Highlights
-
•
Cryo-electron tomography (cryoET) subtomogram averaging has emerged as a structural biology method for sparse and heterogenerous sampls.
-
•
CryoET subtomogram averaging enables in situ structure determination.
-
•
CryoET subtomogram classification can delineate different conformational states of macromolecular complexes.
-
•
Future developments in cryoET and correlative super resolution microscopy promises to bring unprecedented integration of cell biology and structural biology.
Abstract
Cryo-electron tomography (cryoET) can provide 3D reconstructions, or tomograms, of pleomorphic objects such as organelles or cells in their close-to-native states. Subtomograms that contain repetitive structures can be further extracted and subjected to averaging and classification to improve resolution, and this process has become an emerging structural biology method referred to as cryoET subtomogram averaging and classification (cryoSTAC). Recent technical advances in cryoSTAC have had a profound impact on many fields in biology. Here, I review recent exciting work on several macromolecular assemblies demonstrating the power of cryoSTAC for in situ structure analysis and discuss challenges and future directions.
Current Opinion in Structural Biology 2019, 58:249–258
This review comes from a themed issue on Biophysical and computational methods
Edited by Mark Yeager
For a complete overview see the Issue and the Editorial
Available online 5th July 2019
https://doi.org/10.1016/j.sbi.2019.05.021
0959-440X/© 2019 The Author. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Over the past five years, we have witnessed a huge leap in the field of cryoEM, in particular single-particle image analysis (SPA), with which structures of proteins and protein complexes are routinely determined to atomic and near-atomic resolutions [1,2]. It is considered the method of choice for determining the structures of large macromolecular assemblies, as it is more tolerant of structural heterogeneity and requires much less material than crystallographic methods. However, for pleomorphic and heterogeneous biological specimens that are not amenable to SPA, such as intact cells, organelles, pleomorphic viruses and variable macromolecular assemblies, cryo-electron tomography (cryoET) has been the method of choice [3]. In cryoET, a series of projection images from the same object are recorded as the sample is tilted to various angles relative to the incident electron beam. The images are subsequently aligned and reconstructed to generate a 3D tomogram. It provides a 3D volume of a single unique specimen without averaging. CryoET allows 3D imaging of frozen-hydrated biological specimens in a close to native state. Under optimal conditions, structural information to near-atomic resolution can be achieved. CryoET is a versatile technique that can be applied to a broad range of specimens, from isolated protein complexes to large eukaryotic cells [3].
Current practice of cryoET involves two main approaches, namely molecular cryoET and cellular cryoET. Molecular cryoET is typically employed to study in vitro purified ‘single-particle’ samples, often pleomorphic and not amenable for cryoEM SPA. This method has been excellent for generating initial models for cryoEM SPA, particularly when samples are relatively homogeneous. More recently, molecular cryoET has been applied to analyze repeating structures within larger pleomorphic objects using a process called cryoET subtomogram averaging and classification (cryoSTAC), when individual repeating units (i.e. subtomograms) are aligned in 3D and averaged to improve the signal-to-noise ratio (SNR) and the map resolution [4,5]. These 3D subtomograms can be further classified into multiple functional states or conformations [4,6]. Cellular cryoET, in contrast, has been applied to large pleomorphic objects such as intact bacteria and eukaryotic cells. It has been classically used for morphological analysis, until recently when high-resolution in situ structures of cellular complexes and assemblies have been obtained using cryoSTAC [7••]. Compared to SPA, cryoSTAC is arguably the greatest strength of cryoET, because each particle exists as a unique 3D reconstruction and allows for direct analysis of the 3D variance.
In recent years, advances in sample preparation, detector technology, phase plate imaging and image processing tools have enabled unprecedented characterization of protein complexes, in situ and ex situ [3]. CryoET and cryoSTAC have emerged as powerful methods for visualizing the molecular organization within a native cell or organelle, potentially allowing determination of protein complexes in their functional states and native environment to near-atomic resolution. In this review, I describe a typical cryoSTAC workflow and review advances during the past few years, focusing on high-resolution structure determination and classification of functional states. I highlight some exciting cases where near-atomic resolution has been achieved and novel functional insights have been obtained in situ via cryoSTAC.
CryoSTAC workflow
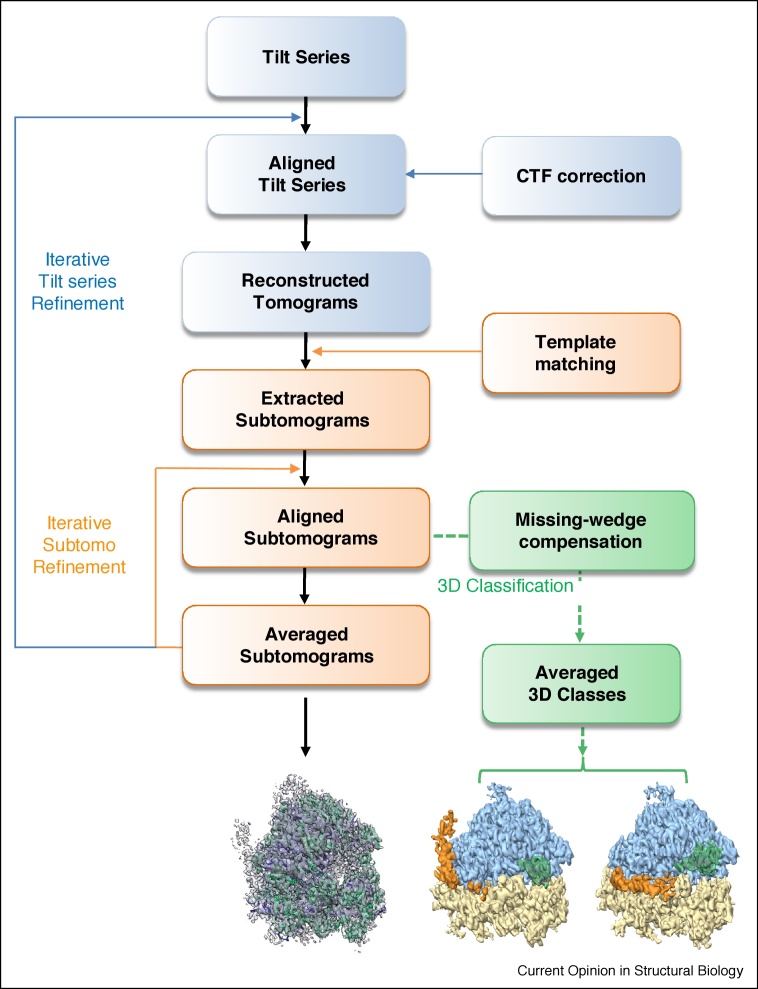
The basic principles of cryoSTAC have been described in previous publications (for recent reviews see Refs. [3,8]). A typical cryoSTAC workflow is illustrated in Figure 1, with three main processing stages: the first stage is processing the tilt series (blue), the second stage is subtomogram averaging (orange), and the third involves 3D classification (green). A number of software packages are available for cryoSTAC processing, and Table 1 presents a comparison of the main features in commonly used packages, including PEET [9], EMAN2 [10,11], RELION [12], Dynamo [13], Jsubtomo [14], PyTom/AV3 [15,16], Protomo/i3 [17] and emClarity [18••].
Figure 1.
The overall workflow for cryoET subtomogram averaging and classification (cryoSTAC).
The processes dealing with tilt series are in blue, subtomograms in orange, and 3D classification in green. The final structures at the bottom of the flowchart are yeast ribosomes.
Table 1.
Comparison of features of major subtomogram averaging software packages
| Major Software | PEET [9] | Eman2 [10,11] | RELION [12] | Dynamo [13] | Jsubtomo [14] | AV3/pyTOM [15,16] | Protomo/i3 [17] | emClarity [18] |
|---|---|---|---|---|---|---|---|---|
| Template Matchinga | Manual | Auto-picking | No | Manual | Yes | Yes | Yes | Yes |
| 3D CTF correctionb | No | Per-particle | Per-particle | No | No | No | No | Yes |
| Missing-wedge compensationc | WMDd | Fourier Intensity | 3D-Sampling | WMD | WMD | WMD | WMD | 3D-Sampling |
| Tilt-series refinement | No | No | No | No | No | No | No | Yes |
| GPU support | No | No | No | Yes | No | No | No | Yes |
| GUI | Yes | Yes | Yes | Yes | No | Partial | No | No |
Method used for extracting subtomograms.
3D CTF correction algorithm such as implemented in NovaCTF [27•].
Handling of missing-wedge in subtomogram alignment and classification.
WMD: wedge masked differences.
The input data for a cryoSTAC workflow are a tilt series, that is, a series of cryoEM projection images recorded from the same specimen area with the specimen tilted over a range of angles, typically ±60°. There are several tilting schemes used for data collection, commonly the unidirectional (from −60 to +60), bidirectional (0 to −60, then 0 to +60), and more recently dose-symmetrical (alternating – and + tilts as the tilt angle increases, 0, −2, +2, −4, +4…) [19•]. The series of tilt images are then aligned, with or without the aid of fiducial beads such as gold particles added to the specimen, and the contrast transfer function (CTF) is determined and compensated [11,20]. A 3D volume (tomogram) can be reconstructed computationally from the aligned tilt series, commonly using a weighted back-projection algorithm as implemented in IMOD [21], although several other reconstruction methods are available [22, 23, 24]. The alignment of a tilt series can be further refined iteratively, using subtomograms as fiducial markers, as implemented in the newly developed emClarity [18••].
The 3D tomogram often contains multiple copies or instances of the complex of interest, which can be extracted, typically using a template-matching algorithm in which a known structure serves as a reference [4]. The angular orientation of the extracted subtomogram is refined iteratively in a way that is conceptually similar to SPA, but in 3D instead of 2D, and is used to generate an improved structure by averaging many copies of the object. Resolutions in the subnanometer range have been attained in situ [25•], and near-atomic ex situ [26••,27•].
These particles, as copies of the complex of interest in a tomogram, often vary in conformation and composition, and thus need to be separated using 3D classification. An important consideration during 3D classification is that tomograms are distorted along the z axis because of the missing data in reciprocal space resulting from the limited tilt range in data collection (also known as the ‘missing-wedge’). This must be taken into account in subtomogram classification, as the missing-wedge artifact tends to obscure real differences between subtomograms [4,9]. A simple approach to compensate for the missing-wedge effect is to apply the same subtomogram wedge to the reference average when these are compared with each other, as implemented in the binary wedge-masked difference (WMD) method [9,16]. More sophisticated approaches have been recently developed to correct for modulation of the CTF, such as Fourier intensity modulation [10] and full 3D sampling function [12,18••].
Recent technical advances in cryoSTAC
In addition to the development of direct electron detectors, which have transformed all modalities of cryoEM, three main areas of recent technical advances have made cryoSTAC an exciting new method for in situ structure determination at subnanometer resolution: sample preparation, data collection, and image processing. Several new sample preparation methods have been developed to overcome the limitation of sample thickness (0.5 μm) for cryoEM imaging of bacteria and large eukaryotic cells in situ. These include mini-bacterial cell preparations [28], controlled bacterial lysis using a phage lysis gene [29], vitreous sectioning (CEMOVIS) [30], and, most significantly cryo-focused ion beam (cryoFIB) micromachining to create a 150–250 nm thick cell lamella, allowing access to any location inside of eukaryotic cells [31,32]. With regard to data acquisition, direct electron detectors, zero-loss imaging with an energy filter and phase plate imaging greatly enhance the SNR in extremely low-dose cryoET images [33]. Furthermore, tilt-series acquisition using the dose-symmetrical scheme ensures an optimal use of limited electron dose [19•]. Lastly, new algorithms have been developed and incorporated into cryoSTAC processing software (Table 1), including template matching for subtomogram extraction [16,17,18••], 3D-CTF for performing CTF corrections [10,18••,27•], tomoCPR for iteratively refining the tilt series alignment using subtomograms as fiducials [18••], use of a 3D-sampling function for improved ‘missing-wedge’ compensation [12,18••], and multiscale principal component analysis (PCA) for robust 3D classification [18••]. These technical improvements in cryoSTAC have had a significant impact on our understanding of the molecular mechanisms and biological functions of macromolecular assemblies, as exemplified in the cases discussed below.
Successful applications
In recent years there have been numerous examples of cryoSTAC applications in various fields. These include membrane-associated and embedded complexes, such as Coat Protein Complex I (COPI) [34•,35], Nuclear Pore Complexes (NPCs) [36•,37], mitochondrial complex I and its supramolecular assemblies [38], mitochondrial ATP synthase [39,40], polysomes [41] and pore forming pneumolysin [42]; large assemblies in bacteria cells, such as chemotaxis signaling arrays [43,44••], the type IV pilus [45••], type III, type IV and Type VI secretion systems [46, 47, 48, 49, 50]; as well as bacteriophages [51,52] and viruses [26••,53, 54, 55,56•,57••]. For the majority of these systems, the resolution has been limited to 2–4 nm. Here, I focus on several recent studies that have achieved close to 1 nm resolution or better, and that have distinguished multiple functional states in situ using cryoSTAC. I show how the application of cryoSTAC has led to new insights into the function of macromolecular complexes that were not previously attainable.
Molecular cryoSTAC at high resolution
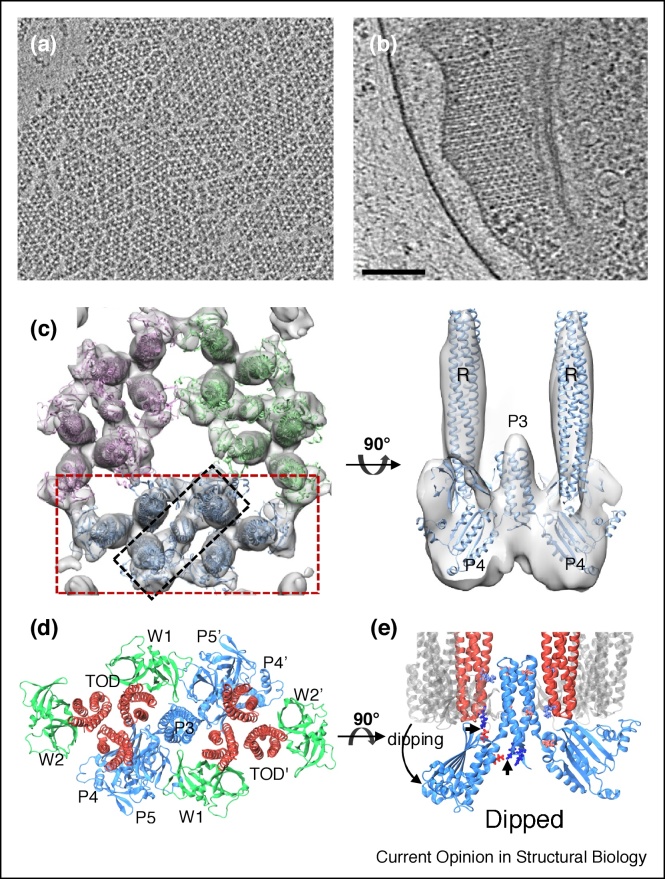
Biological complexes are often heterogeneous and not amenable to the SPA approach. In these cases, molecular cryoSTAC is an ideal approach to obtain their structures, given that the specimens are usually thin and contain many copies. By this approach, components of such biological systems can be isolated and reconstituted in vitro to reduce the complexity. An example of such a reconstituted system is the large and dynamical array comprised of bacterial chemotaxis core signaling complexes, which are responsible for monitoring the chemical environment and directing cell migration towards nutrient sources. Studies of native arrays using cryoSTAC have yielded a great deal of knowledge about the organization of the array [58,59], but with limited resolution. Using purified protein components, the signaling array can be reconstituted on a lipid monolayer, mimicking the structure in native bacterial cells (Figure 2a, b) [44••]. This relatively clean system with a large number of repeating units (∼3000 subtomograms) was amenable to cryoSTAC, from which the structure of the core signaling complex was determined at 11 Å resolution (Figure 2c). Guided by the structural details present in the subtomogram average, an atomic model of the whole signaling array revealed novel interfaces between the component proteins. In addition, molecular dynamics simulations revealed conformational dynamics of the core signaling complex (Figure 2d, e) [60].
Figure 2.
CryoSTAC of a reconstituted bacterial chemotaxis signaling array.
(a) and (b) Tomographic slices of the in vitro reconstituted array of the chemotaxis core signaling complex (a) and of a native array in an Escherichia coli cell (b). Scale bar, 100 nm. (c) The density map of a threefold assembly unit by subtomogram averaging, with a core signaling complex boxed in red and a rotated view shown on the right corresponding to the black box. The receptor, P3 and P4 domains of Che A are labeled as ‘R’, ‘P3’ and ‘P4’, respectively. (d) Pseudo-atomic model of the core signaling complex, consisting of six chemoreceptor dimers (red, labeled ‘TOD’), one CheA dimer (blue, labeled ‘P3–P5’), and four CheW monomers (green, labeled ‘W1’ and ‘W2’). (e) CheA conformational dynamics with a dipping motion, determined by large scale MD simulation. Arrows point to the interacting amino acids in the dipped state [44••].
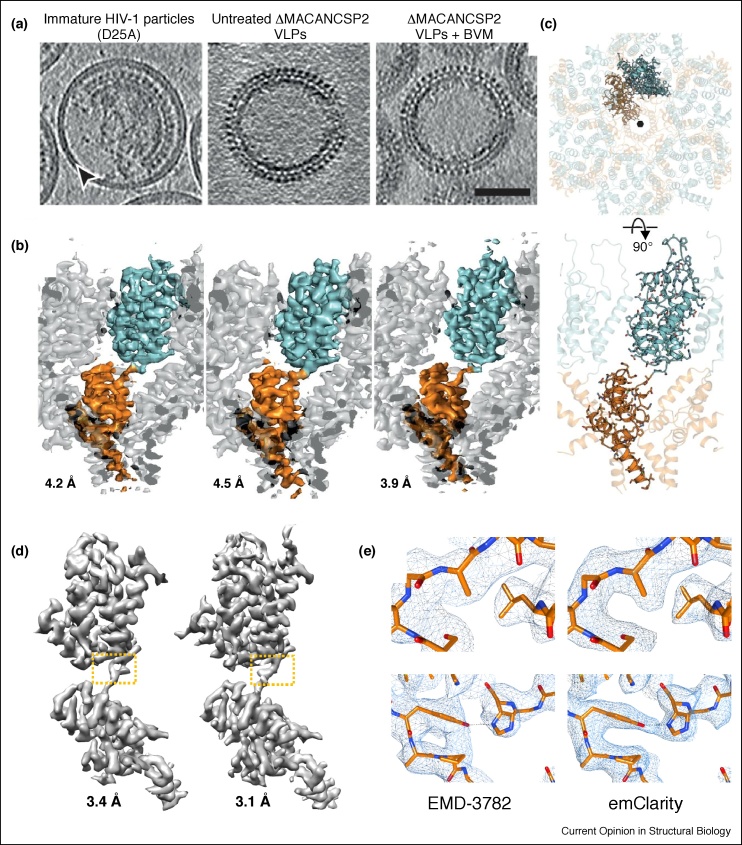
Many virus particles manifest icosahedral or helical symmetry and are amenable to high-resolution structure determination by SPA. Other viruses, such as HIV-1 are pleomorphic. Nevertheless, structure determination via cryoSTAC is still feasible because the pleomorphic capsids comprised many repeating units. By this approach Briggs and colleagues have determined some of the highest resolution subtomogram structures to date [26••,27•]. Examples of their impressive results include the structure of the asymmetric unit of the COPI coat protein assembled in vitro (∼40 000 subtomograms) at 9.2 Å resolution [35], the structure of immature virus-like Rous Sarcoma Virus Gag particles at 8 Å resolution [61]; the structure of Ebola virus nucleocapsid assemblies at 6.6 Å resolution [56•], and most significantly their work on HIV-1 immature and mature capsid assemblies [26••,57••]. The CA portion of immature HIV-1 Gag was initially solved by cryoSTAC to 8.8 Å [62], allowing unambiguous positioning of all α-helices. Optimizing data collection and image processing, as well as increasing the size of the dataset, led to a dramatic improvement of the structure to 3.9 Å resolution, which was further enhanced to 3.4 Å with 3D-CTF correction (Figure 3a–c) [26••,27•]. The near-atomic resolution structure reveals a network of interactions mediating immature HIV-1 assembly and a previously elusive SP1 six-helix bundle stabilized by a maturation inhibitor [26••]. In addition, structures of the CA hexamers and pentamers within mature capsids of native virions have been determined by cryoSTAC, which revealed a different pentamer organization compared to the previous X-ray crystal structures and how the quasi-hexagonal CA lattice flexes to form the variably curved capsid shell [57••].
Figure 3.
CryoSTAC of HIV-1 immature Gag particles.
(a) Tomographic slices of immature HIV-1 particles (with D25A mutation), ΔMACANCSP2 VLPs, in the absence and presence of the maturation inhibitor bevirimat (BVM). Scale bar, 50 nm. (b) CA-SP1 densities from the samples shown in (a) by subtomogram averaging. One CA-SP1 monomer is highlighted, with the CA-NTD in cyan and the CA-CTD and SP1 in orange. (c) The refined atomic model. (d) An improved CA-SP1 density map at 3.1 Å resolution by emClarity (right), compared to the previous structure (left, EMD-3782). (e) Enlarged views of boxed area in (d) overlaid with a real-space refined model [18••,26••].
The highest reported resolution of immature HIV-1 Gag particles determined by cryoSTAC is currently 3.1 Å (Figure 3d, e), owing to the development of emClarity [18••]. This new GPU-accelerated software features a novel iterative tilt-series refinement algorithm, a 3D-sampling function for missing-wedge compensation and a multiscale PCA classification (Figure 1, Table 1). These implementations have enabled a significant improvement in resolution and in 3D classification of different functional states in several test samples [18••]. The prospect of reaching atomic or near-atomic resolution by cryoSTAC and the ability to sort out multiple conformers have generated great interest in the cryoEM field.
Cellular cryoSTAC for in situ structures and functional states
A large body of cellular cryoSTAC studies have been performed on a variety of intact bacterial cells because of their relatively small size. Because of the sparsity and complexity of the object of interest, these studies have resulted in mostly low resolution structures [45••,46, 47, 48, 49, 50]. One exception is the structure of the bacterial S-layer proteins, which has been determined to 7.4 Å by cryoSTAC [25•]. Docking of the X-ray structure into the subtomogram average resulted in a pseudo-atomic model of the S-layer, which revealed that the S-layer is porous and stabilized by multiple Ca2+ ions bound near the interfaces [25•].
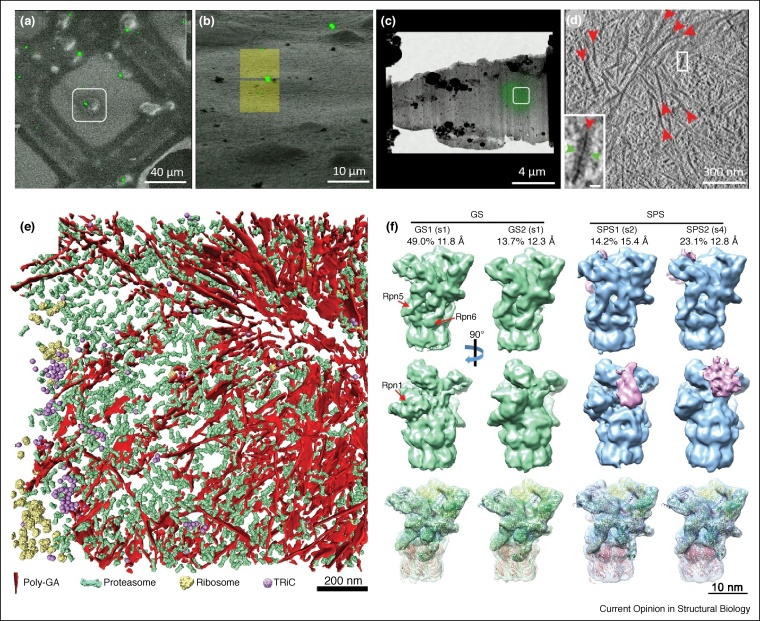
Applications of cryoSTAC to large mammalian cells are much more challenging and usually require cryoFIB milling to produce cell lamellae of 150–250 nm in thickness for cryoEM imaging. In situ low resolution structures of the COPI coat [34•], NPC [63,64], adenovirus particles and microtubules [54], tripeptidyl peptidase II (TPPII) [65] and proteasomes [66] were determined using cryoFIB and cryoSTAC. Most recently, Baumeister’s lab investigated structures of protein aggregates inside neurons, in particular polyglutamine (polyQ)-expanded huntingtin exon 1 and poly-Gly-Ala (poly-GA) aggregates. For this purpose cryo-correlative light and electron microscopy (cryoCLEM) were used to target polyQ and poly-GA inclusions for cryoFIB milling [7••,67•]. They showed that PolyQ inclusions in neurons consist of amyloid-like fibrils, interact with and deform ER membranes and alter ER organization and dynamics without harbouring a significant amount of 26S proteasomes [67•]. In contrast, Poly-GA aggregates consist of more densely packed planar twisted ribbons that recruit numerous 26S proteasome complexes (Figure 4) [7••]. CryoSTAC analysis of recruited 26S proteasomes, in both ground-processing and substrate-processing states, revealed an enrichment of substrate processing conformations and proteasome stalling upon interaction with poly-GA aggregates. The study suggests that poly-GA aggregates may compromise neuronal proteostasis by sequestering and functionally impairing a large fraction of cellular proteasomes. As exemplified in these studies, cellular cryoSTAC combined with 3D classification revealed different conformational states of protein complexes. Their spatial distributions could be mapped in native cells, which changed in response to various perturbations, thus opening a new frontier in structural cell biology.
Figure 4.
CryoSTAC of in situ Poly-GA aggregates with proteasomes recruitment in neurons.
(a) and (b) Correlative cryo-light and cryoFIB/SEM of rat cortical neurons cultured on EM grids and transduced with (GA)175-GFP. SEM (a) and FIB (b) images were aligned and superimposed with the GFP signal from the cryo-LM image. (c) Cryo-TEM low magnification image of the lamella superimposed with the GFP signal. (d) A tomographic slice recorded in the area with GFP signal (white square in (c)). Red arrowheads mark a dense network of poly-GA-GFP. (e) 3D rendering of an aggregate within a neuron transduced with (GA)175-GFP showing different macromolecules found either within or at the periphery of the aggregate. (f) Subtomogram classification of 26S proteasomes reveals enrichment of substrate processing conformations. GS, ground state; SPS, substrate-processing state [7••].
Future perspective
Compared to cryoEM SPA, cryoSTAC is still in its early stages. However, there are examples in which near-atomic resolution structures have been determined, and multiple functional states have been delineated in situ, allowing a direct connection between cellular function and the structure of protein complexes. The greatest strength of cryoSTAC lies in in situ structure determination with 3D classification in native systems. It holds the potential to provide cellular landscapes of macromolecular complexes in near-atomic details with their spatial coordinates (or molecular census) [68]. Yet, the method is limited, as many proteins are too small and too rare, falling below the detection limit. In addition, extreme crowding within the cytoplasm greatly impedes the ability to distinguish individual proteins and protein complexes.
Some of the latest technologies, including direct electron detectors, zero-loss imaging with an energy filter, phase plate imaging to enhance contrast, and cryoFIB milling for access to the interior of thick cells, have proven to be extremely valuable for deriving higher resolution structures by cryoSTAC approaches. There are still many avenues for further improvement and optimization, and many applications to explore. In cryoSTAC processing, algorithms need to be developed to properly handle cryoET data collected using a phase plate. For in situ sample preparation, cryoFIB milling of frozen-hydrated samples is by far the best method, but current applications are limited to samples that are less than 5 μm thick, where proper vitrification can be achieved via plunge freezing. A routine cryoFIB lift-out [69] procedure would greatly facilitate cryoSTAC studies of thicker mammalian cells and even tissues that are high-pressure frozen. CryoCLEM is critical for targeting areas of interest in cryoFIB, but currently its precision is limited to a few hundred nanometers within the imaging plane and much worse in the Z direction. Further enhancement in super resolution cryoCLEM could potentially allow correlations at the single molecule level, which, in combination with improved template matching, will make the localization and identification of macromolecules in 3D tomograms almost entirely unambiguous. And finally, with further development in time-resolved cryoEM [70], we can begin to capture changes of molecular complexes in conformation and localization upon perturbation. By revealing the structures and atlas of macromolecular complexes in situ and in time, cryoET and cryoSTAC approaches will have an immense impact on our mechanistic understanding of biological systems, in normal and pathological physiology.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The author thanks Dr Helen Saibil and Dr Teresa Brosenitsch for critical reading of the manuscript. This work was supported by the Wellcome Trust Investigator Award (206422/Z/17/Z), the BBSRC grant (BB/S003339/1) and the NIH grant (GM082251).
References
- 1.Frank J. Advances in the field of single-particle cryo-electron microscopy over the last decade. Nat Protoc. 2017;12:209–212. doi: 10.1038/nprot.2017.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson R. From electron crystallography to single particle cryoEM (nobel lecture) Angew Chem Int Ed Engl. 2018;57:10804–10825. doi: 10.1002/anie.201802731. [DOI] [PubMed] [Google Scholar]
- 3.Koning R.I., Koster A.J., Sharp T.H. Advances in cryo-electron tomography for biology and medicine. Ann Anat. 2018;217:82–96. doi: 10.1016/j.aanat.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Winkler H., Zhu P., Liu J., Ye F., Roux K.H., Taylor K.A. Tomographic subvolume alignment and subvolume classification applied to myosin V and SIV envelope spikes. J Struct Biol. 2009;165:64–77. doi: 10.1016/j.jsb.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster F., Hegerl R. Structure determination in situ by averaging of tomograms. Methods Cell Biol. 2007;79:741–767. doi: 10.1016/S0091-679X(06)79029-X. [DOI] [PubMed] [Google Scholar]
- 6.Beck M., Baumeister W. Cryo-electron tomography: can it reveal the molecular sociology of cells in atomic detail? Trends Cell Biol. 2016;26:825–837. doi: 10.1016/j.tcb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 7••.Guo Q., Lehmer C., Martinez-Sanchez A., Rudack T., Beck F., Hartmann H., Perez-Berlanga M., Frottin F., Hipp M.S., Hartl F.U. In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell. 2018;172:696–705.e12. doi: 10.1016/j.cell.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, cryoFIB milling, correlative microscopy and cryoSTAC were employed to dissect the molecular architecture of Poly-GA protein aggregates within intact neurons at high resolution. Poly-GA aggregates were found to consist of densely packed twisted ribbons that recruit 26S proteasome complexes. The results suggested a link between protein aggregation and dysfunction of the ubiquitin-proteasome system in neurodegenerative diseases.
- 8.Wan W., Briggs J.A. Cryo-electron tomography and subtomogram averaging. Methods Enzymol. 2016;579:329–367. doi: 10.1016/bs.mie.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Heumann J.M., Hoenger A., Mastronarde D.N. Clustering and variance maps for cryo-electron tomography using wedge-masked differences. J Struct Biol. 2011;175:288–299. doi: 10.1016/j.jsb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galaz-Montoya J.G., Flanagan J., Schmid M.F., Ludtke S.J. Single particle tomography in EMAN2. J Struct Biol. 2015;190:279–290. doi: 10.1016/j.jsb.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galaz-Montoya J.G., Hecksel C.W., Baldwin P.R., Wang E., Weaver S.C., Schmid M.F., Ludtke S.J., Chiu W. Alignment algorithms and per-particle CTF correction for single particle cryo-electron tomography. J Struct Biol. 2016;194:383–394. doi: 10.1016/j.jsb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat T.A., Scheres S.H. Resolving macromolecular structures from electron cryo-tomography data using subtomogram averaging in RELION. Nat Protoc. 2016;11:2054–2065. doi: 10.1038/nprot.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castano-Diez D., Kudryashev M., Arheit M., Stahlberg H. Dynamo: a flexible, user-friendly development tool for subtomogram averaging of cryo-EM data in high-performance computing environments. J Struct Biol. 2012;178:139–151. doi: 10.1016/j.jsb.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Maurer U.E., Zeev-Ben-Mordehai T., Pandurangan A.P., Cairns T.M., Hannah B.P., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Topf M., Huiskonen J.T. The structure of herpesvirus fusion glycoprotein B-bilayer complex reveals the protein-membrane and lateral protein-protein interaction. Structure. 2013;21:1396–1405. doi: 10.1016/j.str.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster F., Pruggnaller S., Seybert A., Frangakis A.S. Classification of cryo-electron sub-tomograms using constrained correlation. J Struct Biol. 2008;161:276–286. doi: 10.1016/j.jsb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Hrabe T., Chen Y., Pfeffer S., Cuellar L.K., Mangold A.V., Forster F. PyTom: a python-based toolbox for localization of macromolecules in cryo-electron tomograms and subtomogram analysis. J Struct Biol. 2012;178:177–188. doi: 10.1016/j.jsb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Winkler H. 3D reconstruction and processing of volumetric data in cryo-electron tomography. J Struct Biol. 2007;157:126–137. doi: 10.1016/j.jsb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 18••.Himes B.A., Zhang P. emClarity: software for high resolution cryo-electron tomography and sub-tomogram averaging. Nature Methods. 2018 doi: 10.1038/s41592-018-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; This GPU-accelerated software is designed for in situ high-resolution structure determination by cryoSTAC. It features an iterative refinement of tilt geometry, a 3D-sampling function for ‘missing-wedge’ compensation and a multiscale PCA for 3D classification.
- 19•.Hagen W.J.H., Wan W., Briggs J.A.G. Implementation of a cryo-electron tomography tilt-scheme optimized for high resolution subtomogram averaging. J Struct Biol. 2017;197:191–198. doi: 10.1016/j.jsb.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a ‘dose-symmetric tilt-scheme’ that maximizes the amount of high-resolution information maintained in the tomogram for subsequent subtomogram averaging. The implementation contains a macro in the environment of SerialEM for automated tilt-series data collection using a dose-symmetric tilt-scheme.
- 20.Winkler H., Taylor K.A. Focus gradient correction applied to tilt series image data used in electron tomography. J Struct Biol. 2003;143:24–32. doi: 10.1016/s1047-8477(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 21.Kremer J.R., Mastronarde D.N., McIntosh J.R. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 22.Turonova B., Marsalek L., Davidovic T., Slusallek P. Progressive stochastic reconstruction technique (PSRT) for cryo electron tomography. J Struct Biol. 2015;189:195–206. doi: 10.1016/j.jsb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert P. Iterative methods for the three-dimensional reconstruction of an object from projections. J Theor Biol. 1972;36:105–117. doi: 10.1016/0022-5193(72)90180-4. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y., Chen Y., Zhang Y., Wang S., Zhang F., Sun F. ICON: 3D reconstruction with’ missing-information’ restoration in biological electron tomography. J Struct Biol. 2016;195:100–112. doi: 10.1016/j.jsb.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 25•.Bharat T.A.M., Kureisaite-Ciziene D., Hardy G.G., Yu E.W., Devant J.M., Hagen W.J.H., Brun Y.V., Briggs J.A.G., Lowe J. Structure of the hexagonal surface layer on Caulobacter crescentus cells. Nat Microbiol. 2017;2:17059. doi: 10.1038/nmicrobiol.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study combines X-ray crystallography of S-layer protein with cryoSTAC of native S-layer. The 2.7 Å X-ray structure shows the hexameric S-layer lattice, which agrees completely with a pseudo-atomic model docked into the 7.4 Å structure of the in situ S-layer from native cells determined by cryoSTAC.
- 26••.Schur F.K., Obr M., Hagen W.J., Wan W., Jakobi A.J., Kirkpatrick J.M., Sachse C., Krausslich H.G., Briggs J.A. An atomic model of HIV-1 capsid-SP1 reveals structures regulating assembly and maturation. Science. 2016;353:506–508. doi: 10.1126/science.aaf9620. [DOI] [PubMed] [Google Scholar]; The structure of CA-SP1 in immature HIV-1 Gag in the presence of a maturation inhibitor was determined at 3.9 Å resolution by cryoSTAC, allowing an atomic model to be reliably built. The high-resolution structure provided detailed intermolecular interactions in the immature HIV-1 capsid.
- 27•.Turonova B., Schur F.K.M., Wan W., Briggs J.A.G. Efficient 3D-CTF correction for cryo-electron tomography using NovaCTF improves subtomogram averaging resolution to 3.4A. J Struct Biol. 2017;199:187–195. doi: 10.1016/j.jsb.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript describes a user-friendly, computationally-efficient 3D-CTF correction tool, NovaCTF, for cryoSTAC. The study showed a significant improvement in resolution using this software tool, as exemplified by the structure of immature HIV-1 Gag assembly at 3.4 Å.
- 28.Farley M.M., Hu B., Margolin W., Liu J. Minicells, back in fashion. J Bacteriol. 2016;198:1186–1195. doi: 10.1128/JB.00901-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X., Himes B.A., Ke D., Rice W.J., Ning J., Zhang P. Controlled bacterial lysis for electron tomography of native cell membranes. Structure. 2014;22:1875–1882. doi: 10.1016/j.str.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Amoudi A., Chang J.J., Leforestier A., McDowall A., Salamin L.M., Norlen L.P., Richter K., Blanc N.S., Studer D., Dubochet J. Cryo-electron microscopy of vitreous sections. EMBO J. 2004;23:3583–3588. doi: 10.1038/sj.emboj.7600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigort A., Bauerlein F.J., Leis A., Gruska M., Hoffmann C., Laugks T., Bohm U., Eibauer M., Gnaegi H., Baumeister W. Micromachining tools and correlative approaches for cellular cryo-electron tomography. J Struct Biol. 2010;172:169–179. doi: 10.1016/j.jsb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang K., Strunk K., Zhao G., Gray J.L., Zhang P. 3D structure determination of native mammalian cells using cryo-FIB and cryo-electron tomography. J Struct Biol. 2012;180:318–326. doi: 10.1016/j.jsb.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoshouei M., Pfeffer S., Baumeister W., Forster F., Danev R. Subtomogram analysis using the Volta phase plate. J Struct Biol. 2017;197:94–101. doi: 10.1016/j.jsb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34•.Bykov Y.S., Schaffer M., Dodonova S.O., Albert S., Plitzko J.M., Baumeister W., Engel B.D., Briggs J.A. The structure of the COPI coat determined within the cell. eLife. 2017;6 doi: 10.7554/eLife.32493. [DOI] [PMC free article] [PubMed] [Google Scholar]; The native structure of the COPI coat within vitrified Chlamydomonas reinhardtii cells was determined by cryoFIB and cryoSTAC. Structural analysis in situ showed that vesicles change their size, membrane thickness, and cargo content as they progress from the cis to trans Golgi apparatus, but the structure of the coat machinery remains constant.
- 35.Dodonova S.O., Aderhold P., Kopp J., Ganeva I., Rohling S., Hagen W.J.H., Sinning I., Wieland F., Briggs J.A.G. 9A structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments. eLife. 2017;6 doi: 10.7554/eLife.26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Mahamid J., Pfeffer S., Schaffer M., Villa E., Danev R., Cuellar L.K., Forster F., Hyman A.A., Plitzko J.M., Baumeister W. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science. 2016;351:969–972. doi: 10.1126/science.aad8857. [DOI] [PubMed] [Google Scholar]; This study reports in situ structures of protein complexes in HeLa cells using cryoFIB and cryoSTAC, including the cytoplasmic translation machinery and the nuclear pore complex, as well as previously elusive structures, such as nucleosome chains and the filaments of the nuclear lamina.
- 37.von Appen A., Kosinski J., Sparks L., Ori A., DiGuilio A.L., Vollmer B., Mackmull M.T., Banterle N., Parca L., Kastritis P. In situ structural analysis of the human nuclear pore complex. Nature. 2015;526:140–143. doi: 10.1038/nature15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies K.M., Blum T.B., Kuhlbrandt W. Conserved in situ arrangement of complex I and III2 in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc Natl Acad Sci U S A. 2018;115:3024–3029. doi: 10.1073/pnas.1720702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muhleip A.W., Dewar C.E., Schnaufer A., Kuhlbrandt W., Davies K.M. In situ structure of trypanosomal ATP synthase dimer reveals a unique arrangement of catalytic subunits. Proc Natl Acad Sci U S A. 2017;114:992–997. doi: 10.1073/pnas.1612386114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muhleip A.W., Joos F., Wigge C., Frangakis A.S., Kuhlbrandt W., Davies K.M. Helical arrays of U-shaped ATP synthase dimers form tubular cristae in ciliate mitochondria. Proc Natl Acad Sci U S A. 2016;113:8442–8447. doi: 10.1073/pnas.1525430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeffer S., Burbaum L., Unverdorben P., Pech M., Chen Y., Zimmermann R., Beckmann R., Forster F. Structure of the native Sec61 protein-conducting channel. Nat Commun. 2015;6 doi: 10.1038/ncomms9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Pee K., Neuhaus A., D’Imprima E., Mills D.J., Kuhlbrandt W., Yildiz O. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. eLife. 2017;6 doi: 10.7554/eLife.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briegel A., Ortega D.R., Mann P., Kjaer A., Ringgaard S., Jensen G.J. Chemotaxis cluster 1 proteins form cytoplasmic arrays in Vibrio cholerae and are stabilized by a double signaling domain receptor DosM. Proc Natl Acad Sci U S A. 2016;113:10412–10417. doi: 10.1073/pnas.1604693113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Cassidy C.K., Himes B.A., Alvarez F.J., Ma J., Zhao G., Perilla J.R., Schulten K., Zhang P. CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling. eLife. 2015;4 doi: 10.7554/eLife.08419. [DOI] [PMC free article] [PubMed] [Google Scholar]; An 11 Å structure of the bacterial chemotaxis core signaling complex was determined using cryoSTAC from data recorded on a CCD camera. A pseudo-atomic model of the signaling array was constructed by cryoEM-based MD simulations, revealing the conformational dynamics of the core signaling complex.
- 45••.Chang Y.W., Rettberg L.A., Treuner-Lange A., Iwasa J., Sogaard-Andersen L., Jensen G.J. Architecture of the type IVa pilus machine. Science. 2016;351 doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the architecture of the type IVa pilus machine (T4PM) in intact Myxococcus xanthus cells using cryoSTAC. Aided by systematic mutations (deletion or tagging), the authors mapped the locations of all 10 T4PM core components and the minor pilins, providing insights into pilus assembly, structure and function.
- 46.Chang Y.W., Rettberg L.A., Ortega D.R., Jensen G.J. In vivo structures of an intact type VI secretion system revealed by electron cryotomography. EMBO Rep. 2017;18:1090–1099. doi: 10.15252/embr.201744072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang Y.W., Shaffer C.L., Rettberg L.A., Ghosal D., Jensen G.J. In vivo structures of the helicobacter pylori cag type IV secretion system. Cell Rep. 2018;23:673–681. doi: 10.1016/j.celrep.2018.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nans A., Kudryashev M., Saibil H.R., Hayward R.D. Structure of a bacterial type III secretion system in contact with a host membrane in situ. Nat Commun. 2015;6 doi: 10.1038/ncomms10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu B., Morado D.R., Margolin W., Rohde J.R., Arizmendi O., Picking W.L., Picking W.D., Liu J. Visualization of the type III secretion sorting platform of Shigella flexneri. Proc Natl Acad Sci U S A. 2015;112:1047–1052. doi: 10.1073/pnas.1411610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chetrit D., Hu B., Christie P.J., Roy C.R., Liu J. A unique cytoplasmic ATPase complex defines the Legionella pneumophila type IV secretion channel. Nat Microbiol. 2018;3:678–686. doi: 10.1038/s41564-018-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farley M.M., Tu J., Kearns D.B., Molineux I.J., Liu J. Ultrastructural analysis of bacteriophage Phi29 during infection of Bacillus subtilis. J Struct Biol. 2017;197:163–171. doi: 10.1016/j.jsb.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu B., Margolin W., Molineux I.J., Liu J. Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proc Natl Acad Sci U S A. 2015;112:E4919–E4928. doi: 10.1073/pnas.1501064112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riedel C., Vasishtan D., Siebert C.A., Whittle C., Lehmann M.J., Mothes W., Grunewald K. Native structure of a retroviral envelope protein and its conformational change upon interaction with the target cell. J Struct Biol. 2017;197:172–180. doi: 10.1016/j.jsb.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grange M., Vasishtan D., Grunewald K. Cellular electron cryo tomography and in situ sub-volume averaging reveal the context of microtubule-based processes. J Struct Biol. 2017;197:181–190. doi: 10.1016/j.jsb.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeev-Ben-Mordehai T., Vasishtan D., Hernandez Duran A., Vollmer B., White P., Prasad Pandurangan A., Siebert C.A., Topf M., Grunewald K. Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B. Proc Natl Acad Sci U S A. 2016;113:4176–4181. doi: 10.1073/pnas.1523234113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Wan W., Kolesnikova L., Clarke M., Koehler A., Noda T., Becker S., Briggs J.A.G. Structure and assembly of the Ebola virus nucleocapsid. Nature. 2017;551:394–397. doi: 10.1038/nature24490. [DOI] [PMC free article] [PubMed] [Google Scholar]; CryoSTAC was used to determine structures of Ebola virus nucleocapsid within intact viruses and recombinant nucleocapsid-like assemblies at 7.3 Å and 6.6 Å resolution, respectively. These structures suggest a mechanistic link between nucleoprotein oligomerization, nucleocapsid condensation, RNA encapsidation and accessory protein recruitment.
- 57••.Mattei S., Glass B., Hagen W.J., Krausslich H.G., Briggs J.A. The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science. 2016;354:1434–1437. doi: 10.1126/science.aah4972. [DOI] [PubMed] [Google Scholar]; In this study, structures of both hexameric and pentameric CA assemblies of HIV-1 mature capsid were determined within intact HIV-1 particles using cryoSTAC, revealing how CA flexes to form the variably curved core shell.
- 58.Briegel A., Li X., Bilwes A.M., Hughes K.T., Jensen G.J., Crane B.R. Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc Natl Acad Sci U S A. 2012;109:3766–3771. doi: 10.1073/pnas.1115719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J., Hu B., Morado D.R., Jani S., Manson M.D., Margolin W. Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. Proc Natl Acad Sci U S A. 2012;109:E1481–1488. doi: 10.1073/pnas.1200781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassidy C.K., Himes B.A., Luthey-Schulten Z., Zhang P. CryoEM-based hybrid modeling approaches for structure determination. Curr Opin Microbiol. 2018;43:14–23. doi: 10.1016/j.mib.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schur F.K., Dick R.A., Hagen W.J., Vogt V.M., Briggs J.A. The structure of immature virus-like Rous sarcoma virus gag particles reveals a structural role for the p10 domain in assembly. J Virol. 2015;89:10294–10302. doi: 10.1128/JVI.01502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schur F.K., Hagen W.J., Rumlova M., Ruml T., Muller B., Krausslich H.G., Briggs J.A. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 A resolution. Nature. 2015;517:505–508. doi: 10.1038/nature13838. [DOI] [PubMed] [Google Scholar]
- 63.Kosinski J., Mosalaganti S., von Appen A., Teimer R., DiGuilio A.L., Wan W., Bui K.H., Hagen W.J., Briggs J.A., Glavy J.S. Molecular architecture of the inner ring scaffold of the human nuclear pore complex. Science. 2016;352:363–365. doi: 10.1126/science.aaf0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mosalaganti S., Kosinski J., Albert S., Schaffer M., Strenkert D., Salome P.A., Merchant S.S., Plitzko J.M., Baumeister W., Engel B.D. In situ architecture of the algal nuclear pore complex. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuda Y., Beck F., Plitzko J.M., Baumeister W. In situ structural studies of tripeptidyl peptidase II (TPPII) reveal spatial association with proteasomes. Proc Natl Acad Sci U S A. 2017;114:4412–4417. doi: 10.1073/pnas.1701367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albert S., Schaffer M., Beck F., Mosalaganti S., Asano S., Thomas H.F., Plitzko J.M., Beck M., Baumeister W., Engel B.D. Proteasomes tether to two distinct sites at the nuclear pore complex. Proc Natl Acad Sci U S A. 2017;114:13726–13731. doi: 10.1073/pnas.1716305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Bauerlein F.J.B., Saha I., Mishra A., Kalemanov M., Martinez-Sanchez A., Klein R., Dudanova I., Hipp M.S., Hartl F.U., Baumeister W. In situ architecture and cellular interactions of PolyQ inclusions. Cell. 2017;171:179–187 e110. doi: 10.1016/j.cell.2017.08.009. [DOI] [PubMed] [Google Scholar]; The structure of inclusions formed by olyglutamine (polyQ)-expanded huntingtin exon 1 within their intact cellular context was characterized by cryoSTAC. PolyQ inclusions consisting of amyloid-like fibrils interact with cellular endomembranes, particularly of the endoplasmic reticulum (ER), and alter ER membrane dynamics.
- 68.Asano S., Fukuda Y., Beck F., Aufderheide A., Forster F., Danev R., Baumeister W. Proteasomes. A molecular census of 26S proteasomes in intact neurons. Science. 2015;347:439–442. doi: 10.1126/science.1261197. [DOI] [PubMed] [Google Scholar]
- 69.Mahamid J., Schampers R., Persoon H., Hyman A.A., Baumeister W., Plitzko J.M. A focused ion beam milling and lift-out approach for site-specific preparation of frozen-hydrated lamellas from multicellular organisms. J Struct Biol. 2015;192:262–269. doi: 10.1016/j.jsb.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Frank J. Time-resolved cryo-electron microscopy: recent progress. J Struct Biol. 2017;200:303–306. doi: 10.1016/j.jsb.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]