF02714161. [DOI] [PubMed] [Google Scholar]
- 37.Sköld C, Lundahl J, Hallden G, Hallgren M, Eklund A. Chronic smoke exposure alters the phenotype pattern and the metabolic response in human alveolar macrophages. Clin Exp Immunol. 1996;106:108–113. doi: 10.1046/j.1365-2249.1996.d01-805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkham PA, Spooner G, Ffoulkes-Jones C, Calvez R. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic Biol Med. 2003;35:697–710. doi: 10.1016/S0891-5849(03)00390-3. [DOI] [PubMed] [Google Scholar]
- 39.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]