Abstract
Novel targeted fluorescent biosensors provide key insights into very local nanodomains of cAMP and PKA activity, and how they respond differently to β-adrenergic activation in cardiac myocytes. This unique spatiotemporal detail in living cells is not available with biochemical measurements of total cellular cAMP and PKA, and provides unique physiological insights.
Introduction
Ca2+ is a ubiquitous intracellular second messenger, and we’ve known for many years how important a synchronized and uniform global Ca2+ transient is in the activation of striated muscle contraction (4). However, it has become increasingly clear that most other Ca2+ signaling is mediated in very specialized nanodomains by target proteins that are specifically located in those domains (5). These domains include the mouths of Ca2+ channels [voltage-gated, ryanodine or InsP3 receptors (RyR, InsP3R)], Ca2+-activated ion channels, neuronal synapses, cardiac myocyte clefts between sarcoplasmic reticulum (SR) and sarcolemma (SL), nuclear envelopes, mitochondrial-SR/ER junctions. Moreover, these very local Ca2+-regulatory signals often operate in relative independence to the average intracellular Ca2+ concentration. Thus global Ca2+ transients are often insufficient to understand Ca2+-dependent signaling in these local domains (65).
The same scenario is true for the sympathetic fight-or-flight response that is activated by β-adrenergic receptor (β-AR) signaling via cAMP and protein kinase A (PKA). Foundational historical studies using what are now traditional destructive biochemical assays of cAMP content, PKA activity, or target phosphorylation in tissue (or cell populations) have driven great progress in understanding these important physiological pathways. And these methods are still important parts of our toolkit for analyzing β-AR signaling via cAMP and PKA. However, during the past 18 years, it has become increasingly clear that individual PKA target proteins may be selectively activated via highly localized cAMP levels and protected from those in the bulk cytosol. This occurs via regulatory processes that compartmentalize cAMP, thus tailoring its concentration to the specific local requirements. That realization came from several sources, including studies using Ca2+ and cAMP-dependent ion channels as local intrinsic biosensors (19, 27, 44) and the parallel development of genetically encoded fluorescent biosensors for the detection of global cAMP levels and PKA activity (36, 50, 72, 75). The application of fluorescent biosensors proved to be particularly informative. These genetically encoded probes are based on fluorescence resonance energy transfer (FRET) between spectral variants of the green fluorescent protein (GFP) that typically sandwich a cAMP binding peptide domain or a PKA target peptide. When expressed in the cell of interest these sensors change their fluorescence properties upon cAMP binding or phosphorylation by PKA and provide a real-time readout of these intracellular signaling events.
Early studies on cAMP compartmentalization focused on hormonal specificity and how different Gs-coupled receptors produce distinct cellular responses. For example, β-AR mainly activates type II isoforms of PKA, whereas prostaglandin activates preferentially type I PKA (via preferential anchoring to distinct A-kinase anchoring proteins, or AKAPs), and phosphorylate different targets (14). That explained how β-AR promotes cardiac inotropy and lusitropy, but the prostaglandin receptor does not, even for similar global cAMP levels (22). β-AR isoforms are also distinctive, with β1-AR and β2-AR leading to differential phosphorylation of multiple targets (3, 52, 66). β2-ARs were also reported to localize exclusively in T-tubules such that cytosolic FRET reporters revealed local cAMP concentration ([cAMP]) increases there. In contrast, β1-ARs were distributed throughout the sarcolemma and produced more diffuse cAMP responses (41). Overall, local [cAMP] gradients in cells must be caused by localized cAMP production [by adenylyl cyclase (AC)], breakdown [by phosphodiesterases (PDEs)], restricted diffusion, and buffering. All four of these aspects may be involved and will be discussed.
In parallel with the progress of real-time imaging technologies, the field of AKAPs has developed (2, 9, 11, 16, 58), again led by pioneering biochemical studies that demonstrated that many aspects of the β-AR-cAMP-PKA signaling components are scaffolded together on AKAPs at PKA targets (FIGURE 1). In addition to PKA, these AKAP complexes can include AC, PDEs, phosphatases (PPs), and other cAMP targets such as exchange protein directly activated by cAMP (Epac). These complexes allow organized and highly localized β-AR-dependent activation of cAMP production by AC, activation of PKA, and both PDEs and PPs that limit local [cAMP] and PKA target phosphorylation. Indeed, ACs were proposed as central foci of cAMP compartmentalization (11) and specificity of cAMP-mediated responses. AKAP complexes coordinate not only distinct local AC isoforms but also specific isoforms of PKA, PDE, and PPs, other modulators, and targets. As such, these nanodomains can have a combinatorial plethora of distinct signalosomes between a specific receptor and defined subcellular targets and responses (19, 53, 73). The role of PDEs often has been emphasized, and they can protect some targets from global cAMP by degradation (18, 24, 34, 54), whereas PP are also critical as the local terminators of PKA target phosphorylation (7, 63).
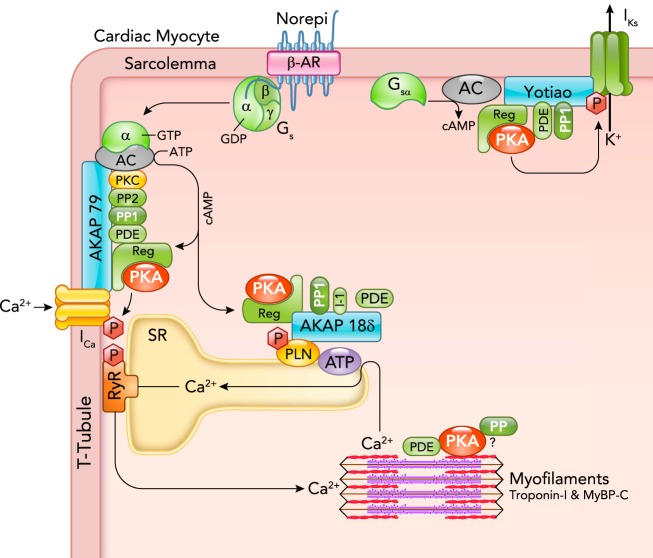
FIGURE 1.
Cardiac myocyte β-AR, cAMP, and PKA signaling proteins are associated with AKAPs
A β-AR activated by norepinephrine (Norepi) activates a GTPase protein (Gs) whose α-subunit activates adenylyl cyclase (AC) to produce cAMP from ATP. That cAMP binds to the regulatory subunit of PKA (Reg) to activate the catalytic subunit (shown as PKA) by relaxing the interaction with Reg. That active PKA can selectively phosphorylate nearby target proteins including sarcolemmal L-type Ca channels, which increases ICa, SR phospholamban (PLN), which increases SR Ca-ATPase activity, and KCNQ1 to enhance delayed rectifier K+ current IKs. PKA is anchored at these three targets, respectively, by AKAP79 (in human or mouse AKAP150; made by AKAP5 gene), AKAP18δ (made by AKAP7 gene), and Yotiao (made by AKAP9 gene). These AKAPs also bind additional cAMP-PKA modulators (PP1, PP2, PDEs, PKC). Troponin T has been proposed to be an AKAP (56), and TPNI and MyBP-C phosphorylation are regulated by PKA, PPs, and PDEs, but those mechanisms are less well resolved. See Ref. 16 for a comprehensive AKAP review.
In addition, recent studies suggest that physiological β-AR activation in cells may not cause complete release of the PKA catalytic subunit (C) from its regulatory subunit (R) that tethers the PKA holoenzyme to the AKAP complex (35, 51, 58). Thus limited AKAP-PKA diffusion may limit greatly the spatial range of PKA target phosphorylation. This challenges the old notion that, once activated, the PKA C subunit can float all over the cell, eventually finding a regulatory subunit someplace else. This loose-tethering idea is attractive because it could allow faster and more robust termination of cAMP-PKA signaling (helped also by local PDEs and PPs). Although attractive, such C-subunit tethering may be incomplete. Indeed, cardiac myocyte studies showed that delayed nuclear PKA activity was mediated, at least in part, by a subset of PKA C subunits that were released from cytosolic R subunits followed by slow nuclear translocation (21, 70). High levels of local R subunit may be another mechanism that helps to spatially constrain active C subunits; a sort of local buffering that helps to restrict cAMP diffusion (61, 71).
The possibility to monitor cAMP/PKA signaling as it occurs in intact, living cells, where the complexity of the intracellular architecture and spatial organization of the biochemical machinery above is preserved, has been instrumental in defining the compartmentalized nature of cAMP signaling and is starting to provide unexpected, novel insight into cell physiology. By using these sensors, it has been possible to establish that the cAMP signal generated by activation of β-AR is not homogeneous throughout the cardiac myocyte but rather that the [cAMP] is regulated very locally, and to attribute such compartmentalization to the local activities of both ACs (that make cAMP) and specific PDE isoforms (that degrade cAMP) that are selectively localized in different cellular domains.
Both [cAMP] and PKA Activity Are Under Very Local Control
The anchoring of PKA very near its specific targets via binding to AKAPs is critical for local signaling. However, to avoid global activation of PKA at all PKA/AKAP complexes, cAMP signals must also be generated in a localized fashion, a property apparently at odds with the highly hydrophilic and diffusible nature of this second messenger. Unlike Ca2+, for which the abundant Ca2+ binding sites and buffering capacity within cell contribute to significantly reduce Ca2+ diffusion and favor localized signaling, only a limited number of cAMP binding proteins have been identified (including PKA, Epac, and PDEs). Given the relatively low abundance of these proteins, they are not expected to substantially buffer [cAMP], at least globally within the cell. A wide range of intracellular cAMP diffusion coefficients have been estimated [10–500 μm2/s, depending on cell type and conditions used (1, 8, 25, 39, 45)], some similar to that of cAMP in water [444 μm2/s (17)]. Although computational diffusion models suggest that PDEs may not suffice to explain nanodomain [cAMP] gradients (48, 71), numerous experimental studies with PDE inhibitors make a compelling case for the involvement of PDEs in nanodomain cAMP and PKA signaling (3, 10, 19, 27, 57, 74). However, there are several reports where PDE inhibition failed to promote uniform cellular target activation, so additional factors are likely involved (41, 46, 62). This remains an unresolved issue in our understanding of cAMP diffusion.
Indeed, evidence of compartmentalized cAMP signaling has emerged from multiple independent investigations by numerous laboratories using a variety of approaches, including local delivery of stimuli (27, 41), local uncaging of cAMP (31, 49), use of targeted ACs (37, 47, 60), and localized probes. Studies using FRET-based reporters that are targeted to defined subcellular sites have been particularly illuminating. The first report showing a localized increase of cAMP in cardiac myocytes treated with norepinephrine used a FRET-based sensor with the R and C subunits of PKA tagged with CFP and YFP, respectively, which could bind with endogenous AKAPs (74). Indeed, the sensor showed a striated sarcomeric localization (as expected for AKAP association), and β-AR activation caused significantly larger [cAMP] increase at the apparent anchoring sites versus that in the bulk cytosol. AKAP-anchored biosensors also showed that activation of β-ARs and prostaglandin receptors result in increases of cAMP levels at distinct subcellular sites (14). Another study used cardiac myocytes from transgenic mice expressing either a cytosolic sensor or a sensor targeted to the SR via fusion to phospholamban (PLN) (53). This demonstrated much higher [cAMP] at the SR compared with the bulk cytosol in response to β1-AR activation and a reversed effect on activation of β2-AR. Analysis of cells other than cardiac myocytes confirms the heterogeneity of local [cAMP] achieved within the cell. For example, HEK293 cells treated with prostaglandin E1 generated at equilibrium a larger cAMP response in the nucleus than in the cytosol, again a difference that requires active PDEs (59). In another study, the distal axon of early polarized hippocampal neurons showed significantly higher [cAMP] on AC activation with forskolin compared with the soma or dendrites (20). In all cases, the compartmentalization of the cAMP signal was shown to be dependent on PDE activity, supporting a role for these enzymes in defining the local domains of cAMP.
PKA is inherently more spatially restricted than cAMP by virtue of its binding to specific AKAPs, where each of many different AKAP isoforms (and splice variants) associate with distinct cellular PKA targets (16). This restriction is especially the case when the PKA C subunit remains AKAP-tethered, which functionally constrains PKA to targets within a radius of 15–25 nm (51). However, this spatial restriction of PKA to fixed AKAPs raises an unresolved question as to how a small pool of myocyte PKA (~200 nM) can rapidly phosphorylate a much larger pool (>100 µM) of key myocyte PKA targets that are widely distributed throughout the cell, including troponin I (TPNI; which regulates myofilament Ca2+ sensitivity) and PLN (which regulates the SR Ca2+-ATPase). Thus important new chapters await detailed exploration regarding precisely how β-AR induces PKA-dependent phosphorylation of many known PKA targets of physiological importance. We posit that targeted cAMP and PKA activity reporters will help to inform these issues.
New Targeted cAMP and PKA Activity Reporters
Molecularly Targeted cAMP Reporters
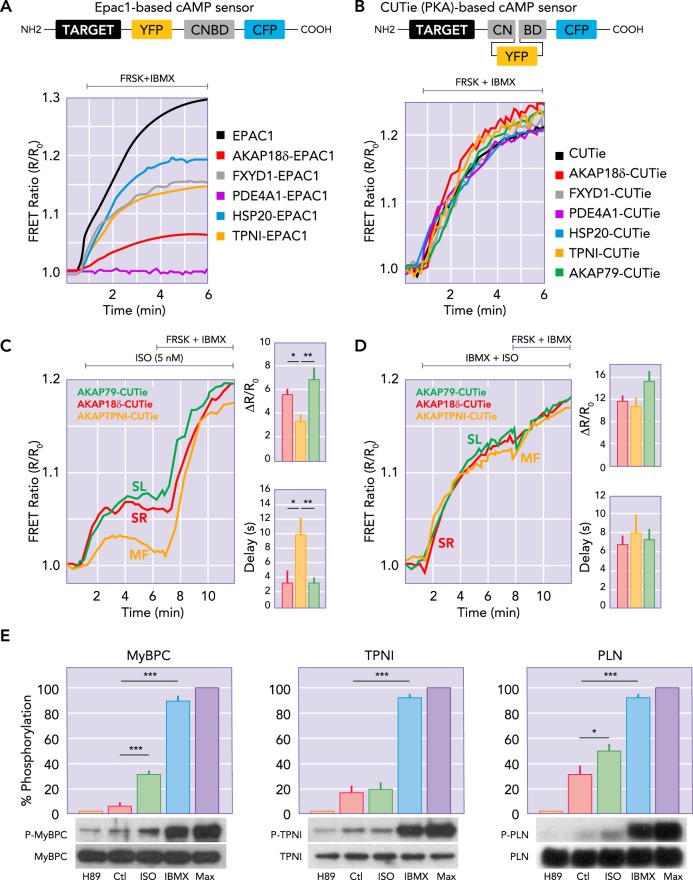
A successful family of FRET-based cAMP reporters used Epac (or its cAMP-binding domain) sandwiched between CFP and YFP variants (15, 39, 43). A drawback to these useful sensors is that when targeting sequences are added to the end of one of the GFPs, to express them at specific cellular domains, it often changes the [cAMP] dependence and FRET signal dynamic range (FIGURE 2A) (29, 57). This limits the ability to compare cAMP signals from different cellular loci. Surdo et al. (57) recently overcame this obstacle by the development of a novel cAMP sensor (named CUTie for cAMP Universal Tag for imaging experiments), engineered to increase the distance between the pair of fluorescent proteins that generate the FRET signal and the targeting moiety and minimize interference. Both the untargeted CUTie and its targeted versions were calibrated “in-cell” to ascertain that they all respond with the same FRET ratio change to a given [cAMP] (FIGURE 2B), and this allows quantitative assessment of cAMP at different subcellular sites (28, 57).
FIGURE 2.
Targeted FRET-based reporters provide direct measurement of [cAMP] at discrete molecular sites in myocytes
The more traditional cAMP reporter (A) has the cAMP binding domain (CNBD) between CFP and YFP, with the targeting domain (TARGET) attached to the NH2 terminal. The newly designed CUTie reporters (B) with different protein arrangement improves the consistency of the reporter affinity and dynamic range, regardless of targeting location (curves in B vs. A). In adult rat ventricular myocytes, targeted CUTies (C) indicated faster initial rise times and larger [cAMP] increases at the SL and SR vs. MF in response to 5 nM isoproterenol (ISO) exposure. When PDE were inhibited and AC was directly activated by forskolin, all three sensors gave the same signal (D), indicating that the nanodomain differences in [cAMP] induced by ISO were due in large part to the presence of PDE. E: PKA-dependent phosphorylation of MyBPC, TNI, and PLN at baseline (Ctl) in response to 0.3 nM ISO with minimum and maximum phosphorylation assessed by treatment with H89 (PKA inhibitor) or IBMX (or IBMX+forskolin; Max). Panels were redrawn based on parts of Figs. 1, 3, and 5 in Ref. 57, with permission from Nature Communications.
For adult ventricular myocyte studies, CUTie was targeted by fusion to AKAP79 for SL localization near L-type Ca2+ channels, to AKAP18δ for SR/PLN localization and to TPNI for myofilament (MF) localization (with respective EC50 values of 7.14, 7.18, and 7.24 µM cAMP, similar to 7.3 µM for untargeted CUTie). When expressed in adult cardiac myocytes, the CUTie reporters revealed that the cAMP response to β-ARs activation was significantly larger and faster at the SR and SL compared with the bulk cytosol and the MF (FIGURE 2C) (57), resulting in an estimated twofold larger PKA activation level at the SL and SR versus the bulk cytosol and MF. The heterogeneous local cAMP signal differences required PDE activity because PDE inhibition (with IBMX) ablated the spatial differences (FIGURE 2D). Notably, PDE inhibition that yields more spatially homogeneous [cAMP] increase resulted in less inotropy compared with ISO that favors compartmentalized cAMP signals. This indicates that spatial coordination of cAMP is required to achieve maximal inotropy.
In cardiac myocytes, the SL, SR, and MF are in very close proximity. The SL has deep transverse invaginations (T-tubules) where the SL is ~15 nm from part of the SR membrane. The MF form bundles of ~0.6 μm in diameter and are tightly surrounded by a network of SR (23). Based on the microarchitecture of the cells, the maximal distance between MF and SL can be estimated to be ~1 µm and from MF to SR only ~300 nm. The fact that sensors at these different sites detect distinct cAMP signals demonstrates that the cAMP domain size is sub-micron in scale (i.e., nanodomains). Mathematical modeling, supported by biochemical evidence (below), suggests that independent regulation of cAMP levels may occur even at TPNI and myosin binding protein C (MyBPC) (57), two PKA targets that are spaced only tens of nanometers apart in the MF. These data suggest that the size of individual cAMP domains may be as small as the volume immediately surrounding functionally relevant cAMP targets.
Imaging of cAMP using non-targeted reporters cannot detect this degree of cAMP compartmentalization (40, 74). This had initially raised skepticism about cAMP compartmentalization (10, 26). However, the apparent spatial scales involved (20–200 nm) may demand direct sensor targeting to those sites because bulk cytosolic probes would require optical spatial resolution beyond that readily available with fluorescence confocal imaging (200 nm).
Physiological Implications of Observed [cAMP] Nanodomains
Surdo et al. (57) used mainly low levels of β-AR activation [0.3–5 nM isoproterenol (ISO)], but the results suggested two intriguing working hypotheses for coordinated orchestration and recruitment of specific PKA targets in the resultant physiological β-AR effects. First, the faster and larger cAMP at SL and SR promote PKA-dependent L-type Ca2+ channel (LTCC) and PLN phosphorylation that rapidly enhances Ca2+ transients and contraction (inotropy), as well as the rate of intracellular Ca2+ concentration ([Ca2+]i) decline (lusitropy). The enhanced SR Ca2+-ATPase function raises SR Ca2+ content (also inotropic). On the other hand, PKA-mediated TPNI phosphorylation reduces MF Ca2+ sensitivity (38) and would limit inotropy. Thus a weaker cAMP signal at the MF versus SL and SR may optimize fast physiological fight-or-flight inotropy (FIGURE 3). This TPNI-dependent decrease in myofilament Ca2+ sensitivity can further enhance the lusitropic effect of faster SR Ca2+ uptake (30, 38), and this may be important in accelerating ventricular relaxation and refilling before the next beat comes along—which is sooner because of β-AR pacemaker effects that increase heart rate and shorten diastolic interval.
FIGURE 3.
Key PKA targets downstream of β-AR activation in ventricular myocytes, their timing, and functional consequences
PKA phosphorylates multiple target proteins in different domains [red hexagons (P)] at Ca2+, Na+, and K+ channels, SR Ca-ATPase and Na/K-ATPase (ATP), and myofilaments (TPNI, MyBP-C, and titin). Each site may be selectively tuned (both timing and kinetics) to optimize the orchestrated and integrated fight-or-flight response activated by the sympathetic nervous system. Table lists list tentative estimate of timing (numbers) and possibly strength, and related to Ca2+ handling (blue) or myofilament (brown).
A second working hypothesis emerged from using a detailed mathematical model (38) to test our quantitative mechanistic understanding of how low [ISO] alters contraction and Ca2+ transients. The model suggested a most likely explanation for the observed combination of contractile and Ca2+ transient effects was if the PKA-dependent acceleration of cross-bridge cycling was more strongly activated than the reduced myofilament Ca2+ sensitivity. PKA phosphorylation of MyBPC may mainly enhance cross-bridge cycling, whereas TPNI may predominantly reduce myofilament Ca2+ sensitivity (55). This raised the hypothesis that the low [ISO] exposure promotes higher phosphorylation of MyBPC versus TPNI. Indeed, immunoblots showed that 0.3 nM ISO caused a strong increase in phosphorylation of MyBPC (and PLN) but no measurable rise in TPNI phosphorylation (FIGURE 2E). Thus an initial enhancement of cross-bridge cycling rate (via MyBPC) may help the larger Ca2+ transients (due to LTCC and SR effects) produce stronger contractions, whereas the TPNI-dependent myofilament Ca2+ desensitization is held in check. This may help maximize (or supercharge) the initial β-AR inotropic effect. At larger or longer β-AR activation levels, where heart rate increases further and diastole is shortened, the lusitropic effect may become more critical for diastolic filling. In that case, the TPNI effect to hasten Ca2+ dissociation from the myofilaments may be preferentially recruited. This is a logical rationale for sequential recruitment of these two MF sites at different β-AR activation levels. That is, at lower levels of β-AR activation, inotropy may be more important than lusitropy, but at higher β-AR activation levels, recruitment of additional lusitropic benefit may be required.
Along these same lines, it has been shown that acute β-AR stimulation of ventricular myocytes caused a rapid increases in LTCC current (ICa) amplitude (τ ~7 s), but a considerably slower activation (τ ~40 s) of the potassium current (IKs) that is critical for limiting action potential duration (APD) prolongation, which would be otherwise induced by the increased ICa (32). This would again initially boost inotropy (high ICa) and slow relaxation (long APD). Computer modeling also demonstrated that delay between ICa and IKs activation causes a transient window of increased vulnerability to cardiac arrhythmias early in β-AR activation, which settles down once the IKs current catches up (67–69).
Phospholemman (PLM) is another sarcolemmal PKA target that is an endogenous Na+-K+-ATPase inhibitor, with which inhibition is relieved by PKA-dependent PLM phosphorylation (12). This is analogous to the well-known SR Ca2+-ATPase regulation by PLN and PKA. It was also shown that this β-AR activation of Na+-K+-ATPase is slower than that of the ICa and SR Ca2+ increase, such that [Na+]i rises initially to higher levels early in β-AR activation than 1–2 min later (13). This transient elevation of [Na+]i also promotes stronger myocyte and SR Ca2+ loading because of the influence of the Na+/Ca2+ exchanger (limiting Ca2+ extrusion, akin to digitalis inotropy). This may also help hasten the initial increase in inotropic effects and arrhythmias, but, since Na+-K+-ATPase is gradually activated, it limits the arrhythmia risk but also the steady-state inotropy (13).
Thus the timing and extent of PKA activation at numerous nanodomains in the myocyte may be exquisitely orchestrated to optimize cardiac function (FIGURE 3). However, that also means that pathological conditions that disturb this optimal nanodomain signaling balance may have serious consequences for dysregulation of β-AR signaling. And the long-term failure of PDE inhibitors used clinically for the treatment of heart failure (HF) (42) may ultimately be a consequence of their spatial homogenization and disruption of the elegant orchestration of nanodomain cAMP signaling (e.g., the differential control of MF targets). Indeed, Surdo et al. (57) used rat models of cardiac hypertrophy and HF, which are characterized by adrenergic downregulation and reduced cAMP synthesis. They found that the MF cAMP signal at the troponin complex was dramatically reduced compared with SL and SR, such that it may limit PKA effects at these key MF targets. This may result in higher myofilament Ca2+ sensitivity, which could contribute to diastolic dysfunction in HF.
These novel insights into the details of local cAMP signaling have important implications for the treatment of cardiac disease. Since local nanodomains of cAMP are regulated by the activity of specific PDEs, identification of the PDE isoforms selectively involved in regulation of cAMP levels at the troponin complex (see next paragraph) would allow their specific targeting and may provide an effective means to reestablish the appropriate level of signaling at the sarcomere and correct Ca2+ sensitization in pathological conditions. In addition, these findings may provide a rationale for the limited success of β-blocker therapy in HF with preserved ejection fraction (HFpEF). In a scenario where the fault in myocyte function involves primarily relaxation, limited TPNI phosphorylation may contribute to the functional deficit.
Molecularly Targeted PKA Activity Reporters
Barbagallo et al. (3) recently studied local PKA regulation, parallel to the Surdo et al. cAMP study above (57). They used an A-kinase activity reporter (AKAR3) (75) with a PKA substrate sandwiched between CFP and YFP plus molecular targeting by fusion with 1) troponin T (MF), 2) the SR transmembrane domain of PLN (SR), and 3) a plasma membrane targeting domain from Kras (SL) (3, 33). These FRET-based reporters measure the PKA phosphorylation level of this substrate at the SR, SL, and MF that results from net local PKA and PP activities. Quantitative comparisons of PKA action at different targets was limited by different dynamic ranges and [cAMP] sensitivities of the three reporters. However, several key functional differences were observed with respect to β-AR and PDE isoform signaling, and changes during HF. In control rabbit ventricular myocytes, the PKA activity around the MF and the SR was entirely dependent on β1-AR (not β2-AR), whereas at the SL roughly a third of the ISO response was β2-AR-dependent. Furthermore, PDE3 was the major isoform that limits [cAMP]-dependent PKA activity at the MF, whereas PDE4 was critical for constraining SL PKA action. In HF rabbits, the β-AR-dependent effects at the SL and SR were depressed (loss of β1-AR signaling), but, at the MF, β2-AR signaling and TPNI phosphorylation were enhanced (and may have been due to reduced local MF PDE3 function). This indicates that specific HF-related changes in MF nanodomain signaling could reduce MF Ca2+ sensitivity (higher TPNI phosphorylation) and limit the inotropic reserve at the same time that reduced β-AR signaling to the SL and SR limits inotropic reserve from the Ca2+-handling perspective. Intriguingly, overexpression of caveolin 3 (which was reduced in HF) restored much of the altered local β-AR signaling (3), and caveolin 3 is a critical component of β2-AR signaling complexes in cardiac myocyte caveolae (6, 64).
Both Barbagallo et al. (3) and Surdo et al. (57) showed decreased PKA and cAMP effects at the SL and SR in HF, which will tend to limit Ca2+-dependent inotropy and lusitropy in HF. However, Surdo et al. (57) found even stronger suppression of MF cAMP responses (versus SL and SR) in HF, whereas Barbagallo et al. (3) found higher ISO-induced PKA activity at MF in HF, especially via β2-AR activation, and suggested that this was partly due to loss of PDE3 at MF in HF. This apparent discrepancy is unresolved but could be related to the different species and HF models used or the much lower ISO concentrations used by Surdo et al. than Barbagallo et al. (~1 vs. 100 nM). That is, at low [ISO], the cAMP level at the MF in HF may not suffice to activate appreciable MF phosphorylation (even with reduced local PDE3 levels). However, at stronger β-AR activation, that same lower PDE3 level may allow higher levels of local PKA activation and MF phosphorylation. The higher [ISO] may also recruit additional signaling (e.g., via arrestin) that further alter cAMP and PKA activity. Although further study is merited to clarify these differences, it illustrates both the complexity and importance of improved understanding of these signaling nanodomains since the physiological balance is perturbed in disease. Our discussion has focused mostly on cardiac myocytes, which are exemplar of β-AR and PKA signaling because of their very organized structures and well-characterized β-AR signaling and functional targets. However, these issues discussed are likely to be broadly relevant for most other cell types, even if the functional nanodomains differ.
Future Perspectives and Therapeutic Opportunities
Indeed, such novel mechanistic insight is likely to enable more precision in therapeutic strategies to target cardiac pathologies. We encourage further studies with targeted fluorescent reporters, high-resolution imaging, molecular manipulations of AKAP complexes, and local PDE and PP environments to help enhance our understanding and clarify some of the unresolved issues. These include elucidation of the true molecular basis of functional nanodomain signaling of cAMP and PKA, whether PKA C subunits really dissociate from the R subunits physiologically, how different isoforms of PDE and PP synergize to constrain the function of AC and PKA in nanodomains, and details as to how all of the players change locally in particular pathologies such as HF. This more complete picture could enable a new array of precision and personalized medicine. This could involve selective manipulation of specific PDE or PP isoforms or their targeting to specific nanodomains to tailor responses. For example, if elevated myofilament Ca2+ sensitivity is a major cause of poor diastolic function in a specific HFpEF patient or one with genetically linked hypertrophic cardiomyopathy, one could selectively promote local PKA phosphorylation of TPNI (to enhance relaxation) by selective inhibition of the local PDE or PP isoform, or disrupt the local PKA anchoring complex with a selective peptide. Conversely, if MF hyperphosphorylation or genetically linked MF desensitization is a factor in limiting cardiac function in a given patient, one might be able to reduce TPNI phosphorylation while promoting MyBPC or PLN phosphorylation by precise molecular targeting. Our future ability to treat clinically with such molecular precision is exciting but requires more complete molecular understanding of this nanodomain signaling.
Acknowledgments
The authors are supported by National Heart, Lung, and Blood Institute Grants R01-HL-30077, R01-HL-127764, R01-HL-133832, and R01-HL-112413, and British Heart Foundation Programme Grant RG/17/6/32944.
No conflicts of interest, financial or otherwise, are declared by the author(s).
D.M.B. and M.Z. conceived and designed research; D.M.B. prepared figures; D.M.B. and M.Z. drafted manuscript; Y.K.X. and M.Z. edited and revised manuscript; Y.K.X. and M.Z. approved final version of manuscript.
References
- 1.Agarwal SR, Clancy CE, Harvey RD. Mechanisms restricting diffusion of intracellular cAMP. Sci Rep 6: 19577, 2016. doi: 10.1038/srep19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin TA, Dessauer CW. Function of adenylyl cyclase in heart: the AKAP connection. J Cardiovasc Dev Dis 5: E2, 2018. doi: 10.3390/jcdd5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbagallo F, Xu B, Reddy GR, West T, Wang Q, Fu Q, Li M, Shi Q, Ginsburg KS, Ferrier W, Isidori AM, Naro F, Patel HH, Bossuyt J, Bers D, Xiang YK. Genetically encoded biosensors reveal PKA hyperphosphorylation on the myofilaments in rabbit heart failure. Circ Res 119: 931–943, 2016. doi: 10.1161/CIRCRESAHA.116.308964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Dynamic imaging in living cells: windows into local signaling. Sci STKE 2003: PE13, 2003. doi: 10.1126/stke.2003.177.pe13. [DOI] [PubMed] [Google Scholar]
- 6.Best JM, Kamp TJ. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J Mol Cell Cardiol 52: 376–387, 2012. doi: 10.1016/j.yjmcc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdyga A, Surdo NC, Monterisi S, Di Benedetto G, Grisan F, Penna E, Pellegrini L, Zaccolo M, Bortolozzi M, Swietach P, Pozzan T, Lefkimmiatis K. Phosphatases control PKA-dependent functional microdomains at the outer mitochondrial membrane. Proc Natl Acad Sci USA 115: E6497–E6506, 2018. doi: 10.1073/pnas.1812383115. A correction for this article is available at 10.1073/pnas.1812383115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J 76: 2861–2867, 1999. doi: 10.1016/S0006-3495(99)77440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol 9: 216–221, 1999. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DM, Tabbasum VG. Adenylate cyclase-centred microdomains. Biochem J 462: 199–213, 2014. doi: 10.1042/BJ20140560. [DOI] [PubMed] [Google Scholar]
- 12.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 13.Despa S, Tucker AL, Bers DM. Phospholemman-mediated activation of Na/K-ATPase limits [Na]i and inotropic state during beta-adrenergic stimulation in mouse ventricular myocytes. Circulation 117: 1849–1855, 2008. doi: 10.1161/CIRCULATIONAHA.107.754051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res 103: 836–844, 2008. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 15.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA 101: 16513–16518, 2004. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diviani D, Dodge-Kafka KL, Li J, Kapiloff MS. A-kinase anchoring proteins: scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol 301: H1742–H1753, 2011. doi: 10.1152/ajpheart.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworkin M, Keller KH. Solubility and diffusion coefficient of adenosine 3′:5′-monophosphate. J Biol Chem 252: 864–865, 1977. [PubMed] [Google Scholar]
- 18.Ercu M, Klussmann E. Roles of A-kinase anchoring proteins and phosphodiesterases in the cardiovascular system. J Cardiovasc Dev Dis 5: E14, 2018. doi: 10.3390/jcdd5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99: 816–828, 2006. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 20.Gorshkov K, Mehta S, Ramamurthy S, Ronnett GV, Zhou FQ, Zhang J. AKAP-mediated feedback control of cAMP gradients in developing hippocampal neurons. Nat Chem Biol 13: 425–431, 2017. doi: 10.1038/nchembio.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haj Slimane Z, Bedioune I, Lechêne P, Varin A, Lefebvre F, Mateo P, Domergue-Dupont V, Dewenter M, Richter W, Conti M, El-Armouche A, Zhang J, Fischmeister R, Vandecasteele G. Control of cytoplasmic and nuclear protein kinase A by phosphodiesterases and phosphatases in cardiac myocytes. Cardiovasc Res 102: 97–106, 2014. doi: 10.1093/cvr/cvu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci USA 76: 1570–1574, 1979. doi: 10.1073/pnas.76.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Y, Crossman DJ, Rajagopal V, Baddeley D, Jayasinghe I, Soeller C. Super-resolution fluorescence imaging to study cardiac biophysics: α-actinin distribution and Z-disk topologies in optically thick cardiac tissue slices. Prog Biophys Mol Biol 115: 328–339, 2014. doi: 10.1016/j.pbiomolbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci 35: 91–100, 2010. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Huang RC, Gillette R. Kinetic analysis of cAMP-activated Na+ current in the molluscan neuron. A diffusion-reaction model. J Gen Physiol 98: 835–848, 1991. doi: 10.1085/jgp.98.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang JY, Falcone JL, Curci S, Hofer AM. Interrogating cyclic AMP signaling using optical approaches. Cell Calcium 64: 47–56, 2017. doi: 10.1016/j.ceca.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA 93: 295–299, 1996. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koschinski A, Zaccolo M. A novel approach combining real-time imaging and the patch-clamp technique to calibrate FRET-based reporters for cAMP in their cellular microenvironment. Methods Mol Biol 1294: 25–40, 2015. doi: 10.1007/978-1-4939-2537-7_3. [DOI] [PubMed] [Google Scholar]
- 29.Koschinski A, Zaccolo M. Quantification and comparison of signals generated by different FRET-based cAMP reporters. Methods Mol Biol 1947: 217–237, 2019. doi: 10.1007/978-1-4939-9121-1_12. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278: H769–H779, 2000. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Gervasi N, Girault JA. Dendritic geometry shapes neuronal cAMP signalling to the nucleus. Nat Commun 6: 6319, 2015. doi: 10.1038/ncomms7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu GX, Choi BR, Ziv O, Li W, de Lange E, Qu Z, Koren G. Differential conditions for early after-depolarizations and triggered activity in cardiomyocytes derived from transgenic LQT1 and LQT2 rabbits. J Physiol 590: 1171–1180, 2012. doi: 10.1113/jphysiol.2011.218164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Li Y, Kim S, Fu Q, Parikh D, Sridhar B, Shi Q, Zhang X, Guan Y, Chen X, Xiang YK. Phosphodiesterases coordinate cAMP propagation induced by two stimulatory G protein-coupled receptors in hearts. Proc Natl Acad Sci USA 109: 6578–6583, 2012. doi: 10.1073/pnas.1117862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 35.Martin BR, Deerinck TJ, Ellisman MH, Taylor SS, Tsien RY. Isoform-specific PKA dynamics revealed by dye-triggered aggregation and DAKAP1alpha-mediated localization in living cells. Chem Biol 14: 1031–1042, 2007. doi: 10.1016/j.chembiol.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Mehta S, Zhang J. Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems. Annu Rev Biochem 80: 375–401, 2011. doi: 10.1146/annurev-biochem-060409-093259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naim N, White AD, Reece JM, Wankhede M, Zhang X, Vilardaga JP, Altschuler DL. Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis. J Biol Chem 294: 1095–1103, 2019. doi: 10.1074/jbc.AC118.004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negroni JA, Morotti S, Lascano EC, Gomes AV, Grandi E, Puglisi JL, Bers DM. β-Adrenergic effects on cardiac myofilaments and contraction in an integrated rabbit ventricular myocyte model. J Mol Cell Cardiol 81: 162–175, 2015. doi: 10.1016/j.yjmcc.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 40.Nikolaev VO, Bünemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res 99: 1084–1091, 2006. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327: 1653–1657, 2010. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 42.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL; The PROMISE Study Research Group . Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 325: 1468–1475, 1991. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 43.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13049–13054, 2001. doi: 10.1073/pnas.221381398. A correction for this article is available at https://doi.org/10.1073/pnas.98.25.14744-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards M, Lomas O, Jalink K, Ford KL, Vaughan-Jones RD, Lefkimmiatis K, Swietach P. Intracellular tortuosity underlies slow cAMP diffusion in adult ventricular myocytes. Cardiovasc Res 110: 395–407, 2016. doi: 10.1093/cvr/cvw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rochais F, Vandecasteele G, Lefebvre F, Lugnier C, Lum H, Mazet JL, Cooper DM, Fischmeister R. Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels. J Biol Chem 279: 52095–52105, 2004. doi: 10.1074/jbc.M405697200. [DOI] [PubMed] [Google Scholar]
- 47.Sample V, DiPilato LM, Yang JH, Ni Q, Saucerman JJ, Zhang J. Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat Chem Biol 8: 375–382, 2012. doi: 10.1038/nchembio.799. A corrigendum for this article is available at . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saucerman JJ, Greenwald EC, Polanowska-Grabowska R. Mechanisms of cyclic AMP compartmentation revealed by computational models. J Gen Physiol 143: 39–48, 2014. doi: 10.1085/jgp.201311044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saucerman JJ, Zhang J, Martin JC, Peng LX, Stenbit AE, Tsien RY, McCulloch AD. Systems analysis of PKA-mediated phosphorylation gradients in live cardiac myocytes. Proc Natl Acad Sci USA 103: 12923–12928, 2006. doi: 10.1073/pnas.0600137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schleicher K, Zaccolo M. Using cAMP sensors to study cardiac nanodomains. J Cardiovasc Dev Dis 5: E17, 2018. doi: 10.3390/jcdd5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith FD, Esseltine JL, Nygren PJ, Veesler D, Byrne DP, Vonderach M, Strashnov I, Eyers CE, Eyers PA, Langeberg LK, Scott JD. Local protein kinase A action proceeds through intact holoenzymes. Science 356: 1288–1293, 2017. doi: 10.1126/science.aaj1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soto D, De Arcangelis V, Zhang J, Xiang Y. Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res 104: 770–779, 2009. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, Nikolaev VO. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat Commun 6: 6965, 2015. doi: 10.1038/ncomms7965. [DOI] [PubMed] [Google Scholar]
- 54.Stangherlin A, Zaccolo M. Phosphodiesterases and subcellular compartmentalized cAMP signaling in the cardiovascular system. Am J Physiol Heart Circ Physiol 302: H379–H390, 2012. doi: 10.1152/ajpheart.00766.2011. [DOI] [PubMed] [Google Scholar]
- 55.Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101: 503–511, 2007. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 56.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Sievert GA, Balke CW, Sumandea MP. Cardiac troponin T, a sarcomeric AKAP, tethers protein kinase A at the myofilaments. J Biol Chem 286: 530–541, 2011. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Surdo NC, Berrera M, Koschinski A, Brescia M, Machado MR, Carr C, Wright P, Gorelik J, Morotti S, Grandi E, Bers DM, Pantano S, Zaccolo M. FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility. Nat Commun 8: 15031, 2017. doi: 10.1038/ncomms15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13: 646–658, 2012. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol 175: 441–451, 2006. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10: 1061–1065, 2014. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker-Gray R, Stengel F, Gold MG. Mechanisms for restraining cAMP-dependent protein kinase revealed by subunit quantitation and cross-linking approaches. Proc Natl Acad Sci USA 114: 10414–10419, 2017. doi: 10.1073/pnas.1701782114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol 580: 765–776, 2007. doi: 10.1113/jphysiol.2006.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber S, Meyer-Roxlau S, Wagner M, Dobrev D, El-Armouche A. Counteracting protein kinase activity in the heart: the multiple roles of protein phosphatases. Front Pharmacol 6: 270, 2015. doi: 10.3389/fphar.2015.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright PT, Nikolaev VO, O’Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol 67: 38–48, 2014. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, Cheng H. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci 25: 358–365, 2004. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Xie Y, Grandi E, Bers DM, Sato D. How does β-adrenergic signalling affect the transitions from ventricular tachycardia to ventricular fibrillation? Europace 16: 452–457, 2014. doi: 10.1093/europace/eut412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie Y, Grandi E, Puglisi JL, Sato D, Bers DM. β-adrenergic stimulation activates early afterdepolarizations transiently via kinetic mismatch of PKA targets. J Mol Cell Cardiol 58: 153–161, 2013. doi: 10.1016/j.yjmcc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Y, Izu LT, Bers DM, Sato D. Arrhythmogenic transient dynamics in cardiac myocytes. Biophys J 106: 1391–1397, 2014. doi: 10.1016/j.bpj.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang JH, Polanowska-Grabowska RK, Smith JS, Shields CW IV, Saucerman JJ. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to β-adrenergic signaling. J Mol Cell Cardiol 66: 83–93, 2014. doi: 10.1016/j.yjmcc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang PC, Boras BW, Jeng MT, Docken SS, Lewis TJ, McCulloch AD, Harvey RD, Clancy CE. A computational modeling and simulation approach to investigate mechanisms of subcellular cAMP compartmentation. PLOS Comput Biol 12: e1005005, 2016. doi: 10.1371/journal.pcbi.1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol 2: 25–29, 2000. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- 73.Zaccolo M, Di Benedetto G, Lissandron V, Mancuso L, Terrin A, Zamparo I. Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochem Soc Trans 34: 495–497, 2006. doi: 10.1042/BST0340495. [DOI] [PubMed] [Google Scholar]
- 74.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci USA 98: 14997–15002, 2001. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]