Abstract
The dramatic rise in overdose deaths linked to synthetic opioids (e.g., fentanyl, carfentanil) may require more potent, longer-duration opiate antagonists than naloxone. Both the high affinity of nalmefene at μ opiate receptors and its long half-life led us to examine the feasibility of developing an intranasal (IN) formulation as a rescue medication that could be especially useful in treating synthetic opioid overdose. In this study, the pharmacokinetic properties of IN nalmefene were compared with an intramuscular (i.m.) injection in a cohort of healthy volunteers. Nalmefene was absorbed slowly following IN administration, with a median time to reach Cmax (Tmax) of 2 hours. Addition of the absorption enhancer dodecyl maltoside (Intravail, Neurelis, Inc., Encinitas, CA) reduced Tmax to 0.25 hour and increased Cmax by ∼2.2-fold. The pharmacokinetic properties of IN nalmefene (3 mg) formulated with dodecyl maltoside has characteristics consistent with an effective rescue medication: its onset of action is comparable to an i.m. injection of nalmefene (1.5 mg) previously approved to treat opioid overdose. Furthermore, the Cmax following IN administration was ∼3-fold higher than following i.m. dosing, comparable to previously reported plasma concentrations of nalmefene observed 5 minutes following a 1-mg i.v. dose. The high affinity, very rapid onset, and long half-life (>7 hours) of IN nalmefene present distinct advantages as a rescue medication, particularly against longer-lived synthetic opioids.
Introduction
A Government Accountability Office report released in October 2018 declared the opioid crisis a public health emergency (https://www.gao.gov/products/GAO-18-685R). The most visible manifestation of this crisis is the rising number of opioid overdose deaths and the dramatic spike in fatalities linked to fentanyl and related synthetic opioids. Thus, based on 2017 estimates (https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates), synthetic opioids (“synthetics”) were linked to more than half of the estimated 49,000 opioid-related deaths, far surpassing fatalities attributed to either heroin or prescription opioids. There are multiple factors responsible for the dangers posed by synthetics, including very high potencies, rapid onset of action, long half-lives, and ease of synthesis; this latter property translates to a low cost of goods relative to heroin and prescription opioids [reviewed by Skolnick (2018)]. Furthermore, the piperidine-based structure of fentanyl is highly mutable. More than 1400 fentanyl analogs have been described in the patent and scientific literature, and a dozen or more are available on the illicit market (Misailidi et al., 2018), adding another layer of complexity for both detection and interdiction by law enforcement.
Naloxone is currently the only Food and Drug Administration (FDA)–approved treatment for suspected or confirmed opioid overdose. The efficacy of naloxone at reversing the pharmacological actions of opioids, including synthetics such as fentanyl, has been well established in both the emergency department and operating room [Glass et al., 1994; Kaplan et al., 1999; reviewed by Boyer (2012)]. There are two FDA-approved naloxone products (an autoinjector and a nasal spray) that are primarily used by first responders (e.g., police, emergency medical service technicians, bystanders) to treat overdose victims (Skolnick, 2018). However, both anecdotal reports (https://www.bloomberg.com/news/articles/2017-08-16/heroin-era-antidotes-can-t-handle-overdoses-in-age-of-synthetics; https://www.washingtonpost.com/amphtml/news/post-nation/wp/2018/04/10/study-despite-decline-in-prescriptions-opioid-deaths-skyrocketing-due-to-heroin-and-synthetic-drugs/) and clinical case studies (Sutter et al., 2017; Uddayasankar, et al., 2018) indicate overdose with synthetics such as fentanyl and carfentanil often requires more naloxone than the standard unit doses [2 mg i.m./4 mg intranasal (IN)] generally available to first responders. Some authors (Li et al., 2018) have recommended parenteral naloxone doses of up to 12–15 mg to successfully reverse a synthetic overdose. While each overdose situation is unique (Skolnick, 2018), the current National Institute on Drug Abuse position states that “Overdoses of fentanyl should be treated immediately with naloxone and may require higher doses to successfully reverse the overdose” (https://www.drugabuse.gov/publications/drugfacts/fentanyl). Moreover, the short half-life of naloxone (t1/2 1.3–2.4 hours) (Ryan and Dunne, 2018) can complicate the management of overdose with long-lived synthetics, including fentanyl (Ahonen et al., 2000; Kharasch, 2015).
In response to the increasing number of overdose deaths linked to synthetics, National Institutes of Health leadership recently called for the development of “…stronger, longer-acting formulations of antagonists” (Volkow and Collins, 2017). At face value, the opiate antagonist nalmefene fulfills these criteria. Thus, multiple studies (Emmerson et al., 1994; Toll et al., 1998; Cassel et al., 2005) have demonstrated that the affinity of nalmefene is ∼5× higher than naloxone at both native and recombinant μ opioid receptors. The half-life (t1/2) of parenterally administered nalmefene is ∼8.2–8.9 hours (Dixon et al., 1986), comparable to the half-lives of synthetics such as fentanyl (7 to 8 hours) and sufentanil (6–9 hours) (Ahonen et al., 2000; Kharasch, 2015). In addition, the efficacy of nalmefene in treating opioid overdose has been established. Thus, parenteral nalmefene was FDA approved (1995) to treat opioid overdose was but withdrawn from the market in 2008 due to low sales, with no significant safety issues (https://www.federalregister.gov/documents/2017/11/03/2017-23952/determination-that-revex-nalmefene-hydrochloride-injection-01-milligram-basemilliliter-and-10). Here, we describe a pilot study in healthy volunteers demonstrating the feasibility of developing an intranasal nalmefene formulation to treat opioid overdose.
Materials and Methods
Study Details.
The study was approved by the MidLands Independent Review Board (Overland Park, KS); all subjects gave written informed consent before participation. The study was conducted at Vince & Associates Clinical Research (Overland Park, KS) and carried out in accordance with the International Conference on Harmonization for Good Clinical Practices guidelines. This trial was registered as NCT03129347 (www.clinicaltrials.gov).
Participant Characteristics.
Male and female volunteers aged 18–55 years with a body mass index of 18–32 kg/m2 were eligible for participation. Subjects were currently not taking either prescription or over-the-counter medications; nonsmokers and subjects who smoked 20 or fewer cigarettes per day were enrolled. Screening procedures conducted within 21 days of study initiation included the following: medical history, physical examination, evidence of nasal irritation, 12-lead electrocardiogram, complete blood count, clinical chemistry, coagulation markers, hepatitis and human immunodeficiency screening, urinalysis, and urine drug screen. Female subjects were tested for pregnancy at screening and admission to the clinic. Subjects were excluded if they had either abnormal nasal anatomy or symptoms (e.g., runny nose, nasal polyps); an upper respiratory tract infection; used opioid analgesics for pain relief within the previous 14 days; or, in the judgment of the investigator, had significant acute or chronic medical conditions. Subjects were required to abstain from alcohol from admission to the end of the last blood draw of the study, from nicotine and from caffeine-containing products and food for at least 1 hour prior to and 2 hours after dose administration, and from caffeine-containing products and food from midnight the day prior to and 4 hours after nalmefene dosing. On days of dosing, a subject’s vital signs were required to be within the normal range before receiving nalmefene (systolic blood pressure >90 and ≤140 mm Hg, diastolic blood pressure >55 and ≤90 mm Hg, resting heart rate >40 and ≤100 beats per minute, and respiratory rate >8 and ≤20 respirations per minute).
Study Design.
The study was an inpatient, double-blind (for IN administration), randomized, four-period, four-treatment, six-sequence crossover. Subjects were randomly assigned to one of six sequences to ensure at least two subjects in each sequence. On the day after clinic admission, participants were administered the study drug in randomized order with a 4-day washout period between doses. Subjects remained at the clinic for 17 days until all four treatments were administered. They were contacted 3–5 days after discharge by a follow-up telephone call. Subjects fasted overnight before each dosing day and received one of the following four treatments:
Treatment A: 3 mg IN (one 0.1-ml spray of a 30-mg/ml nalmefene solution in one nostril).
Treatment B: 3 mg plus 0.25% dodecyl maltoside (DDM; Intravail) IN (one 0.1-ml spray of a 30-mg/ml nalmefene solution containing 0.25% DDM in one nostril).
Treatment C: 1.5 mg IN (one 0.1-ml spray of a 15-mg/ml nalmefene solution in one nostril).
Treatment D: 1.5 mg i.m. (1.5 ml of a 1.0-mg/ml nalmefene solution).
The IN treatments were randomized while the intramuscular dose was the last treatment of all subjects. The high dose (3 mg) of nalmefene was selected based on the relative bioavailability (∼50%) of the structurally related molecule, naloxone (Krieter et al., 2016), and the FDA guidance on parenteral dosing of nalmefene that produces a maximum reversal of a suspected opioid overdose (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf). In phase I studies, intravenous doses of up to 24 mg have been well tolerated in normal volunteers (Dixon et al., 1986).
Study Details.
IN devices were coded so neither the staff nor the subjects knew the treatment administered. IN nalmefene was administered in the supine position, and subjects remained in this position for approximately 1 hour after dosing. Subjects were instructed not to breathe when the drug was administered to simulate an opioid overdose with a patient in respiratory arrest. Nasal passages were examined by medical personnel for irritation using a 6-point scale at predose and at 5 minutes and 0.5, 1, and 4 hours postdose. Nasal irritation was scored as follows: 0 (normal appearing mucosa, no bleeding); 1 (inflamed mucosa, no bleeding); 2 (minor bleeding that stops within 1 minute); 3 (minor bleeding taking 1–5 minutes to stop); 4 (substantial bleeding for 4–60 minutes, does not require medical intervention); and 5 (ulcerated lesions, bleeding that requires medical intervention). Sense of smell was evaluated using “Sniffin’ Sticks” (US Neurologic LLC, Poulsbo, WA) at screening and admission, predose and 4 hours postdose during periods 1–3, and prior to discharge; correct identification of 10 or more odors out of 12 constituted a normal smell test. Subjects were required to identify 10 of 12 odors correctly to be admitted to the study. A subject identifying fewer than 10 odors during the course of study was reported as an adverse event (AE) of a reduced sense of smell. Twelve-lead ECGs were collected predose and at 1 and 8 hours postdose. Venous blood samples (4 ml) were collected for the analyses of plasma nalmefene concentrations predose and at 2.5, 5, 10, 15, 20, 30, 45, and 60 minutes and 2, 3, 4, 6, 8, 12, 16, 24, 30, 36, 48, 60, and 72 hours postdose using Vacutainer tubes containing sodium heparin. Plasma was stored at <−60°C until analyzed.
Study Drugs.
Nalmefene hydrochloride (cGMP grade) was purchased from Rusan Pharma Ltd. (Mumbai, India). The IN and intramuscular solutions were formulated by the Vince & Associates Clinical Research (VACR) pharmacy staff. Nalmefene was dissolved in 0.1 M citrate buffer, pH 4.0, for all IN formulations. Nalmefene was dissolved in saline for intramuscular injection, the pH was adjusted to pH 3.9 using dilute HCl, and the solution was checked for sterility and pyrogenicity prior to administration. This intramuscular formulation is identical to that listed for Revex (nalmefene hydrochloride injection) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf). An Aptar multidose device (Aptar, Louveciennes, France) used for IN administration consisted of a pump and a 10-ml brown glass bottle. Based on solution weights taken before and after dose administration and the analytically determined concentrations of nalmefene, treatments A, B, and C delivered mean doses (S.D.) of 2.97 ± 0.12, 2.96 ± 0.15, and 1.50 ± 0.11 mg, respectively. Plasma concentrations from three subjects (two receiving 1.5 mg of IN nalmefene and one receiving 3 mg of nalmefene + DDM) were not used in the analysis because the amount of solution delivered by these devices was ≤0.057 ml.
Bioanalytical Methods.
Plasma nalmefene concentrations were determined using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay. Plasma samples (0.050 ml) were added to individual wells of a 96-well plate along with 0.050 ml of acetonitrile containing the internal standard (0.5 ng of nalmefene-d3) followed by 0.5 ml of acetonitrile. After vortex mixing, the plate was centrifuged for 5 minutes at 4°C, then 0.50 ml of supernatant was transferred into a new 96-well plate and evaporated to dryness. It was reconstituted with 0.20 ml of methanol:0.1% formic acid in water (15:85) and submitted to LC-MS/MS analysis. Nalmefene was analyzed using an API-5000 LC-MS/MS system (AB Sciex, Framingham, MA) with an atmospheric pressure chemical ionization source operated in the positive ion mode. The mobile phase consisted of a gradient increasing from 0.2% formic acid in water:acetonitrile (9:1) to acetonitrile:methanol (1:1) at a rate of 0.5 ml/min through an Ascentis Express C18 2.7-µm, 50 × 2.1–mm column (Supelco). Nalmefene was eluted at approximately 0.90 minutes. Ions monitored were m/z (mass divided by charge) 340.1 and 268.1 for nalmefene and 343.2 and 268.1 for the internal standard. The calibration curves (peak area ratios) were linear (r2 > 0.994) over the concentration range of 0.200–20.0 ng/ml; the lower limit of quantitation was 0.200 ng/ml. The interday precision of the calibration curves and quality control samples ranged from 2.38% to 5.61%, and the accuracy ranged between −1.20% and 1.11% during the analysis of the samples.
Data Analyses.
The safety population included all subjects who received at least one dose of nalmefene; the pharmacokinetic population included all subjects who received at least one dose of nalmefene with sufficient data to calculate meaningful pharmacokinetic parameters. Pharmacokinetic parameters were calculated using standard noncompartmental methods and a validated installation of WinNonlin Phoenix, version 6.3 (Certara, Princeton, NJ). Descriptive statistics were calculated with R Software version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Values of peak plasma concentrations (Cmax) and the time to reach Cmax (Tmax) were the observed values obtained directly from the concentration-time data. The terminal elimination half-life (t1/2) was estimated by linear regression analysis. The area under the concentration-time curve from time zero to the last quantifiable concentration (AUC0–t) was determined by the linear up/log down trapezoidal method. Within an ANOVA framework, comparisons of ln-transformed dose-normalized pharmacokinetic parameters were performed using a mixed-effects model where sequence, period, and treatment were the independent factors. The 90% confidence interval (CI) for the ratio of the geometric least-squares means of Cmax and AUC0–t was constructed for comparison of the three IN treatments to the intramuscular formulation. The 90% CIs were obtained by exponentiation of the 90% CIs for the differences between the least-squares means based upon an ln scale. Pharmacokinetic comparisons were performed using a mixed-effects model where sequence, period, and treatment were independent factors. All analyses of demographic and safety data were performed using SAS statistical software, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Participant Characteristics.
Ten male and four female participants (Table 1) received at least one dose of nalmefene; 10 subjects completed all four treatments and follow-up procedures. One subject withdrew consent after all three IN doses but before the fourth (intramuscular) dose was administered. Another subject withdrew consent for personal reasons 6 hours after being administered the last dose (intramuscular) of nalmefene. Two subjects left the study 24 hours after the last dose: one withdrew for personal reasons and the other was removed for disruptive behavior.
TABLE 1.
Subject demographics
| All | Female | Male | |
|---|---|---|---|
| N | 14 | 4 | 10 |
| Mean age, yr (range) | 32.9 (18–55) | 30.8 (26–36) | 33.8 (18–55) |
| Race | |||
| White | 6 | 3 | 3 |
| Black/African American | 8 | 1 | 7 |
| Ethnicity | |||
| Hispanic or Latino | 2 | 0 | 2 |
| Not Hispanic or Latino | 12 | 4 | 8 |
| Mean weight, kg (range) | 79.9 (64.1–101.3) | 67.4 (64.1–69.9) | 84.9 (70.0–101.3) |
| Mean BMI,a kg/m2 (range) | 26.4 (20.4–32.2) | 25.4 (22.4–28.5) | 26.8 (20.4–32.2) |
BMI, body mass index.
Pharmacokinetics.
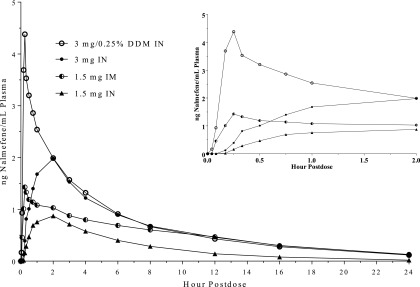
Following IN administration of 3 mg of nalmefene, plasma concentrations were quantifiable for most subjects starting at 15 minutes postdose. When 0.25% DDM was added to the formulation, plasma concentrations were quantifiable in the majority of the samples by 5 minutes postdose (mean 0.93 ng/ml) (Fig. 1, inset). At 15 minutes, nalmefene concentrations following 3 mg of IN with 0.25% DDM were approximately 12-fold higher than in its absence (4.57 vs. 0.392 ng/ml) (Fig. 1, inset). Addition of DDM also reduced the median Tmax from 2 hours to 15 minutes and increased Cmax more than 2-fold (4.45 vs. 1.99 ng/ml) (Table 2). The t1/2 estimates of nalmefene were between 6.6 and 7.8 hours following IN and 8 hours following intramuscular administration; addition of DDM did not appear to alter the t1/2 of IN nalmefene (Table 2). Six hours after IN administration of the 3-mg dose, plasma concentrations of nalmefene (in either the presence or absence of DDM) were ∼0.9 ng/ml. By comparison, 6 hours after a 4-mg dose of IN naloxone, plasma concentrations were ∼0.15 ng/ml (Krieter et al., 2016). IN nalmefene exhibited dose proportionality when the dose increased from 1.5 to 3 mg as evidenced by a doubling of both Cmax and AUC0–t (Table 2). The relative bioavailability of nalmefene after IN administration, when corrected for the dose, was 64%–66% based on Cmax when compared with the intramuscular administration and 55%–63% when AUC0–t was used for this calculation (Table 3). There were modest differences between males and females in some of the pharmacokinetic parameters of nalmefene (Table 4). However, the small sample size of this pilot study precluded any definitive conclusions regarding sex-related differences in the pharmacokinetic properties of nalmefene following both IN and intramuscular dosing.
Fig. 1.
Mean plasma concentrations of nalmefene following single intranasal and intramuscular administration. Doses were as follows: 3 mg IN (closed circles), 3 mg plus 0.25% (w/v) DDM IN (open circles), 1.5 mg IN (triangles), and 1.5 mg i.m. (half-filled circles). Inset: mean plasma concentrations of nalmefene between 2.5 minutes and 2 hours postdose.
TABLE 2.
Pharmacokinetic parameters of nalmefene following intranasal and intramuscular administration
| Parameter (U)a | 3 mg IN (N = 14) | 3 mg IN/0.25% DDM (N = 13) | 1.5 mg IN (N = 11) | 1.5 mg i.m. (N = 13) | ||||
|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | 1.99 | (51.3) | 4.45 | (65.7) | 0.961 | (43.8) | 1.53 | (43.5) |
| Cmax/D (ng/ml/mg) | 0.662 | (51.3) | 1.48 | (65.7) | 0.641 | (43.8) | 1.02 | (43.5) |
| Tmax (h) | 2.00 | (0.33–3.00) | 0.25 | (0.17–1.00) | 2.00 | (1.00–2.07) | 0.33 | (0.25–8.00) |
| AUC0–t (ng·h/ml) | 12.7 | (68.1) | 15.2 | (71.8) | 5.58b | (57.9) | 10.6 | (45.7) |
| AUC0–2.5 min | 0.00 | (0.00) | 0.004 | (0.006) | 0.00 | (0.00) | 0.00 | (0.00) |
| AUC0–5 min | 0.00 | (0.00) | 0.029 | (0.037) | 0.00 | (0.00) | 0.008 | (0.013) |
| AUC0–10 min | 0.005 | (0.010) | 0.257 | (0.239) | 0.00 | (0.00) | 0.072 | (0.053) |
| AUC0–15 min | 0.026 | (0.036) | 0.596 | (0.506) | 0.006 | (0.006) | 0.168 | (0.106) |
| AUC0–20 min | 0.226 | (0.202) | 0.924 | (0.708) | 0.023 | (0.019) | 0.278 | (0.170) |
| AUC0–t/D (ng·h/ml/mg) | 4.24 | (68.1) | 5.06 | (71.8) | 3.72 | (57.9) | 7.07 | (45.7) |
| t1/2 (h) | 7.87c | (40.8) | 7.11 | (45.5) | 6.59d | (53.3) | 8.01b | (39.2) |
AUC0–t/D, AUC0–t divided by the dose; AUC0–x, AUC from time zero to x minutes; Cmax/D, Cmax divided by the dose; %CV, percentage coefficient of variation.
Geometric mean values (%CV) for all except Tmax, which is median (minimum, maximum).
N = 12.
N = 13.
N = 10.
TABLE 3.
Statistical summary of treatment comparisons
| IN Administration (Test) |
Comparison (i.m. as Reference) |
Ratio (Test/Reference) of Adjusted Meansa |
90% CI for Ratio |
|---|---|---|---|
| Cmax/dose (ng/ml/mg) | |||
| 3 mg IN (trt A) | A vs. D | 65.8 | 49.6–87.2 |
| 3 mg/0.25% DDM IN (trt B) | B vs. D | 135 | 100–180 |
| 1.5 mg IN (trt C) | C vs. D | 63.4 | 46.6–86.4 |
| AUC0–inf/dose (ng·h/ml/mg) | |||
| 3 mg IN (trt A) | A vs. D | 63.1 | 53.1–74.9 |
| 3 mg/0.25% DDM IN (trt B) | B vs. D | 69.5 | 58.1–83.2 |
| 1.5 mg IN (trt C) | C vs. D | 55.4 | 45.8–67.1 |
AUC0–inf/dose, AUC per milligram of naloxone administered; Cmax/dose, Cmax per milligram of naloxone administered; trt, treatment.
Geometric least-squares mean ratio between treatments, expressed as a percentage of reference (i.m., treatment D).
TABLE 4.
Pharmacokinetics of nalmefene in males and females following intranasal and intramuscular administration
| Parameter (U)a | 3 mg IN (A) |
3 mg/0.25% DDM IN (B) |
1.5 mg IN (C) |
1.5 mg i.m. (D) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (N = 4) | Male (N = 10) | Female (N = 4) | Male (N = 9) | Female (N = 3) | Male (N = 8) | Female (N = 4) | Male (N = 9) | |||||||||
| Cmax (ng/ml) | 2.20 | (47.0) | 1.90 | (54.9) | 5.51 | (72.7) | 4.04 | (64.3) | 1.19 | (53.1) | 0.888 | (40.8) | 1.13 | (21.7) | 1.75 | (44.2) |
| Tmax (h) | 2.00 (2.00–2.00) | 1.52 (0.33–3.00) | 0.25 (0.25–0.25) | 0.25 (0.17–1.00) | 2.00 (1.00–2.07) | 2.00 (1.00–2.00) | 0.63 (0.25–8.00) | 0.33 (0.25–2.00) | ||||||||
| AUC0–t (ng·h/ml) | 14.6 | (57.1) | 12.0 | (74.7) | 18.4 | (84.1) | 13.9 | (69.8) | 7.38 | (75.9) | 5.03 | (51.8) | 14.0 | (33.1) | 9.38 | (45.8) |
| t1/2 (h) | 8.14b | (46.9) | 7.79 | (41.7) | 9.22 | (10.4) | 6.34 | (45.1) | 7.54 | (92.2) | 6.23c | (41.3) | 10.3 | (43.2) | 7.07d | (32.0) |
%CV, percentage coefficient of variation.
Geometric mean values (%CV) for all except Tmax, which is median (minimum, maximum).
N = 3.
N = 7.
N = 8.
Safety.
Ten subjects experienced at least one AE classified as at least possibly related to nalmefene; all were mild in severity. The AEs reported by more than one participant were nausea (5), vomiting (3), dizziness (3), headache (2), and hyperhidrosis (2). There were no clinically significant laboratory values, and there were no apparent effects of IN nalmefene on the sense of smell (data not shown).
Discussion
Synthetic opioids present multiple challenges for first responders attempting to rescue overdose victims. High-potency synthetics [fentanyl and other synthetics identified in overdose victims can be ≥2 orders of magnitude more potent than morphine (Burns et al., 2018; Misailidi et al., 2018)] may require very high doses of the competitive antagonist naloxone (Sutter et al., 2017; Li et al., 2018; Uddayasankar et al., 2018), the only drug currently approved to treat opioid overdose, to effect a successful rescue. Not only are these high potencies problematic, but the long half-lives of fentanyl (7 to 8 hours) and analogs such as sufentanil (6–9 hours) (Ahonen et al., 2000; Kharasch, 2015) and carfentanil (∼5.7 hours) (Uddayasankar et al., 2018) further complicate rescue with naloxone. Thus, therapeutically effective plasma concentrations of naloxone (t1/2 = 1.3–2.4 hours; Ryan and Dunne, 2018) may not be sustained in the presence of long-duration synthetics, leading to a recurrence of symptoms (renarcotization) including respiratory depression (Kaplan and Marx, 1993; Burns et al., 2018) that complicate management of overdose and may require redosing with naloxone. Finally, synthetic opioids are orders of magnitude more lipophilic than morphine and related semisynthetic opiates such as oxycodone (Drewes et al., 2013). This high lipophilicity favors rapid equilibration between plasma and cerebrospinal fluid (CSF), resulting in a rapid onset of action. While this property is highly valued in an analgesic, it also results in a rapid onset of respiratory depression, effectively reducing the window for rescue.
Based on its high affinity and long half-life relative to naloxone (Table 5), we hypothesized that nalmefene could be useful as an IN rescue medication especially well suited to treat synthetic opioid overdose. Moreover, because parenteral nalmefene was previously approved to treat opioid overdose (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf), development of an IN product is substantially derisked from both a safety and regulatory standpoint compared with a new chemical entity. The structural similarity to naloxone led us to select nalmefene doses (1.5 and 3 mg) based on the hypothesis that its IN bioavailability would also be similar to naloxone (Krieter et al., 2016). To limit the number of arms in this pilot study, we elected to determine if IN nalmefene exhibited dose proportionality and to examine the effects of a single concentration of DDM on one dose of IN nalmefene. The concentration of DDM (0.25%) selected was based on previous studies (Maggio and Pillion, 2013) demonstrating an enhanced IN absorption of other small molecules. Furthermore, this concentration of DDM is used in an FDA-approved IN sumatriptan product (https://www.neurelis.com/neurelis-news/neurelis-announces-first-product-approved-using-intravail-press-release), which de-risks its use in a nalmefene product from a regulatory perspective. The approval of Narcan (naloxone, ADAPT Pharma, Inc.) Nasal Spray for treating opioid overdose was based on achieving both an onset of action and maximum plasma concentration comparable to a previously approved parenteral dose (Krieter et al., 2016). Because parenteral nalmefene was previously approved for the management of known or suspected opioid overdose (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf), by analogy, an intramuscular injection was selected as an appropriate comparator for an IN nalmefene product candidate. The choice of a comparator dose (1.5 mg, i.m.) was based on the FDA label for parenteral nalmefene; for the management of known or suspected opioid overdose, the label states: “A total dose greater than 1.5 mg did not increase the therapeutic response” (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf).
TABLE 5.
Nalmefene and naloxone: a comparison of affinities at μ opioid receptors and pharmacokinetic properties following intranasal administration
| Parameter | Nalmefene | Naloxone |
|---|---|---|
| Ki (nM) | 1.0a | 5.4a |
| t1/2 (h) | 7.11b | 2.08c |
| Tmax (h) | 0.25b | 0.5c |
| Cmax (ng/ml) | 4.45b | 4.83c |
Ki values were estimated using [3H]alvimopan binding to cloned human μ opioid receptors (Cassel et al., 2005). The ∼5-fold higher affinity of nalmefene compared with naloxone is consistent with both Ki values obtained (0.13 and 0.62 nM, respectively) using [3H]DAMGO as a radioligand in monkey brain membranes (Emmerson et al., 1994) and pA2 values of 9.38 and 8.51, respectively, in functional assays using guinea pig ileum and mouse vas deferens (Toll et al., 1998).
Data from Table 2.
The rapid delivery of high plasma concentrations is a cardinal feature of an effective IN rescue product (Krieter et al., 2016; https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM554404.pdf). In the absence of DDM, the onset of IN nalmefene is too slow to be useful as a rescue medication (Fig. 1; Table 2). However, DDM, a member of a class of alkylsaccharide transmucosal absorption enhancers [reviewed by Maggio and Pillion (2013)], reduced the median Tmax of IN nalmefene to a value (0.25 hour) comparable to intramuscular administration (0.33 hour) (Table 2). In an overdose rescue, the first few minutes are critical, perhaps more so when rapid-onset synthetics are involved. It is notable that the Tmax of IN nalmefene indicates that its onset is more rapid than the FDA-approved 4-mg dose of IN naloxone, with a reported Tmax of 0.5 hour (Table 5). Moreover, a comparison of drug exposure at early time points (https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM554404.pdf) demonstrated that in the presence of DDM, the AUC0–t values (t = 5–20 minutes postdose) of IN nalmefene were higher than the reference intramuscular dose (Table 2); exposures were statistically significantly higher following IN dosing at 10, 15, and 20 minutes (P ≤ 0.026, ANOVA). Whereas ∼3-fold higher than following intramuscular administration (1.5 mg), the Cmax value produced by IN nalmefene (3 mg) in the presence of 0.25% DDM is in the range of plasma concentrations observed 5 minutes after a 1-mg i.v. dose in young and elderly males (3.7 and 5.8 ng/ml, respectively) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf). Despite the dramatic effects of DDM on both the Cmax and Tmax of IN nalmefene (Fig. 1; Table 2), overall exposure as measured by AUC0–t was increased by only ∼20%, indicating the principal effect of DDM was to increase the rate of absorption. This latter observation is consistent with the hypothesis that DDM and related alkylsaccharides act as absorption enhancers by transiently opening tight junctions between cells in the nasal epithelium (Maggio and Pillion, 2013).
While multiple studies have reported that nalmefene has a higher affinity than naloxone at both native and recombinant μ opioid receptors (Emmerson et al., 1994; Toll et al., 1998; Cassel et al., 2005), there is no compelling evidence this translates to a clinically significant advantage in a hospital setting (Glass et al., 1994; Kaplan et al., 1999). However, in a study attempting to model overdose rescue in a nonhospital setting, Yong et al. (2014) compared the effects of bolus intramuscular injections of either nalmefene or naloxone to reverse carfentanil-induced loss of righting reflex and respiratory depression. Rats were administered 10 μg/kg of carfentanil (i.v.) and, 5 minutes later, were administered either nalmefene (9.4–150 μg/kg) or naloxone (150 μg/kg). Nalmefene, at doses as low as 9.4 μg/kg significantly reduced the duration of the loss of righting reflex and, at doses between 9.4 and 18.8 μg/kg, reduced the duration of loss of righting reflex to the same extent as 150 μg/kg of naloxone. At a dose of carfentanil (20 μg/kg, i.v.) that depressed respiration, nalmefene (37.5–150 μg/kg) produced a near complete to complete reversal within 10 minutes, restoring both partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) to precarfentanil values. In contrast, naloxone (150 μg/kg) produced a partial, albeit significant, reversal of respiratory depression. While the use of a single dose of naloxone limits interpretation of these results, these data are consistent with a higher potency of nalmefene to reverse the pharmacological effects of carfentanil closely linked to overdose.
Along with therapeutic advantages, a high-potency, long-duration opioid antagonist such as nalmefene has the potential to produce a protracted withdrawal in opioid-dependent individuals. One clinical report compared nalmefene and naloxone in patients admitted to emergency departments with suspected narcotic overdose. In this double-blind study (Kaplan et al., 1999), 156 patients in nine centers were randomized to receive intravenous doses of nalmefene (1 or 2 mg) or naloxone (2 mg) every 5 minutes as needed for up to four doses. Most patients received a single dose of study drug, and in those patients with a confirmed opioid overdose, both drugs produced rapid and robust reversals of respiratory depression. Adverse events in opioid-positive patients were present in all three treatment arms: 12.5% (3/24) in the naloxone group, 10% (3/30) in the 1-mg nalmefene group, and 26.1% (6/23) in the 2-mg nalmefene group. While the incidence of adverse events was highest in the 2-mg nalmefene group, the overall difference among treatment arms was not significant (P > 0.27), and no significant overall time-treatment interactions emerged (Kaplan et al., 1999). It is difficult to extrapolate findings from an emergency department setting to that envisioned for the field use of an IN nalmefene product by first responders. Nonetheless, the overall incidence of adverse events reported in the Kaplan et al. (1999) study is lower than recent case report data provided by both first responders and community-based organizations using a 4-mg naloxone nasal spray (Avetian et al., 2018). Withdrawal symptoms (including nausea, vomiting, irritability, sweating, muscle cramps, piloerection, and diarrhea) precipitated by an overdose rescue are unpleasant and distressing but not life threatening (Boyer, 2012). Given the alarming rise in synthetic opioid-related fatalities over the past 5 years (https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates), the potential for iatrogenic withdrawal symptoms is medically justified weighed against the risk of a fatal overdose.
Abbreviations
- AE
adverse event
- AUC0–t
area under the concentration time curve from time zero to the last measurable concentration
- CI
confidence interval
- DDM
dodecyl maltoside
- FDA
Food and Drug Administration
- IN
intranasal
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- Tmax
time to reach Cmax
- t1/2
elimination phase half-life
Authorship Contributions
Participated in research design: Crystal, Gyaw, Krieter, Skolnick.
Conducted experiments: Gyaw, Krieter.
Performed data analysis: Krieter, Skolnick.
Wrote or contributed to the writing of the manuscript: Crystal, Gyaw, Krieter, Skolnick.
Footnotes
The work was conducted under a Clinical Trial Agreement supported in part by Opiant Pharmaceuticals and National Institute on Drug Abuse contracts N01DA-12-8905, N01DA-13-8920, and N01DA-14-8914.
P.S. and R.C. are employees of Opiant Pharmaceuticals, Inc. P.K. and S.G. are employees of the National Institutes of Health.
References
- Ahonen J, Olkkola KT, Hynynen M, Seppälä T, Ikävalko H, Remmerie B, Salmenperä M. (2000) Comparison of alfentanil, fentanyl and sufentanil for total intravenous anaesthesia with propofol in patients undergoing coronary artery bypass surgery. Br J Anaesth 85:533–540. [DOI] [PubMed] [Google Scholar]
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. (2018) Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin 34:573–576. [DOI] [PubMed] [Google Scholar]
- Boyer EW. (2012) Management of opioid analgesic overdose. N Engl J Med 367:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SM, Cunningham CW, Mercer SL. (2018) DARK classics in chemical neuroscience: fentanyl. ACS Chem Neurosci 9:2428–2437. [DOI] [PubMed] [Google Scholar]
- Cassel JA, Daubert JD, DeHaven RN. (2005) [(3)H]Alvimopan binding to the micro opioid receptor: comparative binding kinetics of opioid antagonists. Eur J Pharmacol 520:29–36. [DOI] [PubMed] [Google Scholar]
- Dixon R, Howes J, Gentile J, Hsu H-B, Hsiao J, Garg D, Weidler D, Meyer M, Tuttle R. (1986) Nalmefene: intravenous safety and kinetics of a new opioid antagonist. Clin Pharmacol Ther 39:49–53. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Jensen RD, Nielsen LM, Droney J, Christrup LL, Arendt-Nielsen L, Riley J, Dahan A. (2013) Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol 75:60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Liu M-R, Woods JH, Medzihradsky F. (1994) Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271:1630–1637. [PubMed] [Google Scholar]
- Glass PS, Jhaveri RM, Smith LR. (1994) Comparison of potency and duration of action of nalmefene and naloxone. Anesth Analg 78:536–541. [DOI] [PubMed] [Google Scholar]
- Kaplan JL, Marx JA. (1993) Effectiveness and safety of intravenous nalmefene for emergency department patients with suspected narcotic overdose: a pilot study. Ann Emerg Med 22:187–190. [DOI] [PubMed] [Google Scholar]
- Kaplan JL, Marx JA, Calabro JJ, Gin-Shaw SL, Spiller JD, Spivey WL, Gaddis GM, Zhao N, Harchelroad FP., Jr (1999) Double-blind, randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Ann Emerg Med 34:42–50. [DOI] [PubMed] [Google Scholar]
- Kharasch ED. (2015) Opioid half-lives and hemlines: the long and short of fashion. Anesthesiology 122:969–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieter P, Chiang N, Gyaw S, Skolnick P, Crystal R, Keegan F, Aker J, Beck M, Harris J. (2016) Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol 56:1243–1253. [DOI] [PubMed] [Google Scholar]
- Li K, Armenian P, Mason J, Grock A. (2018) Narcan or nar-can’t: tips and tricks to safely reversing opioid toxicity. Ann Emerg Med 72:9–11. [DOI] [PubMed] [Google Scholar]
- Maggio ET, Pillion DJ. (2013) High efficiency intranasal drug delivery using Intravail® alkylsaccharide absorption enhancers. Drug Deliv Transl Res 3:16–25. [DOI] [PubMed] [Google Scholar]
- Misailidi N, Papoutsis I, Nikolaou P, Dona A, Spiliopoulou C, Athanaselis S. (2018) Fentanyls continue to replace heroin in the drug arena: the cases of ocfentanil and carfentanil. Forensic Toxicol 36:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SA, Dunne RB. (2018) Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systematic review. Pain Manag 8:231–245. [DOI] [PubMed] [Google Scholar]
- Skolnick P. (2018) On the front lines of the opioid epidemic: rescue by naloxone. Eur J Pharmacol 835:147–153. [DOI] [PubMed] [Google Scholar]
- Sutter ME, Gerona RR, Davis MT, Roche BM, Colby DK, Chenoweth JA, Adams AJ, Owen KP, Ford JB, Black HB, et al. (2017) Fatal fentanyl: one pill can kill. Acad Emerg Med 24:106–113. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178:440–466. [PubMed] [Google Scholar]
- Uddayasankar U, Lee C, Oleschuk C, Eschun G, Ariano R. (2018) The pharmacokinetics and pharmacodynamics of carfentanil after recreational exposure: a case report. Pharmacotherapy 38:e41–e45. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS. (2017) The role of science in addressing the opioid crisis. N Engl J Med 377:391–394. [DOI] [PubMed] [Google Scholar]
- Yong Z, Gao X, Ma W, Dong H, Gong Z, Su R. (2014) Nalmefene reverses carfentanil-induced loss of righting reflex and respiratory depression in rats. Eur J Pharmacol 738:153–157. [DOI] [PubMed] [Google Scholar]