Abstract
The occurrence of pests and diseases can affect plant health and productivity in ecosystems that are already at risk, such as tropical montane cloud forests. The use of naturally occurring microorganisms is a promising alternative to mitigate forest tree fungal pathogens. The objectives of this study were to isolate rhizobacteria associated with five Lauraceae species from a Mexican tropical montane cloud forest and to evaluate their antifungal activity against Fusarium solani and F. oxysporum. Fifty-six rhizobacterial isolates were assessed for mycelial growth inhibition of Fusarium spp. through dual culture assays. Thirty-three isolates significantly reduced the growth of F. solani, while 21 isolates inhibited that of F. oxysporum. The nine bacterial isolates that inhibited fungal growth by more than 20% were identified through 16S rDNA gene sequence analysis; they belonged to the genera Streptomyces, Arthrobacter, Pseudomonas, and Staphylococcus. The volatile organic compounds (VOC) produced by these nine isolates were evaluated for antifungal activity. Six isolates (Streptomyces sp., Arthrobacter sp., Pseudomonas sp., and Staphylococcus spp.) successfully inhibited F. solani mycelial growth by up to 37% through VOC emission, while only the isolate INECOL-21 (Pseudomonas sp.) inhibited F. oxysporum. This work provides information on the microbiota of Mexican Lauraceae and is one of the few studies identifying forest tree–associated microbes with inhibitory activity against tree pathogens.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00094-2) contains supplementary material, which is available to authorized users.
Keywords: Actinobacteria, Pseudomonas, Persea schiedeana, Rhizosphere, Volatile organic compounds
Introduction
Tropical montane cloud forests, despite their limited distribution, have been acknowledged as one of the world’s most diverse ecosystems [10]. They cover about 2.5% of tropical forest land worldwide and are characterized by the frequent presence of clouds and mist, a high level of species endemism, and the presence of very diverse communities [58]. The importance of tropical montane cloud forests as critical providers of ecosystem services is widely recognized, for example, because of their role in the maintenance of the hydrological cycle [9]. However, this ecosystem is considered as one of the most threatened ecosystems globally [13], climate change, illegal logging, and conversion to pastures being the main causes behind the disturbance of tropical montane cloud forests [11].
Another threat facing these forests is the occurrence of pests and diseases that could affect plant health and productivity, and ultimately forest stability. Examples of such diseases are laurel wilt and Fusarium dieback, both caused by fungi associated with invasive ambrosia beetles. Laurel wilt is a disease affecting the Lauraceae family, caused by the fungus Raffaelea lauricola T.C. Harr., Fraedrich & Aghayeva and vectored by the beetle Xyleborus glabratus Eichhoff [28]. On the other hand, Fusarium dieback is caused by various fungi, including Fusarium euwallaceae Freeman, Mendel, Aoki & O’Donnell and F. kuroshium F. Na, J. D. Carrillo & A. Eskalen, which form a symbiotic relationship with the polyphagous shot hole borer (PSHB) and the Kuroshio shot hole borer (KSHB) (Euwallacea spp. nr. fornicatus) respectively [40, 43]. Fusarium dieback affects a large number of plant species, such as those belonging to the Lauraceae, and is considered to present a real threat to natural forests [45]. Since Lauraceae species are relevant members of the tree community of tropical montane cloud forests [39], it is critical to find strategies to protect them against the attack of fungal pathogens.
Different management strategies have been explored in order to mitigate the negative impacts caused by fungal phytopathogens in forest ecosystems. The application of agrochemicals, in addition to silvicultural management, is a common practice [14]. The use of naturally occurring microorganisms as biological control agents is a promising alternative that could protect tree health and productivity while meeting the increasing demand for environment friendly methods [14]. The rhizosphere is a particularly interesting habitat to isolate microorganisms, since it harbors a large microbial diversity which is critical for plant growth and health [50].
Plant–microbe interactions are mediated by the secretion of different chemical compounds, which may present biological activities such as plant growth promotion or fungal inhibition [3, 5, 41]. Rhizobacteria such as Bacillus spp., Pseudomonas spp., and Actinobacteria have been shown to successfully inhibit the growth of pathogenic fungi Fusarium circinatum Nirenberg & O’Donnell and Monilia perniciosa (Stahel) Aime & Phillips-Mora, oomycete Phytophthora infestans (Mont.) de Bary, or bacteria Ralstonia solanacearum (Smith) Yabuuchi et al. [12, 15, 30, 32, 54], through the emission of antifungal diffusible or volatile compounds. Rhizobacteria have shown to be effective antagonists of diverse Fusarium species. For example, avocado rhizobacteria have shown to successfully inhibit the growth of F. euwallaceae and Fusarium sp., causal agents of Fusarium dieback in Persea americana Mill. [25, 26]. Bacterial strain Streptomyces goshikiensis YCXU, isolated from the rhizosphere of cucumber, significantly reduced the incidence of Fusarium wilt caused by F. oxysporum f. sp. niveum, in watermelon, by producing antifungal compounds and inducing stress resistance in the plant [21]. Other reports demonstrated the potential of native rhizobacteria, mainly from the genera Bacillus and Pseudomonas, as biological control agents of F. solani in turmeric or chili plants [16, 56] and as antagonists of F. oxysporum f. sp. cubense, responsible of Fusarium wilt in banana and watermelon, through the emission of volatile organic compounds (VOC) [61, 65].
Although the plant and animal diversity of tropical montane cloud forests has been widely studied [24], little is known about the diversity of its microorganisms and the biotechnological potential they may represent. Cazorla and Mercado-Blanco [14] recently highlighted the scarcity of studies investigating biological control options for tree diseases. Focusing the search for microbial biological control agents on the microorganisms inhabiting tropical montane cloud forests could tackle two issues: gaining information on the existing microbial diversity in this threatened ecosystem and finding naturally occurring microorganisms with inhibitory activity against tree pathogens. The objectives of the present work were therefore to isolate rhizobacteria associated with five Lauraceae species from a tropical montane cloud forest in Xalapa, Mexico, and to evaluate their antifungal activity through in vitro antagonism assays against the ubiquitous fungal phytopathogens F. solani and F. oxysporum.
Materials and methods
Isolation of rhizobacteria
Five trees from different species of Lauraceae were selected within the Santuario del Bosque de Niebla, a protected area of tropical montane cloud forest located in Xalapa, State of Veracruz, Mexico. Species were identified as Ocotea psychotrioides Kunth, Persea schiedeana Nees, Damburneya salicifolia (Kunth) Trofimov & Rohwer, Persea longipes (Schltdl.) Meisn, and Aiouea effusa (Meisn.) R. Rohde & Rohwer.
Four rhizosphere samples were taken per tree, approximately 50 cm away from the trunk and at a depth of 5–10 cm, where most of the feeder roots could be found, following the method reported in Guevara-Avendaño et al. [25]. Samples from a same tree were mixed to obtain one composite sample of rhizosphere soil per tree. Samples were transported to the laboratory in a cooler and immediately processed upon arrival. Loose soil was removed from the roots by shaking them gently, and the remaining soil, which was strongly adhered to the roots, was considered as rhizosphere soil. Solutions were prepared from 1 g of rhizosphere soil and 99 ml of distilled water and homogenized by shaking vigorously. Dilutions of 1 × 10−4 were then streaked onto Petri dishes with Luria–Bertani agar (LB, Sigma–Aldrich), in duplicate, and plates were incubated at 30 °C for 7 days. Bacterial isolates were taken from the plates as they grew and subcultured in LB until pure cultures were obtained. Bacterial isolates from the same tree were then grouped into morphotypes, based on colonial morphology (form, color, and texture) and cellular morpho-anatomical criteria (shape and Gram-staining results) (Online Resource 1).
In vitro direct antagonism assays against F. solani and F. oxysporum
One bacterial isolate per morphotype (56 morphotypes in total) was randomly selected to be screened for antifungal activity against F. solani and F. oxysporum. Strains of F. solani and F. oxysporum, isolated from chili (Capsicum annum L.) and vanilla (Vanilla planifolia Jacks. ex. Andrew) respectively, were provided by Dr. Mauricio Luna-Rodríguez (Universidad Veracruzana, Mexico). Chili is known to be affected by Fusarium wilt and vanilla by root and stem rot caused by these Fusarium species [1, 2, 56]. The fungal strains were cultured on potato dextrose agar (PDA) with 150 ppm chloramphenicol (Sigma–Aldrich) and incubated at 28 °C, 7 days before dual plating. Bacterial isolates were first re-streaked onto LB and incubated at 30 °C, 48 h prior to the implementation of the dual culture assays.
One plug of 5 mm of diameter was taken from the border of the mycelium of each fungus and placed on the center of a PDA plate. Each one of the 56 bacterial isolates to be tested for antifungal activity was then streaked on two opposite sides of the mycelial plug, at a distance of approximately 2 cm from the plug. A duplicate plate with the same combination (bacterial isolate × fungal species) was established so that two Petri dishes were prepared per bacterial isolate. Petri dishes were then incubated at 30 °C for 7 days.
At day 7, mycelium radial growth was measured from the center of mycelial disc towards the bacterial treatment (r) and the control (R), which corresponded to the maximum growth of the fungus away from the bacteria. Two measurements were taken per plate (for the two bacterial streaks), for a total of four measurements per bacterial treatment. The percentage of mycelial growth inhibition was then calculated with the following formula: % inhibition = [(R − r) ∕ R] × 100.
Molecular identification of bacterial isolates with antifungal activity
Bacterial isolates presenting inhibition percentages higher than 20% for at least one of the tested fungi were identified through 16S rRNA gene sequencing. DNA was extracted from bacterial isolates using the DNeasy® Blood and Tissue kit (Qiagen, Germany), as reported in Méndez-Bravo et al. [41] and following the manufacturer’s instructions. The 16S rRNA region was amplified by PCR using universal primers 27F (5´-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5´-TACGGYTACCTTGTTACGACTT-3′), in 50-μl reactions containing 25 ng of template DNA, 1× of Taq buffer, 200 μM of each dNTP, 1.25 mM of MgCl2, 0.4 μM of each primer, and 0.5 U of Taq DNA polymerase (Qiagen, Germany). Sterile milli-Q water was used as template in controls. Reactions were performed in a SureCycler 8800 (Agilent, CA) under the following conditions: initial denaturation at 95 °C for 4 min; 30 cycles of denaturation at 95 °C for 45 s, annealing at 53 °C for 45 s, and extension at 72 °C for 2 min; and a final extension step at 72 °C for 5 min. Successfully amplified DNA products were purified using QiaQuick® Purification kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Purified DNA amplicons were then sent to Macrogen Inc. for sequencing. Sequences were deposited in GenBank (accession numbers MF767291 to MF767299).
In vitro indirect antagonism assays against F. solani and F. oxysporum
The bacterial isolates that showed inhibition of F. solani or F. oxysporum larger than 20% in the direct antagonism assays were further evaluated to determine their antifungal activity against both pathogenic fungi through the emission of VOC. These bacterial isolates were re-streaked onto LB and incubated for 48 h at 30 °C prior to implementing the antagonism assays. The isolates of F. solani and F. oxysporum were incubated on PDA plates, at room temperature, for 7 days. The indirect antagonism assays were carried out with the two-sealed-baseplates method described in Guevara-Avendaño et al. [26]. Briefly, each bacterial isolate (n = 9) was streaked onto a baseplate containing LB medium. Another baseplate was prepared with a disc of 5-mm diameter of fungal mycelium placed in its center, on PDA medium. Both baseplates were sealed with Parafilm® and incubated at 30 °C, during 7 days. Each bacterial isolate was tested for antifungal activity in triplicate against both F. solani and F. oxysporum. Three assays were set up without bacterial treatments (LB only) and used as controls.
At day 7, mycelium diameter was measured in treatments where mycelium was exposed to the bacterial VOC (d) and in control conditions where mycelium was growing in the absence of bacteria (D). The percentage of mycelial growth inhibition was then calculated with the following formula: % inhibition = [(D − d) ∕ D] × 100.
Data analysis
Data from the direct and indirect antagonism assays were analyzed with the SigmaStat v.4 software. Mycelial growth inhibition produced by each bacterial was contrasted against its respective control with a Student t test or a Mann–Whitney U test according to the normality of the data (n = 4 in direct antagonism assays; n = 3 in indirect antagonism assays). Differences were considered as significant when P ≤ 0.05.
Sequences were manually checked in BioEdit 7.2.5. [27] and aligned with the multiple alignment program MUSCLE in MEGA 7 [35]. The edited sequences and their best matches in GenBank nucleotide database (www.ncbi.nlm.nih.gov) were used to construct the alignment. Subsequently, a Neighbor-Joining tree was constructed in MEGA 7, using a Kimura two-parameter model with Gamma distribution and a bootstrap method with 1000 replicates.
Results
A total of 83 bacterial isolates were obtained from the rhizosphere of the five selected Lauraceae species. Nine bacterial isolates were obtained from O. psychotrioides, seven isolates from that of P. schiedeana, 20 isolates from that of D. salicifolia, 21 from that of P. longipes, and 26 from the rhizosphere of A. effusa. After grouping the isolates into morphotypes, 56 isolates (one representative of each morphotype) were selected to be evaluated in antagonism assays against Fusarium spp. (seven isolates obtained from O. psychotrioides and from P. schiedeana respectively, 13 from D. salicifolia, 12 from P. longipes, and 17 from A. effusa).
The mycelial growth of F. solani was significantly inhibited by 33 of the 56 tested bacterial isolates (Online Resource 2), with inhibition percentages ranging from 6 to 31%. On the other hand, 21 isolates significantly reduced the growth of F. oxysporum, with inhibition percentages ranging from 6 to 20%; 18 of these 21 isolates also presented antifungal activity against F. solani (Online Resource 2).
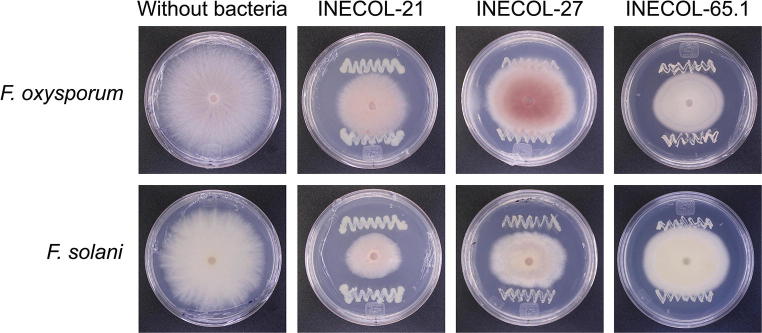
Nine bacterial isolates presented inhibition percentages that were larger than 20%, for at least one of the fungal pathogens (Table 1; Fig. 1). Bacterial isolates INECOL-8, INECOL-11, INECOL-19, INECOL-21, INECOL-27, INECOL-57, INECOL-65.2, and INECOL-100 reduced the growth of F. solani by more than 20%. Five of these isolates also presented significant antagonistic activity against F. oxysporum. However, bacterial isolate INECOL-65.1 was the only isolate to reduce by 20% the growth of F. oxysporum (Figs. 1 and 2). The 16S rDNA sequences from these nine isolates showed that they belonged to the bacterial genera Arthrobacter, Pseudomonas, Staphylococcus, and Streptomyces (Table 2; Fig. 3). Isolates INECOL-21, identified as Pseudomonas sp., and INECOL-27, identified as Arthrobacter sp., exerted the strongest antagonistic activity against F. solani (Fig. 2). Both isolates were obtained from the rhizosphere of P. schiedeana; isolate INECOL-27 was also able to significantly inhibit the mycelial growth of F. oxysporum. Isolate INECOL-65.1, which showed the strongest antagonistic activity against F. oxysporum, was identified as Staphylococcus sp. and was obtained from the rhizosphere of P. longipes.
Table 1.
Mycelial radial growth of F. solani and F. oxysporum confronted with the rhizobacterial isolates that were able to reduce fungal growth by more than 20% in direct antagonism assays
| Bacterial isolate | Mycelial radial growth of F. solani (cm) | Mycelial radial growth of F. oxysporum (cm) | ||
|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |
| INECOL-8 | 2.53 ± 0.40† | 3.15 ± 0.13 | 3.35 ± 0.13 | 3.48 ± 0.21 |
| INECOL-11 | 2.20 ± 0.22* | 2.83 ± 0.24 | 3.28 ± 0.10 | 3.58 ± 0.15 |
| INECOL-19 | 2.10 ± 0.22* | 2.60 ± 0.25 | 3.20 ± 0.16* | 3.50 ± 0.14 |
| INECOL-21 | 2.43 ± 0.10* | 3.35 ± 0.37 | 3.38 ± 0.17 | 3.65 ± 0.17 |
| INECOL-27 | 2.18 ± 0.17† | 3.08 ± 0.15 | 3.20 ± 0.14* | 3.48 ± 0.15 |
| INECOL-57 | 1.83 ± 0.05† | 2.50 ± 0.36 | 3.20 ± 0.18 | 3.45 ± 0.10 |
| INECOL-65.1 | 2.25 ± 0.13* | 2.78 ± 0.10 | 2.00 ± 0.00† | 2.48 ± 0.05 |
| INECOL-65.2 | 2.18 ± 0.05* | 2.73 ± 0.05 | 1.98 ± 0.10* | 2.35 ± 0.13 |
| INECOL-100 | 2.28 ± 0.30* | 3.10 ± 0.22 | 3.05 ± 0.13* | 3.40 ± 0.14 |
Values represent the average of four replicates ± standard error. Italicized values show significant differences between the treatment and its respective control
*P ≤ 0.05, Student’s t test (normal distribution)
†P ≤ 0.05, the Mann–Whitney U test (non-parametric distribution)
Fig. 1.
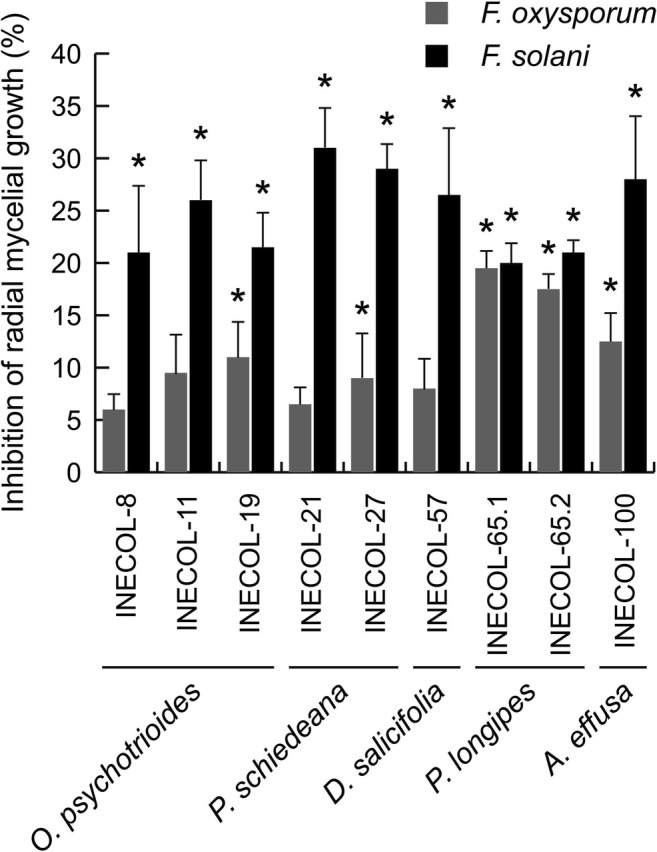
Inhibition percentage of mycelial radial growth of Fusarium solani and F. oxysporum by the nine sequenced bacterial isolates (n = 4 replicates). Bars represent standard errors (s.e.). Asterisk sign represents a significant inhibition of mycelial radial growth in comparison with a control (Student’s t test or the Mann–Whitney U test depending on data distribution, P ≤ 0.05)
Fig. 2.
Dual culture assays to evaluate the antagonism of isolates INECOL-21 (Pseudomonas sp.), INECOL-27 (Arthrobacter sp.), and INECOL-65.1 (Staphylococcus sp.) against F. solani and F. oxysporum. Asterisk sign represents a significant inhibition of mycelial radial growth in comparison with a control. Fungi growing without the presence of bacteria are shown as a reference
Table 2.
Lauraceae rhizobacteria able to reduce the growth of Fusarium solani or F. oxysporum by more than 20% and their closest matches based on the NCBI database “16S ribosomal RNA sequences (bacteria and archaea)”
| Bacterial isolate | GenBank Accession number | NCBI best match | Identity % | |
|---|---|---|---|---|
| Taxonomy | Accession number | |||
| INECOL-8 | MF767291 | Streptomyces cirratus | KU877577.1 | 100 |
| INECOL-11 | MF767292 | Staphylococcus xylosus | KX770743.1 | 100 |
| INECOL-19 | MF767293 | Staphylococcus pasteuri | MF109913.1 | 100 |
| INECOL-21 | MF767294 | Pseudomonas koreensis | MF193902.1 | 100 |
| INECOL-27 | MF767295 | Arthrobacter kerguelensis | HF937010.1 | 100 |
| INECOL-57 | MF767296 | Staphylococcus sp. | KY315825.1 | 100 |
| INECOL-65.1 | MF767297 | Staphylococcus sp. | KY682067.1 | 100 |
| INECOL-65.2 | MF767298 | Staphylococcus pasteuri | MF109913.1 | 100 |
| INECOL-100 | MF767299 | Staphylococcus saprophyticus | KF476046.1 | 99 |
Fig. 3.
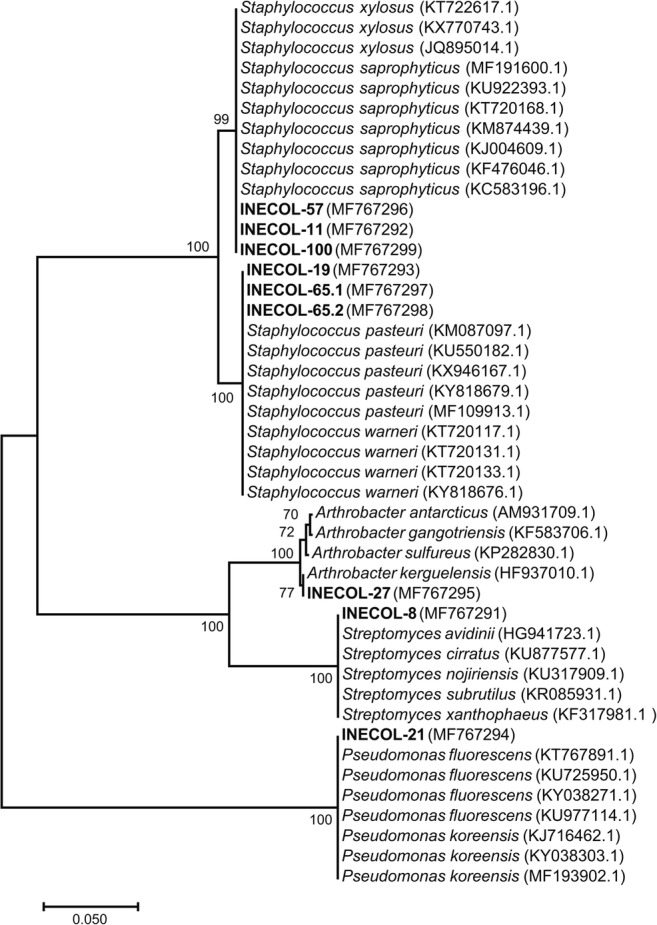
Neighbor-Joining tree of partially sequenced 16S rRNA genes. Bold letters indicate bacterial isolates that were obtained in this study and presented a percentage of inhibition higher than 20% against F. solani or F. oxysporum. Values above nodes correspond to bootstrap values obtained from 1000 replicates
Bacterial VOC produced by six isolates were able to significantly inhibit mycelial growth of F. solani in indirect antagonism assays (Table 3). Bacterial isolates INECOL-8 (Streptomyces sp.). INECOL-11 (Staphylococcus sp.), INECOL-21 (Pseudomonas sp.), INECOL-27 (Arthrobacter sp.), INECOL-57 (Staphylococcus sp.), and INECOL-65.2 (Staphylococcus sp.) reduced F. solani mycelial growth by up to 37%. Isolate INECOL-21 was the only isolate able to inhibit the growth of F. oxysporum through VOC emission. Interestingly, the VOC emitted by four other bacterial isolates, all belonging to the genus Staphylococcus, promoted the growth of F. oxysporum (Table 3).
Table 3.
Mycelial diameter growth of F. solani and F. oxysporum confronted with bacterial VOC in indirect antagonism assays
| Bacterial isolate | Mycelial radial growth of F. solani (cm) | Mycelial radial growth of F. oxysporum (cm) | ||
|---|---|---|---|---|
| Treatment | Control | Treatment | Control | |
| INECOL-8 | 5.77 ± 0.32* | 7.00 ± 0.00 | 8.00 ± 0.00 | 8.00 ± 0.00 |
| INECOL-11 | 4.93 ± 0.23* | 6.20 ± 0.12 | 7.93 ± 0.07 | 8.00 ± 0.00 |
| INECOL-19 | 6.03 ± 0.39 | 6.20 ± 0.12 | 7.53 ± 0.47 | 8.00 ± 0.00 |
| INECOL-21 | 3.90 ± 0.12* | 6.20 ± 0.12 | 7.03 ± 0.77* | 8.00 ± 0.00 |
| INECOL-27 | 5.47 ± 0.12* | 6.67 ± 0.19 | 7.87 ± 0.13 | 8.00 ± 0.00 |
| INECOL-57 | 5.67 ± 0.18* | 6.20 ± 0.12 | 8.00 ± 0.00* | 7.43 ± 0.07 |
| INECOL-65.1 | 6.73 ± 0.28 | 7.00 ± 0.00 | 7.97 ± 0.03* | 7.43 ± 0.07 |
| INECOL-65.2 | 6.40 ± 0.10* | 7.00 ± 0.00 | 8.00 ± 0.00* | 7.43 ± 0.07 |
| INECOL-100 | 6.50 ± 0.50 | 6.20 ± 0.12 | 8.00 ± 0.00* | 7.43 ± 0.07 |
Values represent the average of three replicates ± standard error. Italicized values show significant differences between the treatment and its respective control
*P ≤ 0.05, Student’s t test (normal distribution)
Discussion
The use of rhizobacteria as biocontrol agents against fungal phytopathogens has been increasingly documented [30, 32]. The antifungal compounds that rhizobacteria may produce, and their potential ability to promote plant growth, have made them interesting candidates in the search for more sustainable control methods of fungal diseases [4]. Rhizobacteria belonging to the genera Bacillus [37, 66], Pseudomonas [17], Streptomyces [62], and Paenibacillus [53] have been reported to successfully inhibit the growth of fungi such as Gaeumannomyces graminis (Sacc.) Arx & Olivier, Fusarium verticillioides (Sacc.) Nirenberg, F. solani, and F. oxysporum. More recently, rhizobacteria associated with avocado (a Lauraceae species), all identified as Bacillus spp., were found to exhibit antifungal activities against F. euwallaceae, the causal agent of Fusarium dieback [25]. Another avocado rhizobacterial strain, identified as Bacillus sp. and closely related to B. acidiceler Peak et al., was also reported to reduce the mycelial growth of Phytophthora cinnamomi Rands, through the emission of antifungal diffusible and volatile compounds [41]. While the relevance of rhizobacteria for sustainable management in agricultural settings has been extensively highlighted, few studies have focused on forest tree–associated microbes for biocontrol application in forest management, despite the economic and ecological importance of forest ecosystems [57].
In the present study, we identified nine bacterial isolates with antifungal activity against the widespread fungal pathogens Fusarium spp. These fungi were selected as model organisms, due to the phylogenetic closeness of F. solani to F. euwallaceae [44] and to their frequency on avocado roots [18, 19]. The antagonistic isolates obtained in this study belonged to the bacterial genera Arthrobacter, Pseudomonas, Staphylococcus, and Streptomyces. Surprisingly, unlike the above-mentioned reports of avocado rhizobacteria displaying antifungal activities [25, 41], none of the sequenced isolates belonged to the genus Bacillus.
Most bacterial isolates with antifungal activity that were obtained in this study belonged to the genus Staphylococcus. While Staphylococcus species are mostly known for causing a wide range of human opportunistic diseases, they are also relatively frequent in the soil and in the rhizosphere [7]. Staphylococcus epidermis (Winslow and Winslow) Evans, S. pasteuri Chesneau et al., and S. xylosus Schleifer & Kloos have been isolated from the rhizosphere of potato [8], mangrove [29], and vanilla [2]. In some cases, Staphylococcus strains have been shown to promote plant growth, through the improvement of plant mineral nutrition or the enhancement of plant tolerance to halophilic conditions [31, 46]. The antifungal activity of some Staphylococcus species was also demonstrated, such as that of S. equorum Schliefer et al. against Botrytis cinerea Pers. [55]. The VOC emitted by some Staphylococcus species in our study were able to successfully inhibit the growth of F. solani, but promoted that of F. oxysporum. Fungal growth promotion by VOC produced by S. epidermis had been previously demonstrated for Rhizoctonia solani Kühn and Penicillium waksmanii K.W. Zaleski [34, 60]. As Berg et al. [7] stated, the rhizosphere constitutes a unique, nutrient-rich environment which may select for opportunistic pathogens, as these microorganisms are highly competitive and emit a wide array of antimicrobial substances. However, as these microorganisms are possible human pathogens, it is critical to understand their route of transmission and to assess their potential risk before using them for biotechnological purposes.
Isolate INECOL-21, identified as Pseudomonas sp. and obtained from the rhizosphere of P. schiedeana (a wild relative of P. americana), exhibited the strongest inhibition of F. solani mycelial growth in direct antagonism assays. This isolate also showed the strongest inhibition of F. solani by VOC emission (37%) and was the only one to successfully inhibit F. oxysporum mycelial growth in indirect antagonism assays. Pseudomonas strains have been frequently reported to be plant growth promoters or fungal antagonists [6]. Pseudomonas spp., particularly fluorescent Pseudomonads, have been associated with soil suppressiveness of Fusarium wilt [64], due to their capacity to produce a wide range of antimicrobial compounds such as 2,4-diacetilfloroglucinol (DAPG), phenazines, or pyoluteorin [51]. Pseudomonas species have also been shown to exhibit antifungal activity through the emission of VOC such as aldehydes, alcohols, ketones, and sulfides [22, 26, 48]. Both P. fluorescens Migula and P. koreensis Kwon et al., phylogenetically close relatives of isolate INECOL-21, have been reported to be promising agents for the biological control of Fusarium spp. [36, 52].
Actinobacteria such as Streptomyces spp. or Arthrobacter spp. (isolates INECOL-8 and INECOL-27) are promising candidates for the production of bioactive formulations, due to their capacity to sporulate, to promote plant growth through the production of indole acetic acid, soluble phosphate, and siderophores, and to emit a wide range of antimicrobial compounds [33, 38, 47, 63]. Antifungal compounds produced by Actinobacteria may include volatile molecules such as dimethylpyrazine and dimethylhexadecylamine (activity reported against B. cinerea and Fusarium sp.; [47, 59]), S,S-dipropyl carbonodithioate (activity reported against F. solani and F. oxysporum, among other fungi; [42]), or other diffusible metabolites (activity reported against F. oxysporum; [23]). Streptomyces spp. are also known for their ability to produce fungal cell wall–degrading enzymes such as chitinases, cellulases, and glucanases [20].
This study contributed to gain information about the bacterial diversity associated with Lauraceae species in a tropical montane cloud forest and allowed us to identify several bacterial strains with antagonistic activity against ubiquitous fungal pathogens. The bacterial isolates that successfully inhibited the growth of Fusarium spp., either through the production of diffusible compounds or the emission of VOC, should thus be considered in further evaluations to assess their antagonistic activity in vivo in greenhouse bioassays, in order to confirm their potential application as biocontrol agents. These future studies should focus on those isolates that are not reported as opportunistic pathogens and elucidate whether the selected bacterial isolates also display plant growth–promoting abilities. Combining multiple biocontrol mechanisms such as the production of antibiotics, siderophores, or lytic enzymes, with plant growth promotion through the production of phytohormones or the induction of the plant defense system, has been shown to be critical for rhizobacteria to successfully compete their fungal antagonist [16, 49]. The use in field conditions of rhizobacteria with antifungal activity and plant growth–promoting capacity is therefore a promising approach for the control of Fusarium phytopathogens. The utilization of microorganisms as biological control agents against forest tree diseases is still scarce [14] and little is known about the mechanisms through which fungal inhibition by bacterial biocontrol agents could occur in field conditions. It is therefore necessary to focus future research efforts on the interactions between trees and their associated microbiota, to be able to design new strategies to improve forest tree health in an environmentally friendly manner.
Electronic supplementary material
(DOCX 18 kb)
(DOCX 19 kb)
Acknowledgments
The authors wish to thank Nora Osorio and Julio César García for their help with bacterial isolation and morphotyping and Luis Tlaxcalteco for his assistance with the identification of Lauraceae species. We are also grateful to Alfonso Méndez for his help with image edition.
Funding
This study was funded by the Instituto de Ecología, A.C. as a part of Project no. 2003530890: “Estudio integral 2013-2037 de la Biodiversidad del Jardín Botánico Francisco Javier Clavijero con énfasis en el Santuario del Bosque de Niebla.”
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
N/A
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adame-García J, Rodríguez-Guerra R, Iglesias-Andreu LG, Ramos-Prado JM, Luna-Rodríguez M. Molecular identification and pathogenic variation of Fusarium species isolated from Vanilla planifolia in Papantla Mexico. Bot Sci. 2015;93:669–678. doi: 10.17129/botsci.142. [DOI] [Google Scholar]
- 2.Adame-García J, Luna-Rodríguez M, Iglesias-Andreu LG. Vanilla rhizobacteria as antagonists against Fusarium oxysporum f. sp. vanillae. Int J Agricult Biol. 2016;18:23–30. doi: 10.17957/IJAB/15.0053. [DOI] [Google Scholar]
- 3.Asari S, Matzén S, Petersen MA, Bejai S, Meijer J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol Ecol. 2016;92:fiw070. doi: 10.1093/femsec/fiw070. [DOI] [PubMed] [Google Scholar]
- 4.Beneduzi A, Ambrosini A, Passaglia LM. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol. 2012;35:1044–1051. doi: 10.1590/S1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84:11–18. doi: 10.1007/s00253-009-2092-7. [DOI] [PubMed] [Google Scholar]
- 7.Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol. 2005;7:1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 8.Berg G, Krechel A, Ditz M, Sikora RA, Ulrich A, Hallmann J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol. 2005;51:215–229. doi: 10.1016/j.femsec.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Bruijnzeel LA, Kappelle M, Mulligan M, Scatena FN. Tropical montane cloud forests: state of knowledge and sustainability perspectives in a changing world. In: Bruijnzeel LA, Scatena FN, Hamilton LS, editors. Tropical montane cloud forests. Science for conservation and management. UK: Cambridge University Press; 2010. pp. 691–740. [Google Scholar]
- 10.Bubb P, May I, Miles L, Sayer J. Cloud forest agenda. Cambridge: UNEP-WCMC; 2004. [Google Scholar]
- 11.Calderon-Aguilera LE, Rivera-Monroy VH, Porter-Bolland L, Martínez-Yrízar A, Ladah LB, Martínez-Ramos M, Alcocer J, Santiago-Pérez AL, Hernández-Arana HA, Reyes-Gómez VM, Pérez-Salicrup DR, Díaz-Nuñez V, Sosa-Ramírez J, Herrera-Silveira J, Búrquez A. An assessment of natural and human disturbance effects on Mexican ecosystems: current trends and research gaps. Biodivers Conserv. 2012;21:589–617. doi: 10.1007/s10531-011-0218-6. [DOI] [Google Scholar]
- 12.Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol. 2015;8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayuela L, Golicher DJ, Benayas JMR, González-Espinosa M, Ramírez-Marcial N. Fragmentation, disturbance and tree diversity conservation in tropical montane forests. J Appl Ecol. 2006;43:1172–1181. doi: 10.1111/j.1365-2664.2006.01217.x. [DOI] [Google Scholar]
- 14.Cazorla FM, Mercado-Blanco J. Biological control of tree and woody plant diseases: an impossible task? BioControl. 2016;61:233–242. doi: 10.1007/s10526-016-9737-0. [DOI] [Google Scholar]
- 15.Chaves-López C, Serio A, Gianotti A, Sacchetti G, Ndagijimana M, Ciccarone C, Stellarini A, Corsetti A, Paparella A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J Appl Microbiol. 2015;119:487–499. doi: 10.1111/jam.12847. [DOI] [PubMed] [Google Scholar]
- 16.Chenniappan C, Narayanasamy M, Daniel GM, Ramaraj GB, Ponnusamy P, Sekar J, Ramalingam PV. Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol Control. 2019;129:55–64. doi: 10.1016/j.biocontrol.2018.07.002. [DOI] [Google Scholar]
- 17.Chin-A-Woeng TF, Bloemberg GV, Lugtenberg BJ. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003;157:503–523. doi: 10.1046/j.1469-8137.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 18.Darvas JM (1979) Ecology of avocado root pathogens. South African Avocado Growers’ Association Research Report 3:31–32
- 19.Darvas JM, Kotze JM (1987) Avocado fruit diseases and their control in South Africa. South African Avocado Growers’ Association Yearbook 10:117–119
- 20.Debnath R, Saikia R, Sarma RK, Yadav A, Bora TC, Handique PJ. Psychrotolerant antifungal Streptomyces isolated from Tawang, India and the shift in chitinase gene family. Extremophiles. 2013;17:1045–1059. doi: 10.1007/s00792-013-0587-8. [DOI] [PubMed] [Google Scholar]
- 21.Faheem M, Raza W, Zhong W, Nan Z, Shen Q, Xu Y. Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum f. sp. niveum. Biol Control. 2015;81:101–110. doi: 10.1016/j.biocontrol.2014.11.012. [DOI] [Google Scholar]
- 22.Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem. 2005;37:955–964. doi: 10.1016/j.soilbio.2004.10.021. [DOI] [Google Scholar]
- 23.Getha K, Vikineswary S. Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f. sp. cubense race 4: indirect evidence for the role of antibiosis in the antagonistic process. J Industr Microbiol Biotechnol. 2002;28:303–310. doi: 10.1038/sj.jim.7000247. [DOI] [PubMed] [Google Scholar]
- 24.Gual-Díaz M, Rendón-Correa A (2014) Bosques Mesófilos de Montaña en México: diversidad, ecología y manejo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México. 352 pp.
- 25.Guevara-Avendaño E, Carrillo JD, Ndinga-Muniania C, Moreno K, Méndez-Bravo A, Guerrero-Analco JA, Eskalen A, Reverchon F. Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi. Antonie Van Leeuwenhoek. 2018;111:563–572. doi: 10.1007/s10482-017-0977-5. [DOI] [PubMed] [Google Scholar]
- 26.Guevara-Avendaño E, Bejarano-Bolívar AA, Kiel-Martínez AL, Ramírez-Vázquez M, Méndez-Bravo A, Aguirre von Wobeser E, Sánchez-Rangel D, Guerrero-Analco JA, Eskalen A, Reverchon F. Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol Res. 2019;219:74–83. doi: 10.1016/j.micres.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Hall TA. BioEdit: a friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 28.Harrington TC, Fraedrich SW, Aghayeva DN. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon. 2008;104:399–404. [Google Scholar]
- 29.Holguin G, Bashan Y. Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.) Soil Biol Biochem. 1996;28:1651–1660. doi: 10.1016/S0038-0717(96)00251-9. [DOI] [Google Scholar]
- 30.Hunziker L, Bönisch D, Groenhagen U, Bailly A, Schulz S, Weisskopf L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl Environ Microbiol. 2015;81:821–830. doi: 10.1128/AEM.02999-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ipek M, Pirlak L, Esitken A, Donmez MF, Turan M, Sahin F. Plant growth-promoting rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high calcareous soil conditions. J Plant Nutr. 2011;37:990–1001. doi: 10.1080/01904167.2014.881857. [DOI] [Google Scholar]
- 32.Iturritxa E, Trask T, Mesanza N, Raposo R, Elvira-Recuenco M, Patten CL. Biocontrol of Fusarium circinatum infection of young Pinus radiata trees. Forests. 2017;8:32. doi: 10.3390/f8020032. [DOI] [Google Scholar]
- 33.Jog R, Pandya M, Nareshkumar G, Rajkumar S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology. 2014;160:778–788. doi: 10.1099/mic.0.074146-0. [DOI] [PubMed] [Google Scholar]
- 34.Kai M, Vespermann A, Piechulla B. The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav. 2008;3:482–484. doi: 10.4161/psb.3.7.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:msw054–ms1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leeman M, van Pelt JA, den Ouden FM, Heinsbroek M, Bakker PAHM, Schippers B. Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology. 1995;85:1021–1027. doi: 10.1094/Phyto-85-1021. [DOI] [Google Scholar]
- 37.Li B, Li Q, Xu Z, Zhang N, Shen Q, Zhang R (2014) Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front Microbiol 5(636). 10.3389/fmicb.2014.00636 [DOI] [PMC free article] [PubMed]
- 38.Li M, Guo R, Yu F, Chen X, Zhao H, Li H, Wu J. Indole-3-acetic acid biosynthesis pathways in the plant-beneficial bacterium Arthrobacter pascens ZZ21. Int J Mol Sci. 2018;19:443. doi: 10.3390/ijms19020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorea-Hernández FG. La familia Lauraceae en el sur de México: diversidad, distribución y estado de conservación. Bol Soc Bot Méx. 2002;71:59–70. [Google Scholar]
- 40.Lynch SC, Twizeyimana M, Mayorquin JS, Wang DH, Na F, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A. Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.—two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia. 2016;108:313–329. doi: 10.3852/15-063. [DOI] [PubMed] [Google Scholar]
- 41.Méndez-Bravo A, Cortazar-Murillo EM, Guevara-Avendaño E, Ceballos-Luna O, Rodríguez-Haas B, Kiel-Martínez AL, Hernández-Cristóbal O, Guerrero-Analco JA, Reverchon F. Plant growth-promoting rhizobacteria associated with avocado display antagonistic activity against Phytophthora cinnamomi through volatile emissions. PLoS One. 2018;13(3):e0194665. doi: 10.1371/journal.pone.0194665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munaganti RK, Muvva V, Konda S, Naragani K, Mangamuri UK, Dorigondla KR, Akkewar D. Antimicrobial profile of Arthrobacter kerguelensis VL-RK_09 isolated from mango orchards. Braz J Microbiol. 2016;47:1030–1038. doi: 10.1016/j.bjm.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Na F, Carrillo JD, Mayorquin JS, Ndinga-Muniania C, Stajich JE, Stouthamer R, Huang YT, Lin YT, Chen CY, Eskalen A. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis. 2018;102:1154–1164. doi: 10.1094/PDIS-07-17-1042-RE. [DOI] [PubMed] [Google Scholar]
- 44.O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Na F, Geiser DM, Bateman C, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP. Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genet Biol. 2015;82:277–290. doi: 10.1016/j.fgb.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell K, Libeskind-Hadas R, Hulcr J, Bateman C, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE, Liyanage PNH, Eskalen A, Lynch SC, Geiser DM, Freeman S, Mendel Z, Sharon M, Aoki T, Cossé AA, Rooney AP. Invasive Asian Fusarium—Euwallacea ambrosia beetle mutualists pose a serious threat to forests, urban landscapes and the avocado industry. Phytoparasitica. 2016;44:435–442. doi: 10.1007/s12600-016-0543-0. [DOI] [Google Scholar]
- 46.Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum) Braz J Microbiol. 2016;47:621–627. doi: 10.1016/j.bjm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orozco-Mosqueda M, Valencia-Cantero E, López-Albarrán P, Martínez-Pacheco M, Velázquez-Becerra C. La bacteria Arthrobacter agilis UMCV2 y diversas aminas inhiben el crecimiento in vitro de hongos destructores de madera. Rev Argent Microbiol. 2015;47:219–228. doi: 10.1016/j.ram.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Ossowicki A, Jafra S, Garbeva P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS One. 2017;12:1–13. doi: 10.1371/journal.pone.0174362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passari AK, Mishra VK, Leo VV, Gupta VK, Singh BP. Phytohormone production endowed with antagonistic potential and plant growth promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol Res. 2016;193:57–73. doi: 10.1016/j.micres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 51.Raaijmakers JM, Mazzola M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol. 2012;50:403–424. doi: 10.1146/annurev-phyto-081211-172908. [DOI] [PubMed] [Google Scholar]
- 52.Rafikova GF, Korshunova TY, Minnebaev LF, Chetverikov SP, Loginov ON. A new bacterial strain, Pseudomonas koreensis IB-4, as a promising agent for plant pathogen biological control. Microbiology. 2016;85:333–341. doi: 10.1134/S0026261716030115. [DOI] [Google Scholar]
- 53.Raza W, Yuan J, Ling N, Huang Q, Shen Q. Production of volatile organic compounds by an antagonistic strain Paenibacillus polymyxa WR-2 in the presence of root exudates and organic fertilizer and their antifungal activity against Fusarium oxysporum f. sp. niveum. Biol Control. 2015;80:89–95. doi: 10.1016/j.biocontrol.2014.09.004. [DOI] [Google Scholar]
- 54.Raza W, Ling N, Liu D, Wei Z, Huang Q, Shen Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol Res. 2016;192:103–113. doi: 10.1016/j.micres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Sadfi-Zouaoui N, Essghaier B, Hajlaoui MR, Fardeau ML, Cayaol JL, Ollivier B, Boudabous A. Ability of moderately halophilic bacteria to control grey mould disease on tomato fruits. J Phytopathol. 2008;156:42–52. doi: 10.1111/j.1439-0434.2007.01329.x. [DOI] [Google Scholar]
- 56.Sundaramoorthy S, Raguchander T, Ragupathi N, Samiyappan R. Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol Control. 2012;60:59–67. doi: 10.1016/j.biocontrol.2011.10.002. [DOI] [Google Scholar]
- 57.Terhonen E, Kovalchuk A, Zarsav A, Asiegbu FO. Biocontrol potential of forest tree endophytes. In: Pirttilä A, Frank A, editors. Endophytes of forest trees. Cham: Springer; 2018. pp. 283–318. [Google Scholar]
- 58.Toledo-Aceves T, Meave JA, González-Espinosa M, Ramírez-Marcial N. Tropical montane cloud forests: current threats and opportunities for their conservation and sustainable management in Mexico. J Environ Manag. 2011;92:974–981. doi: 10.1016/j.jenvman.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, Flores-Cortez I, Santoyo G, Hernández-Soberano C, Valencia-Cantero E. The rhizobacterium Arthrobacter agilis produces dimethylhexadecylamine, a compound that inhibits growth of phytopathogenic fungi in vitro. Protoplasma. 2013;250:1251–1262. doi: 10.1007/s00709-013-0506-y. [DOI] [PubMed] [Google Scholar]
- 60.Vespermann A, Kai M, Piechulla B. Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol. 2007;73:5639–5641. doi: 10.1128/AEM.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B, Yuan J, Zhang J, Shen Z, Zhang M, Li R, Ruan Y, Shen Q. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of fusarium wilt of banana. Biol Fert Soils. 2013;49(4):435–446. doi: 10.1007/s00374-012-0739-5. [DOI] [Google Scholar]
- 62.Wang C, Wang Z, Qiao X, Li Z, Li F, Chen M, Cui H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol Lett. 2013;341:45–51. doi: 10.1111/1574-6968.12088. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Zhang M, Gao J, Pu T, Bilal M, Wang Y, Zhang X. Antifungal activity screening of soil actinobacteria isolated from Inner Mongolia, China. Biol Control. 2018;127:78–84. doi: 10.1016/j.biocontrol.2018.07.007. [DOI] [Google Scholar]
- 64.Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, Zhou J, Li C, Ma Y (2019) Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen e813. DOI 10.1002/mbo3.813 [DOI] [PMC free article] [PubMed]
- 66.Zhang D, Gao T, Li H, Lei B, Zhu B. Identification of antifungal substances secreted by Bacillus subtilis Z-14 that suppress Gaeumannomyces graminis var. tritici. Biocontrol Sci Tech. 2017;27:237–251. doi: 10.1080/09583157.2016.1275522. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
(DOCX 19 kb)