Abstract
Acute Megakaryoblastic Leukaemia (AML, M7) is a rare type of acute myeloid leukemia (AML) evolving from primitive megakaryoblasts. It accounted for 1.2% of newly diagnosed AML according to Eastern Cooperative Oncology Group (ECOG) trials between 1984 and 1997. Patients may present with a broad variety of symptoms including low-grade fever, easy bruising, and life-threatening conditions. We report a rare case of AML, M7 in a 19-year-old lady who presented with weakness and fatigue. She was diagnosed as a case of AML, M7 on the basis of peripheral blood finding, bone marrow examination report, radiological findings and immunophenotyping.
Keywords: acute myeloid leukemia, acute megakaryocytic leukaemia, immunophenotyping, myelosclerosis
Introduction
Acute Myeloid Leukaemia (AML) is a group of heterogenous hematological malignancies characterized by a clonal proliferation of myeloid precursors with a reduced capacity to differentiate into more mature cellular elements. As a result, there is an accumulation of leukemic blasts or immature forms in the bone marrow, peripheral blood, and occasionally in other tissues, with a variable reduction in the production of normal red blood cells, platelets, and mature granulocyte1. It accounts for 80% of the acute leukemias in adults and 15–20% of the acute leukaemias in children2. AML constituted 4.9% of all haematological cancers with annual incidence of 1.2 per million in Ilorin, Nigeria3. Patients may present with a broad variety of symptoms including anaemia, low-grade fever and easy bruising. Hepatomegaly or splenomegaly occurs in about 30% of the patients with lymphadenopathy being extremely uncommon4,5. A presumptive diagnosis of AML can be made via examination of the peripheral blood smear when there are circulating leukemic blasts, but a definitive diagnosis usually requires an adequate bone marrow aspiration with immunophenotyping3.
In the French-American-British (FAB) Classification, sub-type classification of AML is based on morphology and cytochemical staining with immunophenotypic data in some instances. Types M0, M1, M2, and M3 are predominantly granulocytic and differ in the stage of maturation. The M4 class is both granulocytic and monocytic, with at least 20% of cells being monocytic. M5 is predominantly monocytic with at least 50% being monocytic. M6 shows primarily differentiation with dysplastic features and megaloblastic changes. The AML classified as acute megakaryocytic leukemia is M7 and is characterized by the presence of megakaryocytic antigens demonstrated by flow cytometry, immunohistochemistry or the presence of platelet peroxides6–7.
Acute megakaryoblastic leukemia (AMegL) is a rare subtype of AML developing from primitive megakaryoblasts, first described by Von Boros et al in 19318. It accounted for 1.2% of newly diagnosed AML by Eastern Cooperative Oncology Group (ECOG) trials between 1984 and 19979. The disease can be identified by antibodies to glycoprotein Ib (CD42), glycoprotein IIb/IIIa (CD41a) and glycoprotein IIIa (CD61), and is often associated with extensive myelofibrosis10–11. This case of a 19-year old lady is reported because of the rarity of the disease and the fact that no such case has been reported in Nigeria.
Case presentation
A 19-year old Nigerian female, secondary school leaver, presented at Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria, with recurrent headache, fever, easy fatiguability and breathlessness of 2 months duration. She was treated at a private hospital for typhoid fever and malaria with intravenous medications and fluid. She was also transfused with 3 units of blood for symptomatic anaemia. The symptoms, however, started again 3 days prior to presentation at our accident and emergency unit.
On examination, she was severely pale with slight tinge of jaundice. There was no significant peripheral lymph node enlargement or evidence of bleeding from orifices or from the gum. There was no bleeding into the skin or pedal oedema. The chest was clinically clear. Liver and spleen were not palpable.
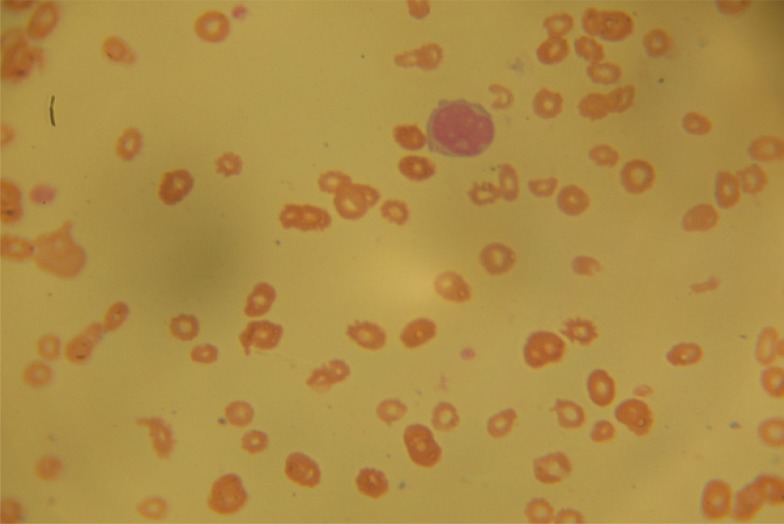
A full blood count (FBC) done by an automated haematology analyzer showed a packed cell volume (PCV) of 10.8 white blood cell (WBC) count of 4.1 × 109 /L (Neutrophil-18.3%, lymphocyte-78%, mid-3.4%) and platelet of 50 × 109/L. Review of peripheral blood film showed normocytic normochromic red blood cells including few nucleated red blood cells and immature mononuclear cells with cytoplasmic blebs (Figure 1).
Figure 1.
Peripheral blood film showing normocytic normochromic red blood cells and immature mononuclear cells with cytoplasmic blebs
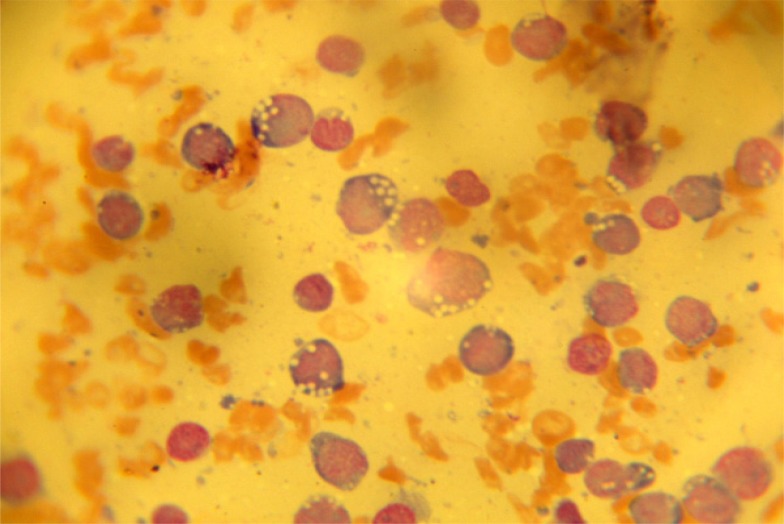
There were giant forms of platelets on the film. The bone marrow aspiration yielded a dry tap which necessitated a bone marrow biopsy. The bone marrow imprint showed increased immature mononuclear cells with abundant basophilic cytoplasm containing vacuoles and hyperchromatic and pleomorphic nuclei. Some of the immature mononuclear cells had cytoplasmic blebs and constituted over 90% of nucleated cells (Figure 2).
Figure 2.
The bone marrow imprint showing increased immature mononuclear cells with abundant basophilic cytoplasm containing vacuoles and hyperchromatic and pleomorphic nuclei, some have cytoplasmic blebs
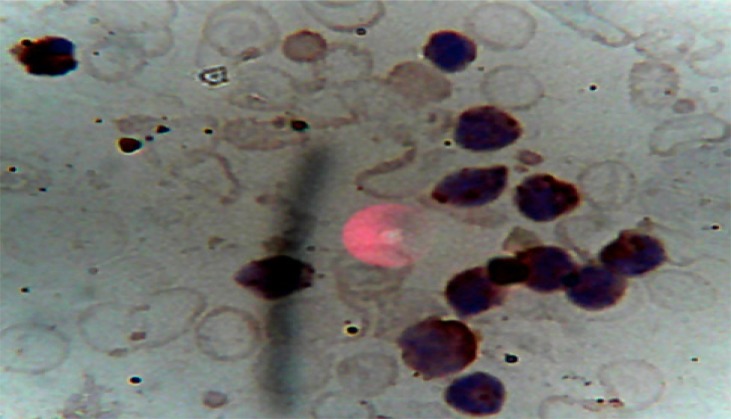
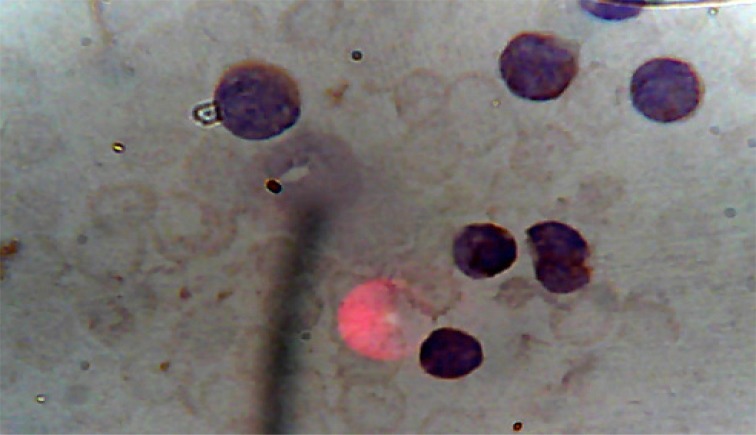
The overall picture was in keeping with AML, most likely Acute Megakaryoblastic Leukaemia (AML, M7), which was confirmed by immunophenotyping (carried out by Safety Molecular Pathology Laboratory, Enugu) of peripheral blood with positivity for CD 33, CD41 and CD 61. CD41 and CD61 are megakaryocyte specific antigens, and CD33 is a myeloid marker. The percentage positivity and micrographs are shown in Table 1 and Figures 3 and 4. The biochemical parameters such as uric acid, bilirubin, creatinine, liver enzymes were normal. X-rays of tibia and fibula showed increased fibrosis (figure 5).
Table 1.
Percentage positivity of CD33, CD41, CD
| CD Marker | % Positivity |
| CD33 | 46 |
| CD41 | 24 |
| CD61 | 20 |
Figure 3.
Peripheral blood with positivity for CD 33
Figure 4.
Peripheral blood with positivity for CD41
Figure 5.
X-rays of tibia and fibula showing increased fibrosis
She was admitted and resuscitated with empirical intravenous antibiotic, intravenous fluid and a transfusion with 5 units of fresh whole blood because the facility for blood component therapy was not available to cater for anaemia and thrombocytopaenia that the patient had. Therapy was initiated thereafter with cytarabine and daunorubicin (7+ 3) regimen.
Repeat FBC on the seventh day of induction showed PCV of 17.7%, WBC of 0.38 × 109/L and platelet of 22 × 109 /L. Peripheral blood film revealed leucopeania, thrombocytopaenia and there was no abnormal mononuclear cell. She was transfused with 2 more units of fresh whole blood. A repeat bone marrow aspiration was done for assessment of remission. Marrow was aspirated with ease and blasts were less than 5%. She was then scheduled for consolidation therapy. Her parents requested for discharge from the hospital due to financial constraint with the promise to return after purchasing the chemotherapeutic drugs. She was discharged and is yet to return to care.
Discussion
AML, M7 is a rare subtype of leukemia accounting to 1.2% of cases of adult AML, compared to 3–10% of childhood AML12. It is as under M7 in the French-American-British classification13. The patient was a 19-year old female with clinical features similar to other types of AML except for organomegaly noted infrequently in adults which was the case with our patient. In our patient, symptoms of anemia were easy fatiguability and breathlessness. Cytopenias are usually present but 30% of patients have platelet counts in excess of 1000 ×109/L.10 In our patient, bicytopaenia (PCV-10.8%, WBC-4.1x 109/L, platelet count-50 x109/L) was found. Osteosclerosis was found in our patient in line with osteosclerotic and osteolytic lesions described in few case reports14,15. This might be due to production of platelet-derived growth factor (PDGF) and transforming growth factor β with their attendant fibroblastic activity. Myelofibrosis has been hypothesized to be a reactive phenomenon secondary to myeloid disorders. It has been postulated that defective or abnormal megakaryocytes and the release of growth factors such as platelet-derived growth factor (PDGF), and transforming growth factors (TGF)-β are responsible for the development of myelofibrosis16. PDGF is a major mitogen for connective tissue cells such as fibroblasts and smooth muscle cells17,18. TGF-β consists of a family of proteins and has broad effects on many types of cells, including fibroblasts and osteoblasts19.
The diagnosis depends on the expression of at least one platelet antigen (CD41, CD42b, and CD61) on the leukemic cells10–11. In our patient, peripheral film showed immature mononuclear cells with cytoplasmic blebs. Bone marrow aspiration was difficult probably due to intense myelofibrosis which has been documented in most patients14–15. Bone marrow biopsy imprint examined for cytology was relied upon for initial diagnosis.
Non-specific, cytogenetic abnormalities are more frequent (>90%) in AML, M7 than in other subtypes of AML.15,20 Cytogenetic analysis was not carried out due to non-availability of the facility. AML, M7 may present as de novo leukemia, secondary leukemia after chemotherapy, or transformed myeloproliferative disorders and myelodysplastic syndromes.21 Our patient had clinical and haematologic remission and remains stable but neutropaenic before discharge against medical advice. This is consistent with the previously observed remission and long term survival in spite of generally poor prognosis of AML, M720.
Conclusion
To our knowledge, this is the first case of AML M7 in the literature in our environment. The lesson here is that proficiency in morphologic diagnosis remains the window through which uncommon diagnosis can be confirmed by molecular technology particularly in resource-limited settings.
Acknowledgement
Safety Molecular Pathology Laboratory, Enugu, for immunocytochemical diagnosis through CD33, CD41 and CD61
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Hoffbrand AV, D Catovsky. Postgraduate Haematology. 5th ed. London: Blackwell Publishing Limited; 2005. EGD Tuddenham. Acute myeloid leukaemia; pp. 104–106. [Google Scholar]
- 2.Liesveld JL, Lichtman . Acute Myelogenous Leukaemia. In: Lichman MA, Beutler E, Kipps TJ, Seligsohn, Kaushansky K, Prchal JT, editors. Williams Haematogy. 7th ed. New York: McGraw-Hill Medical Publishing Division; 2006. pp. 1185–1194. [Google Scholar]
- 3.Babatunde A, Amiwero C, Olatunji P, Durotoye I. Pattern of Haematological Malignancies in Ilorin, Nigeria: A Ten Year Review. Internet J Haematol. 2008;5(2):24. [Google Scholar]
- 4.Hoffbrand AV, Moss PAH, Petit JE. Essential Haematology. 5th ed. Oxford UK: Blackwell Publishing; 2006. Acute myeloid leukaemia; p. 181. [Google Scholar]
- 5.Gollard RP, Robbins BA, Piro L, Saven A. Acute myelogenous leukaemia presenting with bulky lymphadenopathy. Acta Haematol. 1996;95:129. doi: 10.1159/000203861. [DOI] [PubMed] [Google Scholar]
- 6.Catovsky D, Matutes E, Buccheri V, Shetty V, Hanslip J, Yoshida N, et al. A classification of acute leukaemia for the 1990s. Ann of Hematolo. 1991;62(1):16–21. doi: 10.1007/BF01714978. [DOI] [PubMed] [Google Scholar]
- 7.Dohner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 8.Von Boros J, Korenyi A. Uber einen fall von akuter megakaryocyblasten-leukamie, zugleich einige bemerkungen zum Problem der akuten leukemie. Z Klin Med. 1931;118:679–718. [Google Scholar]
- 9.Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, et al. Acute megakaryocytic leukaemia:The Eastern Coperative Oncology Group experience. Blood. 2000;96(7):2405–2411. [PubMed] [Google Scholar]
- 10.Huang M-J, Li CY, Nichols WL, Young JH, Katzmann JA. Acute leukemia with megakaryocytic differentiation: A study of 12 cases identified immunocytochemically. Blood. 1984;64:427–439. [PubMed] [Google Scholar]
- 11.Ruiz-Arguelles GJ, Marin-Lopez A, Lobato-Mendizabal E, Ruiz-Arguelles A, Nichols WL, Katzman JA. Acute megakaryoblastic leukemia: a prospective study of its identification and treatment. Br J Haematol. 1986;62:55–63. doi: 10.1111/j.1365-2141.1986.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 12.Masoumi-Dehshiri R, Hashemi AS, Neamatzadeh H, Zare-Zardeini H. A Case Report: Acute Myeloid Leukemia (FAB M7) Iran J Ped Hematol Oncol. 2014;4(4):188–190. [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson BA, Levine EG. Uncommon subtypes of acute nonlymphocytic leukemia: clinical features and management of FABM5, M6, and M7. Semin Oncol. 1987;14(4):425–434. [PubMed] [Google Scholar]
- 14.Moody A, Simpson E, Shaw D. Florid radiological appearance of megakaryoblastic leukaemia an aid to diagnosis. Pediatr Radiology. 1989;19(6–7):486–488. doi: 10.1007/BF02387668. [DOI] [PubMed] [Google Scholar]
- 15.Muler JH, Valdez R, Hayes C, Kaminski MS. Acute megakaryocytic leukemia presenting as hypercalcemia with skeletal lytic lesion. Euro J Hematology. 2002;68(6):392–396. doi: 10.1034/j.1600-0609.2002.02715.x. [DOI] [PubMed] [Google Scholar]
- 16.Terui T, Niitsu Y, Mahara K, Fujisaki Y, Urushizaki Y, Mogi Y, et al. The production of transforming growth factor-β in acute megakaryoblastic leukemia and its possible implications in myelofibrosis. Blood. 1990;75(7):1540–1548. [PubMed] [Google Scholar]
- 17.Heldin CH. Structural and functional studies on platelet derived growth factor. EMBO J. 1992;11:4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet derived growth factor. Cell. 1986;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AB, Spom MB. Physiological actions and clinical applications of transforming growth factor β. Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 20.Dastugue N, Lafage-Pochitaloff M, Pages MP, Radford I, Bastard C, Talmant P, et al. Cytogenetics profile of childhood and adult megakaryoblastic leukaemia (M7): A study of the Groupe Francais de Cytogenetique Hematologique (GFCH) Blood. 2002;100(2):618–626. doi: 10.1182/blood-2001-12-0241. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute MyeloidLeukemia. J Clin Oncol. 2003;21(24):4642–4646. doi: 10.1200/JCO.2003.04.036. doi: 10:1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]