Abstract
Antibacterial activity of cell-free supernatant from Escherichia coli E against selected pathogenic bacteria in food and aquaculture was the highest against Edwardsiella tarda 3, a significant aquaculture pathogen. Biochemical properties of the bacteriocins were studied and bacteriocin was found to be sensitive to proteinase K, demonstrating its proteinaceous nature. In addition, pH and temperature affected bacteriocin activity and stability. The bacteriocins were partially purified by ammonium sulfate precipitation. The antibacterial activity was only detected in 20% ammonium sulfate fraction and direct detection of its activity was performed by overlaying on the indicator strains. The inhibition zone associated with the antibacterial activity was detected in the sample overlaid by E. tarda 3 and Staphylococcus aureus DMST8840 with the relative molecular mass of about 27 kDa and 10 kDa, respectively. Bacteriocin showed no cytotoxic effect on NIH-3T3 cell line; however, two virulence genes, aer and sfa, were detected in the genome of E. coli E by PCR. The characteristics of bacteriocins produced by E. coli E exhibited the antibacterial activity against both Gram-positive and Gram-negative pathogenic bacteria and the safe use determined by cytotoxicity test which may have interesting biotechnological applications.
Electronic supplementary material
The online version of this article (10.1007/s42770-018-0014-5) contains supplementary material, which is available to authorized users.
Keywords: Bacteriocin, Cytotoxicity assay, Escherichia coli E, Safety evaluation, Virulence genes
Introduction
Despite the growth of antibiotic-resistant bacteria, the development of new and effective antibiotic is steeply declining [1]. The discovery and development of novel antibacterial agents have therefore been implemented by many research groups to develop alternatives to antibiotic treatment [2–4]. New methods are currently under consideration including the development of bacteriocins, which are antibacterial peptides [2, 5, 6]. Bacteriocins are ribosomally synthesized by bacteria that inhibit or kill closely related species [7, 8]. Bacteriocins differ from classical antibiotics in term of synthesis and spectrum of killing activity, and some can be considered pathogen-specific designer drugs [5, 6]. The high level of diversity of bacteriocins produced by Gram-positive and Gram-negative bacteria has attracted great interest because of their potential applications in food preservation, human health, livestock rearing, agriculture, and aquaculture [6, 9–11].
Aquaculture is the fastest-growing food production industry. However, intensive culture has led to outbreaks of various bacterial diseases [12]. Bacterial species belonging to at least 13 genera have been reported to be pathogenic to aquatic animals including Gram-negative bacteria such as Aeromonas, Edwardsiella, Flavobacterium, Francisella, Photobacterium, Piscirickettsia, Pseudomonas, Tenacibaculum, Vibrio, and Yersinia and Gram-positive bacteria such as Lactococcus, Renibacterium, and Streptococcus [12, 13]. Edwardsiella tarda is an intracellular pathogen accounting for enormous economic losses in cultured seawater and freshwater fish [14, 15]. To control bacterial diseases, vaccines and antibiotics have been used [6, 12]. However, these methods can result in high cost, high stress to the animals, the long half-life of drugs, and selection for antibiotic-resistant bacteria [6, 9, 15]. Therefore, the use of bacteriocin-producing probiotic bacteria is an alternative approach to disease prevention in aquaculture [6, 9, 13]. In addition to pathogenic bacteria in aquaculture, food contamination by pathogenic bacteria has been a serious health problem. Salmonella enterica, Escherichia coli O157:H7, Shigella spp., and Staphylococcus aureus are among the major causes of outbreaks of foodborne diseases [16, 17]. Many studies carried out in recent years clearly demonstrate that the application of bacteriocins in food preservation can decrease the risks for transmission of foodborne pathogens through the food chain, extend shelf life, and reduce the application of chemical preservation [18–20].
Escherichia coli is known to produce two types of bacteriocins: colicins and microcins [3, 6, 21]. Bacteriocins produced by nonpathogenic E. coli strains are a potential candidate for inhibition of pathogens such as Salmonella [22]. The study showed that the majority of bacteriocin-producing E. coli strains produce more than one bacteriocin [21]. Colicins are diverse and they are the most extensively studied bacteriocins in E. coli [6]. The colicins of E. coli have served as a model system for investigating the mechanisms of bacteriocin structure and function, genetic organization, ecology, and evolution [6, 8, 23]. They can kill by either membrane permeabilization, nuclease activity, or inhibition of peptidoglycan and lipopolysaccharide biosynthesis [7, 24, 25]. However, colicin is not active against the producing bacteria due to the presence of a specific inhibitor called the immunity protein [7]. Microcins are bacteriocins produced by enterobacteria with molecular masses below 10 kDa [4, 26, 27]. Microcins exhibit a diversity of structures and mechanisms of action such as the inhibition of vital enzymatic functions and damage to the inner membrane [26].
A few studies have reported the cytotoxicity and virulence factors of bacteriocins [5, 28, 29]. Virulence factors have played on important role in the pathogenicity of E. coli strains associated with urinary tract infection by overcoming host defense mechanisms and causing disease [30]. Several genes encoding virulence factors such as hemolysin (hly), cytotoxic necrotizing factor I (cnf I), S-family adhesions (sfa), pyelonephritis-associated pili (pap), and aerobactin (aer) have been detected by PCR and shown to have higher prevalence of the presence of virulence genes in uropathogenic E. coli strains [31, 32]. Therefore, for a safe application of using bacteriocin as an antibacterial agent in aquaculture and food industry, several genetic determinants of virulence factors should be of concern. Thus, the aim of this study was to investigate a new antibacterial agent from bacteriocin-producing bacteria isolated from fermented fruit. E. coli E was previously isolated from fermented pineapple, Ananas comosus and the antibacterial activity against pathogenic bacteria in aquaculture was determined [33]. Due to its potential application as an antibacterial agent, the safety of bacteriocins was evaluated. In addition, virulence factors which play an important role in the pathogenicity of E. coli E were also investigated.
Materials and methods
Bacterial strains and culture conditions
The E. coli E strain was isolated as described previously [33]. Selected pathogenic bacteria in food and aquaculture including Bacillus cereus, Escherichia coli, Streptococcus sp., Salmonella sp., Staphylococcus aureus DMST8840, Shigella sp., Pseudomonas aeruginosa 5, Vibrio cholera 7, Vibrio paraheamolyticus 5, Vibrio alginolyticus 3, Aeromonas hydrophila 9, and Edwardsiella tarda 3 were laboratory strains/isolate which obtained from the culture collection of the Microbiology Laboratory, Faculty of Science and Technology, Prince of Songkla University and Faculty of Agro Based Industry, Universiti Malaysia Kelantan Campus Jeli. Bacteriocin producer E. coli E were cultured in a Luria-Bertani (LB) medium with shaking at 37 °C and maintained in 20% (v/v) glycerol. All pathogenic bacteria were cultured in nutrient broth (NB) in a 37 °C shaker. All Vibrio strains were grown on NB supplemented with 3% NaCl.
Bacteriocin detection and production
The pre-culture of E. coli E strain was inoculated into 250 ml of sterilized LB. Cell culture was then adjusted to the same initial optical density (OD600nm = 0.05) and incubated in a 37 °C shaker at 180 rpm for 6 h. The obtained culture was centrifuged in order to get the cell-free supernatant (CFS).
The activity of bacteriocin expressed in arbitrary units (AU ml−1) was determined through a twofold broth dilution method against E. tarda 3. One AU is defined as the highest serial twofold dilution which prevents the visible turbidity of an indicator strain in a well per 1 ml after 24 h incubation [34].
Antibacterial activity of E. coli E against all pathogenic microorganisms was determined by a well diffusion method [35]. The pathogenic bacteria were inoculated into 25 ml sterilized NA medium (with final concentration 106 CFU ml−1). Wells with the diameter of 5 mm were then filled up with 90 μl of CFS. The bacteriocin activity was determined by measuring the diameter of the inhibition zone around the wells after incubation for 12–36 h at room temperature (28–30 °C) depending on the growth of the pathogenic strains. At least two independent experiments were done for each tested pathogenic bacterium.
Sensitivity to heat, pH, and enzymes
The thermal sensitivity of bacteriocin was tested by incubation of 1 ml CFS at 45 °C, 70 °C, and 90 °C for 15, 30, 60, and 120 min and 121 °C for 20 min then stored immediately on ice followed by a subsequent antibacterial assay. A positive control (no heat treatment at 30 °C) and a negative control (culture medium) were included.
To determine the effect of pH on antibacterial activity, CFS was adjusted to different pH values [2–12] using 1 M HCl and 1 M NaOH and then incubated at room temperature for 30 and 120 min. The agar diffusion method was performed to measure the residual activity after neutralizing treated samples to pH 7.0 [36].
The sensitivity to enzymatic proteolysis was evaluated by treatment of CFS with the final concentration of 1 mg ml−1 of the following proteolytic enzymes: proteinase K (Vivantis, Malaysia) in Tris buffer pH 8.0, trypsin (Sigma, USA) in sodium phosphate buffer (pH 7.0), catalase (Sigma, USA) in phosphate buffer pH 7.0 (potassium phosphate buffer). After treatment, the enzymes were heat inactivated at 100 °C for 3 min. CFS without enzymes and culture medium were used as a positive control and negative control, respectively. The residual activity was then determined by using the well diffusion method against the most sensitive pathogenic bacteria in food and aquaculture including Shigella sp. and E. tarda 3.
Acid and bile tolerance
Acid and bile tolerance was determined using the modified methods according to previously described [37]. For acid tolerance test, 1 ml of the culture was centrifuged at 1677g when the OD600nm reached 1.0. The cell pellets were then washed with 1× PBS (phosphate buffered saline) twice and resuspended in 1 ml of the same buffer. One hundred microliters of cell suspensions was mixed into 900 μl of 1× PBS with the pH values of 1, 2, 3, and 4 (adjusted using 1 mol l−1 HCl). The mixtures were incubated at 37 °C for 0, 1, 2, and 3 h. The serial dilutions were made with 0.9% NaCl and plated onto NA media by the spread plate method. The plates were incubated at 37 °C for 24 h. The acid tolerance of E. coli E was expressed by the number of survival cells.
For bile tolerance test, the culture was collected when the growth of E. coli E reached OD600nm 1.0. One hundred microliters of the culture was spread onto NA agar with bile salt concentrations of 0.1%, 0.2% 0.3%, and 0.6%. The growth of E. coli E was detected using the standard agar plate method after incubation at 37 °C for 24 h.
Partial purification
CFS obtained from 1 L of culture was precipitated with ammonium sulfate at different concentrations (20%, 30%, and 50%) at 4 °C for 24 h using the modified method as previously described [33]. The pellet was collected by centrifugation at 20,130g for 20 min and dissolved with 25 mM Tris-HCl (pH 8) and then dialyzed against the same buffer for 12 h at 4 °C. The partially purified products were concentrated using freeze dryer and lyophilized products were stored in − 20 °C. Each resuspended fraction was filtered with filter membrane (0.45 μm) and screened for its antibacterial activity using the well diffusion method against two indicator strains including Shigella sp. and E. tarda 3. The fraction which exhibited inhibition activity was collected for molecular weight determination.
Direct activity and molecular weight determination
The partially purified bacteriocin was detected through 18% separating gel and 5% stacking gel with 30% acrylamide and 0.8% bis-acrylamide under denaturing and non-denaturing conditions [36]. Duplicate bacteriocin samples and a protein ladder (10–250 kDa, New England BioLabs) were loaded onto a polyacrylamide gel. After electrophoresis, the gel was cut into two parts: the first part containing one of the bacteriocin samples and standard protein was stained with Coomassie brilliant blue G-250 (Sigma-Aldrich, Steinheim, Germany) to determine molecular weight. The second part containing other bacteriocin samples was assayed for direct detection of activity. This part was washed repeatedly for 24 h with distilled water and placed on a pre-poured TSA plate which was then overlaid with 5 ml melted TSA containing indicator strains including Shigella sp., E. tarda 3, and S. aureus DMST8840 [28]. The plate was then incubated at room temperature for 24 h and observed for the clear zone around bands. Protein concentration was determined by the Bradford protein assay (Bio-Rad) using BSA as the standard.
Cell culture
Mouse embryonic fibroblast NIH-3T3 cells (ATCC®CRL1658™) were obtained from the Faculty of Medicine, Khon Kaen University. The cells were cultured in Dulbecco’s minimal Eagle medium (DMEM, Invitrogen, CA, USA) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin, and 100 U ml−1 streptomycin (Invitrogen). Cells were cultured in a humidified 5% CO2 incubator at 37 °C. Cells were subcultured with 0.25% trypsin-EDTA when reaching 90% confluence. Cells with more than 90% viability were used in all experiments.
Cytotoxicity assay
To determine the effect of CFS on NIH-3T3 viability, a cytotoxicity assay was performed using thiazolyl blue tetrazolium bromide (MTT, Sigma) according to previously described [38]. Cells were seeded in 96-well plate at 5 × 105 cells per well in the complete media and incubated for 24 h. The media were replaced by CFS in various dilutions (1/8, 1/16, 1/32, 1/64, and 1/128) and incubated for 72 h. DMEM containing 6.25 mg ml−1 nisin (Sigma) was used as a positive control. All conditions were added with fresh media to 150 μl per well prior to add with 2 mg ml−1 of MTT at 50 μl per well and incubated for 4 h. The supernatants were removed and formazan crystals were dissolved by DMSO. The amount of formazan crystal was measured at 570 nm by a microplate reader (Tecan, Switzerland) and converted to a percentage of viable cells compared to control. All samples were used in three independent experiments.
DNA extraction and PCR amplification of virulence genes
Genomic DNA from E. coli E was isolated with DNAzol® reagent (Invitrogen, USA) as recommended by the manufacturer. The extracted DNA was analyzed with 1% (w/v) agarose gel electrophoresis. Detection of hly, aer, cnf I, sfa, and pap genes was performed by amplifying the genes by PCR. The primers sequences were previously reported in the literatures [31, 32] (Table 1). Primers for hly, aer, cnf I, sfa, and pap amplified DNA fragments of 1177, 602, 498, 410, and 328 bp, respectively. Expected sizes of the amplicons were ascertained by electrophoresis in 1% agarose gel with an appropriate molecular size marker (GeneRuler™ 1 kb, Fermentas). PCR products were visualized under UV after ethidium bromide staining.
Table 1.
Primer pairs used for the detection of virulence genes in E. coli E
| Gene and DNA region amplified | Primer | Primer sequence (5′–3′) | References |
|---|---|---|---|
| hly | F | AACAAGGATAAGCACTGTTCTGGCT | [31, 32] |
| R | ACCATATAAGCGGTCATTCCCGTCA | ||
| cnf I | F | AAGATGGAGTTTCCTATGCAGGAG | |
| R | CATTCAGAGTCCTGCCCTCATTAT | ||
| sfa | F | CTCCGGAGAACTGGGTGCATCTTAC | |
| R | CATCAAGCTGTTTGTTCGTCCGCCG | ||
| pap | F | GACGGCTGTACTGCAGGGTGTGGCG | |
| R | ATATCCTTTCTGCAGGGATGCAATA | ||
| aer | F | TACCGGATTGTCATATGCAGACCG | [31] |
| R | AATATCTTCCTCCAGTCCGGAGAAG |
Statistical analyses
All results were presented as mean ± SD. Multiple comparisons among treatments were made using ANOVA with Tukey’s multiple comparison test.
Results
Bacteriocin production and inhibitory activity
The inhibitory spectrum of the bacteriocins by E. coli strain E against different pathogenic bacteria in food and aquaculture was determined by the well diffusion method. The results indicated that the CFS obtained from E. coli E was able to inhibit various selected pathogens as shown in Table 2. S. aureus DMST8840 and Shigella sp. were the most sensitive to CFS among food pathogens. The highest antibacterial activity was found against E. tarda 3, the most significant pathogen in aquaculture, with an inhibition zone of 25.6 ± 0.58 mm. These indicator stains were then selected for further experiments. Unlike some bacteriocins from E. coli H22 exhibiting inhibitory activity against Gram-negative indicator strains tested of the family Enterobacteriaceae [25], E. coli E exhibited a relatively broad spectrum of antibiotic activity. Culture supernatants from E. coli E were sampled during growth cycle under shaking and non-shaking conditions at 37 °C and tested for bacteriocin activity. The highest bacteriocin activity (1422 AU ml−1) was reached during the early stationary phase after 5–6 h of incubation (Fig. 1). Under static conditions, the highest activity was found after a long period of cultivation (25–30 h) (data not shown). The bacteriocin production strongly decreased during stationary phase in both conditions, probably as a consequence of bacteriocin proteolysis.
Table 2.
Inhibitory spectrum of CFS produced by E. coli E on several pathogenic bacteria in food and aquaculture
| Indicator strains | Diameter of inhibition zone (mm) | Indicator strains | Diameter of inhibition zone (mm) |
|---|---|---|---|
| Bacillus cereus | 0a | P. aeruginosa 5 | 3.5b ± 0.71 |
| Escherichia coli | 0a | V. cholerae 7 | 4.5bc ± 0.71 |
| Streptococcus sp. | 5.0bc ± 1.00 | A. hydrophila 9 | 0a |
| Salmonella sp. | 0a | E. tarda 3 | 25.6e ± 0.58 |
| S. aureus DMST8840 | 14.3d ± 1.53 | V. alginolyticus 3 | 5.7bc ± 0.58 |
| Shigella sp. | 16.0d ± 1.00 | V. parahaemolyticus 5 | 7.0c ± 1.00 |
Data are mean ± SD from three independent replicates (ANOVA, Tukey’s multiple comparison test, P < 0.01). Different superscript letters indicate the significant different of mean value by ANOVA with Tukey's multiple comparison test
Fig. 1.
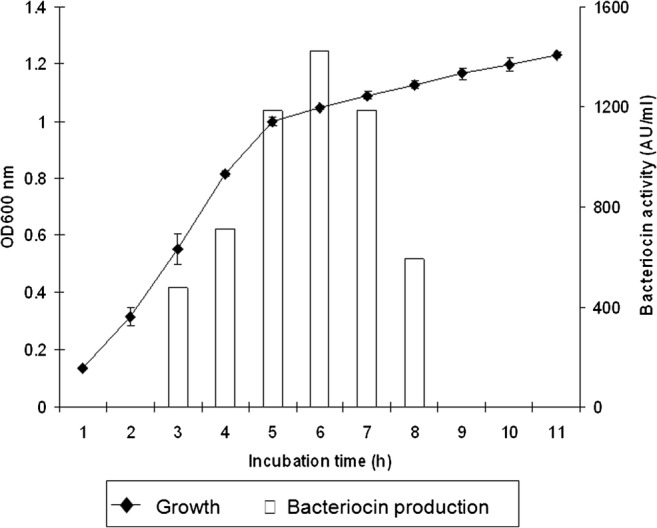
Growth of E. coli E and production of bacteriocin expressed in arbitrary unit (AU ml−1) under shaking condition at 37 °C. The bacterial activity was determined against E. tarda 3. All experiments were performed in triplication
Biochemical properties of bacteriocin
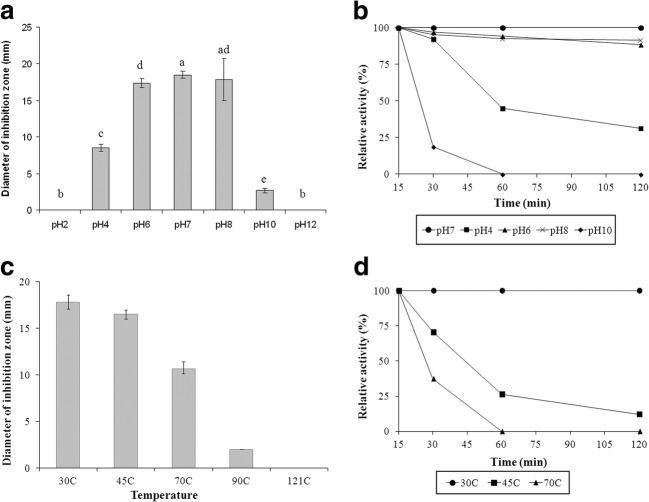
Crude bacterial cultures were collected and treated with various enzymes such as proteinase K, trypsin, and catalase. An enzyme sensitivity assay showed that the antibacterial activity exhibited by E. coli E was completely inactivated by the treatment of proteinase K. However, trypsin and catalase displayed no effect on the activity of CFS from E. coli E. Characterization of the proteinaceous inhibitor confirmed that the antibacterial substances produced by E. coli E were bacteriocins. The effect of pH and temperatures on antibacterial activity against the growth of E. tarda 3 by bacteriocins is shown in Fig. 2. There was no significant difference in bacteriocin activity between control (pH 7) and sample adjusted to pH 8 after the first 15 min (Fig. 2a). In addition, the results indicated that bacteriocins were the most stable at pH range 6.0–8.0 and could retain more than 80% of original activity after 120 min (Fig. 2b). However, bacteriocin activity was totally lost at extreme pH (pH 2.0 and 12.0) within the first 15 min of treatment. To evaluate the thermal stability of bacteriocin, CFS was incubated at 30 °C (control), 45 °C, 70 °C, 90 °C, and 121 °C for various periods. Results indicated that the inhibitory activity significantly decreased with increasing temperature (Fig. 2c). Bacteriocins could maintain their activity after a short treatment period at 45 °C and 70 °C. Its antibacterial activity remained for 120 min at 30 °C (Fig. 2d). However, it was found to be unstable with the loss of the antibacterial activity after autoclave treatment.
Fig. 2.
Effect of pH and temperature on the activity of CFS derived from E. coli E against the growth of E. tarda 3. a Effect of different pH on activity for 15 min. b pH stability profile during 120 min treatment. c Effect of temperature on bacteriocin activity for 15 min. d Temperature stability profile during 120 min of treatment. Values shown are the mean ± SD of three independent experiments. (ANOVA, Turkey’s multiple comparison test, P < 0.05)
Bile salt and acid tolerance
E. coli E was further examined for the probiotic properties including acidic tolerance and bile salt tolerance. Bile salt resistance tests showed that E. coli E was able to survive at bile salt concentrations of 0.1%, 0.2%, and 0.3% at 37 °C for 24 h. As for acid tolerance tests, E. coli E did not tolerate pH 1.0. At pH 2 and 3, cells could survive for 2 h but the viable cell numbers were sharply decreased after 3 h. In this study, pH 4.0 did not affect the cell number after exposure for 3 h.
Partial purification and direct activity determination
In this study, bacteriocins produced by E. coli E were partially purified by mixing them with ammonium sulfate at concentrations of 20%, 30%, and 50%. The obtained fractions were screened for the antibacterial activity by the well diffusion method against both Shigella sp. (Fig. 3a) and E. tarda 3 (Fig. 3b). The antagonistic activity was only detected in 20% ammonium sulfate fraction as shown in Fig. 3. In the active fraction, at least three distinct protein bands were detected by SDS-PAGE (Fig. 4a, lane 2). Direct detection of the bacteriocins activity was performed by overlaying them on the indicator strain (Fig. 4b). Only the apparent molecular masses of about 27 kDa and 10 kDa from 20% ammonium sulfate fraction showed inhibitory activity against E. tarda 3 and S. aureus DMST8840, respectively, whereas other fractions failed to inhibit the growth of tested bacteria (Fig. 4b, lanes 5a and 6b).
Fig. 3.
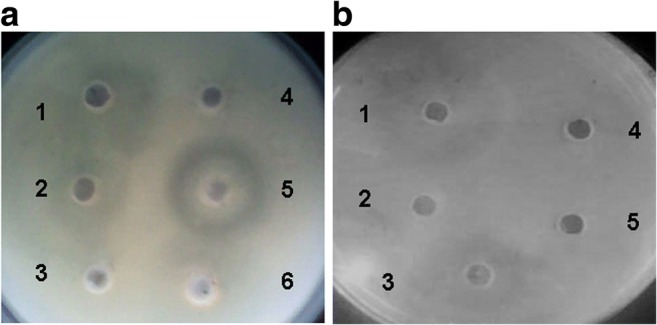
The antibacterial activity of partial purification fractions against the growth of aShigella sp. 1–2: CFS, 3: 50% (NH4)2SO4 saturation, 4: negative control (culture media), 5: 20% (NH4)2SO4 saturation, and 6: 30% (NH4)2SO4 saturation. bE. tarda 3, 1: 20% (NH4)2SO4 saturation, 2: negative control (culture media), 3: CFS, 4: 30% (NH4)2SO4 saturation, 5: 50% (NH4)2SO4 saturation
Fig. 4.
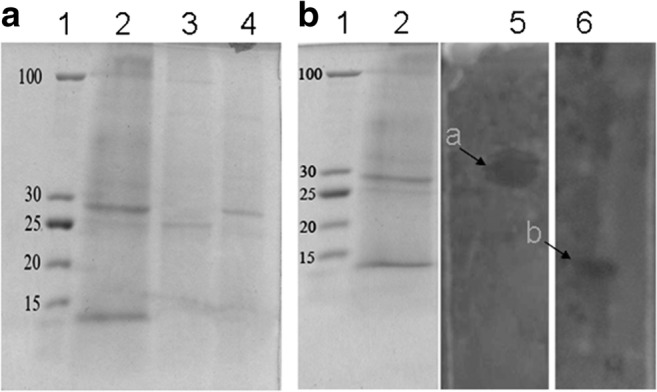
Partial purification and direct activity detection. a SDS-PAGE analysis of ammonium sulfate fractions; lane 1, protein ladder (New England BioLabs); lane 2, 20% (NH4)2SO4 saturation; lane 3, 30% (NH4)2SO4 saturation; and lane 4, 50% (NH4)2SO4 saturation. b Gel containing active fraction overlaid with indicator strain E. tarda 3 (lane 5) and S. aureus DMST8840 (lane 6). Arrows indicate inhibition zones with corresponding active peptides
Cytotoxicity and determination of virulence genes in E. coli E
The effect of CFS on NIH-3T3 viability was measured using an MTT assay. The result showed that CFS at all conditions and nisin did not significantly alter NIH-3T3 viability compared to that of control (Fig. 5). To confirm the safety of E. coli E, PCR was used to detect whether virulence genes were present in the genome. The results revealed that there were two virulence genes including aer (602 bp) and sfa (410 bp) detected in the genome of E. coli E (Fig. 6).
Fig. 5.
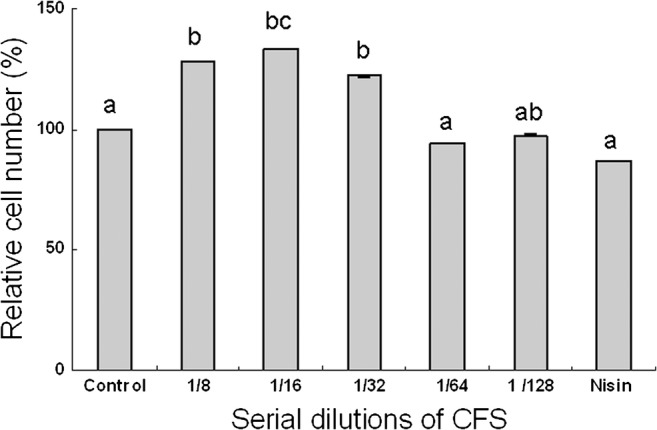
Cytotoxicity of different concentrations of CFS produced by E. coli E against NIH-3T3 determined by MTT assay after 72 h incubation. Values shown are the mean ± SD. (ANOVA, Turkey’s multiple comparison test, P < 0.05)
Fig. 6.
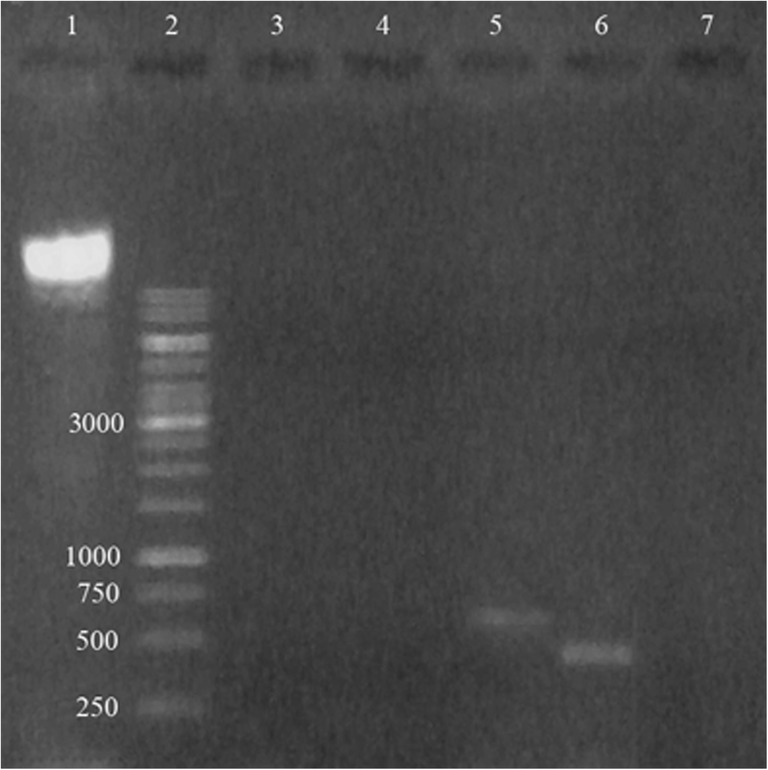
PCR analysis of virulence genes in E. coli E. lane 1, DNA; lane 2, DNA ladder (GeneRuler™ 1kbp); lane 3, pep; lane 4, sfa; lane 5, aer; lane 6, hly; and lane 7 cnf
Discussion
We further characterized E. coli E which isolated from fermented pineapple from the previous study [33] by using the Clermont E. coli phylo-typing method [39]. Base on the quadruplex genotype obtained, an E. coli E is assigned to a phylo-group F (− + − −, indicating arpA−, chuA+, yjaA−, and TspE4.C2−) (Fig. S1). In addition, bacteriocin of the present study exhibited a bacteriocidal mode of action toward S. aureus DMST8840and Shigella sp. which are responsible for foodborne illnesses worldwide. Interestingly, it is highly active against E. tarda 3, the most common pathogens of cultured fish, responsible for edwardsiellosis infection of a wide range of freshwater and marine fish leading to major losses in aquaculture worldwide [15, 40]. E. tada, a Gram-negative rod-shaped bacterium, is found to be an intracellular pathogen and is capable of infecting various types of cells [15, 40]. Currently, the common method to treat edwardsiellosis by antibiotics is not effective as treating extracellular bacterial pathogens such as Vibrio spp. [15]. The previous study showed that E. coli strain H22 exhibited the ability to inhibit the growth of pathogenic or non-pathogenic Gram-negative bacteria including Enterobacter, Escherichia, Klebsiella, Morganella, Salmonella, Shigella, and Yersinia. The growth inhibition was observed against all strains tested of each genus except for the pathogenic E. coli strain p307. However, Gram-positive indicator strains tested such as B. subtilis and S. aureus were not sensitive [25]. Pyocin SA 189, which is a bacteriocin produced by Gram-negative bacteria P. aeruginosa SA 189, showed antibacterial activity against a number of sensitive Gram-positive bacteria including S. aureus, S. pyogenes, and Listeria monocytogenes. However, no inhibitory activity against some species was observed such as Shigella dysenteriae and Salmonella typhi [41]. Microcin 25, excreted by E. coli strain isolated from human feces, showed that the antibiotic was active against E. coli, Salmonella, and Shigella strains of clinical origin by cross-streaking assay [42]. The highest bacteriocin activity was observed to be produced at early stationary phase, which is similar to those in bacteriocin production studies of both Gram-positive and Gram-negative bacteria [28, 43]. The maximum yield of pyocin SA 189 produced by P. aeruginosa SA189 was obtained at late log phase and remained constant until the late stationary phase [41]. The amount of bacteriocin released in the culture medium is correlated with the quantity of biomass produced. However, the bacteriocin production strongly decreased when cells entered the stationary phase. The loss of bacteriocin activity may be caused by proteolytic degradation during this growth phase, protein aggregation, and feedback regulation [44]. The production of active bacteriocin is shown to be affected by environmental factors [41, 44, 45]. In our study, E. coli E could grow and produce bacteriocins in NaCl concentrations up to 4%. The presence of 1.0–4.0% NaCl in culture media did not affect growth compared to control (0% NaCl). The obtained E. coli E displayed a strong tolerance to high salt concentrations. Thus, this strain may be applied to high salt environments such as marine or that of food processing. However, the antibacterial activity of bacteriocin was significantly affected by 1.0–4.0% NaCl compared to control (data not shown). NaCl was found to affect cell growth and bacteriocin activity produced by Lactobacilli species [45].
Probiotic applications employing bacteria have increased both in research and commercial interest for over 20 years [6, 11, 46]. Bacteriocins have demonstrated their remarkable potential in food preservatives, medical uses, and bacterial disease treatment in aquaculture [6, 9, 13, 18, 24, 43]. The digestive processes in marine animals like fish and shrimp are less variable and generally similar to those found in higher animals [47]. The acid and bile are important antimicrobial components of the digestive system. Being tolerant to acid and bile is a fundamental property for the selection of successful probiotic isolates to assure their viability and functionality [37, 46, 48]. To evaluate the probiotic potential, the acidic and bile salt tolerance of E. coli E was determined. In this study, E. coli E was able to grow in NA agar supplemented with up to 0.3% bile salt which is considered to be critical and high enough to screen for resistant strains [37, 46]. Two lactic acid bacteria in a commercial probiotic culture were characterized [46]. The results showed that they were able to tolerate bile salt at a concentration of 0.6% and survive at pH 3 but did not at pH 2.0 [46]. Buntin et al. obtained four LAB strains from 160 isolates based on their survival after exposure to pH 2.5 and resistance to bile salt concentration of 0.4%. None of the strains were able to survive when exposed to pH 1 [37]. B. amyloliquefaciens LN could survive after incubation at pH 2.0 or 3.0 for 3 h and 0.3% bile salt for 12 h [49]. Depending on the species, feeding rate, diet composition, and time after meals, the fish stomach pH varies between 1 and 6 and it is stable until about 6 h after meals then gradually decreases to pH 4 at 24 h [47]. Therefore, the results obtained from this study indicated that E. coli E might be able to resist the digestion process in the gastrointestinal tract where it can function effectively.
The production of more than one bacteriocin by E. coli has been reported previously [3, 21, 24]. Two types of bacteriocins are commonly produced by E. coli, classified by their molecular weight: colicin (25–80 kDa) and microcins (< 10 kDa) [23, 24]. E. coli H22 shows antagonistic activity against several enteric bacteria and it produces colicins E1 and IB and microcin C7 [25]. Our data also indicated that E. coli E produced more than two types of bacteriocins and these two types were associated with the antibacterial activity (Fig. 4b). Bacteriosin LS2 (4 kDa) isolated from the cell pellet of Lactobacillus salivarius BGH01 had antagonistic activity against several indicator strains detected by direct gel detection assay [2]. However, this is the first report to show two specific bacteriocin activities on two different indicator strains. Surprisingly, we could not detect antibacterial activity against Shigella sp. by gel direct detection assay. The in vitro inhibition of Shigella flexneri 4 by E.coli H22 was shown to be mediated by microcin C7 [25]. Bacteriocins produced by E. coli E could carry various inhibitory effects or act synergistically against pathogens. One type of bacteriocin classified by molecular weight as colicins (27 kDa) could inhibit E. tarda 3 as performed by direct detection of activity. Colicin production could be induced by the SOS response system [7, 24]. Mitomycin C that is able to trigger the SOS response was used in our study. The presence of 1 μg ml−1 mitomycin C in culture media could induce about 20% of bacteriocin production compared with control (data not shown). The low molecular weight (10 kDa) could classify it as microcin and it could specifically exhibit antibacterial activity against S. aureus DMST8840. The molecular weight of detected microcin was likely to be microcin V (previously known as ColV) (9 kDa) and microcin S (11.67 kDa) produced by E. coli and E. coli G3/10, respectively [4, 50]. However, further study needs to be carried out to confirm the types of colicin and microcin.
Previous studies showed the co-associations of microcins with other virulence factors such as toxins (usp, hlyA), siderophores (iroN, iroCD, fyuA, iucD), and adhesins (papC, sfa, fimH) [24]. Our study observed two virulence genes, aer and sfa. Miciocins namely H47, M, and ColV were more likely to encode genes associated with siderophores, toxins, and adhesins [24]. However, colicin E1 (57 kDa calculated from the predicted amino acid sequence from the plasmid DNA sequence) was a potentially important virulence factor of certain uropathogenic E. coli strains [3, 51].
Very few studies have been reported on the cytotoxicity upon normal eukaryotic cells [5, 52]. Cytotoxicity studies of bacteriocin (enterocin S37) produced by Enterococcus faecalis S37 on human colon adenocarcinoma cells were performed and dose-dependent cytotoxicity toward tested cells was observed [5]. The result suggested that enterocin S37 caused some loss of plasma membrane integrity of the tested cells [5]. In our study, an MTT assay was performed to indicate the degree of cytotoxicity of CFS. An increase in relative proliferation in fibroblasts treated with higher CFS dilution (1/8–1/32) was observed, whereas there was no difference between control, dilution of 1/64–1/128 and nisin. Moreover, our data also indicated that CFS dilution did not affect the relative cell number in human head and neck cancer cells, HN18 (data not shown). The results obtained indicate the safe use of bacteriocins including secreted metabolites of E. coli E.
Our study demonstrates that bacteriocins produced by E. coli E have great potential to be applied as a new antibacterial agent due to its antagonistic activity against both Gram-positive and Gram-negative pathogenic bacteria. Although two virulence genes were found in the E. coli E genome, bacteriocins in cell-free supernatants are safe for the normal cell lines. Further experiments should be carried out to elucidate the types of bacteriocins.
Electronic supplementary material
Quadruplex PCR profile of Clermont phylo-typing method. Lane 1, group F (E. coli E, - + - -); lane 2 negative control. M, DNA ladder (GeneRuler 100 bp, Thermo ScientificTM). The chuA, yjaA, TspE4.C2 and arpA primers were used for quadruplex PCR. (PNG 392 kb)
Funding information
This work was supported by a grant from Faculty of Science and Technology, Prince of Songkla University, SAT-ASEAN 5602 and SAT-ASEAN scholarship for international students 2013 to V.T. Le. Thanks are due to the Minister of Education, Malaysia, under Niche Research Grant Scheme (NRGS) vot no: R/NRGS/A0.700/00387A/006/2014/00152 for funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nathan C. Antibiotics at the crossroads. Nature. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 2.Busarcevic M, Dalgalarrondo M. Purification and genetic characterisation of the novel bacteriocin LS2 produced by the human oral strain Lactobacillus salivarius BGHO1. Int J Antimicrob Agents. 2012;40:127–134. doi: 10.1016/j.ijantimicag.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Smajs D, Micenkova L, Smarda J, et al. Bacteriocin synthesis in uropathogenic and commensal Escherichia coli: colicin E1 is a potential virulence factor. BMC Micribiol. 2010;10:1–10. doi: 10.1186/1471-2180-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zschuttig A, Zimmermann K, s, et al. Identification and characterization of microcin S, a new antibacterial peptide produced by probiotic Escherichia coli G3/10. PLoS One. 2012;7:e33351. doi: 10.1371/journal.pone.0033351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belguesmia Y, Madi A, Sperandio D, et al. Growing insights into the safety of bacteriocins: the case of enterocin S37. Res Microbiol. 2011;162:159–163. doi: 10.1016/j.resmic.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81:591–606. doi: 10.1007/s00253-008-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascales E, Buchanan SK, Duche D, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley MA. Molecular mechanisms of bacteriocin evolution. Annu Rev Genet. 1998;32:255–278. doi: 10.1146/annurev.genet.32.1.255. [DOI] [PubMed] [Google Scholar]
- 9.Desriac F, Defer D, Bourgougnon N, et al. Bacteriocin as weapons in the marine animal-associated bacteria warfare: inventory and potential applications as an aquaculture probiotic. Marine Drugs. 2010;8:1153–1177. doi: 10.3390/md8041153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol. 2006;17:204–210. doi: 10.1016/j.copbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y-B, Li J-R, Lin J. Probiotics in aquaculture challenges and outlook. Aquaculture. 2008;281:1–4. doi: 10.1016/j.aquaculture.2008.06.002. [DOI] [Google Scholar]
- 12.Pridgeon JW, Klesius PH. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012;7:1–16. doi: 10.1079/PAVSNNR20127048. [DOI] [Google Scholar]
- 13.Sahoo TK, Jena PK, Patel AK, et al. Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: a review. Aquac Res. 2016;47:1013–1027. doi: 10.1111/are.12556. [DOI] [Google Scholar]
- 14.Loch TP, Hawke JP, Reichley SR, et al. Outbreaks of edwardsiellosis caused by Edwardsiella piscicida and Edwardsiella tarda in farmed barramundi (Lates calcarifer) Aquaculture. 2017;481:202–210. doi: 10.1016/j.aquaculture.2017.09.005. [DOI] [Google Scholar]
- 15.Xu T, Zhang X-H. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture. 2014;431:129–135. doi: 10.1016/j.aquaculture.2013.12.001. [DOI] [Google Scholar]
- 16.Ahmed AM, Shimamoto T. Isolation and molecular characterization of Salmonella enterica, Escherichia coli O157:H7 and Shigella spp. from meat and dairy products in Egypt. Int J Food Microbiol. 2014;168-169:57–62. doi: 10.1016/j.ijfoodmicro.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Arfatahery N, Mirshafiey A, Abedimohtasab TP, et al. Study of the prevalence of Staphylococcus aureus in marine and farmed shrimps in Iran aiming the future development of a prophylactic vaccine. Procedia Vaccinol. 2015;9:44–49. doi: 10.1016/j.provac.2015.05.008. [DOI] [Google Scholar]
- 18.Galvez A, Abriouel H, Lopez RL, et al. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120:51–70. doi: 10.1016/j.ijfoodmicro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Hwanhlem N, Ivanova T, Haertle T, et al. Inhibition of food-spoilage and foodborne pathogenic bacteria by a nisin Z-producing Lactococcus lactis subsp lactis KT2W2L LWT. Food Sci Technol. 2017;82:170–175. [Google Scholar]
- 20.Yang S-C, Lin C-H, Sung CT, et al. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014;5:241. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon DM, O’Brien CL. Bacteriocin diversity and the frequency of multiple bacteriocin production in Escherichia coli. Microbiology. 2006;152:3239–3244. doi: 10.1099/mic.0.28690-0. [DOI] [PubMed] [Google Scholar]
- 22.Zihler A, Le Blay G, De Wouters T, Lacroix C, Braegger CP, Lehner A, Tischler P, Rattei T, Hächler H, Stephan R. In vitro inhibition activity of different bacteriocin-producing Escherichia coli against Salmonella strains isolated from clinical cases. Lett Appl Microbiol. 2009;49:31–38. doi: 10.1111/j.1472-765X.2009.02614.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillor O, Kirkup BC, Riley MA. Colicins and microcins: the next generation antimicrobials. Adv Appl Microbiol. 2004;54:129–146. doi: 10.1016/S0065-2164(04)54005-4. [DOI] [PubMed] [Google Scholar]
- 24.Budic M, Rijavec M, Petkovsek Z, et al. Escherichia coli bacteriocins: antimicrobial efficacy and prevalence among isolates from patients with bacteraemia. PLoS One. 2011;6:e28769. doi: 10.1371/journal.pone.0028769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cursino L, Šmajs D, Šmarda J, et al. Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. J Appl Microbiol. 2006;100:821–829. doi: 10.1111/j.1365-2672.2006.02834.x. [DOI] [PubMed] [Google Scholar]
- 26.Duquesne S, Destoumieux-Garzon D, Peduzzi J, et al. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24:708–734. doi: 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 27.Morin N, Lanneluc I, Connil N, et al. Mechanism of bactericidal activity of Microcin L in Escherichia coli and Salmonella enterica. Antimicrob Agents Chemother. 2011;55:997–1007. doi: 10.1128/AAC.01217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherif A, Chehimi S, Limem F, et al. Detection and characterization of the novel bacteriocin entomocin 9, and safety evaluation of its producer, Bacillus thuringiensis ssp. entomocidus HD9. J Appl Microbiol. 2003;95:990–1000. doi: 10.1046/j.1365-2672.2003.02089.x. [DOI] [PubMed] [Google Scholar]
- 29.Maragkoudakis PA, Papadelli M, Georgalaki M, et al. In vitro and in vivo safety evaluation of the bacteriocin producer Streptococcus macedonicus ACA-DC 198. Int J Food Microbiol. 2009;133:141–147. doi: 10.1016/j.ijfoodmicro.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/CMR.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arisoy M, Aysev D, Ekim M, et al. Detection of virulence factors of Escherichia coli from children by multiplex polymerase chain reaction. Int J Clin Pract. 2006;60:170–173. doi: 10.1111/j.1742-1241.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- 32.Farshad S, Emamghorashi F. The prevalence of virulence genes of E. coli strains isolated from children with urinary tract infection. Saudi J Kidney Dis Transpl. 2009;20:613–617. [PubMed] [Google Scholar]
- 33.Lee SW, Wendy W, Leelakriangsak M, et al. The effectiveness of novel bacteriocin derived from Escherichia coli colonized in the fermented pineapple Ananas comosus (L.) Merr. against pathogenic bacteria isolated from aquaculture sites. Vet World. 2014;7:1014–1018. doi: 10.14202/vetworld.2014.1014-1018. [DOI] [Google Scholar]
- 34.Todorov SD, Dicks LM. Bacteriocin production by Pediococcus pentosaceus isolated from Marula (Scerocarya birrea) Int J Food Microbiol. 2009;132:117–126. doi: 10.1016/j.ijfoodmicro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Harrigan WF, MaCance EM. Laboratory methods in food and dairy microbiology. 3. London: Academic Press; 1986. [Google Scholar]
- 36.Mandal V, Sen SK, Mandal NC. Optimized culture conditions for bacteriocin production by Pediococcus acidilactici LAB 5 and its characterization. Indian J Biochem Biophys. 2008;45:106–110. [PubMed] [Google Scholar]
- 37.Buntin N, Chanthachum S, Hongpattarakere T. Screening of lactic acid bacteria from gastrointestinal tracts of marine fish for their potential use as probiotics. SJST. 2008;30:141–148. [Google Scholar]
- 38.Koontongkaew S, Monthanapisut P, Saensuk T. Inhibition of arachidonic acid metabolism decreases tumor cell invasion and matrix metalloproteinase expression. Prostaglandins & Other Lipid Mediat. 2010;93:100–108. doi: 10.1016/j.prostaglandins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Clermont O, Christenson JK, Denamur E, et al. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- 40.Mohanty BR, Sahoo PK. Edwardsiellosis in fish: a brief review. J Biosci. 2007;32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- 41.Naz SA, Jabeen N, Sohail M, et al. Biophysicochemical characterization of Pyocin SA189 produced by Pseudomonas aeruginosa SA189. Braz J Microbiol. 2015;46:1147–1154. doi: 10.1590/S1517-838246420140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salomon RA, Farias RN. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J Bacteriol. 1992;174:7228–7435. doi: 10.1128/jb.174.22.7428-7435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carraturo A, Raieta K, Ottaviani D, et al. Inhibition of Vibrio parahaemolyticus by a bacteriocin-like inhibitory substance (BLIS) produced by Vibrio mediterranei 1. J Appl Microbiol. 2006;101:234–241. doi: 10.1111/j.1365-2672.2006.02909.x. [DOI] [PubMed] [Google Scholar]
- 44.De Vuyst L, Callewaert R, Crabbe K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 45.Rawal K, Bhavsar N, Roal G, et al. Bacteriocin: production and optimization by Lactobacillus species. J Microbiol Biotechnol Res. 2013;3:64–76. [Google Scholar]
- 46.Menconi A, Kallapura G, Latorre JD, et al. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci Microbiota Food Health. 2014;33:25–30. doi: 10.12938/bmfh.33.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Committee on the Nutrient Requirements of Fish and Shrimp . Board on agriculture and natural resources, division on earth and life studies, National Research Council. Nutrient requirements of fish and shrimp. Washington, D.C.: National Academies Press; 2011. p. 393. [Google Scholar]
- 48.Suskovic J, Kos B, Matosic S, et al. The effect of bile salts on survival and morphology of a potential probiotic strain Lactobacillus acidophilus M92. World J Microbiol Biotechnol. 2000;16:673–678. doi: 10.1023/A:1008909505651. [DOI] [Google Scholar]
- 49.Lee A, Cheng K-C, Liu J-R. Isolation and characterization of a Bacillus amyloliquefaciens strain with zearalenone removal ability and its probiotic potential. PLoS One. 2017;12:e0182220. doi: 10.1371/journal.pone.0182220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang J, Manuvakhova M, Tai PC. Characterization of in-frame proteins encoded by cvaA, an essential gene in the colicin V secretion system: CvaA* stabilizes CvaA to enhance secretion. J Bacteriol. 1997;179:689–696. doi: 10.1128/jb.179.3.689-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz SA, Helinski DR. Purification and characterization of colicin E1. J Biol Chem. 1971;246:6318–6327. [PubMed] [Google Scholar]
- 52.Messaoudi S, Madi A, Prevost H, et al. In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe. 2012;18:584–589. doi: 10.1016/j.anaerobe.2012.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quadruplex PCR profile of Clermont phylo-typing method. Lane 1, group F (E. coli E, - + - -); lane 2 negative control. M, DNA ladder (GeneRuler 100 bp, Thermo ScientificTM). The chuA, yjaA, TspE4.C2 and arpA primers were used for quadruplex PCR. (PNG 392 kb)