Highlights
-
•
CryoEM maps at medium (3.5–6 Å) resolution can be challenging to interpret.
-
•
Integration of multiple methods can inform cryoEM studies.
-
•
Mass spectrometry and biochemistry facilitate map interpretation and model building.
Abstract
Electron cryo-microscopy (cryoEM) is used to determine structures of biological molecules, including multi-protein complexes. Maps at better than 3.0 Å resolution are relatively straightforward to interpret since atomic models of proteins and nucleic acids can be built directly. Still, these resolutions are often difficult to achieve, and map quality frequently varies within a structure. This results in data that are challenging to interpret, especially when crystal structures or suitable homology models are not available. Recent advances in mass spectrometry techniques, computational methods and model building tools facilitate subunit/domain fitting into maps, elucidation of protein contacts, and de novo generation of atomic models. Here, we review techniques for map interpretation and provide examples from recent studies of multi-protein complexes.
Current Opinion in Structural Biology 2019, 58:166–174
This review comes from a themed issue on CryoEM
Edited by Matteo D Peraro and Ji-Joon Songo
For a complete overview see the Issue and the Editorial
Available online 27th July 2019
https://doi.org/10.1016/j.sbi.2019.06.009
0959-440X/© 2019 MRC Laboratory of Molecular Biology. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Atomic models are essential for understanding the molecular mechanisms of biological processes. Advances in electron cryo-microscopy (cryoEM) have enabled the elucidation of 3D reconstructions and atomic models of specimens whose structure determination was not feasible only a few years ago. Still, challenges limit the resolution that can be achieved in many cases. For example, difficulties in making suitable specimens, compositional and conformational heterogeneity, and complex stability limit the quality of cryoEM maps. This results in challenges in generating reliable 3D reconstructions, identifying subunits in large assemblies, and building atomic models.
By combining cryoEM with other structural biology techniques and biochemical, biophysical, and mass spectrometry-based methods, it is possible to gain more insight into the mechanism of many complexes. Models generated using this integrative structural biology method can be used to test functional predictions (both in vivo and in vitro) and thereby address specific mechanistic questions.
There are many excellent recent reviews on cryoEM specimen preparation and 3D reconstruction [1, 2, 3, 4, 5] and we will not cover these here. Here, we review recent integrative approaches used to build models from medium resolution (3.5–6 Å) cryoEM maps of macromolecular complexes.
Map quality
Advances in cryoEM software have allowed improved reconstructions of structurally dynamic complexes. One of the major advances has been the implementation of a statistical framework [6] during refinement. This statistical framework has now been extended to pre-processing steps such as per particle motion correction (Bayesian polishing in RELION [7]) and per particle CTF refinement [8••]. Additionally, some of the microscope misalignments (beam tilt) as well as Ewald sphere can be corrected [7,9, 10, 11].
Approaches to refine flexible regions within dynamic complexes have also been implemented, including signal subtraction followed by focused classification or focused refinement for different areas of the map [10,12,13]. This multi-step process has recently been combined into a single task by multi-body refinement [14•]. Principal component analysis can then identify the underlying motions present in the complex. The accessibility and ease-of-use of cryoEM software have also greatly improved [8••,10,15].
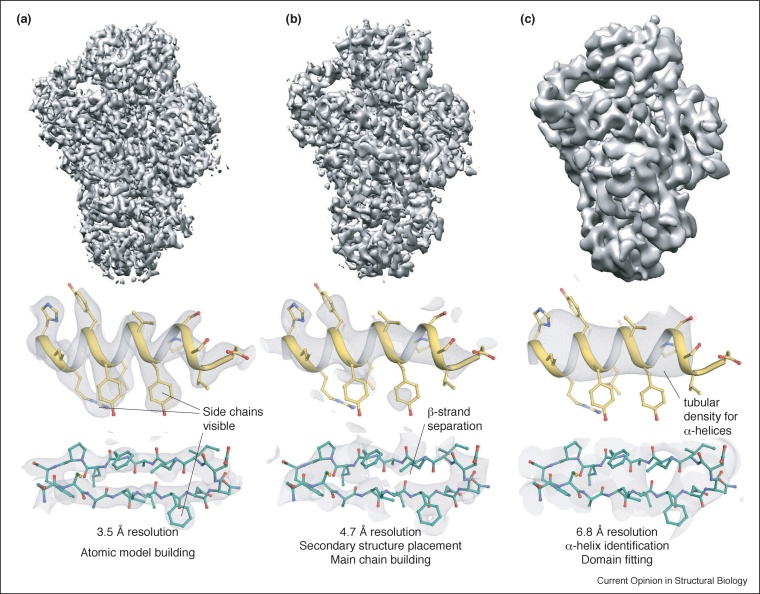
The resolution and quality of a cryoEM map determine the level of biological interpretation that is feasible. Structures with resolutions better than 2.5 Å have good side chain density and atomic models can be built directly into the maps, but these have been determined for only a small number of proteins [16, 17, 18]. With expertise, de novo model-building can be performed at resolutions up to ∼3.8 Å because the backbone and large side chains are visible. At lower resolutions, different structural features are apparent: beta strands are separated at resolutions better than ∼4.5 Å, and alpha helices are resolved as tubular densities at resolutions better than 8 Å (Figure 1).
Figure 1.
Visualization of structural features at different resolutions.
The polymerase module of the Cleavage and Polyadenylation Factor (CPF) [19••] was reconstructed from different numbers of particles to give B-factor sharpened maps at 3.5 Å (a), 4.7 Å (b), or 6.8 Å (c) resolution. The overall reconstructions are shown in surface representation (top). Alpha helical (middle) and beta strand (bottom) regions of the maps with models are also shown.
Visualization of higher resolution features allows a more detailed interpretation of maps (Figure 1). Still, even at relatively low resolutions (6–10 Å), known crystal structures can be positioned within a map with high accuracy, and alpha helical models can be built, giving important functional insight. Notably, the overall resolution of a structure does not imply that all regions can be interpreted equally. Local resolution maps [20] are useful for estimating resolution variability, but manual visual inspection is essential for assessing map quality.
Despite improvements in sample preparation, data collection and computational methods, often the resolution of a cryoEM structure does not go beyond 3.5 Å. Fortunately, even if the specimen cannot be improved biochemically [21] and the map quality cannot be improved with additional data collection and processing, other methods can be used to help interpret maps (Figure 2). Below, we describe such strategies.
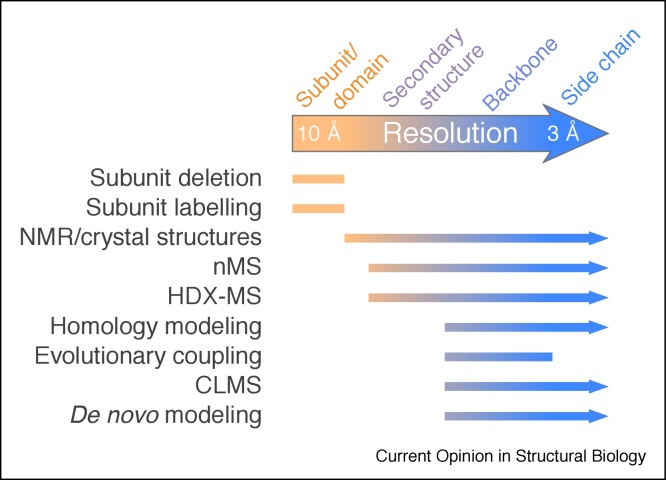
Figure 2.
Multi-resolution modeling of structures of multi-protein complexes.
A selection of methods used in integrative structural biology along with features that can be modeled at different resolutions are shown. Arrows represent the resolution range where highlighted methods are useful. nMS, native mass spectrometry; HDX-MS, hydrogen-deuterium exchange mass spectrometry; CLMS, cross-linking mass spectrometry.
Methods to assist with subunit identification
The first step in interpreting medium resolution (3.5–6 Å) cryoEM maps of multi-protein complexes is to identify the locations of individual subunits. In some cases, high-resolution structures are available, for example, from X-ray crystallography, and these can be fit into the maps, initially using rigid body fitting. In recent studies of the yeast Cleavage and Polyadenylation Factor (CPF), the characteristic shape of a beta propeller subunit was visible, allowing us to locate the position of the Pfs2 subunit [19••]. In another example, a crystal structure of the dimerization domain of the cytoplasmic motor protein dynein was fit into a cryoEM structure of the dynein tail bound to its cofactor dynactin, to explain how dynein's two chains are held together [22•].
Subunits can also be located within cryoEM maps by labelling strategies. For example, imaging of complexes after adding a bulky tag, binding of an antibody, or deletion of a specific subunit can be used to identify its location. In a recent 10 Å resolution structure of the COMPASS complex, a globular eGFP tag [23], the rod-like dynein light chain-interacting domain (DID) [24], and a high affinity Fab-epitope tag (PA-NZ) [25] were used as subunit-specific labelling strategies to determine the overall subunit organization [26•]. In a 4.0 Å resolution map of the dynactin complex [27•], previous rotary-shadowed EM images of antibody-labelled subunits [28] helped to identify subunit locations. In another example, analysis of the 500 kDa core CPF complex in the presence and absence of the Ysh1 nuclease subunit allowed identification of the position of Ysh1 within a low-resolution negative stain map [29••].
Mass spectrometry
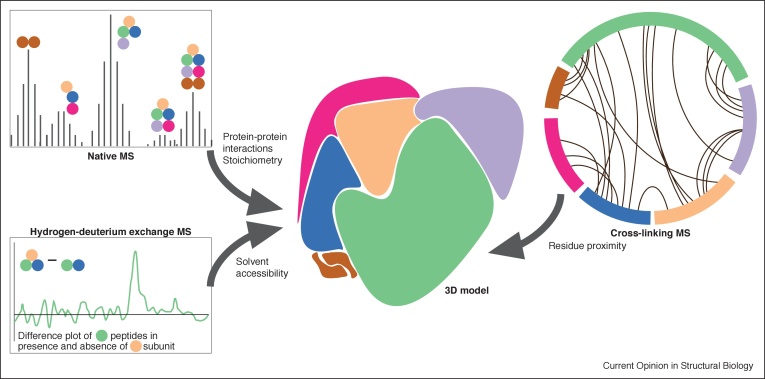
Mass spectrometry (MS) has emerged as one of the most powerful techniques to complement structural biology. It can reveal information about the stoichiometry and composition of protein complexes, interaction surfaces, dynamic regions and the presence of small molecules [30]. Importantly, it can provide information on all parts of a complex, including those that are less well-ordered or not visible in cryoEM structures. There are several types of MS that are used in combination with cryoEM, including native MS, hydrogen-deuterium exchange MS, and cross-linking MS (Figure 3) [31].
Figure 3.
Interpretation of cryoEM maps of multi-protein complexes using mass spectrometry.
Mass spectrometry (MS) can be instrumental in interpreting medium-resolution cryoEM maps. Types of MS and the information they yield are shown.
Native mass spectrometry
Native MS (nMS) is used to analyze intact protein complexes [32,33•]. Because the protein assemblies are preserved in the gas phase, intact masses can be determined, and therefore subunit stoichiometries can be calculated. For example, nMS was used to determine subunit stoichiometry in a 17 Å resolution structure of RNA polymerase II bound to a capping enzyme [34]. An elegant study of the Kai circadian oscillator used nMS to guide cryoEM sample preparation, to assist with model building, and to verify the structural models using mutational analysis [35••]. nMS can also be used to identify ligands, such as lipids [33•].
Protein complexes can be dissociated into subcomplexes in the mass spectrometer. After analysis of the resulting subcomplexes/subunits by nMS and computational network analysis, it is possible to build a protein–protein interaction network of multi-component assemblies. We recently used nMS to elucidate a subunit interaction map of CPF [19••]. The complex had been initially purified as a fifteen-subunit complex, but nMS revealed that only fourteen subunits were part of CPF. The fifteenth protein was part of a separate complex with overlapping subunits called APT. Further analysis of the nMS data revealed that CPF comprises three modules, each incorporating one of the enzymatic activities. These nMS data were used to define appropriate purification protocols for CPF subcomplexes, and for subunit identification in cryoEM maps.
Hydrogen-deuterium exchange mass spectrometry
Hydrogen-deuterium exchange MS (HDX-MS) can define the dynamics and interaction interfaces of proteins within a complex [36]. HDX-MS involves exposure of a protein to deuterium oxide (D2O), resulting in rapid exchange of hydrogen for deuterium. After the exchange reactions are quenched at different time points, proteins are digested and the relative quantities of hydrogen and deuterium can be measured by MS. This is most informative for backbone amide hydrogens and occurs faster in disordered or exposed regions (those not involved in stable hydrogen bonding interactions) than in structured regions. The rate of exchange can provide information on secondary structure, protein–protein interactions, ligand binding sites and conformational changes. HDX-MS is particularly useful in comparative studies. For example, interaction surfaces and conformational changes can be mapped by HDX-MS analysis of proteins in the presence and absence of a binding partner. This reveals peptides that are protected or exposed upon interaction.
HDX-MS has been successfully used to assist in the interpretation of cryoEM maps. For example, it was used to confirm interfaces between subunits of the Kai circadian oscillator derived from a cryoEM map at 4.7 Å resolution [35••]. Also, it has been used to examine the conformation, dynamics and ligand binding of insulin degrading enzyme [37] and the Hsp104 AAA+ ATPase [38].
Cross-linking mass spectrometry
Chemical crosslinking coupled to MS (CLMS) can be used to identify protein segments that are in close spatial proximity within macromolecular complexes [39,40•,41•]. In CLMS, pairs of functional groups (most commonly the primary amine group in lysines) are covalently cross-linked. The reaction products are then enzymatically digested, cross-linked peptides are enriched, analyzed by MS, and identified by database searching. CLMS therefore reveals residues in close proximity within and between protein subunits. These can be used as distance restraints when building atomic models into cryoEM maps and to define conformational changes [39,40•,42].
CLMS has been used in many studies to confirm, guide or actively model protein structures in cryoEM maps. For instance, CLMS was crucial in generating models of the nuclear pore complex in combination with other structural data including low resolution maps from cryo-electron tomography [43, 44, 45,46••]. It was also important for generating a structure of the 26S proteasome [47], an RNA polymerase II – Mediator complex [48•] and the mammalian mitochondrial complex I [49]. For CPF, CLMS defined contacts between proteins that were not visible by cryoEM and crystallography [19••].
Homology modeling, structure prediction and evolutionary covariance
To generate initial models, homology modelling of domains, subunits or heteromeric complexes can be very helpful. Since structure is usually more conserved than sequence, homology modelling can be useful even for remote homologs. Recent methods combine sequence alignments, predicted secondary structure and template-based modeling to generate new models. If distance constraints are available, for example from CLMS, these can sometimes be used as restraints. A number of programs are available to generate homology models, including PHYRE2 [50], I-TASSER [51], SWISS-MODEL [52], Robetta [53], and MODELLER [54]. Webservers make these programs easily accessible.
Other computational tools can also be extremely useful. For example, evolutionary covariance of individual residues detects correlated evolutionary sequence changes to identify amino acids that are likely to form direct contacts [55,56]. This requires the availability of a sufficient number of diverse sequences. In studies of bovine mitochondrial ATP synthase, evolutionary covariance analysis allowed model building for one of the subunits into cryoEM maps at subnanometer resolution [57•]. Programs/webservers include EVcouplings/EVcomplex and Gremlin [55,56].
Subunit modeling
All structural and biochemical data can be combined to generate (atomic) models that agree with the cryoEM structure of a multi-protein complex. In some cases, existing structures or models can be fit into the map but if that is not possible, de novo modelling can be performed [58••]. This can be challenging and is best performed by integrating data from multiple sources.
Initial models
At resolutions worse than 3 Å, automatic de novo model-building programs typically result in incomplete solutions. Still, methods are improving and programs such as ARP/wARP [59], buccaneer [60], Rosetta [61••], phenix.map_to_model [62], pathwalking [63] and EMBuilder [64] are in development. Some cryoEM projects that have successfully integrated initial models generated by these programs include amyloid fibrils (ARP/wARP EM, [65]), the imidazoleglycerol-phosphate dehydratase (IGPD) enzyme of the histone biosynthesis pathway (buccaneer and Rosetta [66]), Paramecium bursaria chlorella virus 1 (PBCV-1), viral RNA-polymerase, and mechanosensitive ion channels (EMbuilder, [67, 68, 69]).
Molecular replacement methods, such as MOLREP [58••,70], or RosettaES [71] can be used for density-based fold recognition using a database of known protein domains/fragments. These can provide suitable templates for de novo model building or automated rebuilding [72].
Model building and refinement
Coot [58••,73] has been extensively used for both crystallography and cryoEM model-building and it now provides a set of improved tools for model building into cryoEM maps including morphing, jiggle fit, Cα baton-mode, de-novo model building (including helices, beta-strands, RNA and DNA), loop fitting, and local distance restraints. For example, one can combine jiggle fitting and morphing of a domain with placing idealized secondary structure elements (SSEs, alpha helices and beta strands) and baton-building of new main chain atoms.
Helices and beta-strands can be first identified in a given map either visually or with the aid of the ‘Find Secondary Structure’ functionality followed by their automatic building using the ‘Place Helix Here’ or ‘Place Strand Here’ tools. The SSE and modeled domains can then be refined using appropriate alpha helix, beta-strand, ProSMART [74•] or Rama restraints [75]. By using secondary structure prediction, it may be possible to determine how the SSEs are connected to each other when the resolution in those regions does not allow complete visualization of connecting loops.
Next, if the map offers enough resolution to identify the side chains of some residues, these can be used as starting points for amino acid sequence assignment. It is important to ensure that the amino acid chemistry makes sense, for example, residues fit within appropriate hydrophobic and hydrophilic environments. Constraints from other methods (e.g. CLMS) should also be satisfied. After tracing the density and building the structure of an unknown domain, one can use the model to search the PDB or the DALI server [76] for homologous structures that may further facilitate model building.
When manually inspecting maps, it is often useful to simultaneously view multiple blurred/sharpened maps which can be generated using a combination of Coot and REFMAC [74•]. This facilitates interpretation of intermediate resolution maps as features of the main chain (blurring) and side-chains (sharpening) can both be visualized with more detail. Confidence maps [77] and locally sharpened maps [78] may also assist model-building.
Once a round of model building has come to an end, models are typically refined with REFMAC5 in Fourier space [79] or phenix.real_space_refine in real space [80]. At intermediate resolutions, Rosetta [72,81] provides an automated approach for de novo model building and to improve the geometry and sidechain placement of atomic models. Other fitting and refinement programs available are Cryo-Fit [82], MDFF [83,84], DireX [85] and iMODFIT [86].
Validation and assessment
Validation of atomic models is essential. Validation tools are available in Coot, Molprobity and EMRinger [87, 88, 89] to analyze geometry, density fit, rotamers, and Ramachandran outliers. These can identify problematic regions that can be modified or improved to ensure the fitness of the final model. It is often the case that not all outliers can be fixed. If a sequence can be reliably assigned but density for side chains is not apparent, side chains can be removed (stubbed) from the models. If the sequence cannot be assigned, models can be built as poly-alanine. Model quality can also be assessed using a recently described multi-model approach [90].
Integrative modelling
Although manual or semi-automated methods may work for interpretation of many cryoEM datasets at medium resolution, methods also exist to computationally integrate many different types of data simultaneously. These can provide a more unbiased and comprehensive approach. For example, models can be generated using distance restraints from CLMS, subunit stoichiometries and connectivities from nMS, and subunit locations from labelling, mutational and deletion experiments. Computational approaches to integrate such data include integrative modelling platform (IMP, [91]), HADDOCK [92,93] and XL-MOD [94].
Conclusions and future perspectives
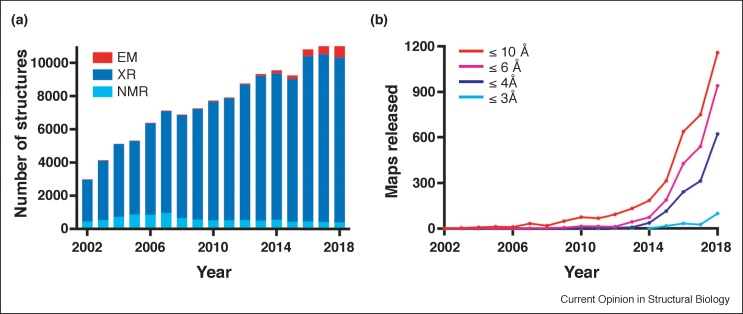
Recent progress in cryoEM has led to a substantial increase in the number of cryoEM structures that can be interpreted with atomic models (Figure 4). Still, many structures are of medium resolution and are therefore more difficult to interpret (Figure 4b). Integrative methods allow multi-resolution modelling, including regions that cannot be visualized by cryoEM because they are flexibly tethered within complexes. Additional new methods, for example, using manifold embedding to map continuous conformational changes [95], will facilitate our understanding of the molecular motions within dynamic complexes.
Figure 4.
Number of structures and maps released in the PDB and EMDB. (a) Number of structures released in the PDB from NMR, X-ray crystallography (XR) and cryoEM between 2002 and 2018. The number of cryoEM structures has rapidly increased since 2013. (b) EMDB map releases at given resolutions between 2002 and 2018. Of a total of 7885 maps deposited in the EMDB at the submission time of this manuscript, 1587 (20%) and 246 (3%) have a resolution better than 4 Å and 3 Å, respectively. In 2018, 1770 maps were released in the EMDB, 624 (35%) and 98 (5.5%) achieved a resolution better than 4 Å and 3 Å, respectively.
The interplay between cryoEM and crystallography may become increasingly important, with cryoEM guiding construct design for crystallization and crystal structures becoming invaluable to interpreting cryoEM maps (especially for small domains or regions where cryoEM does not lead to high enough resolution). The building of atomic models into maps of 3.5–6 Å resolution remains a challenge but additional developments in MS, computational methods, automatic model generation, and de novo model building will continue to improve cryoEM structure determination. More efforts towards integrating information from diverse experimental and theoretical data will simplify and speed-up interpretation of the structures of macromolecular complexes. Importantly, integrative methods not only facilitate interpretation of cryoEM maps by providing a more complete description of protein complex architecture and assembly, but they also broaden our biochemical and mechanistic understanding of cellular machines.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Andrew Carter, Paul Emsley, Glenn Masson and Mark Skehel for comments on the manuscript. This work was supported by the European Union’s Horizon 2020 research and innovation programme (ERC grant 725685); and Medical Research Council grant MC_U105192715 (both to L.A.P.).
Contributor Information
Ana Casañal, Email: acasanal@mrc-lmb.cam.ac.uk.
Lori A Passmore, Email: passmore@mrc-lmb.cam.ac.uk.
References
- 1.Fernandez-Leiro R., Scheres S.H.W. Unravelling biological macromolecules with cryo-electron microscopy. Nature. 2016;537:339–346. doi: 10.1038/nature19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo C.J., Passmore L.A. Progress towards an optimal specimen support for electron cryomicroscopy. Curr Opin Struct Biol. 2016;37:81–89. doi: 10.1016/j.sbi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drulyte I., Johnson R.M., Hesketh E.L., Hurdiss D.L., Scarff C.A., Porav S.A., Ranson N.A., Muench S.P., Thompson R.F. Approaches to altering particle distributions in cryo-electron microscopy sample preparation. Acta Crystallogr D Struct Biol. 2018;74:560–571. doi: 10.1107/S2059798318006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y. Single-particle cryo-EM-how did it get here and where will it go. Science. 2018;361:876–880. doi: 10.1126/science.aat4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyumkis D. Challenges and opportunities in cryo-EM single-particle analysis. J Biol Chem. 2019;294:5181–5197. doi: 10.1074/jbc.REV118.005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheres S.H.W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zivanov J., Nakane T., Scheres S.H.W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 2019;6:5–17. doi: 10.1107/S205225251801463X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:163. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes RELION3. Major algorithms implemented in this release are Bayesian particle polishing and multibody refinement.
- 9.Wolf M., DeRosier D.J., Grigorieff N. Ewald sphere correction for single-particle electron microscopy. Ultramicroscopy. 2006;106:376–382. doi: 10.1016/j.ultramic.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Grant T., Rohou A., Grigorieff N. cisTEM, user-friendly software for single-particle image processing. eLife. 2018;7 doi: 10.7554/eLife.35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo C.J., Henderson R. Ewald sphere correction using a single side-band image processing algorithm. Ultramicroscopy. 2018;187:26–33. doi: 10.1016/j.ultramic.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai X.-C., Rajendra E., Yang G., Shi Y., Scheres S.H.W. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife. 2015;4:1485. doi: 10.7554/eLife.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilbach S., Hantsche M., Tegunov D., Dienemann C., Wigge C., Urlaub H., Cramer P. Structures of transcription pre-initiation complex with TFIIH and Mediator. Nature. 2017;551:204–209. doi: 10.1038/nature24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Nakane T., Kimanius D., Lindahl E., Scheres S.H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife. 2018;7:1485. doi: 10.7554/eLife.36861. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a multi-body refinement algorithm to improve maps of flexible multidomain complexes.
- 15.Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 16.Merk A., Bartesaghi A., Banerjee S., Falconieri V., Rao P., Davis M.I., Pragani R., Boxer M.B., Earl L.A., Milne J.L.S. Breaking Cryo-EM resolution barriers to facilitate drug discovery. Cell. 2016;165:1698–1707. doi: 10.1016/j.cell.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartesaghi A., Aguerrebere C., Falconieri V., Banerjee S., Earl L.A., Zhu X., Grigorieff N., Milne J.L.S., Sapiro G., Wu X. Atomic resolution cryo-EM structure of β-galactosidase. Structure. 2018;26:848–856.e3. doi: 10.1016/j.str.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Y.Z., Aiyer S., Mietzsch M., Hull J.A., McKenna R., Grieger J., Samulski R.J., Baker T.S., Agbandje-McKenna M., Lyumkis D. Sub-2 Å Ewald curvature corrected structure of an AAV2 capsid variant. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Casañal A., Kumar A., Hill C.H., Easter A.D., Emsley P., Degliesposti G., Gordiyenko Y., Santhanam B., Wolf J., Wiederhold K. Architecture of eukaryotic mRNA 3′-end processing machinery. Science. 2017;358:1056–1059. doi: 10.1126/science.aao6535. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used nMS, CLMS, HDX-MS and cryo-EM to understand the structure and function of eukaryotic mRNA 3′ end processing.
- 20.Kucukelbir A., Sigworth F.J., Tagare H.D. Quantifying the local resolution of cryo-EM density maps. Nat Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passmore L.A., Russo C.J. Specimen preparation for high-resolution cryo-EM. Methods Enzymol. 2016;579:51–86. doi: 10.1016/bs.mie.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Urnavicius L., Lau C.K., Elshenawy M.M., Morales-Rios E., Motz C., Yildiz A., Carter A.P. Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature. 2018;554:202–206. doi: 10.1038/nature25462. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used cryo-EM and crystallography to generate models in a multi-resolution map.
- 23.Zang Y., Wang H., Cui Z., Jin M., Liu C., Han W., Wang Y., Cong Y. Development of a yeast internal-subunit eGFP labeling strategy and its application in subunit identification in eukaryotic group II chaperonin TRiC/CCT. Sci Rep. 2018;8 doi: 10.1038/s41598-017-18962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flemming D., Thierbach K., Stelter P., Böttcher B., Hurt E. Precise mapping of subunits in multiprotein complexes by a versatile electron microscopy label. Nat Struct Mol Biol. 2010;17:775–778. doi: 10.1038/nsmb.1811. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Han W., Takagi J., Cong Y. Yeast inner-subunit PA-NZ-1 labeling strategy for accurate subunit identification in a macromolecular complex through cryo-EM analysis. J Mol Biol. 2018;430:1417–1425. doi: 10.1016/j.jmb.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 26•.Wang Y., Ding Z., Liu X., Bao Y., Huang M., Wong C.C.L., Hong X., Cong Y. Architecture and subunit arrangement of the complete Saccharomyces cerevisiae COMPASS complex. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used multiple labelling methods to identify subunit locations in a 10 Å resolution cryo-EM map.
- 27•.Urnavicius L., Zhang K., Diamant A.G., Motz C., Schlager M.A., Yu M., Patel N.A., Robinson C.V., Carter A.P. The structure of the dynactin complex and its interaction with dynein. Science. 2015;347:1441–1446. doi: 10.1126/science.aaa4080. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used cryo-EM, crystallography, nMS and labelling methods to generate models in a multi-resolution map.
- 28.Schroer T.A. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 29••.Hill C.H., Boreikaitė V., Kumar A., Casañal A., Kubík P., Degliesposti G., Maslen S., Mariani A., Loeffelholz von O., Girbig M. Activation of the endonuclease that defines mRNA 3′ ends requires incorporation into an 8-subunit core cleavage and polyadenylation factor complex. Mol Cell. 2019;73:1217–1231. doi: 10.1016/j.molcel.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a combination of crystallography, cryo-EM, CLMS, HDX-MS and biochemistry, the authors elucidated the architecture of a 500 kDa protein complex.
- 30.Liko I., Allison T.M., Hopper J.T., Robinson C.V. Mass spectrometry guided structural biology. Curr Opin Struct Biol. 2016;40:136–144. doi: 10.1016/j.sbi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Marcoux J., Robinson C.V. Twenty years of gas phase structural biology. Structure. 2013;21:1541–1550. doi: 10.1016/j.str.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Rostom A.A., Robinson C.V. Disassembly of intact multiprotein complexes in the gas phase. Curr Opin Struct Biol. 1999;9:135–141. doi: 10.1016/s0959-440x(99)80018-9. [DOI] [PubMed] [Google Scholar]
- 33•.Allison T.M., Bechara C. Structural mass spectrometry comes of age: new insight into protein structure, function and interactions. Biochem Soc Trans. 2019;47:317–327. doi: 10.1042/BST20180356. [DOI] [PubMed] [Google Scholar]; This review highlights the latest advances of nMS and their importance in the context of structural biology.
- 34.Martinez-Rucobo F.W., Kohler R., van de Waterbeemd M., Heck A.J.R., Hemann M., Herzog F., Stark H., Cramer P. Molecular basis of transcription-coupled pre-mRNA capping. Mol Cell. 2015;58:1079–1089. doi: 10.1016/j.molcel.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 35••.Snijder J., Schuller J.M., Wiegard A., Lössl P., Schmelling N., Axmann I.M., Plitzko J.M., Förster F., Heck A.J.R. Structures of the cyanobacterial circadian oscillator frozen in a fully assembled state. Science. 2017;355:1181–1184. doi: 10.1126/science.aag3218. [DOI] [PubMed] [Google Scholar]; This paper uses nMS and cryo-EM to gain insight into the Kai circadian oscillator.
- 36.Konermann L., Pan J., Liu Y.-H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem Soc Rev. 2011;40:1224–1234. doi: 10.1039/c0cs00113a. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Liang W.G., Bailey L.J., Tan Y.Z., Wei H., Wang A., Farcasanu M., Woods V.A., McCord L.A., Lee D. Ensemble cryoEM elucidates the mechanism of insulin capture and degradation by human insulin degrading enzyme. eLife. 2018;7:464. doi: 10.7554/eLife.33572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye X., Lin J., Mayne L., Shorter J., Englander S.W. Hydrogen exchange reveals Hsp104 architecture, structural dynamics, and energetics in physiological solution. Proc Natl Acad Sci U S A. 2019;116:7333–7342. doi: 10.1073/pnas.1816184116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt C., Urlaub H. Combining cryo-electron microscopy (cryo-EM) and cross-linking mass spectrometry (CX-MS) for structural elucidation of large protein assemblies. Curr Opin Struct Biol. 2017;46:157–168. doi: 10.1016/j.sbi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 40•.O’Reilly F.J., Rappsilber J. Cross-linking mass spectrometry: methods and applications in structural, molecular and systems biology. Nat Struct Mol Biol. 2018;25:1000–1008. doi: 10.1038/s41594-018-0147-0. [DOI] [PubMed] [Google Scholar]; The authors present a comprehensive review on the use and application of CLMS in structural biology.
- 41•.Iacobucci C., Götze M., Ihling C.H., Piotrowski C., Arlt C., Schäfer M., Hage C., Schmidt R., Sinz A. A cross-linking/mass spectrometry workflow based on MS-cleavable cross-linkers and the MeroX software for studying protein structures and protein-protein interactions. Nat Protoc. 2018;13:2864–2889. doi: 10.1038/s41596-018-0068-8. [DOI] [PubMed] [Google Scholar]; This paper provides a detailed protocol to perform CLMS on macromolecular complexes.
- 42.Schmidt C., Robinson C.V. A comparative cross-linking strategy to probe conformational changes in protein complexes. Nat Protoc. 2014;9:2224–2236. doi: 10.1038/nprot.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 44.Alber F., Dokudovskaya S., Veenhoff L.M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B.T. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 45.Kosinski J., Mosalaganti S., Appen von A., Teimer R., DiGuilio A.L., Wan W., Bui K.H., Hagen W.J.H., Briggs J.A.G., Glavy J.S. Molecular architecture of the inner ring scaffold of the human nuclear pore complex. Science. 2016;352:363–365. doi: 10.1126/science.aaf0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Kim S.J., Fernandez-Martinez J., Nudelman I., Shi Y., Zhang W., Raveh B., Herricks T., Slaughter B.D., Hogan J.A., Upla P. Integrative structure and functional anatomy of a nuclear pore complex. Nature. 2018;555:475–482. doi: 10.1038/nature26003. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this work, the authors used an integrative approach to determine the structure of the entire nuclear pore complex, defining the mass, composition, stoichiometry and protein–protein interactions.
- 47.Lasker K., Förster F., Bohn S., Walzthoeni T., Villa E., Unverdorben P., Beck F., Aebersold R., Sali A., Baumeister W. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci U S A. 2012;109:1380–1387. doi: 10.1073/pnas.1120559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Plaschka C., Larivière L., Wenzeck L., Seizl M., Hemann M., Tegunov D., Petrotchenko E.V., Borchers C.H., Baumeister W., Herzog F. Architecture of the RNA polymerase II-mediator core initiation complex. Nature. 2015;518:376–380. doi: 10.1038/nature14229. [DOI] [PubMed] [Google Scholar]; This study used cryo-EM, crystallography and MS to understand the structure of Mediator bound to RNA polymerase II.
- 49.Fiedorczuk K., Letts J.A., Degliesposti G., Kaszuba K., Skehel M., Sazanov L.A. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D.E., Chivian D., Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–31. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parida B.K., Panda P.K., Misra N., Mishra B.K. MaxMod: a hidden Markov model based novel interface to MODELLER for improved prediction of protein 3D models. J Mol Model. 2015;21:30. doi: 10.1007/s00894-014-2563-3. [DOI] [PubMed] [Google Scholar]
- 55.Hopf T.A., Schärfe C.P.I., Rodrigues J.P.G.L.M., Green A.G., Kohlbacher O., Sander C., Bonvin A.M.J.J., Marks D.S. Sequence co-evolution gives 3D contacts and structures of protein complexes. eLife. 2014;3:65. doi: 10.7554/eLife.03430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ovchinnikov S., Kamisetty H., Baker D. Robust and accurate prediction of residue-residue interactions across protein interfaces using evolutionary information. eLife. 2014;3 doi: 10.7554/eLife.02030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Zhou A., Rohou A., Schep D.G., Bason J.V., Montgomery M.G., Walker J.E., Grigorieff N., Rubinstein J.L. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife. 2015;4 doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, the authors used evolutionary coupling analysis to trace a polypeptide chain in an ATP synthase structure.
- 58••.Brown A., Long F., Nicholls R.A., Toots J., Emsley P., Murshudov G. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Cryst. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes some of the widely used tools that facilitate interpretation of EM data with atomic models, including fold recognition (BALBES/MOLREP), model building (Coot), refinement (ProSMART, REFMAC) and validation.
- 59.Langer G., Cohen S.X., Lamzin V.S., Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc. 2008;3:1171–1179. doi: 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Cryst. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 61••.Wang R.Y.-R., Kudryashev M., Li X., Egelman E.H., Basler M., Cheng Y., Baker D., DiMaio F. De novo protein structure determination from near-atomic-resolution cryo-EM maps. Nat Methods. 2015;12:335–338. doi: 10.1038/nmeth.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors present an approach to refine and improve the quality of manually traced cryoEM maps using Rosetta.
- 62.Terwilliger T.C., Adams P.D., Afonine P.V., Sobolev O.V. A fully automatic method yielding initial models from high-resolution electron cryo-microscopy maps. Nat Methods. 2018;15:905–908. doi: 10.1038/s41592-018-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen M., Baldwin P.R., Ludtke S.J., Baker M.L. De Novo modeling in cryo-EM density maps with Pathwalking. J Struct Biol. 2016;196:289–298. doi: 10.1016/j.jsb.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou N., Wang H., Wang J. EMBuilder: a template matching-based automatic model-building program for high-resolution cryo-electron microscopy maps. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radamaker L., Lin Y.-H., Annamalai K., Huhn S., Hegenbart U., Schönland S.O., Fritz G., Schmidt M., Fändrich M. Cryo-EM structure of a light chain-derived amyloid fibril from a patient with systemic AL amyloidosis. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rawson S., Bisson C., Hurdiss D.L., Fazal A., McPhillie M.J., Sedelnikova S.E., Baker P.J., Rice D.W., Muench S.P. Elucidating the structural basis for differing enzyme inhibitor potency by cryo-EM. Proc Natl Acad Sci U S A. 2018;115:1795–1800. doi: 10.1073/pnas.1708839115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang Q., Zhu D., Agarkova I., Adhikari J., Klose T., Liu Y., Chen Z., Sun Y., Gross M.L., Van Etten J.L. Near-atomic structure of a giant virus. Nat Commun. 2019;10 doi: 10.1038/s41467-019-08319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Zhang F., Su R., Li X., Chen W., Chen Q., Yang T., Wang J., Liu H., Fang Q. Structure of RNA polymerase complex and genome within a dsRNA virus provides insights into the mechanisms of transcription and assembly. Proc Natl Acad Sci U S A. 2018;115:7344–7349. doi: 10.1073/pnas.1803885115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Wang D., Kang Y., Wu J.-X., Yao F., Pan C., Yan Z., Song C., Chen L. Structure of the mechanosensitive OSCA channels. Nat Struct Mol Biol. 2018;25:850–858. doi: 10.1038/s41594-018-0117-6. [DOI] [PubMed] [Google Scholar]
- 70.Vagin A., Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- 71.Frenz B., Walls A.C., Egelman E.H., Veesler D., DiMaio F. RosettaES: a sampling strategy enabling automated interpretation of difficult cryo-EM maps. Nat Methods. 2017;14:797–800. doi: 10.1038/nmeth.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang R.Y.-R., Song Y., Barad B.A., Cheng Y., Fraser J.S., DiMaio F. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife. 2016;5:352. doi: 10.7554/eLife.17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Nicholls R.A., Tykac M., Kovalevskiy O., Murshudov G.N. Current approaches for the fitting and refinement of atomic models into cryo-EM maps using CCP-EM. Acta Crystallogr D Struct Biol. 2018;74:492–505. doi: 10.1107/S2059798318007313. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work reviews the tools available for macromolecular structure refinement into cryo-EM reconstructions that are available via CCP-EM, with a focus on REFMAC5.
- 75.Pertsemlidis A., Zelinka J., Fondon J.W., Henderson R.K., Otwinowski Z. Bayesian statistical studies of the Ramachandran distribution. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1165. [DOI] [PubMed] [Google Scholar]
- 76.Holm L., Laakso L.M. Dali server update. Nucleic Acids Res. 2016;44:W351–W355. doi: 10.1093/nar/gkw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beckers M., Jakobi A.J., Sachse C. Thresholding of cryo-EM density maps by false discovery rate control. IUCrJ. 2019;6:18–33. doi: 10.1107/S2052252518014434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jakobi A.J., Wilmanns M., Sachse C. Model-based local density sharpening of cryo-EM maps. eLife. 2017;6 doi: 10.7554/eLife.27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murshudov G.N., Skubák P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Afonine P.V., Poon B.K., Read R.J., Sobolev O.V., Terwilliger T.C., Urzhumtsev A., Adams P.D. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiMaio F., Song Y., Li X., Brunner M.J., Xu C., Conticello V., Egelman E., Marlovits T., Cheng Y., Baker D. Atomic-accuracy models from 4.5-Å cryo-electron microscopy data with density-guided iterative local refinement. Nat Methods. 2015;12:361–365. doi: 10.1038/nmeth.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirmizialtin S., Loerke J., Behrmann E., Spahn C.M.T., Sanbonmatsu K.Y. Using molecular simulation to model high-resolution cryo-EM reconstructions. Methods Enzymol. 2015;558:497–514. doi: 10.1016/bs.mie.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 83.Trabuco L.G., Villa E., Mitra K., Frank J., Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph A.P., Malhotra S., Burnley T., Wood C., Clare D.K., Winn M., Topf M. Refinement of atomic models in high resolution EM reconstructions using Flex-EM and local assessment. Methods. 2016;100:42–49. doi: 10.1016/j.ymeth.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schröder G.F., Brunger A.T., Levitt M. Combining efficient conformational sampling with a deformable elastic network model facilitates structure refinement at low resolution. Structure. 2007;15:1630–1641. doi: 10.1016/j.str.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopéz-Blanco J.R., Chacón P. iMODFIT: efficient and robust flexible fitting based on vibrational analysis in internal coordinates. J Struct Biol. 2013;184:261–270. doi: 10.1016/j.jsb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 87.Chen V.B., Arendall W.B., Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams C.J., Headd J.J., Moriarty N.W., Prisant M.G., Videau L.L., Deis L.N., Verma V., Keedy D.A., Hintze B.J., Chen V.B. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 2018;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barad B.A., Echols N., Wang R.Y.-R., Cheng Y., DiMaio F., Adams P.D., Fraser J.S. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat Methods. 2015;12:943–946. doi: 10.1038/nmeth.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herzik M.A., Fraser J.S., Lander G.C. A multi-model approach to assessing local and global cryo-EM map quality. Structure. 2019;27:344–358.e3. doi: 10.1016/j.str.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webb B., Viswanath S., Bonomi M., Pellarin R., Greenberg C.H., Saltzberg D., Sali A. Integrative structure modeling with the integrative modeling platform. Protein Sci. 2018;27:245–258. doi: 10.1002/pro.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Zundert G.C.P., Melquiond A.S.J., Bonvin A.M.J.J. Integrative modeling of biomolecular complexes: HADDOCKing with cryo-electron microscopy data. Structure. 2015;23:949–960. doi: 10.1016/j.str.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 93.van Zundert G.C.P., Rodrigues J.P.G.L.M., Trellet M., Schmitz C., Kastritis P.L., Karaca E., Melquiond A.S.J., van Dijk M., de Vries S.J., Bonvin A.M.J.J. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Ferber M., Kosinski J., Ori A., Rashid U.J., Moreno-Morcillo M., Simon B., Bouvier G., Batista P.R., Müller C.W., Beck M. Automated structure modeling of large protein assemblies using crosslinks as distance restraints. Nat Methods. 2016;13:515–520. doi: 10.1038/nmeth.3838. [DOI] [PubMed] [Google Scholar]
- 95.Frank J., Ourmazd A. Continuous changes in structure mapped by manifold embedding of single-particle data in cryo-EM. Methods. 2016;100:61–67. doi: 10.1016/j.ymeth.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]