Abstract
This study focuses on the prevalence of Listeria monocytogenes (Lm) in pork meat and on inert surfaces from slaughterhouses in Sonora, Mexico. A total of 21 Lm were obtained from 103 samples, giving a prevalence of 20.3%. The prevalence of Lm in pork loin was 15.9% and 20.8% for inert surfaces in Federal Inspection Type (FIT) slaughterhouses. For non-FIT slaughterhouses, the prevalence was 25.7%. PCR amplification of genomic DNA from the Lm isolates revealed the presence of the hlyA gene, suggesting a pathogenic nature for these isolates. The isolates obtained in this work all clustered with Lm, according to our phylogenetic analysis based on the 16S rDNA sequence. This Lm cluster indicates that Lm isolates 7-2, 4, 2-1, 10B, 8, 3, 3-3, and 9 share 16S rRNA identity with other Lm isolates that have been reported as foodborne pathogens (rR2-502, J1817, J1816, J1926) and that are involved in foodborne outbreaks. The most commonly detected serotypes were 1/2a and 1/2b. All isolates displayed differential responses to the assayed antibiotics, and most isolates were able to grow in the presence of penicillin G, or both penicillin and penicillin-derived (oxacillin) antibiotics.
Keywords: Pathogen occurrence, Listeria monocytogenes, Pork meat, Antibiotics
Introduction
The genus Listeria includes the foodborne pathogen L. monocytogenes (Lm) and 14 other species: L. grayi, L. innocua, L. welshimeri, L. seeligeri, L. ivanovii, L. marthii, L. rocourtiae, L. fleischmannii, L. weihenstephanensis, L. floridensis, L.aquatica, L. cornellensis, L. riparia, and L. grandensis [1].
Globally, Lm is closely linked to the contamination of various food sources [1]. Importantly, Lm can infect humans and cause the disease listeriosis, which affects the elderly, immuno-compromised people and pregnant women, all with a high mortality rate [2]. Lm is ubiquitous, and human health responses after pathogen exposure vary depending on the pathogen virulence and dosage as well as the immune status [3].
Upon infection, Lm can cause meningitis, miscarriage, perinatal infections, encephalitis, meningoencephalitis, septicemia, stillbirth, and gastroenteritis [4].
One main source of Lm is ready-to-eat food products. Occasionally, a dose with low infectivity can cause an outbreak and in some cases only one bacterial cell is needed to cause disease, although the microorganism inoculum can reach levels > 1000 CFU g−1 in listeriosis outbreaks [3]. The Codex Alimentarius established in their Listeria’s directories of ready-to-eat foods in which growth of L. monocytogenes can occur a microbiological criteria where in a 2-class plan the maximum number allowed of defective sample unit is zero and the m value is absence in 25 g (<0.04 cfu g−1), (CAC/GL 61–2007) [5].
Food safety during manufacturing and food processing is a vital concern. Lm, which is known to be persistent during food processing, is well adapted to aggressive environmental conditions such as low temperature [6], low pH [7], and high salt concentrations [8]. More recently, Lm has been reported as being resistant to different sanitizers, making it more difficult to eliminate when biofilms are formed [9]. Lm frequently occurs in raw pork meat and the main sites of raw pork meat contamination are the slaughterhouse and the cutting room, suggesting that the equipment coming into contact with the raw meat may be the primary source of contamination [3]. The equipment used in pork meat preparation also has a determinant role in the spread of Listeria in slaughterhouses, which includes surfaces such as pluck sets, tongues, tonsils, saws, drains, and cutting tables [10]. Furthermore, it has been reported that the pathogen has a 71–100% occurrence in chilling and cutting areas [10]. Finally, poor quality control during handling and packaging in the food production process can also cause Lm contamination.
The CDC (USA) has reported that different foods contaminated with Lm resulted in 1600 cases of illness, about 1500 hospitalizations, and 260 deaths per year [11]. One of the most lethal outbreaks occurred in Canada, which was due to the consumption of contaminated processed products, resulting in 57 sick patients and 22 deaths [12].
Mexico exports pork to several different Asian countries (Japan, South Korea, and China). Sonora is the second largest pork-producing region in Mexico (234,639 t produced in 2016), part of which is exported to China [13]. In Mexico, there is no exact data on the incidence of listeriosis but Lm has a similar incidence compared with other countries, ranging from 1 to 20%. In Mexico, also there are no precise estimates on the economic costs that Lm represents [14]. The Lm prevalence in samples of Mexican beef and imported beef in Mexico has been reported at 18% and 8.8%, respectively [15]. In Sonora, collected cheese samples have been found with an Lm prevalence of 3.4%, using two different methods NOM-FDA and USDA-FIS for sample recovery and bacterial isolation [16]. Although there are no reports of Lm incidence or occurrence in processed pork meat in Mexico, the pork meat industry in Sonora must focus on the control of this pathogen by minimizing its prevalence on inert surfaces that come into contact with fresh meat. The aim of this study was to evaluate Lm prevalence in pork loin and on inert surfaces, as well as to perform the molecular identification of Lm isolates obtained from Federal Inspection Type (FIT) and non-FIT slaughterhouses in Sonora, Mexico.
Materials and methods
Sample collection
Pork samples were collected from March 2014 through February 2015 at one FIT slaughterhouse and one non-FIT slaughterhouse (N/FIT) in southern Sonora, Mexico. A total of 103 samples were obtained, including 79 pork loin samples (44 from FIT and 35 N/FIT) and 24 samples from FIT inert surfaces including conveyor belts, polyamide tables, knives, and hooks. No samples from N/FIT slaughterhouse inert surfaces were taken. All samples collected were stored at low temperature (4–8 °C) and analyzed on the same day of the collection.
Bacterial isolation and biochemical characterization of Listeria monocytogenes
Listeria spp. isolation was performed according to Annex C of the standard norm regulation 210 for Mexico [17]; this norm is fully equivalent to the ISO11290 protocol [18]. An individual meat (pork loin) sample (25 g) was mixed with 25 ml of phosphate buffer saline (1× PBS; with a final concentration of 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, and pH 7.4) and homogenized with a sterile blender under sterile conditions. For inert surface sampling, sterile pre-wetted gauze was used to rub a surface area of 10 cm2. Individual gauze sections were submerged in 25 ml of PBS, and enrichments were performed for each sample with 225 ml of Fraser Broth (Biocontrol, Cat. 63017-500), combined with Fraser supplement (Sigma-Aldrich, Cat. 90836-10VL; Darmstadt, Germany), and incubated for 48 h at 36 °C. The enrichment culture was then plated onto Oxford medium (Condalab, Cat. 1133; Torrejón de Ardoz, Madrid, Spain) with supplements (Oxoid, Cat. SR0140E; Waltham, Massachusetts, USA) and incubated for 48 h at 36 °C for selective isolation of Lm. Subsequently, the round grayish colonies surrounded by dark halos were taken to the following tests.
The following tests were performed to each recovered isolate: Gram staining; catalase and oxidase tests, motility test at 25 °C, acid production from rhamnose and xylose, the β-hemolytic activity on sheep blood agar test, and the CAMP test (Christie, Atkins, Munch-Peterson test). They were performed according to Momtaz and Yadollahi [19]. For the CAMP test, the Staphylococcus aureus ATCC 29213 (SA+) and Rhodococcus equi ATCC 6939 (RE−) were used as controls [19]. Listeria monocytogenes ATCC 7644 was used as the positive control for all tests. All recovered isolates were stored at − 70 °C with glycerol (15%) until use.
Molecular analysis: hlyA detection, molecular serotyping, and phylogenetic analysis
Lm bacterial isolates were cultured for 24 h on nutrient agar at 37 °C. Cells were suspended in 200 μl of lysis buffer (20 mM Tris–Cl, pH 8.0; 2 mM sodium EDTA; 1.2% Triton X-100; lysozyme at 20 mg/ml) and incubated at 37 °C for 30 min. Next, 25 μl of proteinase K (15 mAU/ml) was added and incubated at 56 °C for 30 min. Genomic DNA was extracted using the DNeasy Blood & Tissue Kit (QIAGEN; Hilden, Germany) following the manufacturer’s instructions for Gram-positive bacteria. The DNA concentration and purity were measured using a Nanodrop 2000c UV–Vis spectrophotometer (ThermoFisher, Inc.; Wilmington, DE, USA).
Primers LmA (5′-CGG AGG TTC CGC AAA GAT G-3′) and LmB (5′-CCT CCA GAG TGA TCG ATG TT-3′) were used to amplify a 230-bp product from the hlyA gene [20]. The PCR reaction for the hlyA gene was performed in a total volume of 25 μl containing 1× PCR buffer, 2 mM of MgCl2, 0.2 μM of dNTPs, 0.2 μM of each oligonucleotide, 1 U of Taq DNA polymerase, and 10 ng of DNA. The PCR (Labnet, MultiGene optiMAX; Edison, NJ, USA) conditions used for the hlyA gene were an initial denaturation step at 95 °C for 5 min, followed by 30 cycles at 95 °C for 30 s, annealing at 57.5 °C for 30 s, and extension at 72 °C for 30 s, and a final step at 72 °C for 10 min.
Primers F2C (5′-AGA GTT TGA TCC TGG CTC-3′) and C (5′-GTA CAC ACC GCC CGT-3′) were used to amplify a 1408-bp product from the16S rDNA gene [21]. The PCR reaction for 16S rDNA gene was performed in a total volume of 25 μl containing 1X PCR buffer, 1.5 mM of MgCl2, 0.2 μM of dNTPs, 0.2 μM of each oligonucleotide, 1 U of Taq DNA polymerase, and 10 ng of DNA. The PCR conditions were an initial denaturation step at 95 °C for 5 min, followed by 30 cycles at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 90 s, and a final step at 72 °C for 10 min. L. monocytogenes ATCC 7644 gDNA was used as positive control.
The PCR assay for the detection of Lm serotypes was carried out with the primers reported previously [19]. Three pairs of primers were used for the three major serovars of Lm; lmo0737F (5′-AGG GCT TCA AGG ACT TAC CC-3′) and lmo0737R (5′-ACG ATT TCT GCT TGC CAT TC-3′) were used to amplify 691-pb of the putative protein gene with unknown function in order detect the serotype 1/2a; ORF2819F (5′-AGC AAA ATG CCA AAA CTC GT-3′) and ORF2819R (5′-CAT CAC TAA AGC CTC CCA TTG-3′) were used to amplify 471-pb fragment of the putative transcriptional regulator in order to detect the serotype 1/2b; and the primers ORF2110F (5-AGT GGA CAA TTG ATT GGT GAA-3′) and ORF2110R (5′-CAT CCA TCC CTT ACT TTG GAC-3′) were used to amplify 597-pb fragment of the putative secreted protein in order to identify the 4b serotype. The PCR reaction was performed in a total volume of 12.5 μl containing 1× PCR buffer, 2.5 mM of MgCl2, 0.8 mM of dNTPs, 0.4 μM of each oligonucleotide, 1 U of Taq DNA polymerase, and 10 ng of DNA. The PCR conditions were an initial denaturation step at 95 °C for 7 min, followed by 30 cycles at 95 °C for 15 s, annealing at 51 °C for 15 s, and extension at 72 °C for 45 s, and a final step at 72 °C for 10 min. PCR products were loaded and electrophoresed in a 1% agarose gel in 0.5 X Tris-acetate EDTA buffer, stained with ethidium bromide, and visualized on a Minibis Pro DNR system (Bio-Imaging systems).
Sequencing of the 16S rDNA gene
16S rDNA amplicons were purified using the QIAquick Gel Extraction kit (QIAGEN; Hilden, Germany) according to the manufacturer’s instructions. The internal primer U1 (5′ CCA GCA GCC GCG GTA ATA CG 3′) was used for sequencing the F2C/C amplicons with an ABI 3730 XL automated sequencer at the National Laboratory of Genomics and Biodiversity (LANGEBIO; Irapuato, Mexico) [21].
Phylogenetic analysis
The sequence files were analyzed using Chromas freeware (ver. 2.01; Chromas lite Technelysium Pvt. Ltd.; South Brisbane, Australia). Reference sequences were obtained from the GenBank database (National Center for Biotechnology Information). The alignment was constructed using Clustal-W with the MEGA 6.0 software. The phylogenetic relatedness was estimated using the neighbor-joining method. The evolutionary distances were computed using the Kimura 2-parameter method. The bootstrap consensus tree inferred from 1000 replicates represents the evolutionary history of the sequences analyzed. The phylogenetic analysis was performed using MEGA software version 6.0 [22].
Antibiotic susceptibility
Isolates presenting the hlyA gene (as detected by PCR) were assayed for antibiotic susceptibility by the disk diffusion assay according to the Clinical and Laboratory Standards Institute (CLSI) guidelines as described in de Vasconcelos Byrne et al. [23]. Briefly, the bacterial isolates were grown in tryptic soy broth at 35 °C for 24 h. After this, the cultures were diluted on saline solution and adjusted to 0.5 according to the McFarland scale. The assays were carried out in Muller–Hinton agar (Becton Dickinson and Company, Sparks, MD USA.), and disks impregnated with the following antibiotics were analyzed: penicillin G (Pcn., 10 U; Cat # CT0043B), oxacillin (Oxa., 1 μg; CT0159B), tetracycline (Tet., 30 μg; CT0054B), clarithromycin (Clar., 15 μg; CT0693B), and sulfamethoxazole/trimethoprim (Smx/Tpm, 1.25/23.75 μg; CT0052B), these antibiotics were purchased from Oxoid (Cambridge, UK). The Staphylococcus aureus ATCC 29213 was used as a control strain. The inhibition halos were interpreted following the breakpoints criteria established in the CLSI guidelines for Staphylococcus aureus [23, 24].
Results
Biochemical characterization of bacterial isolates as Lm
Twenty-one biochemically confirmed Lm isolates were obtained from the 103 samples analyzed (Table 1). All isolates showed results identical to the positive control (Lm ATCC 7644). Seven isolates were obtained from 44 pork loin samples from FIT slaughterhouse. Nine isolates were obtained from 35 pork loin samples from N/FIT slaughterhouse, and five isolates were obtained from inert surfaces samples from FIT slaughterhouse (Table 1).
Table 1.
Prevalence of Lm strains isolated from different samples. All samples were collected in pork slaughterhouses
| Type of sample | Samples | Prevalence % | Lm isolates obtained | |
|---|---|---|---|---|
| Pork loins | FIT | 44 | 15.9 | 7 |
| N/FIT | 35 | 25.7 | 9 | |
| Inert surfaces | FIT | 24 | 20.8 | 5 |
| N/FIT | 0 | 0 | 0 | |
| TOTAL | 103 | 20.3 | 21 |
Federal Inspection Type slaughterhouse is a place for slaughter, processing, and sanitary industrialization of beef, pork, and poultry meat. These slaughterhouses have complied with the most stringent international standards of quality and hygiene, with the purpose of producing meat of good quality and in optimal sanitary conditions (definition taken and translated from https://www.gob.mx/firco/articulos/sabes-que-es-un-rastro-tipo-inspeccion-federal?idiom=es)
N/FIT: The samples were taken from a non-FIT slaughterhouse, which does not comply with the international standards of quality and hygiene. Inert surface samples were not collected in the N/FIT slaughterhouse
Prevalence of Lm in FIT and non-FIT slaughterhouses
Twenty-one samples out of a total of 103 samples from FIT and non-FIT slaughterhouses were found contaminated with Lm, giving 20.3% of total prevalence (Table 1). A total of 7 contaminated samples resulted from 44 pork loin samples analyzed giving a prevalence of 15.9% for FIT slaughterhouse. The 9 pork loin samples from N/FIT slaughterhouse give a 25.7% of prevalence, resulting in 9 confirmed isolates. And the prevalence for inert surfaces from FIT was 20.8% (Table 1).
Molecular identification, serotyping, and phylogenetic analysis of Lm isolates
Here, we detected by PCR amplification the presence of the hlyA gene in all 21 Lm isolates, confirming the identity as Lm.
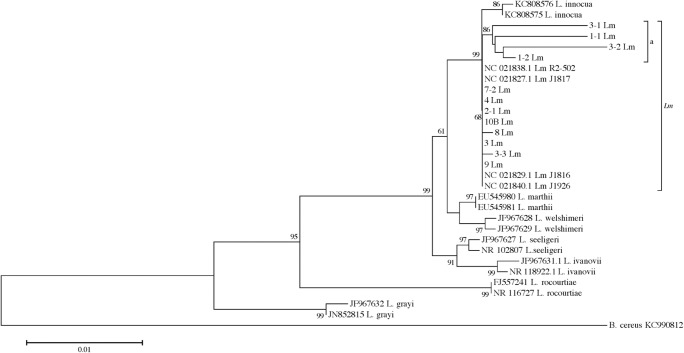
We used 16S rDNA sequencing to know the phylogenetic relationship of Lm isolates with other Lm isolates previously reported. The 16S rDNA 1406 bp amplicon sequences were registered in GenBank, and the accession numbers are KY952637 to KY952657. For the phylogenetic analysis, several 16S rDNA gene sequences were retrieved from NCBI, as well as two sequences each from several species of Listeria (L. innocua, L. marthii, L. welshimeri, L. seeligeri, L ivanovii, L. rocourtiae, and L. grayi) and four Lm sequences from strains that were previously reported as pathogenic to humans. The Lm isolates were located in a separate cluster from other Listeria species, but showing identity to L. innocua (68%) (Fig. 1). A sub-cluster composed of the four remaining isolates (3-1, 1-1, 3-2, and 1-2) was formed in the Lm cluster group (a) (Fig. 1). All tested isolates displayed a shared identity (ranging from 97 to 100%) between them and other Lm pathogenic isolates (Lm R2-502, Lm J1817, Lm J1816, Lm J1926) (Fig. 1).
Fig. 1.
Distance tree derived from 16S rDNA gene sequences. The tree was constructed with MEGA 6.0 (bootstraps = 1000), using the Kimura 2-parameter method (K2P + G). The rate variation among sites was modeled using a gamma distribution (shape parameter = 105). Bootstrap values are indicated as percentages. Twenty-one Lm isolates obtained from pork loin samples and inert surfaces in addition to 19 reference sequences were used to build the tree. NJ neighbor joining. 10B Lm represents 10 isolates of Lm with identical sequences (1-3 Lm, 7 Lm, 7-1 Lm, 10 Lm, 11 Lm, 12 Lm, 13 Lm, 17 Lm, 19 Lm, and 20 Lm). (a) indicates a subgroup inside the Lm branch of the tree
The most frequent serotype detected was 1/2b, resulting in 17/21 (80.95%) samples. The frequency of 1/2a was 19.04% (4/21), the serotype 4b was not detected (Table 2).
Table 2.
Frequency of Listeria monocytogenes (Lm) serotypes
| Number of isolates (%) | ||||
|---|---|---|---|---|
| Isolation source | No. of Listeria monocytogenes | 1/2a | 1/2b | 4b |
| Pork loin | 16 | – | 16 (100) | – |
| Inert surface | 5 | 4(80) | 1(20) | – |
| Total | 21 | 4(19.04) | 17 (80.95) | – |
Antibiotic susceptibility
The 21 isolates identified as Lm showed differential responses to the antibiotic susceptibility. All 21 isolates were resistant to Oxa and Pcn in this study (Table 3). For Smx/Tpm, 12 isolates were susceptible, surprisingly 9 isolates were resistant (1 resistant isolate and 8 intermediate ones) (Table 3). All the isolates were susceptible to Clar, and a total of 20 isolates showed Tet resistance. One isolate displayed intermediate Tet resistance (Table 3).
Table 3.
Antibiotic susceptibility of Listeria monocytogenes isolates obtained from raw pork meat and inert surfaces from slaughterhouses
| Antibiotic | Breakpoints (mm) | Isolates (number) | ||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| Tetracycline | ≥ 19 | 15–18 | ≤ 14 | 20 | 1 | 0 |
| Clarithromycin | ≥ 18 | 14–17 | ≤ 13 | 21 | 0 | 0 |
| Smx/Tpm | ≥ 16 | 11–15 | ≤ 10 | 12 | 8 | 1 |
| Oxacillin | ≥ 18 | ND | ≤ 17 | 0 | 0 | 21 |
| Penicillin G | ≥ 29 | ND | ≤ 28 | 0 | 0 | 21 |
ND means not defined
Discussion
Prevalence of Lm in FIT and non-FIT slaughterhouses
Food safety is an international concern, and food contamination (especially by Lm) can cause wide-ranging issues. Specifically, Lm contamination can inflict economic losses on the food industry, in addition to its severe human health repercussions, including miscarriage and food poisoning. The persistent nature of this pathogen highlights the need for vigilance through cleaning and disinfection cycles in the food industry [25]. Twenty-one isolates were obtained from pork loin and inert surfaces on pork industry, evidencing the presence of Lm on raw pork meat.
The FIT slaughterhouse includes slaughter facilities and processing and industrialization sanitary facilities for beef, pork, and poultry meat, and they are regulated with the strictest International Standards of Quality and Hygiene with the purpose of supplying the consumers with meat of good quality and in optimum sanitary conditions in comparison with a non-FIT slaughterhouse that does not follow similar standards of quality. Lm is capable of contaminating pork and meat products, as well as other processed food products for human consumption [26]. The Lm prevalence reported here on raw pork samples from pork processing plants is less to the 37% prevalence reported in a previous study [27]. Other studies have reported 55% [28] and even up to 70–100% prevalence in pork chilling and cutting areas [10]. Our results regarding Lm presence on inert surfaces from slaughterhouses (20.8%) as reported other studies, 17% prevalence reported from meat producing plants in Finland [29], or the ~ 3% Lm prevalence in deli meat samples in China [30]. The prevalence present in the non-FIT slaughterhouse can be attributed to the lack of adequate cleaning and disinfecting procedures. This allows pathogens to establish on surfaces and equipment, thereby contaminating food processing lines [3]. Importantly, the contamination detected in the FIT slaughterhouse can be eliminated simply by following preventive measures such as more stringent manufacturing and hygiene practices. Indeed, the Lm prevalence can be reduced or eliminated by applying techniques like HACCP (Hazard Analysis and Critical Control Points) that help to identify contaminant sources and how they are disseminated [3]. The presence of Lm in pork loin samples and on inert surfaces suggests that these isolates are well-adapted to various elements of the meat processing industry including chilling, post-slaughter processing, and the cutting room [26]. Lm prevalence is common in food processing plants, and its ability to adhere to inert surfaces makes this bacteria persistent in these environments, possibly due to biofilm formation [3]. Our findings from quantifying the presence of Lm suggest that sanitizing methods and cleaning chemicals are insufficient to eradicate this pathogen in slaughterhouses, necessitating the implementation of a more thorough sanitization procedure. In Mexico, the lack of epidemiological surveillance systems leads to the need for accurate data on the incidence of listeriosis and its association with foodborne disease [14]. In 2009, 120,806 spontaneous abortions were reported, in 2011, 5,283,896 cases of ill-defined gastroenteritis, 44,467 cases of bacterial food poisoning, and 957 cases of meningitis; paradoxically, in none of these cases was the etiological diagnosis established [14]. Given this, there is a need to inform and warn the appropriate entities, to define strategies for the mandatory search of Lm through the whole food production chain and clinical suspects, for the epidemiological importance and control of listeriosis [14].
Molecular identification and phylogenetic analysis of Lm isolates
The hlyA encoding for a listeriolysin O (LLO) was the virulence factor selected to identify Lm isolates. The LLO toxin is a secreted pore-forming protein necessary for the lysis of the vacuole and spreading cell to cell in a host during Lm life cycle. This was demonstrated with a mutation assay on the hlyA gene resulting in LLO negative mutants, this modification shows that the LLO is required for the development of listeriosis [31]. This result confirms the presence of the gene that encodes the LLO, an essential toxin related to Lm pathogenic mechanism, suggesting the possible virulent nature of these isolates [32]. Indeed, the hlyA gene is central to the identification of Lm, and it is the most frequently chosen target (among other virulence genes) for PCR detection of this pathogen [32]. Conventional biochemical methods of identification are time-consuming in comparison with molecular analysis, limiting the number of samples that can be effectively tested. By contrast, the implementation of molecular techniques for Lm detection allows an easy and fast identification method for confirming the presence of this pathogen [33].
Food products contaminated with Lm have serious implications for human health, but also economic losses within the industry [26]. Recently, the growing need to identify foodborne pathogens has made it necessary to use PCR in order to amplify virulence-associated Lm genes [34] and differentiate isolates taken from raw and processed meat samples [35], as well as other non-food samples such as environmental or clinical samples that can be used to confirm the presence of this pathogen [34]. Nevertheless, rapid methods to identify Lm in food and other samples require ongoing development [36].
Although 16S rDNA is a highly conserved DNA region among Listeria species, it was still possible to separate the species included in the distance tree. The Lm cluster revealed that 17 out of the 21 isolates share an identity with Lm isolates previously reported as foodborne pathogens and involved in foodborne outbreaks [37]. This finding suggests that the isolates analyzed here could be potential human pathogens that cause listeriosis. Previously, it was reported that isolates from the same Listeria species share more identity among themselves than with other species [33]. 16S rDNA gene sequencing is commonly used to enable bacterial phylogenetic analyses for different genera and species [38]. We successfully used this DNA region to differentiate Lm isolates, confirming previous studies in which Lm differentiation was performed with food-related isolates and from a variety of environmental and clinical samples [31].
The serotypes 1/2a and 1/2b were detected in Mexico which has previously reported the serotypes 1/2a, 1/2b, 1/2c, 3b, and 4b for chicken and raw beef [39]. These serotypes have also been isolated from cheese samples in Mexico.
Antibiotic susceptibility
Pcn resistance has previously been reported in Lm isolates obtained from clinical, foods, and environmental samples [40] as well the Oxa resistance in isolates obtained from pork meat in slaughter environments [41]. However, in contrast with the study realized by de Vasconcelos Byrne [23], their Lm isolates obtained from vegetable samples showed susceptibility to Pcn and Oxa. This resistance could be due to the extensive use of the drugs and their perseverance and thoughtful problematic situation; the development of microbial resistance is a major challenge that the clinicians face presently [42]. Similar to this work obtained for Lm isolates reported in Lebanon, the authors found that Lm isolates were able to grow in the presence of Oxa and Pcn, showing a resistance [43].
The first choice for treating a disease caused by Lm (listeriosis) in Mexico is penicillin in combination with gentamicin [14, 44]; here, we report a resistance of all isolates for ß-lactam antibiotics. The Smx/Tpm is the second choice for the clinical listeriosis therapy and other infectious diseases in human and animal treatments, this could indicate an increase of antibiotic resistance of wildtype Lm as the isolates reported here. The susceptibility to Smx/Tpm was observed previously in Lm strains isolated from cheese in Lebanon as well as the resistance for single isolate [43], and this pattern was observed in isolates from food and food processing environments in Italy, showing one resistance isolate to Smx/Tpm [45].
It is possible that the development of antibiotic resistance in Lm isolates could be due to the extensive use of these antibiotics in human bacterial infections, the resistance observed here could be a problem for listeriosis related with some of these isolates due to the fact that β-lactams antibiotics and Smx/Tpm are the choices for treating this infection [14].
In this work, all isolates were susceptible to Clar as observed in Listeria isolates from retail raw food samples [46], whereas most isolates were susceptible to Tet. None of the isolates reported were resistant to Clar or Tet, these antibiotics could be a treatment for possible listeriosis cases in this region. The drugs tested in this work are commonly used to treat listeriosis [47, 48], and we observed varying degrees of susceptibility to these antibiotics.
Conclusions
To the best of our knowledge, the scientific literature regarding Lm contamination of pork meat and its production processes in Mexico is currently limited. In this report, we have determined that both non-FIT and FIT slaughterhouses require improved hygiene procedures as well as the ability to localize contamination sources. Indeed, the unequivocal identification of Lm strains suggests that current hygiene procedures are not completely efficient. Each of the 21 isolates was considered Lm after biochemical testing and was further molecularly identified and confirmed by PCR as Lm. This was confirmed by the phylogenetic analyses, where these isolates show identity with other Lm reporter as pathogenic.
We recommend the use of strategies such as PCR for the continuous monitoring of foodborne pathogens of the pork-producing industry and its slaughterhouses. Since the conventional biochemical methods of identification are time-consuming in comparison with molecular analysis, the application of these kinds of techniques will help to identify this foodborne more accurately.
The coupling of traditional biochemical methods with more current methods such as PCR offers new tools that are necessary for the rapid and efficient identification of food pathogens. This study thus demonstrates the importance of modern control methods and effective sanitizing procedures to the food industry, as a means to avoid pork contamination by Lm and its associated high risks to human health.
Acknowledgements
We thank Dr. Brandon Loveall of Improvence for English proofreading of the manuscript.
Funding
This work was supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (CONACyT), the FESE Foundation (FESE/267/14), and the Instituto Tecnológico de Sonora (PROFAPI 2016-0016).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weller D, Andrus A, Wiedmann M, den Bakker H. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int J Syst Evol Microbiol. 2015;65(1):286–292. doi: 10.1099/ijs.0.070839-0. [DOI] [PubMed] [Google Scholar]
- 2.Hyden P, Pietzka A, Lennkh A, Murer A, Springer B, Blaschitz M, Indra A, Huhulescu S, Allerberger F, Ruppitsch W, Sensen CW. Whole genome sequence-based serogrouping of Listeria monocytogenes isolates. J Biotechnol. 2016;235:181–186. doi: 10.1016/j.jbiotec.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Thévenot D, Dernburg A, Vernozy-Rozand C. An updated review of Listeria monocytogenes in the pork meat industry and its products. J Appl Microbiol. 2006;101(1):7–17. doi: 10.1111/j.1365-2672.2006.02962.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhama K, Karthik K, Tiwari R, Shabbir MZ, Barbuddhe S, Malik SVS, Singh RK. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: a comprehensive review. Vet Q. 2015;35(4):211–235. doi: 10.1080/01652176.2015.1063023. [DOI] [PubMed] [Google Scholar]
- 5.Alimentarius C. Guidelines on the application of general pronciples of food hygiene to the control of Listeria monocytogenes in foods.http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/es/
- 6.Puga C, SanJose C, Orgaz B. Biofilm development at low temperatures enhances Listeria monocytogenes resistance to chitosan. Food Control. 2016;65:143–151. doi: 10.1016/j.foodcont.2016.01.012. [DOI] [Google Scholar]
- 7.O’Driscoll B, Gahan C, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62(5):1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poimenidou SV, Chatzithoma D-N, Nychas G-J, Skandamis PN. Adaptive response of Listeria monocytogenes to heat, salinity and low pH, after habituation on cherry tomatoes and lettuce leaves. PLoS One. 2016;11(10):e0165746. doi: 10.1371/journal.pone.0165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Du L, Zhao T, Zhao P, Doyle MP. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control. 2017;78:72–78. doi: 10.1016/j.foodcont.2017.02.050. [DOI] [Google Scholar]
- 10.Van den Elzen A, Snijders J. Critical points in meat production lines regarding the introduction of Listeria monocytogenes. Vet Q. 1993;15(4):143–145. doi: 10.1080/01652176.1993.9694393. [DOI] [PubMed] [Google Scholar]
- 11.Scallan E, Hoekstra R, Angulo F, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weatherill S. Report of the independent investigator into the 2008 Listeriosis outbreak. Canada H, ed. 2009:vii. http://publications.gc.ca/collections/collection_2009/agr/A22-508-2009E.pdf
- 13.SAGARPA. Crecen 9.5 por ciento exportaciones de carne de porcino: SAGARPA. social U de comunicación, ed. 2017. http://www.sagarpa.gob.mx/Delegaciones/jalisco/boletines/Paginas/2017B03015.aspx
- 14.Castañeda-Ruelas G, Eslava-Campos C, Castro-del Campo N, León-Félix J, Chaidez-Quiroz C. Listeriosis en México: importancia clínica y epidemiológica. Salud Publica Mex. 2014;56:654–659. [PubMed] [Google Scholar]
- 15.Rubio-Lozano M, Martínez-Bruno J, Hernández-Castro R, et al. Detección de Listeria monocytogenes, Salmonella y Yersinia enterocolitica en carne de res en puntos de venta en México. Rev Mex Cienc Pecu. 2013;4(1):107–115. [Google Scholar]
- 16.Moreno-Enriquez R, Garcia-Galaz A, Acedo-Felix E, et al. Prevalence, types, and geographical distribution of Listeria monocytogenes from a survey of retail Queso Fresco and associated cheese processing plants and dairy farms in Sonora, Mexico. J Food Prot. 2007;70(11):2596–2601. doi: 10.4315/0362-028X-70.11.2596. [DOI] [PubMed] [Google Scholar]
- 17.SSA. 2014 Productos y servicios. Métodos de prueba microbiológicos. Determinación de microorganismos indicadores. Determinación de microorganismos patógenos. (SSA1) C de R y FS, ed. ;NOM-210-SS. http://www.economia-noms.gob.mx/normas/noms/2010/210ssa12015.pdf
- 18.Scotter SL, Langton S, Lombard B, Schulten S, Nagelkerke N, in‘t Veld PH, Rollier P, Lahellec C. Validation of ISO method 11290 part 1 — detection of Listeria monocytogenes in foods. Int J Food Microbiol. 2001;64(3):295–306. doi: 10.1016/S0168-1605(00)00462-1. [DOI] [PubMed] [Google Scholar]
- 19.Momtaz H, Yadollahi S. Molecular characterization of Listeria monocytogenes isolated from fresh seafood samples in Iran. Diagn Pathol. 2013;8(1):149. doi: 10.1186/1746-1596-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furrer B, Candrian U, Hoefelein C, Luethy J. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J Appl Bacteriol. 1991;70(5):372–379. doi: 10.1111/j.1365-2672.1991.tb02951.x. [DOI] [PubMed] [Google Scholar]
- 21.Cordero-Ramírez JD, López-Rivera R, Figueroa-Lopez AM, Mancera-López ME, Martínez-Álvarez JC, Apodaca-Sánchez MÁ, Maldonado-Mendoza IE. Native soil bacteria isolates in Mexico exhibit a promising antagonistic effect against Fusarium oxysporum f. sp. radicis-lycopersici. J Basic Microbiol. 2013;53(10):838–847. doi: 10.1002/jobm.201200128. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vasconcelos Byrne V, Hofer E, Vallim DC, de Castro Almeida RC. Occurrence and antimicrobial resistance patterns of Listeria monocytogenes isolated from vegetables. Braz J Microbiol. 2016;47(2):438–443. doi: 10.1016/j.bjm.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI (2018) Performance standards for antimicrobial disk susceptibility tests. CLSI standar:M02
- 25.Carpentier B, Cerf O. Review-persistence of Listeria monocytogenes in food industry equipment and premises. Int J Food Microbiol. 2011;145(1):1–8. doi: 10.1016/j.ijfoodmicro.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Thévenot D, Delignette-Muller M, Christieans S, Vernozy-Rozand C. Prevalence of Listeria monocytogenes in 13 dried sausage processing plants and their products. Int J Food Microbiol. 2005;102(1):85–94. doi: 10.1016/j.ijfoodmicro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Chasseignaux E, Gérault P, Toquin M, Salvat G, Colin P, Ermel G. Ecology of Listeria monocytogenes in the environment of raw poultry meat and raw pork meat processing plants. FEMS Microbiol Lett. 2002;210(2):271–275. doi: 10.1111/j.1574-6968.2002.tb11192.x. [DOI] [PubMed] [Google Scholar]
- 28.Salvat G, Toquin M, Michel Y, Colin P. Control of Listeria monocytogenes in the delicatessen industries: the lessons of a listeriosis outbreak in France. Int J Food Microbiol. 1995;25(1):75–81. doi: 10.1016/0168-1605(94)00087-M. [DOI] [PubMed] [Google Scholar]
- 29.Peccio A, Autio T, Korkeala H, Rosmini R, Trevisani M. Listeria monocytogenes occurrence and characterization in meat-producing plants. Lett Appl Microbiol. 2003;37(3):234–238. doi: 10.1046/j.1472-765X.2003.01384.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Lu L, Pan Y, Sun X, Hwang CA, Zhao Y, Wu VCH. Rapid detection and differentiation of Listeria monocytogenes and Listeria species in deli meats by a new multiplex PCR method. Food Control. 2015;52:78–84. doi: 10.1016/j.foodcont.2014.12.017. [DOI] [Google Scholar]
- 31.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68(2):999–1003. doi: 10.1128/IAI.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law J, Ab Mutalib N, Chan K, Lee L (2015) An insight into the isolation, enumeration, and molecular detection of Listeria monocytogenes in food. Front Microbiol 6(1227). 10.3389/fmicb.2015.01227 [DOI] [PMC free article] [PubMed]
- 33.Hellberg R, Martin K, Keys A, Haney C, Shen Y, Smiley R. 16S rRNA partial gene sequencing for the differentiation and molecular subtyping of Listeria species. Food Microbiol. 2013;36(2):231–240. doi: 10.1016/j.fm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Liu D. Identification, subtyping and virulence determination of Listeria monocytogenes, an important foodborne pathogen. J Med Microbiol. 2006;55(6):645–659. doi: 10.1099/jmm.0.46495-0. [DOI] [PubMed] [Google Scholar]
- 35.Al-Nabulsi A, Osaili T, Awad A, Olaimat A, Shaker R, Holley R. Occurrence and antibiotic susceptibility of Listeria monocytogenes isolated from raw and processed meat products in Amman, Jordan. CyTA-J Food. 2015;13(3):346–352. doi: 10.1080/19476337.2014.982191. [DOI] [Google Scholar]
- 36.Beumer R, Hazeleger W. Listeria monocytogenes: diagnostic problems. FEMS Immunol Med Microbiol. 2003;35(3):191–197. doi: 10.1016/S0928-8244(02)00444-3. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Strain E, Allard M, Brown E. Genome sequences of Listeria monocytogenes strains J1816 and J1-220 associated with human outbreaks. J Bacteriol. 2011;193(13):3424–3425. doi: 10.1128/JB.05048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa-López AM, Cordero-Ramírez JD, Martínez-Álvarez JC, López-Meyer M, Lizárraga-Sánchez GJ, Félix-Gastélum R, Castro-Martínez C, Maldonado-Mendoza IE. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. Springerplus. 2016;5(1):330. doi: 10.1186/s40064-016-1780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castañeda-ruelas GM, Campo NC, Félix JL, et al. Prevalence , levels , and relatedness of Listeria monocytogenes isolated from raw and ready-to-eat foods at retail markets in Culiacan , Sinaloa , Mexico. J Microbiol Res. 2013;3(2):92–98. doi: 10.5923/j.microbiology.20130302.06. [DOI] [Google Scholar]
- 40.Davis JA, Jackson CR. Comparative antimicrobial susceptibility of Listeria monocytogenes, L. innocua, and L. welshimeri. Microb Drug Resist. 2009;15(1):27–32. doi: 10.1089/mdr.2009.0863. [DOI] [PubMed] [Google Scholar]
- 41.Moreno LZ, Paixao R, Gobbi DD, et al. Characterization of antibiotic resistance in Listeria spp. isolated from slaughterhouse environments, pork and human infections. J Infect Dev Ctries. 2014;8(4):416–423. doi: 10.3855/jidc.4188. [DOI] [PubMed] [Google Scholar]
- 42.Dajani AS. Beta-lactam resistance: clinical implications for pediatric patients. J Int Med Res. 2002;30(1_suppl):2A–9A. doi: 10.1177/14732300020300S102. [DOI] [PubMed] [Google Scholar]
- 43.Harakeh S, Saleh I, Zouhairi O, Baydoun E, Barbour E, Alwan N. Antimicrobial resistance of Listeria monocytogenes isolated from dairy-based food products. Sci Total Environ. 2009;407(13):4022–4027. doi: 10.1016/j.scitotenv.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Ramaswamy V, Cresence VM, Rejitha JS, et al. Listeria review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40(1):4–13. [PubMed] [Google Scholar]
- 45.Conter M, Paludi D, Zanardi E, Ghidini S, Vergara A, Ianieri A. Characterization of antimicrobial resistance of foodborne Listeria monocytogenes. Int J Food Microbiol. 2009;128(3):497–500. doi: 10.1016/j.ijfoodmicro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Wang X-M, Lü X-F, Yin L, Liu HF, Zhang WJ, Si W, Yu SY, Shao ML, Liu SG. Occurrence and antimicrobial susceptibility of Listeria monocytogenes isolates from retail raw foods. Food Control. 2013;32(1):153–158. doi: 10.1016/j.foodcont.2012.11.032. [DOI] [Google Scholar]
- 47.Katzung BG. Chapter 34. Chloramphenicol, tetracyclines, macrolides, clindamycin, & streptogramins. In: Trevor AJ, Katzung BG, Kruidering-Hall M, editors. Katzung & Trevor’s pharmacology: examination & broad review. 11th ed. San Francisco, United States of America: McGraw-Hill; 2007. [Google Scholar]
- 48.Gómez D, Azón E, Marco N, Carramiñana JJ, Rota C, Ariño A, Yangüela J. Antimicrobial resistance of Listeria monocytogenes and Listeria innocua from meat products and meat-processing environment. Food Microbiol. 2014;42:61–65. doi: 10.1016/j.fm.2014.02.017. [DOI] [PubMed] [Google Scholar]