Abstract
Optical imaging is commonly used to investigate biological flows and cardiovascular disease using compliant silicone polydimethysiloxane (PDMS) Sylgard 184 geometries. However, selecting the working fluid with blood density and viscosity, and PDMS index of refraction (RI) for such experiments is challenging. Currently, water-glycerol is commonly used and sodium iodide (NaI) often added to increase the index of refraction without changing fluid viscosity. But the resulting fluid density is well above blood. Moreover, NaI is expensive, has safety and material discoloration concerns, and has been reported to affect non-Newtonian fluid behavior. Here, we present a new blood analog alternative based on urea. Urea is approximately five to fifteen times less expensive than NaI, safe and easy to handle, optically clear, and causes no discoloration. Water-glycerol-urea solutions, unlike those with NaI, simultaneously matched the density and viscosity of blood and RI of PDMS. Water-xylitol and water-xylitol-urea solutions are also possible blood analog solutions. Xanthan gum (XG)-water-glycerol non-Newtonian solutions maintained similar viscoelastic properties throughout the range of weight percent (about 15–25%) of urea and NaI used here. The results showed that the XG weight percent affected viscoelastic properties more than the weight percent of urea or NaI tested in this study. Overall, we demonstrate urea is useful for PDMS blood analog experiments and should also be considered as an inexpensive additive, and an alternative to NaI.
Keywords: index matching, blood analog, PDMS, urea, sodium iodide
Graphical Abstract
1. Introduction
In vitro flow experiments are used to evaluate a range of vascular diseases. Optical in vitro imaging measurement techniques, such as particle image velocimetry (PIV), are used because they provide well-controlled flow fields with high spatiotemporal resolution. For such experiments, the test geometries are often made of silicone polydimethysiloxane (PDMS) Sylgard 184 because the versatility in the curing process allows for specific shapes and compliances to be represented (Wright et al. 2017). Although 3D printing and plastic materials are also used, they are limited to rigid test sections. Detailed evaluation and review of in vitro experimental techniques, including test section manufacturing, are presented by Wright et al. (2017) and Yazdi et al. (2018).
For optical experiments using PDMS models, selecting the proper working fluid is critical, but difficult even for Newtonian fluids (Yazdi et al. 2018). Imaging requires the fluid to be optically clear and have the same index of refraction (RI) as the material of the geometry section. For complex geometries, even small differences in RI can result in significant optical distortion (Patil and Liburdy 2012). The working fluid must also simultaneously match the fluid properties of blood (ρ = 1060 kg/m3, μ = 2.9–4.37 mPa-s) (Mayer 1964; Yazdi et al. 2018). While dynamic scaling may be used in cases where the working fluid does not match that of blood, this is not always possible and can add complexity to the experiment and the calculation of properly scaled spatiotemporal gradients. A 60/40 (by volume) water-glycerol mixture is one of the most widely used blood analogue solutions for use with PDMS. However, a 60/40 water-glycerol mixture yields an index of refraction of approximately 1.39, well below that of PDMS at 1.414 (Yousif et al. 2011). A water-glycerol mixture with the same RI of PDMS has a viscosity about 2–3 times higher than blood. To increase the RI of a 60/40 water glycerol solution, sodium iodide (NaI) is often added because it increases index but does not change the kinematic viscosity of the fluid (Long et al. 2005; Yousif et al. 2011; Najjari et al. 2016). However, NaI is expensive, corrosive to equipment, and can cause discoloration of material upon direct contact (Yousif et al. 2011; Bai and Katz 2014; Najjari et al. 2016). NaCl has also been used to increase the index of refraction of water-glycerol, but this increases the solution viscosity above that of blood (Shuib et al. 2010). Najjari et al. (2016) tested sodium thiocyanate (NaSCN) and potassium thiocyanate (KSCN) as additives to water-glycerol, but safety of both chemicals is a concern, particularly when heated. Other proposed Newtonian blood analog solutions include a mixture of ethanol and diethylphthalate (Nguyen 2004) and a mixture of isopropyl alcohol and glycerol (Bale-Glickman et al. 2003). However, alcohols have densities lower than water (and thus blood) and are flammable, presenting safety issues for use with high-power lasers.
Among Newtonian blood analog solutions, fluid density is rarely considered. For example, the density of water-glycerol-NaI solutions matching the RI of Sylgard 184 is typically about 1250 kg/m3 (Najjari et al. 2016; Wright et al. 2017; Yazdi et al. 2018), substantially higher than the density of blood. NaI is often added because of the convenience of not changing the viscosity, but its high density increases fluid density significantly, a concern for PIV studies where tracer particles must be neutrally buoyant. While water-glycerol-NaI solutions can simultaneously match the RI of PDMS and viscosity of blood, it is not able to simultaneously match these two parameters and the density of blood.
The effect of each additive on non-Newtonian fluids must also be considered. Most often a very small amount (<0.06% by weight) of xanthan gum (XG) is added to the base solution mixture, to yield a non-Newtonian fluid. Long et al. (2005) reported that NaI does not affect viscoelastic properties of an XG-water-glycerol solution. However, Najjari et al. (2016) reported NaI significantly reduced the shear-thinning properties of such solutions. Deplano et al. (2014) also reported that the rheology of XG solutions is sensitive to the addition of salts.
In this work, we present a novel blood analog solution—a mixture of water-glycerol and urea—which is able to simultaneously match the index of refraction of PDMS, and the density and viscosity of blood. Like NaI, urea increases the index of refraction of water and water-glycerol linearly (Warren and Gordon 1966). Urea is less expensive (about 5–15x) and safer than NaI. By comparison, urea causes small increases in the kinematic viscosity and density of water-glycerol solutions, while NaI causes minimal change in kinematic viscosity and a large increase in density. We tested different concentrations of water-glycerol-urea solutions and compared these solutions to mixtures of water-glycerol-NaI. To test the effect of urea on XG solutions, we added various concentrations of XG to water-glycerol-urea and compared the viscoelastic properties to XG-water-glycerol-NaI solutions. Xylitol was also tested as a substitute for glycerol. Although in this work, we limit evaluation to blood analog solutions for use with PDMS, urea should be considered as a cost-effective and safe additive for index matching in any PIV experiment.
2. Materials and Methods
2.1. Working fluid preparation
Fluids were prepared using ultrapure water (Thermo Scientific Barnstead NANOpure Water Purification System) and mixed with a magnetic stir bar at room temperature. Additives included urea (Fisher Chemical), xylitol (99%, Alfa Aesar), sodium iodide (Technical Grade 9080, IodiTech), sodium chloride (Fisher Chemical), glycerol (99%, PTI Process Chemicals), and xanthan gum (Hodgson Mill). All chemicals were used as received from the supplier, with no additional purification.
To obtain baseline information, two-component Newtonian mixtures, where each additive was added incrementally to the ultrapure water, were first tested. Solutions were magnetically stirred for approximately 30 minutes before testing. The temperature, index of refraction, density, and dynamic viscosity were recorded during each iteration of Newtonian testing, as described in Section 2.2.
Three-component Newtonian mixtures were then tested using ultrapure water, glycerol, and NaI or urea. Three ratios of water-glycerol were used to represent low, medium (average), and high values of reported blood viscosity. The three water-glycerol base mixtures were allowed to stir for 24 hours and subsequently split into two 200 mL sub-volumes, resulting in six total solutions. Incremental amounts of NaI or urea were added to the water-glycerol solutions until the refractive index of PDMS Sylgard 184 was reached. Each incremental addition was allowed to stir for about 30 minutes before testing.
Newtonian testing was done using the six final PDMS-index matched water-glycerol-urea and water-glycerol-NaI solutions. Each of the six solutions were split into four 50 mL sub-volumes, resulting in 24 total samples. Xanthan gum was added at 0.02, 0.04 and 0.06 wt%. All samples were stirred constantly for 3–4 days to ensure well-mixed solutions. Temperature, index of refraction, density, and oscillatory elasticity and viscosity were obtained for all non-Newtonian fluids.
2.2. Fluid Property Measurement
Refractive indices were measured at 589 nm with a handheld, digital Abbe Refractometer (Model PA202) with range of 1.3330–1.5000 nD and +/−0.0001 precision. Densities were calculated as the average of three mass/volume measurements. Volumes were measured using a 1000 μL pipette for highest accuracy. Rheological properties were measured with a Discovery HR-2 Hybrid Rheometer (TA Instruments) using a cone (1°:00’:11” and 40-mm diameter) geometry. These tests were performed under controlled temperature conditions (23.6°C ±0.02°C) correlating with the initial fluids at room temperature.
2.2.1. Newtonian
The steady shear (sweep mode) experiments were conducted using the cone geometry with a gap of 66 μm and shear rate ranging from 1 to 500 s−1. In sweep mode, a steady and increasing shear rate is applied to the fluid to evaluate variations of viscosity with shear rate. Newtonian fluids plateaued to a constant viscosity at higher shear rates, and these values were used to obtain the dynamic viscosity, μ∞ (Pa-s), of the fluid. For the chosen shear rate range, the kinematic viscosity remained constant throughout.
2.2.2. Non-Newtonian
Non-Newtonian fluids were tested using the cone geometry with a 66 μm gap and an oscillatory shear stress of 2 Hz to obtain the storage modulus, G’ (Pa), and loss modulus, G” (Pa). These parameters were used to compute the elasticity and viscosity of the fluids respectively. Oscillatory elasticity, ζ (m2/s), was calculated according to the Equation 1:
| (1) |
where ω is angular frequency (ω = 12.566 rad/s) and ρ is the density of the fluid (kg/m3). Oscillatory viscosity, ν (m2/s), was calculated using Equation 2:
| (2) |
Triplicate oscillatory shear rate runs were performed, and the G’ and G” values averaged. The averaged values were used to compute the oscillatory viscosity and elasticity. The results are presented with uncertainty bars showing ±1 standard deviation of the three run average.
3. Results and discussion
3.1. Two-component Newtonian Fluids
Properties of the two-component Newtonian fluids were characterized at room temperature to identify feasible alternatives to NaI for the purpose of increasing the index of refraction of blood analogs. Alternatives to water-glycerol solutions were also investigated. Urea and xylitol were identified as low cost, low density, RI increasing additives. Both urea and xylitol are safer and about 5x less-expensive per unit mass than NaI. Urea solutions are also more stable than NaI, improving the safety and usability of long-term stored solutions. Table 1 summarizes the properties of each additive used in this study.
Table 1.
Properties of chemical additives used in two-component solutions.
| Material | Chemical Formula | Density (kg/m3) | Solubility (g/L) | Cost per 500g (USD)† | Safety‡ |
|---|---|---|---|---|---|
| Urea | CH4N2O | 1320 | 545 (25°C) |
$8.95 – 58.90 | Health: 1 |
| Flammability: 0 | |||||
| Instability: 0 | |||||
| Sodium Iodide | NaI | 3670 | 1840 (25°C) |
$141.80 – 264.00 | Health: 2 |
| Flammability: 0 | |||||
| Instability: 1 | |||||
| Xylitol | C5H12O5 | 1520 | 1700 (20°C) |
$68.30 – 84.00 | Health: 1 |
| Flammability: 0 | |||||
| Instability: 1 | |||||
| Sodium Chloride | NaCl | 2160 | 100 (20°C) |
$7.35 – 39.50 | Health: 1 |
| Flammability: 0 | |||||
| Instability: 1 | |||||
| Glycerol | C3H8O3 | 1261 | Miscible | $10.29 – 92.86 | Health: 1 |
| Flammability: 1 | |||||
| Instability: 1 |
Prices ranges based on Fischer Scientific Alfa Aesar 99+%, Sigma-Aldrich ACS reagent grade, and Carolina Biological laboratory grade. Xylitol is not available from Carolina Biological and based on other two prices only.
As defined by NFPA 704 on chemical SDS; 0 – least hazardous and 4 – most hazardous
The index of refraction, density and viscosity of each aqueous solutions of each additive are shown in Figure 1a, 1b, and 1c, respectively. Figure 1a shows that by weight percent (wt%), both xylitol and urea were about as effective as NaI at increasing the index of refraction of water. NaCl was most effective, but the solution quickly lost clarity and reached saturation at an index of refraction of 1.3793. Figure 1b shows that the increase in fluid density as a function of additive weight percent was about five times less for xylitol and urea than NaI. Specifically, an aqueous 50.21 wt% NaI solution had a density of 1416 kg/m3, while at similar weight percent, aqueous urea (49.83 wt%) had a density of 1167 kg/m3 and aqueous xylitol (49.94 wt%) had a density of 1190 kg/m3. Figure 1c indicates that xylitol caused a larger increase in viscosity of the fluid than both urea and NaI. A 49.94 wt% solution of xylitol had a viscosity of 8.493E-3 Pa-s, while similar weight percent solutions of urea (49.83 wt%) and NaI (50.21 wt%) had viscosities of 1.857E-3 Pa-s and 1.213E-3 Pa-s, respectively.
Figure 1.
Effect of aqueous solutions of glycerol, xylitol, urea, sodium chloride, and sodium iodide on the index of refraction (a), density (b), and viscosity (c) of water.
These results demonstrate that urea can be used as a low-density additive for changing the refractive index of working fluids. Among additives tested, NaI and urea had the smallest and similar effect on dynamic viscosity. These results agreed with the general consensus that NaI has negligible effect on kinematic viscosity. For blood analogs matching refractive indices in the range of 1.38 to 1.40, a mixture of water-xylitol should be considered. For example, you can achieve a water-xylitol solution where the RI = 1.3915, ρ = 1139 kg/m3, and μ = 3.619E-3 Pa-s.
3.2. Three-component Newtonian Fluids
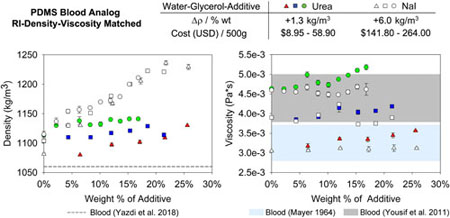
Urea and NaI were used to make three-component Newtonian solutions of water-glycerol-additive to develop blood analogs. Xylitol was not included here because the resulting solution when mixed with glycerol has a viscosity 2–3 times that of blood. Figure 2 shows the RI (2a), density (2b) and viscosity (2c) of these fluids. Water-glycerol solutions were prepared to represent low (3.0E-3 Pa-s), medium (3.8E-3 Pa-s), and high (4.6E-3 Pa-s) values of reported blood viscosity (Mayer 1964; Yousif et al. 2011). The corresponding water-glycerol volume ratios for the low, medium, and high viscosity solutions were 67/33, 62/38 and 58/42, respectively.
Figure 2.
Index of refraction (a), density (b), and viscosity (c) variations of three-component Newtonian solutions when urea and NaI were added to water-glycerol. Three starting ratios of water-glycerol were tested (low, medium, high) to span the range of reported human blood viscosities.
As shown in Figure 2a, the RI of both the urea and NaI solutions increased at a similar rate as a function of weight percent. The change of index as a function of weight percent of urea was independent of the starting ratio of water-glycerol in this range. Thus, when using urea, the desired fluid properties and RI can be planned and adjusted based on weight percent of glycerol and urea. Considering the solution density, as observed in Figure 2b, the addition of urea caused a density increase of about 1.3 kg/m3 per wt%, while NaI caused a density increase of approximately 6.0 kg/m3 per wt%, a nearly five-fold difference. Thus, when adding NaI, the increase in density is substantial and should be considered. In terms of viscosity, urea increased the fluid viscosity of the low, medium, and high water-glycerol solutions by 17, 7, and 12%, respectively. Thus, when adding urea to water-glycerol solutions, a small increase of viscosity is expected, but the fluid viscosity can still remain within the range of blood viscosity. Table 2 summarizes the final properties of all three-component solutions. As previously indicated in Table 1, the urea solutions are approximately 5 times less expensive than the NaI solutions. Further, urea solutions are safe and easy to make and use. A water-glycerol-urea solution was left in an experimental flow loop for approximately 6 weeks and in a Pyrex container for approximately four months. No discoloration of the fluid or any flow loop components was observed.
Table 2.
Final properties of three-component Newtonian fluids.
| Starting Viscosity | Material | Wt% Water | Wt% Glycerol | Wt% Additive | Refractive Index | Viscosity (Pa-s) | Density* (kg/m3) |
|---|---|---|---|---|---|---|---|
| Blood; | -- | -- | -- | 1.4118 | 2.81E-3 – 3.72E-31 | 1060 | |
| PDMS | 3.8E-3 – 5.0E-32 | ||||||
| LOW | Urea | 45.64 | 28.77 | 25.58 | 1.4118 | 3.564E-3 | 1130 |
| NaI | 45.51 | 28.70 | 25.79 | 1.4138 | 3.117E-3 | 1229 | |
| MEDIUM | Urea | 44.07 | 34.52 | 21.41 | 1.4124 | 4.184E-3 | 1114 |
| NaI | 44.07 | 34.52 | 21.41 | 1.4143 | 3.898E-3 | 1221 | |
| HIGH | Urea | 43.21 | 39.96 | 16.83 | 1.4132 | 5.178E-3 | 1141 |
| NaI | 43.21 | 39.96 | 16.82 | 1.4131 | 4.616E-3 | 1211 |
Mayer GA (1964) Blood Viscosity in Healthy Subjects and Patients With Coronary Heart Disease. Can Med Assoc J 91:951–4.
Yousif MY, Holdsworth DW, Poepping TL (2011) A blood-mimicking fluid for particle image velocimetry with silicone vascular models. Exp Fluids 50:769–774. doi: 10.1007/s00348-010-0958-1
Density uncertainty was 0.61% or less for all reported values.
Although xylitol increased viscosity too much to be used in a water-glycerol-xylitol solution, it can instead replace glycerol in three-component Newtonian solutions. Because of the oily nature of glycerol, it can be difficult to handle and leaves slippery residue when spilled. Conversely, xylitol is easy to handle, a common and safe product, and can be bought in a technical grade inexpensively. Table 3 provides fluid composition and properties for three water-xylitol-urea solutions. Table 3 confirms that water-xylitol-urea solutions can be used as a blood analog with PDMS.
Table 3.
Properties of water-xylitol-urea solutions.
| Wt% Water | Wt% Xylitol | Wt% Urea | Tempterature (°C) | Refractive Index | Viscosity (Pa-s) (23.6°C) | Density (kg/m3) |
|---|---|---|---|---|---|---|
| 50.6 | 20.0 | 29.4 | 23.6 | 1.4118 | 2.957E-3 | 1154 |
| 50.7 | 25.3 | 24.0 | 22.4 | 1.4120 | 3.547E-3 | 1154 |
| 50.9 | 30.3 | 18.8 | 24.2 | 1.4118 | 4.033E-3 | 1152 |
Optical experiments using glass or acrylic models require a higher fluid RI of between 1.47–1.49. To test for such experiments, urea was added to a 57/43 by volume solution of water-glycerol. The highest achievable index of refraction before saturation of urea was 1.4428. Thus, water-glycerol-urea alone cannot be used for high index of refraction optical experiments. However, urea can be combined with NaI to achieve high RI fluids at a cheaper cost, through the fluid and stability properties of such solutions should be explored in future work.
3.3. Non-Newtonian Fluids
For flow in small vessels (diameter < 0.5 – 0.6 mm), in addition to RI, density and viscosity, the Non-Newtonian behavior of blood must also be considered (Ramnarine et al. 1998). Previous studies have reported that non-Newtonian solutions using xanthan gum lose shear-thinning properties with the addition of salts, such as NaI (Deplano et al. 2014; Najjari et al. 2016). Therefore, the effect of urea on non-Newtonian XG solutions was tested. Xanthan gum was added at 0.02, 0.04 and 0.06 wt% to the final three-component Newtonian solutions to introduce non-Newtonian properties. Fluid testing was conducted for a strain percent range of 5E-3 to 5000, corresponding to an oscillation strain rate of approximately 8E-4 to 650. The resulting oscillatory viscosity (m2/s) and oscillatory elasticity (m2/s) as a function of oscillatory strain rate (s−1) are shown in Figure 3 in comparison to blood (Thurston 1979; Long et al. 2005). Increased uncertainty in oscillatory elasticity at high oscillatory strain rates was observed, possibly due to the large gap size used, however, this increased uncertainty does not change the general observations and conclusions presented here.
Figure 3.
Oscillatory elasticity (a-c) and viscosity (d-f) with 0.02 (a, d), 0.04 (b, e), and 0.06 (c, f) wt% xanthan gum added to the low, medium, and high water-glycerol ratios with urea and NaI added. Blood viscosity and elasticities reported in Thurston (1979) and Long et al. (2005) were extracted using WebPlotDigitizer.
Overall, as observed in Figure 3, urea and NaI maintained similar viscoelastic properties in XG-water-glycerol solutions, confirming that urea can also be used for non-Newtonian solutions. At constant weight percent XG, the weight percent of urea or NaI in this range (about 15–25 wt%) had small effect on the shear thinning non-Newtonian behavior. It is important to note, because the index of refraction of PDMS is of primary interest in this work, the range of additive used here was limited compared to other studies that investigate fluids of high refractive index (1.47–1.49). Based on Figure 3, adding 0.02 wt% XG to the studied solutions resulted in the closest match to the non-Newtonian properties of blood.
Figure 4 shows the low starting viscosity solutions with varying weight percent XG (0–0.06 wt%) added. Because the weight percent XG added was so low, the weight percent of water, glycerol, and additive were effectively unchanged. The weight percent of XG from 0.02–0.06 wt% had a significant effect on the magnitude of the oscillatory elasticity and viscosity of the fluid. Also, at higher weight percent XG, the slope of the shear-thinning property increased slightly. It is important to note, in our method the XG was added last, allowing for the weight percent of XG added to be precisely controlled for each solution. However, it is typical for the XG to be added directly to glycerol prior to mixing all other components together, to reduce mixing time. The latter method changes the weight percent of XG in the final solution, often significantly, because of the small weight percent of XG used. For example, as reported in Najjari et al. (2016), the wt% of XG was 0.06 with no NaI added but decreased to 0.044 and 0.028 wt% XG for 26.87 and 55.46 wt% NaI added, respectively. Further, the change in NaI shear-thinning observed in that work closely resembles the change observed in Figure 4 when only the XG weight percent is reduced, though it cannot be specifically determined from these observations to what degree the reported change in shear-thinning viscoelastic properties in that work was caused by the addition of NaI versus the reduction in XG weight percent. Therefore, future studies are needed to explore this and investigate the viscoelastic properties of higher wt% NaI, urea, and other additives with a fixed weight percent of XG across all solutions.
Figure 4.
Oscillatory elasticity (a-b) and viscosity (c-d) at various weight percent xanthan gum added to the low water-glycerol ratio solution with urea (a and c) and NaI (b and d) added. In (a), high uncertainty in the 0 wt% XG yielded an abnormal elasticity trend, but was still included for completeness.
4. Conclusion
Two-component and three-component Newtonian and non-Newtonian blood analog solutions for use with PDMS and optical imaging were studied here. In this work, we characterized urea and xylitol as additives to water and water-glycerol blood analog mixtures as low-density alternatives to NaI. The addition of urea to Newtonian water-glycerol solutions resulted in a blood analog that simultaneously matched blood density, viscosity, and the RI of PDMS. In contrast, NaI increased fluid density by about 6.0 kg/m3 per weight percent added, making water-glycerol-NaI solutions with densities well above that of blood. Urea is relatively inexpensive, safe, and easy to work with. Water-xylitol and water-xylitol-urea were also found to be adjustable for a wide range of refractive indices while meeting blood analog criteria, particularly low blood viscosities. For non-Newtonian testing using XG-water-glycerol solutions, urea and NaI maintained similar shear-thinning properties. These results also showed that the viscoelastic properties of the non-Newtonian fluids were primarily affected by weight percent XG, while weight percent additive in the studied range (about 15–25%) had a smaller effect. These results demonstrate that urea should be considered as an affordable and simple way to adjust the RI of working fluids, while only slightly altering the fluid density and viscosity, for PIV experiments.
5. Acknowledgements
The support of the American Heart Association pre-doctoral fellowship (17PRE33670268) to Melissa Brindise is gratefully acknowledged.
6. References
- Bai K, Katz J (2014) On the refractive index of sodium iodide solutions for index matching in PIV. Exp Fluids. doi: 10.1007/s00348-014-1704-x [DOI] [Google Scholar]
- Bale-Glickman J, Selby K, Saloner D, Savasş O (2003) Experimental Flow Studies in Exact-Replica Phantoms of Atherosclerotic Carotid Bifurcations Under Steady Input Conditions. J Biomech Eng 125:38. doi: 10.1115/1.1537734 [DOI] [PubMed] [Google Scholar]
- Deplano V, Knapp Y, Bailly L, Bertrand E (2014) Flow of a blood analogue fluid in a compliant abdominal aortic aneurysm model: Experimental modelling. J Biomech 47:1262–1269. doi: 10.1016/j.jbiomech.2014.02.026 [DOI] [PubMed] [Google Scholar]
- Long JA, Ündar A, Manning KB, Deutsch S (2005) Viscoelasticity of pediatric blood and its implications for the testing of a pulsatile pediatric blood pump. ASAIO J 51:563–566. doi: 10.1097/01.mat.0000180353.12963.f2 [DOI] [PubMed] [Google Scholar]
- Mayer GA (1964) Blood Viscosity in Healthy Subjects and Patients With Coronary Heart Disease. Can Med Assoc J 91:951–4. [PMC free article] [PubMed] [Google Scholar]
- Najjari MR, Hinke JA, Bulusu KV., Plesniak MW (2016) On the rheology of refractive-index-matched, non-Newtonian blood-analog fluids for PIV experiments. Exp Fluids 57:1–6. doi: 10.1007/s00348-016-2185-x [DOI] [Google Scholar]
- Nguyen TT (2004) A Method for Matching the Refractive Index and Kinematic Viscosity of a Blood Analog for Flow Visualization in Hydraulic Cardiovascular Models. J Biomech Eng 126:529. doi: 10.1115/1.1785812 [DOI] [PubMed] [Google Scholar]
- Patil VA, Liburdy JA (2012) Optical measurement uncertainties due to refractive index mismatch for flow in porous media. Exp Fluids 53:1453–1468. doi: 10.1007/s00348-012-1369-2 [DOI] [Google Scholar]
- Ramnarine KV., Nassiri DK, Hoskins PR, Lubbers J (1998) Validation of a new blood-mimicking fluid for use in Doppler flow test objects. Ultrasound Med Biol 24:451–459. doi: 10.1016/S0301-5629(97)00277-9 [DOI] [PubMed] [Google Scholar]
- Shuib AS, Hoskins PR, Easson WJ, Model ASA (2010) Flow Regime Characterization in a Diseased Artery Model. 4:87–91. [Google Scholar]
- Thurston GB (1979) Rheological parameters for the viscosity viscoelasticity and thixotropy of blood. Biorheology 16:149–162. [DOI] [PubMed] [Google Scholar]
- Warren JR, Gordon JA (1966) On the refractive indices of aqueous solutions of urea. J Phys Chem 70:297–300. doi: 10.1021/j100873a507 [DOI] [Google Scholar]
- Wright SF, Zadrazil I, Markides CN (2017) A review of solid–fluid selection options for optical-based measurements in single-phase liquid, two-phase liquid–liquid and multiphase solid–liquid flows. Springer Berlin; Heidelberg [Google Scholar]
- Yazdi SG, Geoghegan PH, Docherty PD, et al. (2018) A Review of Arterial Phantom Fabrication Methods for Flow Measurement Using PIV Techniques. Ann Biomed Eng. doi: 10.1007/s10439-018-2085-8 [DOI] [PubMed] [Google Scholar]
- Yousif MY, Holdsworth DW, Poepping TL (2011) A blood-mimicking fluid for particle image velocimetry with silicone vascular models. Exp Fluids 50:769–774. doi: 10.1007/s00348-010-0958-1 [DOI] [PubMed] [Google Scholar]






