Abstract
Plants colonised by dark septate endophytic (DSE) fungi show increased uptake of nutrients available in the environment. The objective of the present study was to evaluate the impact of DSE fungi on the activity of proton pumps, nitrogen (N) recovery from ammonium sulphate, and nutrient accumulation in rice plants. Treatments consisted of non-inoculated plants and plants inoculated with two isolates of DSE fungi, A101 and A103. To determine N recovery from the soil, ammonium sulphate enriched with 15N was added to a non-sterile substrate while parameters associated with the activity of proton pumps and with NO3− uptake were determined in a sterile environment. The A101 and A103 fungal isolates colonised the roots of rice plants, promoting 15N uptake, growth, and accumulation of nutrients as compared with the mock control. A103 induced the expression of the plasma membrane H+-ATPase (PM H+-ATPase) isoforms OsA5 and OsA8, the activity of the PM H+-ATPase and H+-pyrophosphatase. Our results suggest that the inoculation of rice plants with DSE fungi represents a strategy to improve the N recovery from ammonium sulphate and rice plant growth through the induction of OsA5 and OsA8 isoforms and stimulation of the PM H+-ATPase and H+-pyrophosphatase.
Keywords: Fertiliser-15N, Oryza sativa L., Pleosporales, Vacuolar H+-pyrophosphatase, OsA8, PM H+-ATPase
Introduction
The dark septate endophytic (DSE) fungi represent a diverse and cosmopolitan group of conidial or sterile ascomycetes found in more than 600 plant species in 320 genera and 11 families, in which they can act to promote growth. They present melanised and septate hypha and form brown to dark-coloured colonies. In the plant host, they colonise the epidermis and the cortex of the roots both intra- and extracellularly, eventually producing melanised microsclerotia without causing any disease symptoms [1–3].
Different mechanisms have been proposed to explain the promotion of plant growth by DSE fungi. The first involves the synthesis of phytohormones such as indoleacetic acid and gibberellin by these fungi [4, 5]. The second suggests the protection of the host plant against soil pathogens [6], and the third is associated with the production of a series of extracellular enzymes that digest complex carbon, nitrogen, and phosphorus compounds that are commonly found in soils, thereby providing the host plant with nutrients otherwise inaccessible to them [2, 7–10]. Even though other plant growth–promoting fungi, such as arbuscular mycorrhizal fungi (AMF), Trichoderma sp., and Piriformospora indica, induce plasma membrane H+-ATPase (PM H+-ATPase) as the central mechanism of growth stimulation [11–15], the behaviour of PM H+-ATPase when plants are inoculated with DSE fungi remains unknown.
Rice represents one of the most important sources of grains for human nutrition, and it is a model for molecular biology studies. Its genome contains 10 PM H+-ATPase isoforms, which are encoded by a multigenic family and show distinct and/or partially overlapping expression profiles and physiological roles, possibly to ensure the correct transport of substances, a function of particular importance in plants [16–19].
PM H+-ATPase is the most prominent pump in the plant plasma membrane. It is ubiquitous in all cell types and is a member of the P-type ATPase protein family, which is characterised by the synthesis of phosphorylated intermediates during the catalysis of transmembrane movement [18, 20, 21]. This glycoprotein comprises 10 transmembrane domains that hydrolyse ATP on the cytosolic region in order to electrogenically pump H+ out of the cell. The hydrolysis establishes an electrochemical gradient of H+ across the plasma membrane which is necessary for several physiological processes essential to plant growth, such as nutrient uptake, ion homeostasis, the regulation of intracellular pH and stomata aperture, and cellular expansion [22–27].
PM H+-ATPase activity is regulated by its auto-inhibitory domain, located on the C-terminal region of the polypeptide chain, which can change conformation depending on its phosphorylation state and association with the 14-3-3 protein or through interaction with certain lipids present in the surroundings. Therefore, post-translational modifications allow this enzyme to alternate between two states: active and inactive [28–30].
Vacuolar H+-ATPase (V-H+-ATPase) and vacuolar H+-pyrophosphatase (V-H+-PPase) consist of electrogenic pumps that simultaneously transport protons from the cytosol into the vacuole and generate the proton-motive force across the vacuolar membrane (tonoplast) which energises the accumulation and/or the remobilisation of anions such as NO3−. In order to transport a H+ ion, the V-H+-PPase utilises the free energy released by the hydrolysis of inorganic pyrophosphate (PPi) while V-H+-ATPase hydrolyses ATP to presumably transport two H+ ions [17, 19].
There is a large diversity of substances that can affect PM H+-ATPase activity, including the fungal toxin fusicoccin, which is secreted by Fusicoccum amygdali to activate the enzyme by stabilising the association of the 14-3-3 protein with the C-terminal domain [28, 31, 32]. Other fungal substances such as B1 fumonisin, produced by Fusarium verticillioides, inhibit PM H+-ATPase activity [33]. Auxins, lysophospholipids, and fatty acids affect ATPase activity through mechanisms involving the conformational change of the auto-inhibitory C-terminal domain, while coumarin, a plant secondary metabolite with allelopathic effects, apparently alters ATPase activity at the transcriptional level [29, 34]. Plant root colonisation by AMF, Trichoderma sp., and P. indica also affects PM H+-ATPase activity [11–15]. Therefore, root colonisation by DSE fungi possibly impacts not only PM H+-ATPase activity but also the activities of V-H+-ATPase, V-H+-PPase, and nitrate transporters (NRTs).
In aerated soils, ammonium (NH4+) is promptly converted into NO3−, which thus exists as the most abundant N form available to plants, with losses of approximately 50% [35–37]. In plants, NO3− uptake transits between the high (HATS) and low (LATS) affinity transport system. HATS is encoded by genes of the NRT2 protein family to act under external concentrations below 1 mM, while LATS is encoded by genes of the NRT1 protein family to act at external concentrations above 1 mM [38]. The transport of NO3− and other nutrients across the plasma membrane and the tonoplast depends on the availability of free energy stored in the form of a H+ gradient generated respectively by PM H+-ATPase and V-H+-ATPase/V-H+-PPase [37, 39–42].
AMF is the most studied group amongst fungi that promote plant growth. Besides optimising NO3− uptake by their plant hosts preferentially by strongly inducing the expression of the NO3− transporters NRT2.3, NFP1.3, and NFP6.3 and of the accessory protein NAR 2.2, which is responsible for redirecting the NRT2.2 transporter to the membrane, these fungi also enable plants to access other nutrients present in the soil, such as NH4+, K, P, and Zn, a process in which the PM H+-ATPase isoform OsA8 plays an important role [11, 15, 43–45]. Similarly, in previous studies using rice plants (Piauí variety) inoculated with the DSE fungal isolates A101 (unknown taxon) and A103 (order Pleosporales, suborder Massarineae), we observed a lower Michaelis-Menten constant (or high affinity) for the uptake of NO3− associated with greater depletion of this nutrient from the environment. Furthermore, plants inoculated with A103 showed higher accumulation of N, P, K, S, Ca, Mg, and dry matter in the root and in the aerial parts, as well as more numerous tillers [46], contrary to previous studies [47–50] that observed negative effects of DSE fungi inoculation. However, as in the majority of the studies carried out to date [51], the effects of inoculation with DSE fungi were evaluated by Vergara et al. [46] under controlled growth conditions. Since these effects are still poorly understood, further studies should be carried out not only with non-sterile soil enriched with labelled 15N but also with the goal to investigate physiological and molecular mechanisms by which DSE fungi promote growth, similar to what occurs when AMF are analysed.
In this context, our hypotheses were as follows: (i) DSE fungi improve the efficiency of nitrogen recovery from ammonium sulphate and other nutrients present in rice plants; (ii) these fungi promote plant growth; and (iii) DSE fungi stimulate the activity of proton pumps and NO3− transporters in the roots of rice plants. The objective of this study was to evaluate the activity of proton pumps, the accumulation of macro- and micronutrients and dry matter, and the efficiency of N recovery from ammonium sulphate in inoculated rice plants. For this purpose, rice plants (Piauí variety) were inoculated with the A101 and A103 fungal isolates and supplemented with ammonium sulphate enriched with 15N. The accumulation of N, P, K, Ca, Mg, Fe, Mn, and Zn, dry matter, tillers number, total leaf number, recovery efficiency, and levels of nitrogen derived from 15N-labelled ammonium sulphate were determined in rice plants 54 days after transplantation. In addition, the expression of six genes coding for PM H+-ATPase isoforms (OsA1, OsA2, OsA3, OsA5, OsA7, and OsA8), two genes encoding NO3− transporters (NRT2.1 and NRT2.2), and one gene encoding the NAR2.1 accessory protein was also determined in Piauí rice plants inoculated with the Pleosporales fungus (A103) and supplemented with 0.2 mM NO3− after 72 h of N deprivation.
Material and methods
Experiment 1: under non-sterilised sand soil to evaluate plant growth promotion
Fertilisation and soil liming
The soil used in this work to evaluate the growth promotion of rice plants inoculated with DSE fungi and supplemented with an inorganic N source was the same as mentioned previously [52], and it was collected in an organic production system located at Seropédica Municipality, RJ, Brazil, at 0–20-cm depth. Fertilisation and soil liming were described by Vergara et al. [52].
The soil was classified as Haplic Planosol (according to Brazilian Soil Taxonomy, or Planosol, based on World Reference Base-FAO). Soil analysis showed the following characteristics: pH = 5.47 in water; Al3+ = 0.03 exchangeable and H + Al = 1.86 cmolc dm−3; Ca+2 = 1.21 and Mg+2 = 0.41 cmolc dm−3; available P = 6.74 and K+ = 36.00 mg L−1; total N = 0.05% and C = 0.47%. Soil texture was a typical sandy soil (3% clay, 5% silt, and 92% sandy fraction). The soil sample was sieved and homogenised, and 12 kg was distributed in pots (14 L), which were the experimental units. In order to correct Ca+2 and Mg+2 deficiencies 2 months prior the planting, lime was added to each pot (equivalent of 1.62 t ha−1; MineralCal). Just before planting, the soil was fertilised with simple superphosphate (equivalent of 27 kg P2O5 ha−1), with potassium sulphate (equivalent of 13 kg K2O ha−1), and 7 kg ha−1 of micronutrient as F.T.E BR-12 (fritted trace elements), following the recommendations to rice crops [53].
15N-labelled nitrogen for fertilisation
Ammonium sulphate enriched with 1 atom % 15N (ammonium sulphate-15N) was the inorganic nitrogen source. For distribution, 15N-labelled ammonium sulphate (0.12 g per pot, equivalent to 20 kg N ha−1) was dissolved in distilled water and 500 mL of the homogenised solution was applied to the pots for soil labelling [54]. All treatments received ammonium sulphate-15N as the sole N source.
Fungal inoculum preparation and inoculation
The two fungal lineage used (A101 and A103) and their origin and phylogeny were mentioned elsewhere [46, 55]. The inoculum was obtained following the instructions from Andrade-Linares et al. [56] and Vergara et al. [54].
Each isolate was grown in a 300-mL Erlenmeyer flask containing 150 mL of potato dextrose agar medium (PDA) during 2 weeks at 28 °C in 80 rpm shaking. The fresh mycelium was filtered and washed with sterilised distilled water until the liquid was cleared to avoid transfer of any material from the medium to the inoculum. Thereafter, in laminar flow, the fungi mycelium was weighed and part of it was added of distilled sterilised water and mixed (1 min at the minimum speed using a mixer). The viability of each isolate was verified by plating the mycelium in the PDA medium. For inoculation, the suspensions were adjusted to 1% of mycelium concentration (w/v) in sterilised distilled water.
Experimental, treatments, and growth conditions
Experiment with rice seedlings was conducted in randomised complete block design, with five replicates with one plant each (n = 5), under greenhouse conditions at Embrapa Agrobiologia located in Seropédica, Rio de Janeiro, Brazil. Treatments consisted of a variety of rustic rice (Oryza sativa [L.] Piauí) plants grown with DSE fungi A101 and A103 inoculation and no inoculation (control). Rice seeds were washed with 70% alcohol (5 min), disinfected with sodium hypochlorite (2.5%; 10 min), followed by eight successive washes with sterilised distilled water. Then, seeds were pre-germinated in water agar (8 g L−1) at 28 °C to select homogenous plants [54]. Six-day rice seedlings were inoculated with DSE fungi by immersion of the roots in the mycelial suspension (1% w/v), while control plants only received sterilised distilled water. Additionally, the soil in the pots destined to inoculation treatments was also drenched by the 500 mL of fresh mycelium suspension (1% w/v), while control treatment only received sterilised distilled water [54]. The soil moisture was maintained near field capacity through daily watering (500 mL of distilled water per pot).
Measurements
Shoot biomass, tillers and leaf number, total leaf area (LI-3100C area meter, LI-COR, NE, USA), and chlorophyll levels (SPAD-502 m, Konica-Minolta, Japan) of rice plants were measured 54 days after the plant transplantation. Shoot dry biomass (dried at 65 °C, 72 h) were macerated in laboratory mill (< 40 mesh) followed by a rolling mill to powder the sample [57]. In the aboveground tissues, the concentrations of N, P, K, Ca, Mg, Fe, Mn, and Zn and the 15N abundance (atom % 15N excess) were measured. Micronutrient concentrations were determined in an aqua regia extract [58] by a plasma detector (PerkinElmer® Optima™ 8300), while macronutrient concentrations were quantified according to Tedesco [59].
15N abundance was obtained using continuous-flow isotope ratio mass spectrometry (Finnigan DeltaPlus mass spectrometer coupled to the output of a Carlo Erba EA 1108 total C and N analyser—Finnigan MAT, Bremen, Germany) [60]. The contents of macro- (mg plant−1) and micronutrients (μg plant−1), the fraction of 15N in the plant derived from 15N-labelled ammonium sulphate (%fNdfAS), and the recovery efficiency of 15N (%) were calculated following the International Atomic Energy Agency [61] and our previous work [52].
Experiment 2: to analyse the gene expression of plasma membrane (PM) H+-ATPase isoforms and NO3− transporters and the activity of the H+ pumps
The experiment was carried out in an entirely randomised design with split-plots divided into two treatments (non-inoculated Piauí rice plants and plants inoculated with A103), five harvest time points (0, 3, 6, 9, and 24 h), and four repetitions consisting of four independent pots with four rice plantlets each, for a total of two treatments × five harvest time points × four repetitions = 40 pots, in a growth chamber belonging to Embrapa Agrobiologia, in the municipality of Seropédica, RJ, Brazil.
Disinfestation, sowing and inoculation of the seeds, and cultivation conditions and growth of rice plants were performed as proposed by Vergara et al. [46]. The rice seeds were washed with 70% ethanol for 5 min followed by disinfestation with 2.5% sodium hypochlorite for 10 min and 10 successive washes with sterile distilled water. Disinfested seeds were sown on plastic Petri dishes (four seeds on each) previously perforated and filled with 10 g L−1 sterile water agar in order to allow for mycelial growth inside and outside of the nutritive solution, as proposed by Vergara et al. [46]. A disc of the potato dextrose agar (PDA) culture medium containing fungal mycelium previously grown for 7 days at 28 °C was placed beside each seed. In the control plates, a PDA disc without mycelium was placed on the medium. After sowing, the plates were incubated for 3 days at 28 °C in order to stimulate seed germination and fungal establishment. After this time, the Petri dishes containing uniform plants were selected, and these plants were transferred to glass jars filled with distilled water autoclaved for 1 h at 120 °C and cultivated in a growth chamber with a light intensity of 384 μmol m−2 s−1 from photosynthetically active photon fluxes, a photoperiod of 13 h/11 h (light/dark), 70% relative humidity, and temperatures of 28 °C/24 °C (light/dark). Five days after germination, the water present in the pots was substituted with a nutritive solution with 1/4 ionic strength and 1.5 mM NO3− (KNO3 as N source) formulated based on Hoagland and Arnon [62]. Three days later, this solution was replaced by another one with 1/2 ionic strength and 2.0 mM NO3− and 0.5 mM NH4+, with Ca(NO3)2 and (NH4)2SO4 as N sources, at a pH of 5.6. This strategy was adopted in order to avoid salt stress for the rice plantlets [63]. The 1/2 strength solution was then replaced every 3 days.
Thirty-eight days after germination (DAG), the plants were subjected to N starvation for a 72-h period with the purpose of increasing N uptake by the roots [64]. Then, a nutritive solution containing 0.2 mM NO3− and again KNO3 as a N source was provided to the plants. The roots of rice plants were harvested 41 DAG at 0, 3, 6, 9, and 24 h after N starvation and stored at − 80 °C for total RNA extraction and the isolation of the microsomal fraction to measure the activity of H+ pumps.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
The total RNA of rice plants subjected or not subjected to inoculation with the fungal isolate A103 was extracted from 100 mg fresh roots pulverised in liquid N2 using an RNeasy Plant Mini Kit (Qiagen, Milan, Italy) according to the manufacturer’s instructions. Total RNA was quantified in duplicate in a NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and qualitatively evaluated by the A260/A230 and A260/A280 absorption ratios and visualisation in a 1.5% agarose gel stained with GelRed (Biotium, Hayward, CA, USA).
Total RNA samples used for cDNA synthesis were treated with DNAse I (Invitrogen™ Carlsbad, CA, USA) according to the manufacturer’s instructions. The single-strand cDNA was synthesised using the High Capacity RNA to cDNA Kit (Applied Biosystems™, Carlsbad, CA, USA) and oligodT primers following the manufacturer’s recommendations.
The real-time polymerase chain reactions (real-time PCRs) were performed in duplicate using Fast EvaGreen® qPCR Master Mix (Biotium, Hayward, CA, USA) in a StepOne Real-Time PCR System (Applied Biosystems™, Carlsbad, CA, USA) according to the manufacturer’s instructions. Each reaction was carried out as follows: 2 min at 95 °C followed by 45 amplification cycles of 95 °C for 15 s and 60 °C for 1 min. At the end, the primer dissociation temperature was applied to generate the melting curves in order to identify eventual unspecific amplifications.
PCRs were performed to evaluate the expression of genes encoding six PM H+-ATPase isoforms (OsA1, OsA2, OsA3, OsA5, OsA7, and OsA8) previously detected in roots of rice plants [24], as well as to determine the expression of genes that codify NO3− transporters (OsNRT2.1 and OsNRT2.2) or the accessory protein OsNAR2.1. The sequences of the primers used to amplify cDNA from the PM H+-ATPases and the NRTs were described by Sperandio et al. [24], while the primers used to amplify the OsNAR2.1 cDNA were described by Bucher et al. [65]. The expression of elongation factor 1 [66] was used as an endogenous control in order to calculate the relative expression of the target genes by the 2−ΔΔCT method [67].
Vesicle extraction from the plasma membrane and assessment of proton pump activity
Roots of rice plants treated or not treated with the A103 fungal isolate and harvested 0, 3, 6, 12 and 24 h after inoculation were used as source material to extract vesicles from the plasma membrane according to the method developed by Facanha and De Meis [68] and Santos et al. [69], while protein was quantified with the Bradford assay [70], using bovine serum albumin as a standard. The activities of H+ pumps were calculated based on the inorganic phosphorus (Pi) released by the hydrolysis of ATP (PM H+-ATPase and V-H+-ATPase) and PPi (pyrophosphate) (V-H+-PPase), applying the method proposed by Yan et al. [71] and adjusted by Santos et al. [69] and Sperandio et al. [24].
Colonisation, disease symptoms, and DSE structures
The observation of the symptoms of the disease, the preparation of the fresh root for the DSE structures visualisation, and the quantification of DSE colonisation were performed as described by Vergara et al. [52].
The roots of rice plants inoculated with A101 and A103 DSE fungal isolates were washed and fixed in 50% ethanol. To confirm the colonisation, the roots were then treated with 2.5% potassium hydroxide and 1% hydrochloric acid and stained with 0.002% (m/v) of methyl blue (a mixture of 10:9:1 glycerol/distilled water/hydrochloric acid; Grace and Stribley [72]). Root segments with length around 4 cm were placed on slides with glycerine and the fungal hyphae and other structures observed in Axioplan optical microscope (Carl Zeiss, Jena, 151 Germany) equipped with an Axiocam MRC5 (Carl Zeiss) digital camera. Intraradical microsclerotia and hyphae were counted in 100 microscopic fields per root system under a × 200 magnification. Disease symptoms were evaluated on a 0–3 scale (0: absence of disease symptoms; 1: slight yellowing of the plant; 2: yellowing and late plant growth; 3: wilt or plant death) [50, 73].
Statistical analysis
The data from each experiment were individually subjected to Bartlett’s test (homogeneity of variance) and to the Shapiro-Wilk test of normality as well as to analysis of variance (ANOVA). For experiment 2 (genetic expression), the ANOVAs were independently performed for each harvest time point (0, 3, 6, 9, and 24 h). When the differences were significant according to ANOVA, the means of the treatments were compared using a t test at a 95% confidence level. All statistical analyses were carried out using R software version 3.4.1 [74]. Results are shown as mean ± standard error.
Results
Colonisation rates
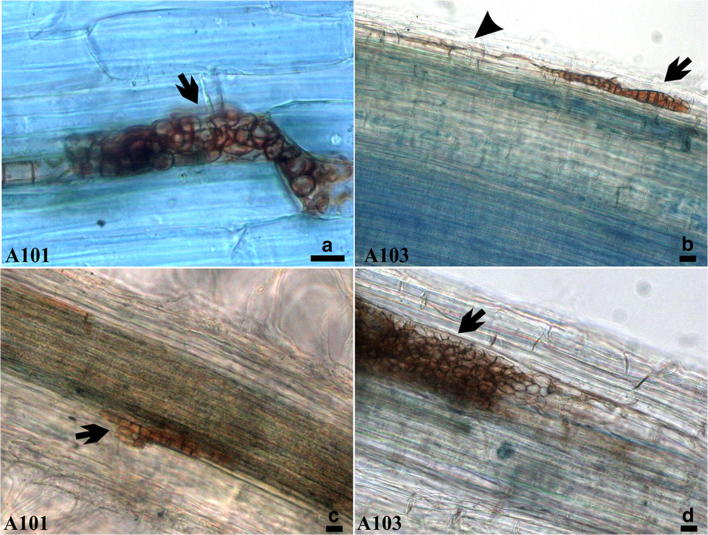
The hyphae (Fig. 1b) of the two isolates of DSE fungi, A101 and A103, colonised the roots of rice plants (Fig. 1a–d) and formed abundant microsclerotia in the epidermis (Fig. 1a, b) and cortex (Fig. 1c, d), without causing any visible symptoms of disease. Intraradical hyphae colonisation of 43 ± 1.5 and 41 ± 0.9% and intraradical microsclerotial colonisation of 44 ± 3.0 and 42 ± 0.9% were observed in the roots of rice plants inoculated with A101 and A103 isolates, respectively, resulting in root colonisation ratios of 88 ± 3.5 and 84 ± 0.3%.
Fig. 1.
Morphological features of rice roots (Piauí variety) inoculated with the dark septate endophytic fungi (isolates A101and A103). Melanised septate hyphae (arrowheads) in the epidermis cells (b). Microsclerotia structures (arrow) formed by the fungi in the epidermis (a and b), and in the cortex (c and d). Samples were stained with 0.002% methyl blue. Bar = 20 μm
Plant growth promotion
Both DSE fungal isolates (A101 and A103) promoted growth in rice crops supplemented with 15N-ammonium sulphate as the N source (Table 1; Fig. 2). The rice plants presented significant increases in the aerial part biomass (approximately 16%), tiller number (62 and 77% for A101 and A103, respectively), leaf number, total leaf area (19 and 23% for A101 and A103, respectively), and chlorophyll levels in comparison with the mock treatment (non-inoculated plants) (Table 1).
Table 1.
Indicators of rice plants (Piauí variety) growth at 54 days after transplanting. Plant roots were inoculated with dark septate endophytic fungi (isolates A101 and A103) or left non-inoculated and fertilised with ammonium sulphate-15N as the sole inorganic source of N
| Treatment | Aerial part biomass (g plant−1) | Leaf number (unit plant−1) | Tiller number (unit plant−1) | Total leaf area (cm2 plant−1) | Chlorophyll levels |
|---|---|---|---|---|---|
| Control | 5.1 ± 0.1b | 17.0 ± 0.3b | 2.6 ± 0.2b | 474 ± 28b | 41.0 ± 0.6b |
| A101 | 5.9 ± 0.1a | 20.5 ± 0.2a | 4.2 ± 0.2a | 564 ± 36a | 44.1 ± 0.9a |
| A103 | 6.0 ± 0.2a | 21.0 ± 0.3a | 4.6 ± 0.2a | 582 ± 17a | 45.1 ± 0.9a |
| CV (%) | 6.81 | 3.31 | 13.59 | 11.68 | 4.45 |
Means ± SE (n = 5) followed by the same lowercase letter in the same column do not differ significantly, as indicated by the t test (p < 0.05). Absence of a letter indicates no significant difference, as determined by the F test (p < 0.05). SE, standard error
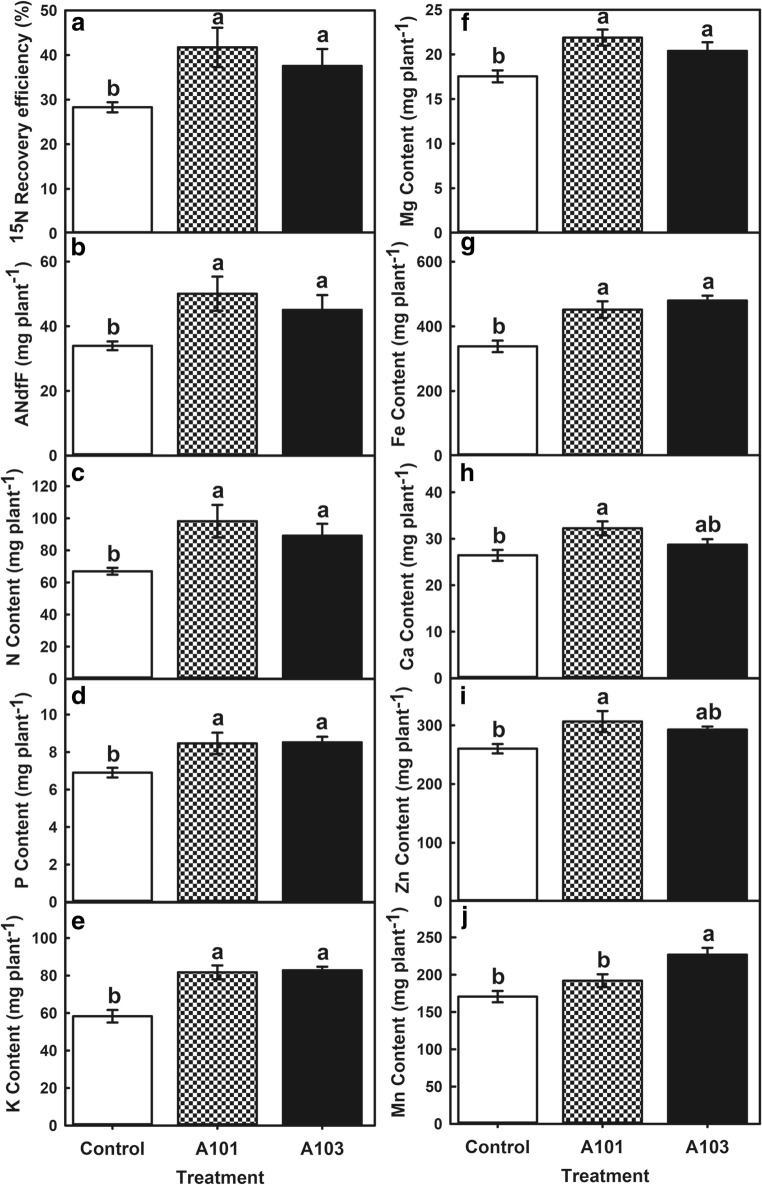
Fig. 2.
Recovery efficiency (a) and amount of nitrogen (b) in the plant derived from ammonium sulphate-15N, contents of N (c), P (d), K (e), Mg (f), Fe (g), Ca (h), Zn (i), and Mn (j) at 54 days after transplanting of rice plants (Piauí variety). Plant roots were inoculated with DSE fungi (isolates A101 and A103) or left non-inoculated (control group), and fertilised with ammonium sulphate-15N as the sole inorganic source of N. In each bar chart, values followed by the same lowercase letter do not differ between treatments at LSD t test (p < 0.05). Error bars indicate standard error of mean (n = 5)
Efficiency of nitrogen recovery from 15N-labelled ammonium sulphate
After investigating 15N abundance in the dry matter, the efficiency of N recovery and the amount of N derived from ammonium sulphate-15N were determined in mock-treated rice plants and in plants inoculated with DSE fungi. Compared with the control, plants inoculated with the A101 and A103 fungal isolates were more efficient at recovering N from the ammonium sulphate-15N provided as the sole N source; they exhibited significant respective increments of 47% and 33% for recovery efficiency and amount of nitrogen derived from ammonium sulphate-15N (Fig. 2a, b).
Effect of DSE on nutrient accumulation
The inoculation of rice plants with both A101 and A103 promoted a significant accumulation of N, P, K, Mg, and Fe in the aerial parts as compared with the control plants (Fig. 2c–g). Furthermore, inoculation with A101 stimulated the content of Ca and Zn in the aerial parts at similar levels to plants treated with A103 and higher than in the control plants (Fig. 2h, i). In addition, Mn contents in the aerial organs of plants inoculated with the isolate A103 were higher than in plants subjected to either of the other two treatments (Fig. 2j). N, P, and K levels increased 47 and 33%; 23%; and 40 and 42% in plants treated with A101 and A103, respectively, corroborating the higher 15N recovery efficiency observed. Besides, Ca and Mg contents increased 22 and 9% and 25 and 16%, respectively, while Fe, Zn, and Mn levels were 34 and 42%; 46 and 32%; and 12 and 33% higher upon inoculation with A101 and A03, respectively.
Activity of proton pumps
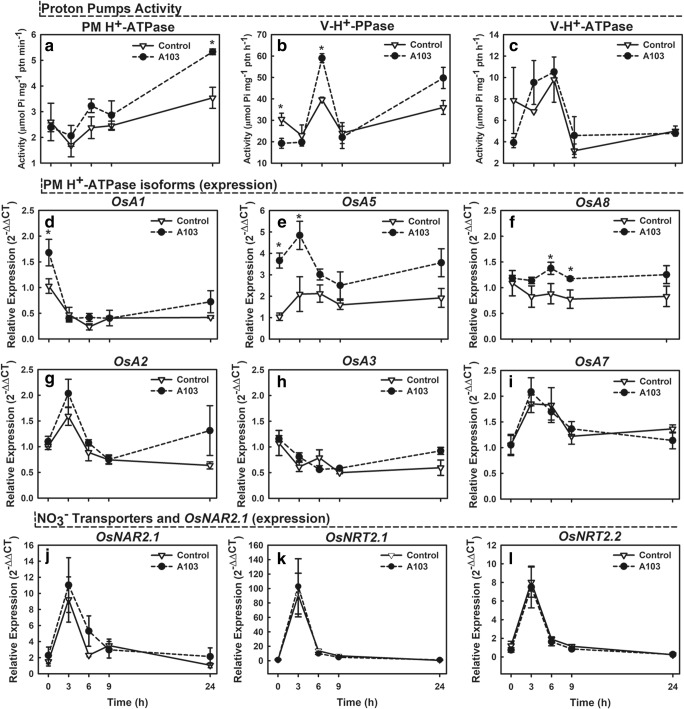
The exposure of plants inoculated with the fungal isolate A103 to 0.2 mM NO3− significantly increased the hydrolytic activity of ATP on PM H+-ATPase by 50% relative to the control 24 h after exposition to that compound (Fig. 3a). Similarly, inoculation with the same isolate also increased the hydrolytic activity of PPi on V-H+-PPase by 49 and 38% at 6 h (significant) and 24 h (not significant), respectively. On the other hand, V-H+-PPase activity significantly decreased by 37% in the absence of NO3− (0 h) (Fig. 3b), whereas V-H+-ATPase was not affected by inoculation (Fig. 3c).
Fig. 3.
Time course of PM H+-ATPase activity (a), V-H+-PPase activity (b), and V-H+-ATPase activity (c) and relative expression of gene encoding for NO3− transporters (OsNRT2.1 and OsNRT2.2), OsNAR2.1 protein (OsNAR2.1) (j–l) and PM H+-ATPase (OsA1, OsA2, OsA3, OsA5, OsA7, and OsA8) isoforms (d–i) from rice root (Piauí variety; 41-day old). Plants were inoculated with dark septate endophytic fungus A103 or left non-inoculated and exposed to 0.2 mM NO3−-N after 72 h of nitrogen starvation. Evaluations were performed at 0, 3, 6, 9, and 24 h after the addition of 0.2 mM NO3−-N. The bars indicate the standard error of the mean of four biological replicates. Asterisk (*) indicates significant differences between non-inoculated and inoculated treatment (LSD t test at p < 0.05)
Gene expression analysis
Analyses of quantitative real-time PCR with genes involved in NO3− uptake (OsNRT2.1, OsNRT2.2, and OsNAR2.1) and PM H+-ATPase activity (OsA1, OsA2, OsA3, OsA5, OsA7, and OsA8) were performed in order to evaluate the relative abundance of transcripts 0, 6, 9, and 24 h after the exposition of inoculated and non-inoculated plants to 0.2 mM NO3−. In the absence of NO3−, inoculation of plants with the isolate A103 significantly induced transcript abundance of the PM H+-ATPase isoform OsA1 by approximately twofold as compared with the control treatment. In contrast, no induction was observed when the nutritive solution was supplemented with NO3− (Fig. 3d). The OsA5 isoform was induced approximately fourfold in the absence of NO3−. When this compound was added to the medium, transcription increased about twofold as compared with the control treatment at time points 0 and 3 h, when it reached the highest levels (Fig. 3e). Supplementation of the nutritive solution with NO3− resulted in significantly increased (approximately twofold) levels of OsA8 transcript at time points 6 and 9 h (Fig. 3f). In contrast, the PM H+-ATPase isoforms OsA2, OsA3, and OsA7 (Fig. 3g–i), both NO3− transporters (OsNRT2.1 and 2.2), and OsNAR2.1 protein (Fig. 3j–l) were not induced by fungal inoculation.
Discussion
Piauí rice cultivar used in this study is a local landrace from Maranhão-Brazil that is used to cropping with low N fertilisation. Piauí has lower Michaelis-Menten constant (or high affinity) to nitrate uptake [75], especially when it is inoculated with the dark septate endophytic fungi A101 and A103 [46] and higher nitrogen remobilisation efficiency [76]. As previously reported [46, 77, 78], the hyphae of the fungal isolates A101 and A103 colonised the root of rice plants and formed abundant intracellular microsclerotia in the epidermis and cortex with no disease symptoms. These fungi were isolated from wild rice (O. glumaepatula) and belong to Pleosporales order (suborder Massarineae) (A103) and to an unknown taxon (A101) [46, 55].
In grasses [4, 46, 78, 79] and other plant species [4, 80] grown under controlled conditions after inoculation with DSE fungi and supplementation with sources of inorganic minerals, nutrient uptake is more efficient than in non-inoculated plants, resulting in a positive effect on dry matter accumulation in the roots and aerial parts. Likewise, in the present study, which was carried out in non-sterile substrate, inoculation of rice plants with DSE fungi (A101 and A103 isolates) significantly increased the 15N recovery efficiency and accumulation of macro- (N, P, K, Ca, and Mg) and micronutrients (Fe, Zn, and Mn) as well as chlorophyll, total leaf area, tiller number, and dry matter in the aerial parts in comparison with non-inoculated controls. Furthermore, inoculation with A103 also increased PM H+-ATPase and H+-pyrophosphatase activities and the transcription levels of the PM H+-ATPase isoforms OsA1, OsA5, and OsA8. These results indicate that inoculation with DSE fungi increases the efficiency of nutrient uptake and utilisation, although further investigation is required in order to evaluate grain yield and quality.
Twenty-four hours after exposure to 0.2 mM NO3−, plants inoculated with the fungal isolate A103 did not show increased transcript levels of OsNRT2.1 or OsNRT2.2, encoding NO3− transporters strictly involved in the activity of the inducible high affinity transport system (iHATS) [81, 82], or of the gene encoding the accessory protein OsNAR2.1 in comparison with non-inoculated plants. Therefore, it is necessary to evaluate the expression of other NO3− transporters induced by AMF, such as NRT2.3, NFP1.3, and especially the dual-affinity NFP6.3 transceptor (previously known as NRT1.1) [43–45]. NFP6.3 operates under both low and high NO3− concentrations, is able to detect this anion in the external medium, and regulates root growth in order to increase the likelihood of finding NO3− [83] and/or another fungus that helps the host plant to take up this nutrient from the soil, similar to the function of LjPT4, which is induced by mycorrhizal fungi in Lotus japonicus plants [84].
Under the conditions used in the present study, sandy soil irrigated to field capacity, it is expected that rice plants take up predominantly NO3−, since NH4+ is rapidly converted to NO3− in aerated soils [36]. The NO3− present in soil penetrates cells by symport with two protons (2H+:NO3−) generated by PM H+-ATPase [85, 86], where it can be incorporated into amino acids through the activity of a series of enzymes, such as nitrate and nitrite reductases and glutamine synthase, or stored in vacuoles [37, 87]. Unreduced NO3− and amino acids are exported across the xylem to the aerial parts for the synthesis of proteins, nucleic acids, and other cellular constituents required for crop growth and productivity, since nitrogen is one of the essential macronutrients that most limits productivity [87, 88]. Roots, particularly the growing root tips, also obtain amino acids and amides from mature plant organs through the phloem in order to produce proteins necessary for their growth [89]. Thus, the increments of 47% and 33% observed for recovery efficiency and amount of N derived from 15N-ammonium sulphate, as well as the increased contents of N in general, are in line with the greater amount of dry matter in the aerial parts of plants inoculated with the fungal isolates A101 and A103, respectively.
Significant elevation of approximately 40% in the K levels of plants inoculated with A101 and A103 in comparison with the control plants was observed. As observed previously by Vergara et al. [54], our fungal isolates could help the rice plant hosts to absorb K by transposing the depletion zone to sites away from the root surface. The authors also speculated that the increase of K and N was responsible for the greater leaf area observed in plants inoculated with fungal isolates of DSE. Indeed, in our study, we observed elevation of K and N levels associated with increases of approximately 20% in the total leaf area. This positive effect of fungal DSE isolates on the leaf area was previously observed by other studies [54, 90].
The higher P contents observed in the inoculated plants relative to the controls are a good indicator that the interaction of DSE fungi with rice plants allows them to better compete with the reactions for P fixation in the soil, which restrict P recovery to a mere 20% or less of the applied dose, even under adequate fertilisation [91]. P is therefore often very limiting for plant growth and development [92].
Because Ca ensures plasma membrane integrity and selectivity [93, 94], confers structural rigidity to the cell wall [95], and improves plant tissue resistance [95–97], it is possible that the accumulation of this mineral in inoculated plants is associated with improved protection of the host plants against biotic and abiotic stresses [98, 99].
The DSE fungi are also able to make the uptake of Mg and micronutrients such as Fe, Zn, and Mn, which are important for photosynthetic activity [54, 100, 101]. In this study, as compared with the control plants, inoculation with the fungal isolates A101 and A103 was associated with an elevation of Mg, Fe, Zn, and Mn levels. This observation results from a better recovery of these minerals at the source (soil, FTE BR12, and MineralCal). Vergara et al. [54] speculated that the increased micronutrient content due to the inoculation with DSE fungi is probably related to the ability of these fungi to produce siderophores. In fact, the DSE fungus Phialocephala fortinii produces the siderophore hydroxamate and improves Fe (III) uptake by the host plant [100]. An adequate Mg supply improves rice plant health and increases aerial part biomass, grain yield, chlorophyll levels, photosynthetic rate, and the activities of antioxidant enzymes. Nevertheless, its deficiency affects key chloroplast enzymes and leads to chlorophyll degradation [102–104].
The higher 15N recovery efficiency, together with the nutrient and dry matter accumulation observed in inoculated plants, especially with the fungus Pleosporales A103, might be associated with the stimulation of PM H+-ATPase, an enzyme involved in cellular expansion, nutrition, and intracellular pH regulation [30]. It could alternatively result from V-H+-PPase stimulation and its role in PM H+-ATPase activity, auxin transport, growth of roots and the aerial parts during the vegetative state, N and P uptake, resistance to drought and salt stress, and photosynthetic activity [105–109]. Indeed, plants inoculated with the fungal isolate A103 exposed to 0.2 mM NO3− showed significantly increased V-H+-PPase and PM H+-ATPase activities of 50 and 49% relative to the control treatment at 6 and 24 h, respectively. This elevated PM H+-ATPase activity is crucial to the uptake of anions such as NO3− and H2PO4−, which require the proton-motive force (H+ gradient) in order to overcome the negative electric potential (electrical gradient) that favours cation uptake across the plasma membrane of the root cells [86, 110].
The increased PM H+-ATPase activity was linked with an induction in the PM H+-ATPase isoforms OsA1, OsA5, and OsA8, members of families I, II, and V [111], respectively, but not with the OsA2, OsA3, and OsA7 isoforms. Transcripts of the isoforms OsA1, OsA5, and OsA8 were differentially expressed in inoculated plants. Inoculation with the fungal isolate A103 in the absence or the presence of NO3− significantly promoted the accumulation of OsA5 transcripts as compared with the control treatment. On the other hand, it stimulated OsA1 only in the absence and OsA8 in the presence of NO3, stressing out the specific transcription of OsA5 upon fungal inoculation. The protein encoded by rice OsA8/OsHA1 [24, 111], an orthologue of Medicago truncatula MtHA1 and Solanum lycopersicum (L.) SlHA8, stimulates root colonisation by AMF, improves H2PO4− uptake by the host plant, and creates an electrochemical potential gradient across the periarbuscular membrane to allow for bidirectional nutrient transport [11, 14, 15]. In addition, isoforms OsA5 and OsA8 are associated with NO3− uptake [24], while OsA8 is involved in P uptake and translocation to the aerial parts [112]. These data thus suggest that the isoforms OsA5 and OsA8 may be important in the establishment of the interaction between DSE fungi and rice plants.
It has already been reported that DSE fungi exude auxins and other metabolites [4, 5], increase the biomass of plant roots and aerial parts [4, 46, 78, 79], decrease the Michaelis-Menten constant for NO3− uptake, deplete greater amounts of this anion in the environment [46], and promote macro- [46, 80] and micronutrient accumulation [54, 100, 101]. Therefore, based on these findings and on our results, the present study proposes a model in which DSE fungi exudate metabolites stimulate the hydrolysis of ATP by PM H+-ATPase and/or enzyme accumulation, with V-H+-PPase optimising ATP utilisation (by means of PPi) and increasing PM H+-ATPase trafficking to the plasma membrane, which culminates with in an increased activity of the enzyme or in a larger extracellular acidification. The auxins and PM H+-ATPases would promote root hair elongation and lateral root ramification, enlarging the root surface to be colonised by the fungi and enhancing water and nutrient absorption. Therefore, inoculated plants would exhibit better performance in several physiological processes and growth indicators, which would translate into greater productivity. However, further studies on plant and fungal mutants are required in order to elucidate the exact role of PM H+-ATPase isoforms OsA5 and OsA8 and other nutrient transporters possibly responsive to the plant-fungus interaction, and to evaluate the eventual contribution of nutrient uptake pathways of DSE fungi and their transcriptome to plant host fitness.
Conclusion
The present study indicates that rice plants inoculated with isolates of dark septate endophytic fungi, A101 and A103, more efficiently recover macro- and micronutrients from the soil. They also present increased 15N amounts and recovery efficiency, as well as larger tiller number and improved growth. These effects are mediated by increased activities of plasma membrane H+-ATPase (PM H+-ATPase) and vacuolar H+-pyrophosphatase (V-H+-PPase) and by elevated transcript levels of the PM H+-ATPase genes OsA5 and OsA8 in rice plants inoculated with Pleosporales isolate A103. In addition, this study provides a more comprehensive perspective on the molecular (NO3− transporters, accessory protein NAR2.1, and PM H+-ATPase isoforms) and physiological (V-H+-ATPase, PM H+-ATPase, and V-H+-PPase activities) entities acting in rice plants inoculated with the DSE fungal isolate A103.
Acknowledgements
We are indebted to the University Federal Rural do Rio de Janeiro (UFRRJ), especially for the Laboratory Nutrition of Plants, the state University of Norte Fluminense Darcy Ribeiro (UENF), especially for LBCT, the Brazilian Agricultural Research Corporation (Embrapa), for their help with the work. This work was supported by the Foundation for Support of Research in the State of Rio de Janeiro (FAPERJ), the Brazilian National Council for Scientific and Technological Development (CNPq), and the Coordination for the Improvement of Higher Education Personnel (CAPES).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jumpponen A. Dark septate endophytes-are they mycorrhizal? Mycorrhiza. 2001;11:207–211. doi: 10.1007/s0057201001. [DOI] [Google Scholar]
- 2.Jumpponen A, Trappe JM. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. 1998;140:295–310. doi: 10.1046/j.1469-8137.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 3.Knapp DG, Kovács GM, Zajta E, Groenewald J, Crous PW. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia. 2015;35:87–100. doi: 10.3767/003158515X687669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthelot C, Leyval C, Foulon J, Chalot M, Blaudez D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol Ecol. 2016;92:fiw144. doi: 10.1093/femsec/fiw144. [DOI] [PubMed] [Google Scholar]
- 5.Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandyam K, Jumpponen A. Abundance and possible functions of the root-colonizing dark septate endophytic fungi. Stud Mycol. 2005;53:173–190. doi: 10.3114/sim.53.1.173. [DOI] [Google Scholar]
- 7.Upson R, Read DJ, Newsham KK. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza. 2009;20:1–11. doi: 10.1007/s00572-009-0260-3. [DOI] [PubMed] [Google Scholar]
- 8.Usuki F, Narisawa K. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia. 2007;99:175–184. doi: 10.3852/mycologia.99.2.175. [DOI] [PubMed] [Google Scholar]
- 9.Mandyam K, Loughin T, Jumpponen A. Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie. Mycologia. 2010;102:813–821. doi: 10.3852/09-212. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell BA, Jumpponen A, Trappe JM. Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia. 2000;92:230–230. doi: 10.2307/3761555. [DOI] [Google Scholar]
- 11.Krajinski F, Courty PE, Sieh D, Franken P, Zhang H, Bucher M, Gerlach N, Kryvoruchko I, Zoeller D, Udvardi M, Hause B. The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell. 2014;26:1808–1817. doi: 10.1105/tpc.113.120436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felle HH, Waller F, Molitor A, Kogel KH. The mycorrhiza fungus Piriformospora indica induces fast root-surface pH signaling and primes systemic alkalinization of the leaf apoplast upon powdery mildew infection. Mol Plant-Microbe Interact. 2009;22:1179–1185. doi: 10.1094/mpmi-22-9-1179. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Coria M, Hernandez-Mendoza JL, Sanchez-Nieto S. Trichoderma asperellum induces maize seedling growth by activating the plasma membrane H+-ATPase. Mol Plant-Microbe Interact. 2016;29:797–806. doi: 10.1094/mpmi-07-16-0138-r. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Liu J, Chen A, Ji M, Chen J, Yang X, Gu M, Qu H, Xu G. Analysis of tomato plasma membrane H+-ATPase gene family suggests a mycorrhiza-mediated regulatory mechanism conserved in diverse plant species. Mycorrhiza. 2016;26:645–656. doi: 10.1007/s00572-016-0700-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang E, Yu N, Zhang X, Liu C, Miller AJ, et al. A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell. 2014;26:1818–1830. doi: 10.1105/tpc.113.120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Q, Ouyang S, Wang A, Zhu W, Maiti R, Lin H, Hamilton J, Haas B, Sultana R, Cheung F, Wortman J, Buell CR. The institute for genomic research Osa1 rice genome annotation database. Plant Physiol. 2005;138:18–26. doi: 10.1104/pp.104.059063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaxiola RA, Palmgren MG, Schumacher K. Plant proton pumps. FEBS Lett. 2007;581:2204–2214. doi: 10.1016/j.febslet.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Janicka-Russak M (2011) Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In: Abiotic stress response in plants-physiological, biochemical and genetic perspectives. InTech
- 19.Taiz L, Zeiger E, Møller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal. Porto Alegre: Artmed. 888–888 p
- 20.Okumura M, Inoue S, Takahashi K, Ishizaki K, Kohchi T, Kinoshita T. Characterization of the plasma membrane H+-ATPase in the liverwort Marchantia polymorpha. Plant Physiol. 2012;159:826–834. doi: 10.1104/pp.112.195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanczewska J, Marco S, Vandermeeren C, Maudoux O, Rigaud JL, Boutry M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci U S A. 2005;102:11675–11680. doi: 10.1073/pnas.0504498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morsomme P, Boutry M. The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim Biophys Acta. 2000;1465:1–16. doi: 10.1016/S0005-2736(00)00128-0. [DOI] [PubMed] [Google Scholar]
- 23.Morth JP, Pedersen BP, Buch-Pedersen MJ, Andersen JP, Vilsen B, Palmgren MG, Nissen P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol. 2011;12:60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- 24.Sperandio MVL, Santos LA, Bucher CA, Fernandes MS, SRd S. Isoforms of plasma membrane H+-ATPase in rice root and shoot are differentially induced by starvation and resupply of NO3− or NH4+ Plant Sci. 2011;180:251–258. doi: 10.1016/j.plantsci.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Haruta M, Sussman MR. The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol. 2012;158:1158–1171. doi: 10.1104/pp.111.189167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem. 2010;285:17918–17929. doi: 10.1074/jbc.M110.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi Y, Takahashi K, Inoue S, Kinoshita T. Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:845–853. doi: 10.1093/pcp/pcu028. [DOI] [PubMed] [Google Scholar]
- 28.Falhof J, Pedersen Jesper T, Fuglsang Anja T, Palmgren M. Plasma membrane H+-ATPase regulation in the Center of Plant Physiology. Mol Plant. 2016;9:323–337. doi: 10.1016/j.molp.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Morales-Cedillo F, Gonzalez-Solis A, Gutierrez-Angoa L, Cano-Ramirez DL, Gavilanes-Ruiz M. Plant lipid environment and membrane enzymes: the case of the plasma membrane H+-ATPase. Plant Cell Rep. 2015;34:617–629. doi: 10.1007/s00299-014-1735-z. [DOI] [PubMed] [Google Scholar]
- 30.Haruta M, Gray WM, Sussman MR. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr Opin Plant Biol. 2015;28:68–75. doi: 10.1016/j.pbi.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson F, Sommarin M, Larsson C. Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal inhibitory domain. Plant Cell. 1993;5:321–327. doi: 10.1105/tpc.5.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korthout HA, de Boer AH. A fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. Plant Cell. 1994;6:1681–1692. doi: 10.1105/tpc.6.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez-Najera N, Munoz-Clares RA, Palacios-Bahena S, Ramirez J, Sanchez-Nieto S, et al. Fumonisin B1, a sphingoid toxin, is a potent inhibitor of the plasma membrane H+-ATPase. Planta. 2005;221:589–596. doi: 10.1007/s00425-004-1469-1. [DOI] [PubMed] [Google Scholar]
- 34.Lupini A, Araniti F, Mauceri A, Princi MP, Sorgona A, et al. Coumarin enhances nitrate uptake in maize roots through modulation of plasma membrane H+ -ATPase activity. Plant Biol (Stuttg) 2017;20:390–398. doi: 10.1111/plb.12674. [DOI] [PubMed] [Google Scholar]
- 35.Lassaletta L, Billen G, Grizzetti B, Anglade J, Garnier J. 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ Res Lett. 2014;9:105011. doi: 10.1088/1748-9326/9/10/105011. [DOI] [Google Scholar]
- 36.Cole J. Controlling environmental nitrogen through microbial metabolism. Trends Biotechnol. 1993;11:368–372. doi: 10.1016/0167-7799(93)90160-b. [DOI] [PubMed] [Google Scholar]
- 37.De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 38.Aslam M, Travis RL, Huffaker RC. Comparative induction of nitrate and nitrite uptake and reduction systems by ambient nitrate and nitrite in intact roots of barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1993;102:811–819. doi: 10.1104/pp.102.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krebs M, Beyhl D, Gorlich E, Al-Rasheid KA, Marten I, et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci U S A. 2010;107:3251–3256. doi: 10.1073/pnas.0913035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmgren MG. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 41.Miller AJ, Smith SJ. Nitrate transport and compartmentation in cereal root cells. J Exp Bot. 1996;47:843–854. doi: 10.1093/jxb/47.7.843. [DOI] [Google Scholar]
- 42.Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–2306. doi: 10.1093/jxb/erm066. [DOI] [PubMed] [Google Scholar]
- 43.Drechsler N, Courty PE, Brule D, Kunze R. Identification of arbuscular mycorrhiza-inducible nitrate transporter 1/peptide transporter family (NPF) genes in rice. Mycorrhiza. 2017;28:93–100. doi: 10.1007/s00572-017-0802-z. [DOI] [PubMed] [Google Scholar]
- 44.Hildebrandt U, Schmelzer E, Bothe H. Expression of nitrate transporter genes in tomato colonized by an arbuscular mycorrhizal fungus. Physiol Plant. 2002;115:125–136. doi: 10.1034/j.1399-3054.2002.1150115.x. [DOI] [PubMed] [Google Scholar]
- 45.Saia S, Rappa V, Ruisi P, Abenavoli MR, Sunseri F, Giambalvo D, Frenda AS, Martinelli F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci. 2015;6:815. doi: 10.3389/fpls.2015.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vergara C, Araujo KEC, Alves LS, Souza SR, Santos LA, et al. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol. 2018;49:67–78. doi: 10.1016/j.bjm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saikkonen K, Faeth SH, Helander M, Sullivan TJ. Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst. 1998;29:319–343. doi: 10.1146/annurev.ecolsys.29.1.319. [DOI] [Google Scholar]
- 48.Mandyam KG, Jumpponen A (2015) Mutualism–parasitism paradigm synthesized from results of root-endophyte models. Front Microbiol 5. 10.3389/fmicb.2014.00776 [DOI] [PMC free article] [PubMed]
- 49.Wilcox HE, Wang CJK. Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings. Can J For Res. 1987;17:884–899. doi: 10.1139/x87-140. [DOI] [Google Scholar]
- 50.Diene O, Wang W, Narisawa K. Pseudosigmoidea ibarakiensis sp. nov., a dark septate endophytic fungus from a cedar Forest in Ibaraki, Japan. Microbes Environ. 2013;28:381–387. doi: 10.1264/jsme2.ME13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newsham KK. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011;190:783–793. doi: 10.1111/j.1469-8137.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 52.Vergara C, Araujo KEC, Urquiaga S, Santa-Catarina C, Schultz N, da Silva Araújo E, de Carvalho Balieiro F, Xavier GR, Zilli JÉ. Dark septate endophytic fungi increase green manure-(15)N recovery efficiency, N contents, and micronutrients in rice grains. Front Plant Sci. 2018;9:613. doi: 10.3389/fpls.2018.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freire L, Balieiro FdC, Zonta E, Anjos Ld, Pereira M, et al. (2013) Manual de calagem e adubação do Estado do Rio de Janeiro. Seropédica, RJ: Universidade Rural do Rio de Janeiro: Editora Universidade Rural. 430 p
- 54.Vergara C, Araujo KEC, Urquiaga S, Schultz N, FdC B et al (2017) Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front Microbiol 8. 10.3389/fmicb.2017.02437 [DOI] [PMC free article] [PubMed]
- 55.Ribeiro KG (2011) Fungos endofíticos dark septates em arroz silvestre Oryza glumaepatula Steund [Dissertation]. Boa Vista,RO: Universidade Federal de Roraima. 68–68 p
- 56.Andrade-Linares DR, Grosch R, Restrepo S, Krumbein A, Franken P. Effects of dark septate endophytes on tomato plant performance. Mycorrhiza. 2011;21:413–422. doi: 10.1007/s00572-010-0351-1. [DOI] [PubMed] [Google Scholar]
- 57.Smith J, Um MH. Rapid procedures for preparing soil and KCl extracts for 15N analysis. Communications in Soil Science & Plant Analysis. 1990;21:2173–2179. doi: 10.1080/00103629009368368. [DOI] [Google Scholar]
- 58.ISO 12914 . Soil quality-microwave-assisted extraction of the aqua regia soluble fraction for the determination of elements. Geneva: International Organization for Standardization; 2012. [Google Scholar]
- 59.Tedesco MJ (1982) Extração simultânea de N, P, K, Ca, e Mg em tecido de plantas por disgestão com H2O2-H2SO4. Porto Alegre, RS: UFRGS. 23–23 p
- 60.Boddey RM, Alves BJR, Urquiaga S. Quantificação da fixação biológica de nitrogênio associada a plantas utilizando o isótopo 15N. In: Hungria M, Araujo RS, editors. Manual de métodos empregados em estudos de microbiologia agrícola. Brasília: Embrapa-SPI; 1994. pp. 471–494. [Google Scholar]
- 61.IAEA . Training course series No 14. Use of isotope and radiation methods in soil and water management and crop nutrition. Vienna: IAEA; 2001. [Google Scholar]
- 62.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Stn Bull. 1950;347:1–32. [Google Scholar]
- 63.Furlani AMC, Furlani PR (1988) Composição e pH de soluções nutritivas para estudos fisiológicos e seleção de plantas em condições nutricionais adversas. Campinas
- 64.Lee RB, Rudge RA. Effects of nitrogen deficiency on the absorption of nitrate and ammonium by barley plants. Ann Bot. 1986;57:471–486. doi: 10.1093/oxfordjournals.aob.a087129. [DOI] [Google Scholar]
- 65.Bucher CA, Santos LA, Nogueira EM, Rangel RP, de Souza SR, et al. The transcription of nitrate transporters in upland rice varieties with contrasting nitrate-uptake kinetics. J Plant Nutr Soil Sci. 2014;177:395–403. doi: 10.1002/jpln.201300086. [DOI] [Google Scholar]
- 66.Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- 67.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 68.Facanha AR, De Meis L. Inhibition of maize root H+-ATPase by fluoride and fluoroaluminate complexes. Plant Physiol. 1995;108:241–246. doi: 10.1104/pp.108.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos LA, Bucher CA, Souza SR, Fernandes MS. Effects of nitrogen stress on proton-pumping and nitrogen metabolism in rice. J Plant Nutr. 2009;32:549–564. doi: 10.1080/01904160802714953. [DOI] [Google Scholar]
- 70.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 71.Yan F, Zhu Y, Muller C, Zorb C, Schubert S. Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 2002;129:50–63. doi: 10.1104/pp.010869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grace C, Stribley DP. A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res. 1991;95:1160–1162. doi: 10.1016/S0953-7562(09)80005-1. [DOI] [Google Scholar]
- 73.Mahmoud RS, Narisawa K. A new fungal endophyte, Scolecobasidium humicola, promotes tomato growth under organic nitrogen conditions. PLoS One. 2013;8:e78746–e78746. doi: 10.1371/journal.pone.0078746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.(2017) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing
- 75.Santos LA, Santos WA, Sperandio MVL, Bucher CA, SRd S, et al. Nitrate uptake kinetics and metabolic parameters in two rice varieties grown in high and low nitrate. J Plant Nutr. 2011;34:988–1002. doi: 10.1080/01904167.2011.555581. [DOI] [Google Scholar]
- 76.Souza SR, Stark EMLM, Fernandes MS (1998) Nitrogen remobilization during the reproductive period in two Brazilian rice varieties. J Plant Nutr:2049–2063
- 77.Qin Y, Pan X, Kubicek C, Druzhinina I, Chenthamara K, Labbé J, Yuan Z. Diverse plant-associated pleosporalean fungi from saline areas: ecological tolerance and nitrogen-status dependent effects on plant growth. Front Microbiol. 2017;8:158. doi: 10.3389/fmicb.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan ZL, Llin FC, Zhang CL, Kubicek CP. A new species of Harpophora (Magnaporthaceae) recovered from healthy wild rice (Oryza granulata) roots, representing a novel member of a beneficial dark septate endophyte. FEMS Microbiol Lett. 2010;307:94–101. doi: 10.1111/j.1574-6968.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- 79.Santos SG, Silva PR, Garcia AC, Zilli JE, Berbara RL. Dark septate endophyte decreases stress on rice plants. Braz J Microbiol. 2017;48:333–341. doi: 10.1016/j.bjm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jumpponen A, Mattson KG, Trappe JM. Mycorrhizal functioning of Phialocephala fortinii with Pinus contorta on glacier forefront soil: interactions with soil nitrogen and organic matter. Mycorrhiza. 1998;7:261–265. doi: 10.1007/s005720050190. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto M, Kumar A, Li W, Wang Y, Siddiqi MY, Crawford NM, Glass ADM. High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 2006;140:1036–1046. doi: 10.1104/pp.105.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuo D, Okamoto M, Vidmar JJ, Glass ADM. Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999;17:563–568. doi: 10.1046/j.1365-313X.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 83.Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volpe V, Giovannetti M, Sun XG, Fiorilli V, Bonfante P. The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots. Plant Cell Environ. 2016;39:660–671. doi: 10.1111/pce.12659. [DOI] [PubMed] [Google Scholar]
- 85.Thibaud JB, Grignon C. Mechanism of nitrate uptake in corn roots. Plant Sci Lett. 1981;22:279–289. doi: 10.1016/0304-4211(81)90241-8. [DOI] [Google Scholar]
- 86.Sondergaard TE, Schulz A, Palmgren MG. Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol. 2004;136:2475–2482. doi: 10.1104/pp.104.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoneyama T, Ito O, Engelaar WMHG. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem Rev. 2003;2:121–132. doi: 10.1023/B:PHYT.0000004198.95836.ad. [DOI] [Google Scholar]
- 88.Yoneyama T, Fujita K, Yoshida T, Matsumoto T, Kambayashi I, Yazaki J. Variation in natural abundance of 15N among plant parts and in 15N/14N fractionation during N2 fixation in the legume-rhizobia symbiotic system. Plant Cell Physiol. 1986;27:791–799. doi: 10.1093/oxfordjournals.pcp.a077165. [DOI] [Google Scholar]
- 89.Tatsumi J, Kono Y. Root growth of rice plants in relation to nitrogen supply from shoot. Jpn J Crop Sci. 1980;49:112–119. doi: 10.1626/jcs.49.112. [DOI] [Google Scholar]
- 90.Valli PPS, Muthukumar T. Dark septate root endophytic fungus Nectria haematococca improves tomato growth under water limiting conditions. Indian J Microbiol. 2018;58:1–7. doi: 10.1007/s12088-018-0749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Russell DW. Soil conditions and plant growth. New York: Longman Group Ltd; 1973. [Google Scholar]
- 92.Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 93.Hanson JB. The functions of calcium in plant nutrition. In: Tinker PB, Lauchli A, editors. Advances in plant nutrition. New York: Praeger Publishers; 1984. pp. 149–208. [Google Scholar]
- 94.Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hepler PK, Winship LJ. Calcium at the cell wall-cytoplast interface. J Integr Plant Biol. 2010;52:147–160. doi: 10.1111/j.1744-7909.2010.00923.x. [DOI] [PubMed] [Google Scholar]
- 96.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 97.Hirschi KD. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong-Bo S, Li-Ye C, Ming-An S, Shi-Qing L, Ji-Cheng Y. Bioengineering plant resistance to abiotic stresses by the global calcium signal system. Biotechnol Adv. 2008;26:503–510. doi: 10.1016/j.biotechadv.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartholdy B, Berreck M, Haselwandter K. Hydroxamate siderophore synthesis by Phialocephala fortinii, a typical dark septate fungal root endophyte. BioMetals. 2001;14:33–42. doi: 10.1023/A:101668702. [DOI] [PubMed] [Google Scholar]
- 101.Haselwandter K (2009) Mycorrhizal fungi: colonisation pattern of alpine plants and ecological significance of siderophore release. Asp Appl Biol:105–108
- 102.Ding Y-C, Chang C-R, Luo W, Wu Y-S, Ren X-L, et al. High potassium aggravates the oxidative stress induced by magnesium deficiency in rice leaves. 1 1Project supported by the Dead Sea Works Ltd. Israel Pedosphere. 2008;18:316–327. doi: 10.1016/S1002-0160(08)60021-1. [DOI] [Google Scholar]
- 103.Shaul O. Magnesium transport and function in plants: the tip of the iceberg. Biometals. 2002;15:309–323. doi: 10.1104/pp.112.199778. [DOI] [PubMed] [Google Scholar]
- 104.Hermans C, Vuylsteke M, Coppens F, Cristescu SM, Harren FJ, et al. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010;187:132–144. doi: 10.1111/j.1469-8137.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 105.Li J, Yang H, Peer WA, Richter G, Blakeslee J, et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 106.Li X, Guo C, Gu J, Duan W, Zhao M, Ma C, du X, Lu W, Xiao K. Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under pi and N deprivation, high salinity, and drought. J Exp Bot. 2014;65:683–696. doi: 10.1093/jxb/ert442. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Paez-Valencia J, Sanchez-Lares J, Marsh E, Dorneles LT, Santos MP, Sanchez D, Winter A, Murphy S, Cox J, Trzaska M, Metler J, Kozic A, Facanha AR, Schachtman D, Sanchez CA, Gaxiola RA. Enhanced proton translocating pyrophosphatase activity improves nitrogen use efficiency in Romaine lettuce. Plant Physiol. 2013;161:1557–1569. doi: 10.1104/pp.112.212852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci U S A. 2005;102:18830–18835. doi: 10.1073/pnas.0509512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang H, Zhang X, Gaxiola RA, Xu G, Peer WA, Murphy AS. Over-expression of the Arabidopsis proton-pyrophosphatase AVP1 enhances transplant survival, root mass, and fruit development under limiting phosphorus conditions. J Exp Bot. 2014;65:3045–3053. doi: 10.1093/jxb/eru149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duby G, Boutry M. The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch. 2009;457:645–655. doi: 10.1007/s00424-008-0457-x. [DOI] [PubMed] [Google Scholar]
- 111.Arango M, Gevaudant F, Oufattole M, Boutry M. The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta. 2003;216:355–365. doi: 10.1007/s00425-002-0856-8. [DOI] [PubMed] [Google Scholar]
- 112.Chang C, Hu Y, Sun S, Zhu Y, Ma G, Xu G. Proton pump OsA8 is linked to phosphorus uptake and translocation in rice. J Exp Bot. 2009;60:557–565. doi: 10.1093/jxb/ern298. [DOI] [PubMed] [Google Scholar]