Abstract
Sporotrichosis is an infection of the skin caused by traumatic inoculation of the fungus Sporothrix schenckii. Definitive diagnosis relies on direct visualization of the fungus or its isolation on culture medium, although both have low sensitivity. Alternatively, the detection of the antibody response offers a more rapid alternative for diagnosis. Although the available immunoassays possess good sensitivity and specificity, cross-reactivity is still a problem. This study aimed to evaluate the effect of sodium metaperiodate and 6 M urea solutions on the serological diagnosis of sporotrichosis using an enzyme-linked immunosorbent assay (ELISA) test. Ninety-six-well plates were sensitized with exoantigens from the yeast phase of S. schenckii. Sera of patients with confirmed sporotrichosis, sera of patients with paracoccidioidomycosis, and sera of individuals with a sporotrichin-negative skin test were tested. Two strategies were used; the first consisted of treating the antigen with sodium metaperiodate solution for different incubation times, and the second consisted of treating the serum with 6 M urea solution for different incubation times. ROC curve analysis revealed that the best discrimination parameters were obtained using 6 M urea solution incubated for 5 min and serum dilution at 1/600. The use of 6 M urea solution improves the performance of the ELISA test in the diagnosis of sporotrichosis.
Keywords: Human sporotrichosis, ELISA, Sodium metaperiodate, Urea, Sporothrix schenckii
Introduction
Sporotrichosis is a chronic granulomatous disease caused by different species of the hyphomycete genus Sporothrix, by traumatic inoculation of the in the skin or subcutaneous tissue; this usually occurs through thorns, barbs, scratches, or bites. Sporothrix spp. exist as a mycelial form in the environment and as a yeast form in humans and other infected mammals [1]. Medically relevant Sporothrix species now include Sporothrix brasiliensis, S. schenckii, S. globosa, S. Mexicana, and S. luriei [2]. Clinically, sporotrichosis may manifest as lymphocutaneous, fixed cutaneous, disseminated cutaneous, extracutaneous, or disseminated forms and also very rarely as a primary pulmonary disease [3]. The disease has been reported worldwide and, in recent years, most of the reported cases have come from Central and South America, particularly Brazil, Colombia, Mexico, Peru, and China [4]. In Rio de Janeiro, Brazil, epidemic transmission of the disease occurred through scratches or bites from S. brasiliensis–infected cats [5]. Direct mycological examination using potassium hydroxide (KOH) or differential staining is low sensitive for the diagnosis of human sporotrichosis due to the scarcity of fungal elements in the lesions, particularly in lymphocutaneous and fixed cutaneous forms. However, Gram, Giemsa, Periodic-Schiff (PAS), and Grocott-Gomori’s staining (silver staining) can be successfully used in disseminated manifestations. The gold standard for diagnosis of sporotrichosis is based on conventional culture of clinical specimens that can be obtained from active lesions and cultured on Sabouraud agar. Positive cultures appear in the first 2 weeks of incubation; however, in some cases, it will be necessary to observe for up to 30 days before discarding them as negative [6]. Many authors related that sporotrichosis is the only subcutaneous mycosis for which direct examination or histology is of little or no value for diagnosis [4]. However, researchers with more experience in isolating the fungus from clinical lesions may get more positivity in the isolation. Methods that can detect anti-S. schenckii antibodies in patient serum offer an alternative to the laboratory diagnostic tools that exist for sporotrichosis identification; among these methods, precipitation and agglutination techniques were the first to be described [7–10]. Nevertheless, the above-mentioned methods provide satisfactory specificity but low sensitivity values. More recently, enzyme-linked immunosorbent assay (ELISA)–based tests have been developed for the diagnosis of human or feline sporotrichosis [11–14]. Although the ELISA immunoassays present higher sensitivity and specificity than the precipitation and agglutination techniques, they still show cross-reactivity among fungal antigens and other infectious agents. Therefore, this study aimed to evaluate the effects of sodium metaperiodate and 6 M urea solutions on the serological diagnosis of sporotrichosis using an ELISA test.
Material and methods
Production of crude exoantigens of Sporothrix schenckii
S. schenckii s. str exoantigens prepared from the yeast phase of the strain #118 (isolated from a human case of lymphocutaneous sporotrichosis) were obtained, as previously described, using Sabouraud medium (Difco) [15]. The protein content was measured by the Bradford method [16]. Molecular characterization was performed by sequencing the calmodulin gene (Genbank accession number: JX077126) and showed that strain Ss118 is a S. schenckii s. str specie [17]. This isolate was deposited at the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands) under the code CBS 132974.
Serum samples
Four serum samples from patients with clinical and laboratory diagnosis of sporotrichosis were obtained from the Hospital of the Universidade Federal do Triângulo Mineiro, Brazil (four men and one woman; two lymphocutaneous, one cutaneous fixed, and one disseminated form). Eleven serum samples from individuals with a sporotrichin-negative skin test (six men and five women), and eight serum samples (six men and two women) from patients with clinical and serological diagnosis of chronical adult form of paracoccidioidomycosis (PCM) were used in the optimization of the ELISA assay described herein. The blood collected in vacutainer SST II advance tube for serum collection (BD, Plymouth, UK) were centrifuged for 10 min at 400g, room temperature. The sera was transferred to 3-mL sterile tubes and kept at − 20 °C. To validate the ELISA, we also used 20 serum samples from patients proven (by means of serological and mycologic tests from the lesions) to be harboring different clinical forms of sporotrichosis (15 lymphocutaneous, three cutaneous fixed, two disseminated forms); these were obtained from a serum collection at the Universidade do Estado do Rio de Janeiro, RJ, Brazil [12]. This study was approved by the local Ethics Committee (CAAE 00752212.1.0000.5142).
Double immunodiffusion test
A double immunodiffusion test was performed using crude exoantigen of S. schenckii, to assess the presence of anti-S. schenckii antibodies [18]. Sera collected from rabbits that were previously immunized with crude exoantigens of S. schenckii strain #118 were used as a positive control.
Enzyme-linked immunosorbent assay
The indirect ELISA assay was performed as described by Camargo et al. [19] with some modifications. Flat-bottomed 96-well polystyrene plates (NUNC) were sensitized with 100 μL/well with S. schenckii antigen at 5 μg/mL in carbonate-bicarbonate buffer (0.1 M, pH 9.6) at 4 °C for 16 h. After this time, the plates were washed three times with 0.9% NaCl-0.05% Tween 20 (washing solution). Subsequently, the plates were blocked with 200 μL/well with 5% skim milk (Molico®) solution in phosphate-buffered saline and Tween 20 (PBS 0.05% Tween) for 1 h at 37 °C. The plate was washed again three times with wash solution. Next, 100 μL of the 1/50, 1/100, 1/200, 1/400, 1/800, 1/100, 1/1600, and 1/3200 dilutions of sera from patients with sporotrichosis, patients with PCM, and sporotrichinin-negative patients in 3% gelatin in PBS-0.05% Tween solution were added to the plates and then incubated for 1 h at 37 °C. After this time, the plates were washed three times with washing solution. Then, 100 μL of goat anti-human total IgG peroxidase–conjugated antibodies (SIGMA) diluted 1/8000 in 3% gelatin solution in PBS 0.05% Tween for 1 h at 37 °C. After, the plate was washed three times with washing solution. Then, 100 μL/well of the ortho-phenylenediamine (OPD) (SIGMA) enzyme substrate prepared in citrate buffer (0.1 M pH 4.5) was added and allowed to develop color for about 8 min in a darkroom. The reaction was stopped by the addition of 30 μL/well of 2 N H2SO4. The plate reading was performed at 490 nm on a microplate reader (Zenyth 200 rt). We used two different strategies in order to improve the sensibility and specificity of the ELISA assay. The first strategy consisted of the addition of 100 μL per well of a 40 mM sodium metaperiodate (Merk) solution in 0.1 M acetate buffer solution, for 30 min at 37 °C, in a darkroom; this was performed after the step of sensitizing the plate with S. schenckii antigens in order to eliminate carbohydrate epitopes sensitive to periodate oxidation [20]. The second strategy consisted of the addition of 100 μL/well of 6 M urea solution in phosphate buffer saline-Tween 20(PBS-T) for 1, 5, 10, and 20 min, at room temperature, after the incubation period with serum samples and prior to addition of the conjugate antibody. Six molars of urea solution has the ability to break the low-affinity binding between the antigen and antibody, thereby reducing the occurrence of cross-reactivity [21]. Cutoff values for serum reactivity to S. schenckii yeast-phase exoantigens were calculated as the mean of the optical density readings plus two times the standard deviation of negative samples in the presence or absence of metaperiodate or 6 M urea solution, in each sera dilution. Serum samples with absorbance values above the cutoff were considered to be positive.
Statistical analysis
Statistical analysis was performed with GraphPad Prism® 4.0 software. Comparisons between patients with sporotrichosis, patients with paracoccidioidomycosis, and individuals with sporotrichin-negative skin tests were performed using the Kruskal-Wallis multivariate analysis, followed by Dunn’s post-tests. The minimum significance level accepted was 5% (P < 0.05). In addition, receiver operating characteristic (ROC) curves were constructed, for which sensitivity and specificity were calculated as a function of the cutoff values [22]. From the results obtained, each treatment with sodium metaperiodate or 6 M urea solution became a point on the ROC curve, and the treatment that was closer to the ideal (100% sensitivity and 100% specificity) was considered the best for diagnosing sporotrichosis.
Results
Double immunodiffusion test
The results of the double immunodiffusion test indicated that 75% of sera from sporotrichosis patients tested negative, suggesting a very low sensitivity. Further, 100% of sera samples from PCM patients and individuals with sporotrichin-negative skin tests were also negative, which suggested high specificity for this test (Table 1).
Table 1.
Results in percentage of double immunodiffusion test using sera from different patients
| Double immunodiffusion test | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Sporotrichosis | 25% (1) | 75% (3) | 100% (4) |
| Paracoccidioidomycosis | 0% (0) | 100% (8) | 100% (8) |
| Sporotrichin-negative | 0% (0) | 100% (11) | 100% (11) |
Enzyme-linked immunosorbent assay
The mean and standard deviation of the ELISA readings using sera from patients with sporotrichosis, sera from individuals with sporotrichin-negative skin tests, and sera from patients with PCM are shown in the Fig. 1. All samples showed higher readings at low dilutions. There were statistically significant differences between the readings from patients with sporotrichosis and those from individuals with sporotrichin-negative skin tests, at 1/400 to 1/1600 dilutions. There were no statistically significant differences between readings from sporotrichosis patients and those from PCM patients, or between sporotrichosis patients and individuals with sporotrichin-negative skin tests (P > 0.05) at 1/400 and 1/3200 dilutions.
Fig. 1.

ELISA results of IgG (means and standard deviations of O.D. at 490 nm) obtained from the sera from patients with sporotrichosis (Ss+, n = 8), sera from individuals with sporotrichin-negative skin tests (Ss−, n = 8), and sera from patients with paracoccidioidomycosis (PCM, n = 8) against yeast-phase S. schenckii exoantigens. Comparison using the Kruskal-Wallis multivariate analysis, followed by a Dunn’s post-test, was made between Ss+, Ss−, and PCM, for each dilution. *P < 0.05
ELISA with sodium metaperiodate solution
As shown in Fig. 1, sera from individuals with sporotrichin-negative skin tests, patients with sporotrichosis, and PCM patients showed some cross-reactivity to S. schenckii exoantigens. Part of this cross-reactivity may be due to common carbohydrate epitopes. Sodium metaperiodate oxidizes carbohydrates present in glycoproteins or polysaccharides, typical constituents of fungal exoantigens, decreasing the cross-reactions between carbohydrates and antibodies. Using specificity and sensitivity parameters calculated with ROC curves for each sera dilution, we found that sodium metaperiodate solution decreased the discriminative power of the ELISA test for the diagnosis of sporotrichosis (Fig. 2).
Fig. 2.

Receiver operating characteristic (ROC) curves for the crude S. schenckii exoantigens comparing different dilutions of sera in the presence or absence of sodium metaperiodate solution. The ROC is plotted between the true-positive rate (sensitivity) on the y-axis, and the false-positive rate (1-specificity) on the x-axis. The area under the curve (AUC) represents the accuracy of the ELISA test, which was 0.9
ELISA with 6 M urea solution
Published literature indicates that the serum of patients with different infections may have antibodies with high and low affinity for a determinate antigen/epitope, and the presence of these low-affinity antibodies may increase the rate of false-negative diagnosis, mainly due to the non-specific binding [21]. Six molars of urea solution breaks down low-affinity binding between antibodies and antigens, thus reducing cross-reactivity [23]. After treatment with 6 M urea solution and the subsequent determination of sensitivity and specificity using ROC curves, we found that treatment with 6 M urea solution improved the performance of the ELISA. Higher values of sensitivity and specificity were obtained in comparison to the protocol that did not use urea. The best discriminative parameters were obtained using a serum diluted at 1/1600 for a duration of 5 min (Fig. 3). Furthermore, other combinations of dilution factors and incubation times, for example, serum diluted at 200- or 400-fold for 20 min, and serum diluted at 800-fold for 5 min, were statistically (and numerically) equal to the best dilution/incubation combinations identified (serum diluted at 1/1600 and incubation for 5 min) (data not shown).
Fig. 3.
Receiver operating characteristic (ROC) curves for the crude S. schenckii exoantigens comparing different sera dilutions and incubation periods, in the presence or absence of 6 M urea solution. The ROC is plotted between the true-positive rate (sensitivity) on the y-axis, and the false-positive rate (1-specificity) on the x-axis. The area under the curve (AUC) represents the accuracy of the ELISA test, which was 0.9
ELISA assay validation
Once we had determined the best working conditions for the assay (serum diluted at 1/1600 and incubation time of 5 min), we used 20 sera samples from well-characterized sporotrichosis patients harboring different clinical forms of the disease. As can be seen in Fig. 4, the assay showed good discrimination among sporotrichin-negative individuals, PCM patients, and sporotrichosis-positive control sera used in the standardization and sera test (P < 0.05). Similar results were observed using other serum dilutions (data not shown).
Fig. 4.
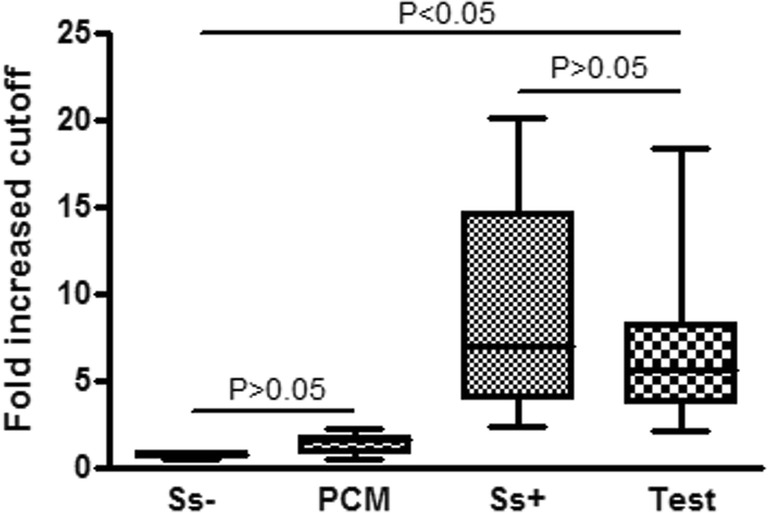
ELISA results of IgG (fold increased cutoff) obtained from the sera of patients with sporotrichosis (Ss+, n = 8), sera from individuals with sporotrichin-negative skin tests (Ss−, n = 8), sera from patients with paracoccidioidomycosis (PCM, n = 8), and test sera from patients with different clinical forms of sporotrichosis (test, n = 20) against yeast-phase S. schenckii exoantigens. Comparison using the Kruskal-Wallis multivariate analysis, followed by a Dunn’s post-test. Ss− compared to PCM, and Ss+ compared to test, P > 0.05; Ss− and PCM compared to Ss+ and test, P < 0.05. Cutoff values for serum reactivity were calculated by dividing the mean of the optical density readings of each serum sample at 1/1600 dilution by negative samples in the presence of 6 M urea solution
Discussion
Sporotrichosis is a subcutaneous mycosis with a worldwide distribution. It is caused by the traumatic inoculation of material contaminated with the environmental fungus S. schenckii, or other pathogenic species in this complex [24, 25]. Latin America is one of the most common areas with high incidence of fungal infections. It is estimated that the annual incidence in South America is 48 to 60 cases of sporotrichosis per hundred thousand inhabitants; the number of cases of men and women varies from region to region [26]. Although there have been several advances in the diagnosis of feline and human sporotrichosis [14, 27, 28], to date, the definitive diagnosis of sporotrichosis is based on the isolation of the fungus in a culture with Sabouraud agar. However, the in vitro culture outcome takes days to develop; meanwhile, there is a risk of culture contamination. Further, a trained professional is necessary to identify the fungus correctly. Another problem is the occurrence of false-negative results due to the difficulty of growing the fungus in culture medium [6]. Given these difficulties, it is important to develop immunological techniques for the diagnosis of this pathology based on the detection of antibodies in patients. Similarly, techniques that allow for a faster result, thus facilitating the diagnosis and monitoring of patients, are needed. Furthermore, such developments may contribute to epidemiological assessment, as an easy tool to determine the real prevalence of this disease.
Precipitation or agglutination techniques of antibody-antigen complexes, for the detection of anti-S. schenckii antibodies, were proposed by Blumer et al. [8] These authors found low sensitivity but satisfactory specificity values. In the present work, the results of double radial immunodiffusion showed low sensitivity for sporotrichosis (25%) and high specificity (100%) for both PCM and individuals with a sporotrichin-negative skin test (Table 1); this is consistent with the findings of Blumer et al. [8] and Casserone et al. [9]. In general, immunoenzymatic assays have a detection threshold that is higher than agglutination or precipitation methods, which thus leads to greater sensitivity and specificity. The literature describes a variety of immunoassays for the diagnosis of infections caused by other dimorphic fungi, such as PCM and histoplasmosis. However, traditional ELISA has not been shown to have satisfactory sensitivity and specificity for the diagnosis of these diseases [29, 30].
Many studies have used ELISA immunoassays to help diagnose sporotrichosis. In the first such published work, an enzyme immunoassay using a yeast form of S. schenckii antigen was developed to diagnose sporotrichosis; however, the test showed high sensitivity but low specificity [31]. Similarly, Bernardes-Engemann et al. [13] developed an ELISA using an antigen preparation isolated from the yeast cell wall, called SsCBF, prepared by concanavalin A affinity chromatography. The ELISA test showed 90% sensitivity and 86% specificity. Aiming to improve the time and cost of antigen preparation, Almeida-Paes et al. [11] developed an ELISA for the diagnosis of sporotrichosis using crude extract of the filamentous form of S. schenckii and obtained 97% sensitivity and 89% specificity. The same group also investigated the presence of IgG, IgM, and IgA in patients with sporotrichosis without treatment and during treatment with itraconazole; they concluded that IgM and IgA also participate in the pathogenesis of sporotrichosis [32]. However, all these studies had cross-reactions with other fungal diseases. Fernandes et al. [14] standardized an ELISA to diagnose sporotrichosis in cats using the SsCBF antigen, showing 96% sensitivity and 98% specificity. In the current study, we used crude exoantigens derived from isolate Ss118 to develop our ELISA. Although sporotrichosis is known to be caused by several agents, Rodrigues et al. [33] reported that different Sporothrix species, i.e., S. brasiliensis, S. schenckii, and S. globosa, exhibit convergent antigenic signatures in human [33], feline [5], and murine sporotrichosis [34], supporting the hypothesis that antigenic epitopes may be conserved among closely related agents such as those embedded in the S. schenckii clade. In addition, the antigenic similarities among S. brasiliensis, S. schenckii, and S. globosa probably can be explained by their phylogenetic proximity in the S. schenckii complex [17]. In this study, the isolate Ss118 was selected because it has been previously characterized at the molecular level, and its crude exoantigen was successfully used to diagnose feline sporotrichosis via an ELISA assay [5].
We found high O.D. readings in the lower dilutions of sera from patients with sporotrichosis. Sera from patients with PCM and sera from individuals with sporotrichin-negative skin tests showed cross-reactivity against antigens of S. schenckii (Fig. 1). Similar results were found by Almeida-Paes and collaborators [11] and Scott and Muchmore [31]. Regarding ELISA tests performed with crude antigen preparations, cross-reactions were observed with sera from patients with other mycoses and even other infectious diseases [35–37]. Indeed, carbohydrate epitopes may be responsible for cross-reactions between antigens and antibodies. Antigen treatment with sodium metaperiodate is a strategy used by some researchers to reduce or eliminate non-specific reactions due to carbohydrate epitopes; this technique can lead to a considerable increase in the sensitivity and specificity of the test, as has been observed for diagnosis of PCM [29] and histoplasmosis [30]. However, in the current work, for the diagnosis of sporotrichosis, the strategy of treating the antigen with sodium metaperiodate did not improve the discrimination between sera from patients with sporotrichosis and sera from the other groups (Fig. 2). Even when glycosylated, the carbohydrate residues of the S. schenckii antigens seem to induce anti-IgM antibodies preferably [32]. In turn, Guimarães et al. [30] showed no decrease in the cross-reactivity of sera from patients with histoplasmosis and other fungal infections against glycosylated or non-glycosylated histoplasmin antigens. The authors explained that the immune response could be mainly driven by protein epitopes. Similar to the results shown in Fig. 2, the work of Albuquerque et al. [29] showed that sodium metaperiodate was not able to eliminate all cross-reacting antibodies in an ELISA test specific for PCM diagnosis. On the other hand, Ferreira et al. [20] and Noya et al. [38] showed improvement in an ELISA test using antigens treated with sodium metaperiodate for PCM and schistosomiasis, respectively. The main reason for this discrepancy between our results and those mentioned above could be attributed to the different nature of the antigens used. Similarly, other authors have shown the presence of antibodies that recognize carbohydrate epitopes in the sera of patients with sporotrichosis [39].
A further feature of the human immune response is the avidity of antibody binding, which may be regarded as an estimate of the average affinity of antibodies for a complex antigen [40]. The avidity of antibody-antigen binding varies greatly according to the time of infection and the class of antibodies produced [21]. As described in the literature, low-affinity antibodies may be responsible for cross-reactions observed in several immunoassays, including ELISA, or even for failure in the serodiagnosis [41]. The affinity of antibodies can be evaluated by adding 6 M urea [21, 23], as this solution is able to break low-affinity bonds between a weak antigen-antibody complex and therefore reduce the chemical bounds responsible for non-specific reactions. Evaluation of the ROC curve comparing the sensitivity and specificity of ELISA, and using the cutoff points for each treatment, showed that this strategy was suitable to improve the ELISA parameters for the diagnosis of sporotrichosis. In this working condition, a panel of 20 sera samples from sporotrichosis patients showed 100% sensitivity and specificity (Fig. 4). Therefore, the use of 6 M urea solution proved to be a reliable tool to improve the diagnosis of sporotrichosis through ELISA.
Acknowledgments
We thank Prof. Dr. Mario Leon Silva Vergara from Universidade Federal do Triângulo Mineiro (UFTM) for providing sera and information about the medical status of the patients included in this study. We also thank Dr. Rosane Orofino Costa of Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil for her clinical contribution for the sera collection supported by Faperj/PPSUS project. Also, we thank Dr. Felipe Fornias Sperandio and Dr. Anderson Messias Rodrigues for their critical review of the manuscript and Dr. Jorge Kleber Chavasco in the production of rabbit antibodies against S. schenckii antigens.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/14/2019
In the article mentioned above an author’s name was misspelled. The correct author name reads as follows: Leila Maria Lopes-Bezerra. We apologize for the inconvenience.
References
- 1.Ramos-e-Silva M, Vasconcelos C, Carneiro S, Cestari T. Sporotrichosis. Clin Dermatol. 2007;25(2):181–187. doi: 10.1016/j.clindermatol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Marimon R, Gené J, Cano J, et al. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44:3251–3256. doi: 10.1128/JCM.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vásquez-del-Mercado E, Arenas R, Padilla-Desgarenes C. Sporotrichosis. Clin Dermatol. 2012;30(4):437–443. doi: 10.1016/j.clindermatol.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Schechtman RC. Sporotrichosis: part I. Skinmed. 2010;8(4):216–220. [PubMed] [Google Scholar]
- 5.Rodrigues AM, de Melo TM, de Hoog GS, Schubach TM, Pereira SA, Fernandes GF, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7(6):e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues AM, Fernandes GF, de Camargo ZP. Sporotrichosis. In: Bayry J, editor. Emerging and re-emerging infectious diseases of livestock. Cham: Springer; 2017. [Google Scholar]
- 7.de Albornoz MB, Villanueva E, de Torres ED. Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis. Mycopathologia. 1984;85(3):177–183. doi: 10.1007/BF00440950. [DOI] [PubMed] [Google Scholar]
- 8.Blumer SO, Kaufman L, Kaplan W, McLaughlin DW, Kraft DE. Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl Microbiol. 1973;26(1):4–8. doi: 10.1128/am.26.1.4-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casserone S, Conti-Díaz IA, Zanetta E, Peña de Pereira ME. Serology of cutaneous sporotrichosis. Sabouraudia. 1983;21(4):317–321. doi: 10.1080/00362178385380461. [DOI] [PubMed] [Google Scholar]
- 10.Karlin JV, Nielsen HS., Jr Serologic aspects of sporotrichosis. J Infect Dis. 1970;121(3):316–327. doi: 10.1093/infdis/121.3.316. [DOI] [PubMed] [Google Scholar]
- 11.Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PC, Peralta JM, Nosanchuk JD, et al. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin Vaccine Immunol. 2007;14(3):244–249. doi: 10.1128/CVI.00430-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardes-Engemann AR, Costa RC, Miguens BR, Penha CV, Neves E, Pereira BA, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43(6):487–493. doi: 10.1080/13693780400019909. [DOI] [PubMed] [Google Scholar]
- 13.Bernardes-Engemann AR, Loureiro y Penha CV, Benvenuto F, Braga JU, Barros ML, Orofino-Costa R, et al. A comparative serological study of the SsCBF antigenic fraction isolated from three Sporothrix schenckii strains. Med Mycol. 2009;47(8):874–878. doi: 10.3109/13693780802695520. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes GF, Lopes-Bezerra LM, Bernardes-Engemann AR, Schubach TM, Dias MA, Pereira SA, et al. Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet Microbiol. 2011;147(3–4):445–459. doi: 10.1016/j.vetmic.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes GF, do Amaral CC, Sasaki A, Godoy PM, De Camargo ZP. Heterogeneity of proteins expressed by brazilian Sporothrix schenckii isolates. Med Mycol. 2009;47(8):855–861. doi: 10.3109/13693780802713216. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues AM, de Hoog GS, de Cássia PD, Brihante RS, Sidrim JJ, Gadelha MF, et al. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14(1):219. doi: 10.1186/1471-2334-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Camargo ZP, Unterkircher C, Campoy SP, Travassos LR. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988;26(10):2147–2151. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo ZP, Guesdon JL, Drouhet E, Improvisi L. Enzyme-linked immunosorbent assay (ELISA) in the paracoccidioidomycosis. Comparison with counterimmunoelectrophoresis and erythro-immunoassay. Mycopathologia. 1984;88(1):31–37. doi: 10.1007/BF00439292. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira AP, Côrrea T, Cunha R, Marques MJ, Montesano MA, Souza MA, Teixeira HC. Human serum antibody reactivity towards Paracoccidioides brasiliensis antigens treated with sodium metaperiodate. Rev Soc Bras Med Trop. 2008;41(4):325–329. doi: 10.1590/S0037-86822008000400001. [DOI] [PubMed] [Google Scholar]
- 21.Lappalainen M, Koskela P, Koskiniemi M, Ammälä P, Hiilesmaa V, Teramo K, et al. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167(3):691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 22.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 23.Lappalainen M, Hedman K. Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity. Ann Ist Super Sanita. 2004;40(1):81–88. [PubMed] [Google Scholar]
- 24.Lopes-bezerra LM, Schubach A, Costa RO. Sporothrix schenckii and sporotrichosis. An Acad Bras Cienc. 2006;78(2):293–308. doi: 10.1590/S0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 25.Marimon R, Cano J, Gen J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45(10):3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti Díaz IA. Epidemiology of sporotrichosis in Latin America. Mycopathologia. 1989;108(2):113–116. doi: 10.1007/BF00436061. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues AM, Fernandes GF, Araujo LM, Della Terra PP, dos Santos PO, Pereira SA, et al. Proteomics-based characterization of the humoral immune response in sporotrichosis: Toward discovery of potential diagnostic and vaccine antigens. PLoS Negl Trop Dis. 2015;9(8):e0004016. doi: 10.1371/journal.pntd.0004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardes-Engemann AR, De Lima BM, Zeitune T, Russi DC, Orofino-Costa R, Lopes-Bezerra LM. Validation of a serodiagnostic test for sporotrichosis: a follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med Mycol. 2014;53(1):28–33. doi: 10.1093/mmy/myu058. [DOI] [PubMed] [Google Scholar]
- 29.Albuquerque CF, Marques Silva SH, Camargo ZP. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J Clin Microbiol. 2005;43(4):1944–1946. doi: 10.1128/JCM.43.4.1944-1946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães AJ, Pizzini CV, De Matos Guedes HL, Albuquerque PC, Peralta JM, Hamilton AJ, et al. ELISA for early diagnosis of histoplasmosis. J Med Microbiol. 2004;53(Pt 6):509–514. doi: 10.1099/jmm.0.05469-0. [DOI] [PubMed] [Google Scholar]
- 31.Scott EN, Muchmore HG. Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol. 1989;27(2):300–304. doi: 10.1128/jcm.27.2.300-304.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida-Paes R, Pimenta MA, Monteiro PCF, Nosanchuk JD, Zancopé-Oliveira RM. Immunoglobulins G, M, and A against Sporothrix schenckii exoantigens in patients with sporotrichosis before and during treatment with itraconazole. Clin Vaccine Immunol. 2007;14(9):1149–5117. doi: 10.1128/CVI.00149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues AM, Kubitschek-Barreira PH, Fernandes GF, de Almeida SR, Lopes-Bezerra LM, de Camargo ZP. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J Proteome. 2015;115:8–22. doi: 10.1016/j.jprot.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes GF, dos Santos PO, Rodrigues AM, Sasaki AA, Burger E, de Camargo ZP. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence. 2013;4(3):241–249. doi: 10.4161/viru.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizaki H, Nakamura Y, Wheat RW. Serological cross-reactivity between sporothrix schenckii and various unrelated fungi. Mycopathologia. 1981;73(2):65–68. doi: 10.1007/BF00562591. [DOI] [PubMed] [Google Scholar]
- 36.Ishizaki H, Wheat RW, Kiel DP, Conant NF. Serological cross-reactivity among Sporothrix schenckii, Ceratocystis, Europhium, and Graphium species. Infect Immun. 1978;21(2):585–593. doi: 10.1128/iai.21.2.585-593.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y, Ishizaki H, Wheat RW. Serological cross reactivity between group B Streptococcus and Sporothrix schenckii, Ceratocystis species, and Graphium species. Infect Immun. 1977;16(2):547–549. doi: 10.1128/iai.16.2.547-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noya BA, Colmenares C, Lanz H, Caracciolo MA, Losada S, Noya O. Schistosoma mansoni: immunodiagnosis is improved by sodium metaperiodate which reduces cross-reactivity due to glycosylated epitopes of soluble egg antigen. Exp Parasitol. 2000;95(2):106–112. doi: 10.1006/expr.2000.4515. [DOI] [PubMed] [Google Scholar]
- 39.Penha CV, Bezerra LM. Concanavalin A-binding cell wall antigens of Sporothrix schenckii: a serological study. Med Mycol. 2000;38(1):1–7. doi: 10.1080/mmy.38.1.1.7. [DOI] [PubMed] [Google Scholar]
- 40.Vermont CL, Van Dijken HH, Van Limpt CJP, De Groot R, Van Alphen L, Van den Dobbelsteen GPJM. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect Immun. 2002;70(2):584–590. doi: 10.1128/IAI.70.2.584-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neves AR, Mamoni RL, Rossi CL, de Camargo ZP, Blotta MH. Negative immunodiffusion test results obtained with sera of paracoccidioidomycosis patients may be related to low-avidity immunoglobulin G2 antibodies directed against carbohydrate epitopes. Clin Diagn Lab Immunol. 2003;10(5):802–807. doi: 10.1128/CDLI.10.5.802-807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]



